Introduction
Neuroblastoma (NB) is one of the most frequently
occurring pediatric solid tumors and is derived from ganglionic
lineage precursors of the sympathetic nervous system. Patients with
high-risk NB have very poor outcomes with 5-year disease-free
survival rates between 25 and 35% (1,2).
Distant metastasis, particularly bone metastasis, is one of the
leading causes of mortality in patients with NB.
Previous studies have shown that most NB cell lines
express rearranged during transfection (RET), and overexpression or
activation of RET alters cell adhesion and enhances metastatic
behavior and non-adherent proliferation of NB cells, which may
contribute to NB metastasis (3,4). The
RET proto-oncogene, which encodes a receptor tyrosine kinase known
to be expressed on the surface of neuronal and neural crest-derived
cells, is critical for development of the enteric nervous system
(ENS) and kidneys. However, gain-of-function mutation of RET is
associated with the development of various types of human cancer,
including medullary thyroid carcinoma, multiple endocrine
neoplasias type 2A and 2B, pheochromocytoma and NB (5). During kidney development, Lu et
al found that the ETS transcription factors Etv4 and Etv5 are
positively regulated by RET/GDNF signaling in the ureteric bud
tips, and they identified several genes whose expression in the
ureteric bud depends on Etv4 and Etv5, including C-X-C chemokine
receptor type 4 (CXCR4), Myb, Met and Mmp14 (6). CXCR4 is an α-chemokine receptor
specific for stromal-derived-factor-1 (SDF-1, also called CXCL12),
and both are involved in metastasis in various malignancies, such
as NB, prostate and breast cancer (7–9).
Matrix metalloproteinases (MMPs), including MMP14, are capable of
degrading several types of extracellular matrix proteins. MMPs are
also thought to play a major role in cell invasion in tumor
metastasis (10,11).
Vandetanib (ZD6474) is an orally active small
molecule tyrosine kinase inhibitor with activity against the RET
tyrosine kinase, VEGF receptor 2 (VEGFR2), VEGF receptor 3 (VEGFR3)
and epidermal growth factor receptor (EGFR) (12,13).
Current studies have shown that vandetanib is effective against NB
tumor cells and reduces NB xenograft tumor growth rates, whereas
bevacizumab and erlotinib, inhibitors of the VEGFR and EGFR
pathways, respectively, do not hinder tumor growth (14,15).
Moreover, the results of the present study showed that SK-N-SH
cells lack EGFR and VEGFR2, and phosphorylated RET was consistently
expressed at high levels in NB cell lines including SK-N-SH and
SH-SY5Y, with undetectable levels of phosphorylated EGFR and
VEGFR2. These results indicate antitumor activity by vandetanib
should be mainly mediated by inhibition of phosphorylated RET.
The aim of the present study was to investigate the
activity and potential mechanisms of vandetanib against the
migration and invasion of NB in vitro. Due to the evidence
of the roles of RET signaling in NB pathogenesis and CXCR4 and
MMP14 being downstream of RET signaling, we hypothesized that
vandetanib-induced inhibition of NB migration and invasion may be
associated with downregulation of the SDF-1/CXCR4 axis and
MMP14.
Materials and methods
Reagents
Vandetanib was purchased from LC Laboratories
(Woburn, MA, USA). A stock solution (50 mM) was produced in
dimethyl sulfoxide (DMSO; Sigma-Aldrich) and stored at −20°C.
AMD3100 (Sigma; cat: 155148-31-5) was soluble in water at 22 mg/ml
and stored at −20°C. Recombinant human GDNF (cat: 450-10) and
recombinant human SDF-1α/CXCL12 (cat: 300-28A) (both from
PeproTech) were diluted in buffer containing 0.1% bovine serum
albumin (BSA) and stored in working aliquots at −20°C.
Cell lines and culture conditions
SK-N-SH cells were maintained in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum
(FBS) (Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin.
SH-SY5Y cells were maintained in DMEM-F12 supplemented with 10%
FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. The two NB
cell lines were maintained at 37°C in a humidified atmosphere with
5% carbon dioxide.
In vitro proliferation, apoptosis and
cell cycle assay
A cell proliferation assay was performed with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(Sigma; cat: M2128). Before determining proliferation, SK-N-SH or
SH-SY5Y cells were seeded into 96-well plates in quintuplicate and
allowed to adhere in complete medium overnight. The medium was
removed and 100 μl of fresh medium containing the control vehicle
or vandetanib (0.625, 1.25, 2.5, 5, 10 and 20 μM) was added. After
48 h, 15 μl of MTT was added to each well. After 4 h of incubation,
the medium was removed, 200 μl of DMSO was added, and the optical
density was measured at 570 nm in a multi-well plate reader (Thermo
Scientific). The background absorbance of the medium in the absence
of cells was subtracted, and the results were expressed as a
percentage of the control, which was defined as 100%.
Apoptosis was measured using the Annexin V-FITC
Apoptosis Detection kit (KeyGen Biotech, China; cat: KG107)
according to the manufacturer’s instructions. Apoptotic cells were
differentiated from viable or necrotic cells by the combined
application of Annexin V-FITC and propidium iodide (PI). Briefly,
cells were incubated in the presence of vehicle (DMSO), vandetanib
(5 μM), vandetanib (10 μM) or vandetanib (20 μM) for 48 h. The
samples were harvested and washed with 4°C PBS. A total of
1×106 cells were resuspended in 500 μl of 1X binding
buffer, incubated with 5 μl of Annexin V-FITC and 5 μl of PI
solution and finally incubated for 15 min at room temperature in
the dark. The fluorescence of the cells was determined immediately
and analyzed with a flow cytometry (FCM) analyzer (BD Biosciences,
USA). This assay was performed in triplicate.
The cell cycle assays were analyzed with an FCM
analyzer. Both floating and adherent cells were harvested after
incubating in the presence of vehicle (DMSO), vandetanib (5 μM),
vandetanib (10 μM) or vandetanib (20 μM) for 48 h, washing with 4°C
PBS and fixing overnight with ice-cold 70% ethanol at 4°C. Cells
were centrifuged to obtain a pellet, resuspended in 500 μl of
hypotonic buffer (0.5% Triton X-100 and 0.5 g/ml RNase A in
ice-cold PBS) and incubated for 30 min at 37°C. Then, 1 ml of PI
solution (50 μg/ml) was added and incubated for 30 min at 4°C to
form a PI-DNA complex. The percentage of the cell population in
each phase of the cell cycle was measured using FACStar flow
cytometer, and the results were analyzed using CellQuest software
(Becton-Dickinson and Company, Franklin Lakes, NJ, USA).
Detection of RET phosphorylation
NB cells were treated with vehicle (DMSO),
vandetanib (1, 5 and 10 μM, respectively) for 48 h, rinsed twice
with ice-cold PBS and lysed in protein lysis buffer (0.5% Nonidet
P-40, 10 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA and 1 mM
Na3VO4) supplemented with protease
inhibitors, calcineurin inhibitors and 1 mM phenylmethylsulfonyl
fluoride (PMSF). A total of 30 μg of protein was separated by 6–12%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred
to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica,
MA, USA). The membranes were blocked for 1 h with 5% BSA (Bovine
Serum Albumin Fraction V; Roche, 10735078001) in TBS-T [10 mM
Tris-HCl pH 7.5, 100 mM NaCl and 0.1% (w/v) Tween-20]. The
membranes were then incubated overnight at 4°C with primary
antibodies against β-actin, p-EGFR (cat: 3777s), p-VEGFR2 (cat:
2478s) (both from Cell Signaling Technology, Beverly, MA, USA) or
p-RET (Santa Cruz Biotechnology, Santa Cruz, CA, USA; cat:
sc-20252-R). The membranes were rinsed three times with TBS-T and
incubated with an HRP-conjugated goat anti-rabbit or goat
anti-mouse IgG (H+L) secondary antibody. The blots were visualized
using an ECL kit (Tiangen Biotech Co., Ltd., Beijing, China).
Transwell cell migration assay
The in vitro migration of vandetanib-treated
NB cells was assessed using a Transwell chemotaxis system. To
analyze the role of the SDF-1/CXCR4 axis in the migration of
vandetanib-treated NB cells, AMD3100, a highly specific chemokine
receptor CXCR4 antagonist, served as a control. Non-coated culture
plate well inserts (Millipore) with 8-μm pore size polycarbonate
membranes (upper chambers) were inserted into the 24-well plates
(lower chambers), and DMEM or DMEM-F12 supplemented with 10% FBS
containing 100 ng/ml hSDF-1a was added to each lower chamber.
Before migration, NB cells were cultured for 72 h in the presence
of vehicle (DMSO), vandetanib (5 μM), vandetanib (10 μM), AMD3100
(10 μM), vandetanib (5 μM) or vandetanib plus AMD3100. Then, the
floating apoptotic cells were rinsed away, the cells were harvested
and 100 μl of cells at a concentration of 3×105 cells/ml
was seeded into the upper chamber of each Transwell and allowed to
migrate for 24 h at 37°C in 5% CO2. After incubation, NB
cells on the upper surface of the insert membrane were gently
removed with cotton tips. Cells on the lower surface of the
membrane were fixed with 100% methanol for 15 min and then stained
with DAPI (DAPI-dilactose; Sigma) for 15 min. Each treatment was
independently repeated three or four times in duplicate. The total
number of cells in four random fields of each membrane was counted
at 10×10 magnification with a fluorescence microscope (Nikon
Instruments Inc., Japan).
Transwell invasion assay
A Transwell invasion assay was performed using a BD
BioCoat Matrigel™ 24-well invasion chamber with 8-μm pores (BD
Biosciences). The Matrigel BioCoat inserts were initially
rehydrated by adding serum-poor medium in both the upper and lower
chambers and incubated in a tissue culture incubator for 2 h. After
removal of the rehydrating medium, DMEM or DMEM-F12 supplemented
with 10% FBS containing 100 ng/ml hSDF-1a was added to each lower
chamber. The invasion assays were designed in the same five
configurations as the Transwell cell migration assays. After being
treated with vehicle (DMSO), vandetanib (5 μM), vandetanib (10 μM),
AMD3100 (10 μM), vandetanib (5 μM) or vandetanib plus AMD3100 for
72 h, the floating apoptotic cells were rinsed away, the cells were
harvested and 100 μl of cells at a concentration of
3×105 cells/ml was seeded into the upper chamber insert
with the Matrigel coated membrane. The plates were then incubated
at 37°C in 5% CO2 for 48 h. After incubation,
non-invading cells and the Matrigel matrix on the upper surface of
the membrane were gently removed with cotton tips. The cells on the
lower surface of the membrane were fixed with 100% methanol for 15
min and then stained with DAPI (DAPI-dilactose) for 15 min. Each
treatment was independently repeated three times in duplicate. The
total number of cells in four random fields of each membrane was
counted at 10×20 magnification with a fluorescence microscope
(Nikon Instruments Inc.).
RT-qPCR analysis
Briefly, total RNA from NB cells was isolated with
TRIzol reagent (Invitrogen; cat: 15596-026) and reverse transcribed
with the PrimeScript™ RT reagent kit (Takara; cat: DRR037A).
Quantitative real-time PCR (qPCR) was performed in triplicate in
10-μl reactions using the CFX96 Real-Time System (Bio-Rad, USA).
The reaction solution contained 20 ng of reverse transcribed total
RNA, 0.4 μM of each paired primer and 5 μl of SYBR Premix Ex Taq™
II (Takara; cat: DRR820A). Amplification was performed using the
following conditions: 95°C for 30 sec followed by 40 cycles of 95°C
for 10 sec and 60°C for 30 sec. The specificity of each amplified
qPCR product was examined by dissociation curve analysis. The
temperature range to detect the melting temperature of the PCR
product was set from 65 to 95°C. The relative expression was
calculated using the comparative Ct (threshold cycle) method with
the arithmetic formula 2−ΔΔCt. A standard curve of
serially diluted PCR products of cDNA was used to correlate changes
in ΔCt with fold changes in the mRNA levels of each gene.
Amplification of the housekeeping gene β-actin was measured for
each sample as an internal control for sample loading and
normalization. The results are representative of at least three
independent experiments. Primers used for PCR analysis were: RET
forward, 5′-TTTG CCTGGCAGATCTCACA-3′ and reverse, 5′-GATGTTTCTG
GCTGCCAAGTC-3′; CXCR4 forward, 5′-TTCTACCCCAAT GACTTGTG-3′ and
reverse, 5′-ATGTAGTAAGGCAGCCA ACA-3′; MMP14 forward,
5′-TGCCTGCGTCCATCAACA-3′ and reverse, 5′-ATCTTGTCGGTAGGCAGC-3′;
β-actin forward, 5′-AAGATGACCCAGATCATGTTTGAGACC-3′ and reverse,
5′-GCCAGGTCCAGACGCAGGAT-3′.
Western blotting
Total cell protein extracts were obtained as
described above from NB cells, which were cultured in the presence
of vehicle (DMSO), vandetanib (5 μM) for 48 h or vandetanib (5 μM)
for 72 h. A total of 30 μg of protein was separated by 6–12%
SDS-PAGE and transferred to PVDF membranes. The membranes were
blocked for 1 h with 5% BSA in TBS-T, then incubated overnight at
4°C with primary antibodies against β-actin, p-RET, RET (Santa Cruz
Biotechnology, Inc.; cat: sc-167), CXCR4 (Abcam; cat: ab2074) or
MMP14 (Santa Cruz Biotechnology, Inc.; cat: sc-30074). The
membranes were rinsed three times with TBS-T and incubated with an
HRP-conjugated goat anti-rabbit or goat anti-mouse IgG (H+L)
secondary antibody. The blots were visualized using an ECL kit
(Tiangen Biotech Co., Ltd.).
Immunofluorescence
For the immunofluorescence staining,
1×105 cells were cultured on glass coverslips in 24-well
plates overnight, and then treated with 5 μM vandetanib or vehicle
(DMSO) for 48 h. All the samples were washed twice with PBS and
fixed with ice-cold methanol for 15 min. The samples were blocked
with 5% BSA for 1 h at room temperature, incubated overnight at 4°C
with primary antibody against p-RET, and then incubated with
TRITC-conjugated secondary antibody in the dark for 1 h at room
temperature. All the subsequent procedures were performed in the
dark. The samples were incubated overnight at 4°C with primary
antibody against CXCR4, followed by an FITC-conjugated secondary
antibody for 1 h at room temperature and counterstaining with DAPI
for 15 min at room temperature. The coverslips were sealed to
microscope slides with DABCO, and the cells were imaged with a
fluorescence microscope (Nikon Instruments Inc.).
Statistical analysis
Significant differences between groups were
determined for apoptosis, cell cycle, migration, invasion and mRNA
expression studies using an ANOVA test with multiple comparisons
analyzed by SPSS (version 17.0) software. The results are presented
as the means ± SD. The minimal level of significance was set to
p<0.05.
Results
Vandetanib inhibits the proliferation of
human NB cell lines
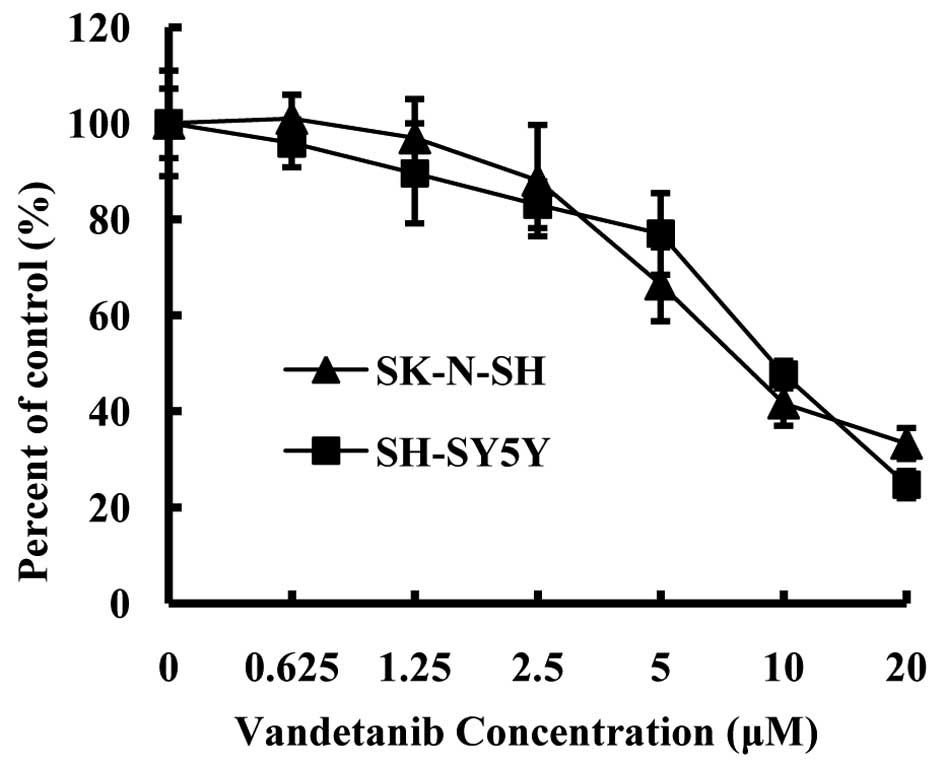
To determine the IC50 value of vandetanib
on NB cells in vitro, we treated SK-N-SH and SH-SY5Y cells
with the control vehicle or vandetanib (0.625, 1.25, 2.5, 5, 10 and
20 μM) for 48 h. We then evaluated the antiproliferative effect
with an MTT assay. In the present study, the IC50 value
of vandetanib on SK-N-SH and SH-SY5Y cells was ~10 μM (Fig. 1), which is consistent with previous
studies (14,15).
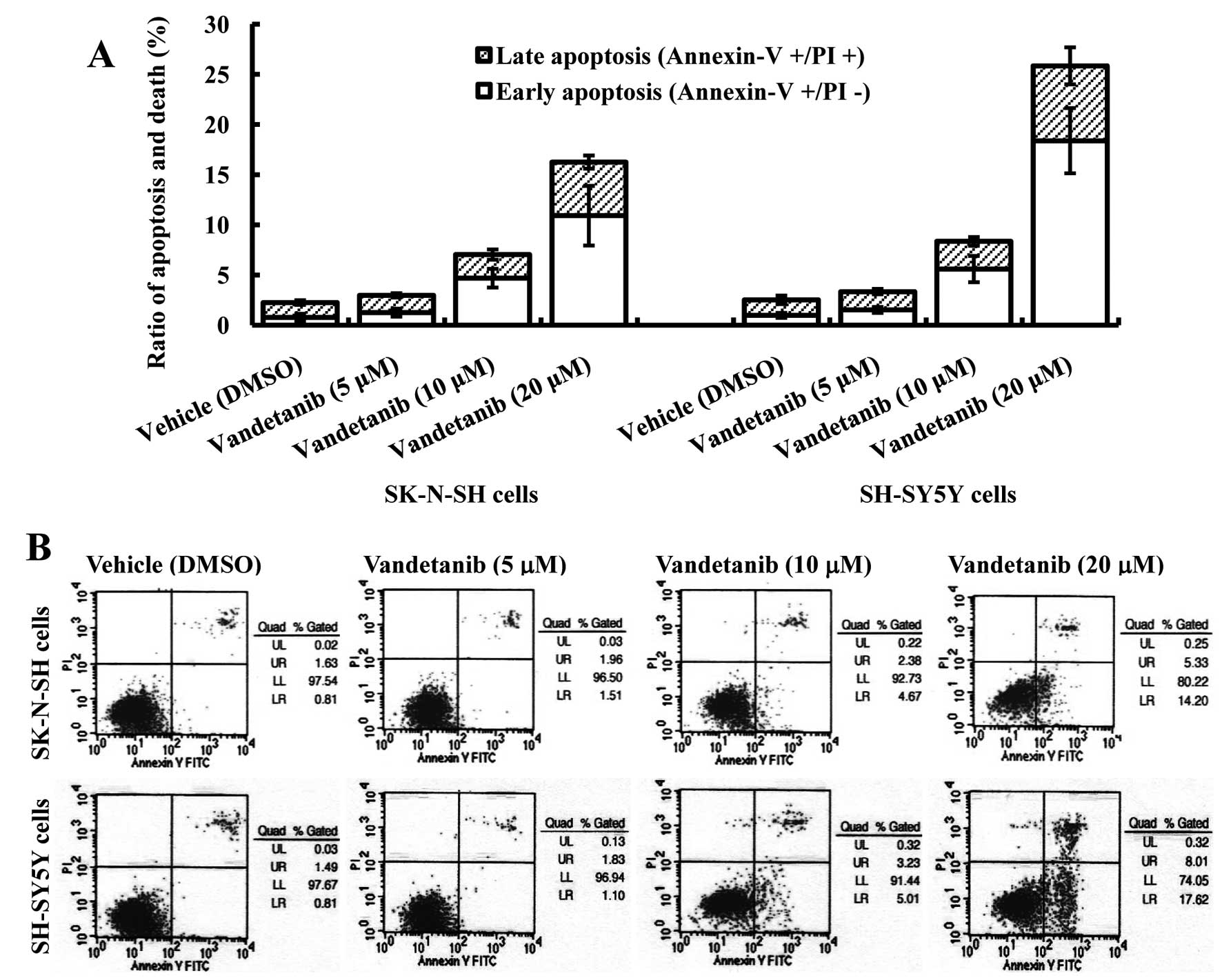
Vandetanib induces apoptosis in human NB
cell lines
Based on the results of the MTT assay, vandetanib
concentrations of 5, 10 and 20 μM, which inhibited the growth of
the two cell lines by ~25–75% over 48 h, were used for apoptosis
and cell cycle assay. To elucidate the mechanism by which
vandetanib exerts its antiproliferative effect on NB cell lines, an
Annexin V/PI binding assay was performed. Our data revealed an
increased percentage of early apoptotic cells (Annexin
V+/PI−) after treatment of SK-N-SH cells with
10 μM vandetanib for 48 h (p<0.05), and the percentage of early
apoptotic cells increased significantly after increasing the
vandetanib concentration to 20 μM (p<0.01). Vandetanib treatment
of SK-N-SH cells also induced an increase in the fraction of late
apoptotic/necrotic cells (Annexin V+/PI+) at
concentrations of 10 μM (p<0.05) and 20 μM (p<0.01). There
was no significant difference in the percentage of early apoptotic
cells between the control group and the 5 μM vandetanib group
(p>0.05). Similar results were obtained with SH-SY5Y cells
(Fig. 2).
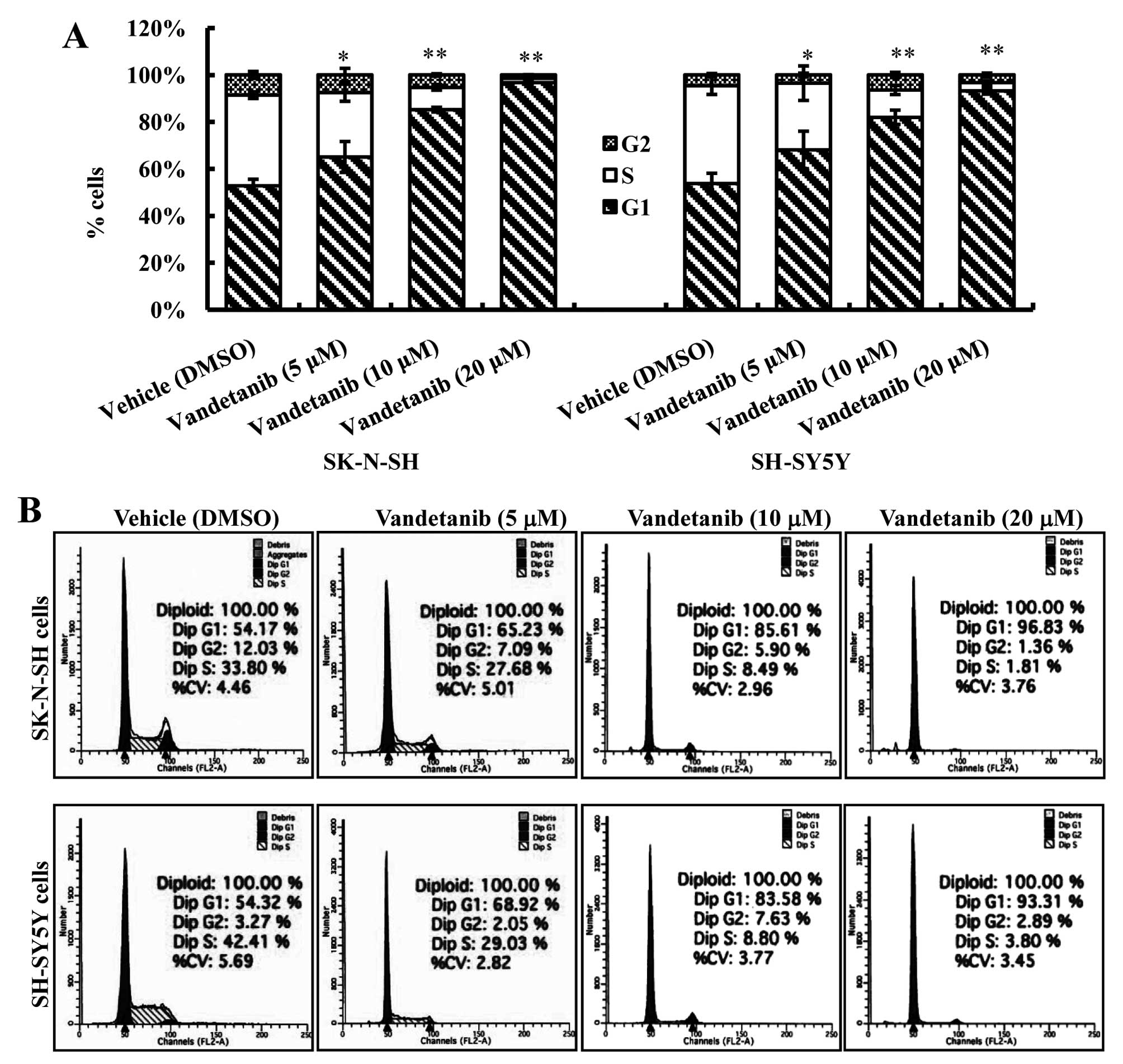
Vandetanib induces G1 phase cell cycle
arrest in human NB cell lines
To investigate whether the antiproliferative effect
of vandetanib on the NB cell lines was partly mediated via specific
cell cycle arrest, we examined the cell cycle phase distribution of
cells treated with vandetanib by flow cytometric analysis. Our data
showed that vandetanib treatment caused a significant accumulation
of cells in the G1 phase in the two cell lines (Fig. 3). The percentage of SK-N-SH cells in
the G1 phase after treatment with 5 or 10 μM vandetanib
(65.10±6.58%, 85.25±0.99%) increased markedly compared with the
cells treated with vehicle (52.80±2.77%) (p<0.01). The
percentage of G1 phase cells continued to increase in SK-N-SH cells
treated with 20 μM vandetanib (96.23±0.41%) (p<0.01). Similar
results were obtained in SH-SY5Y cells treated with vandetanib.
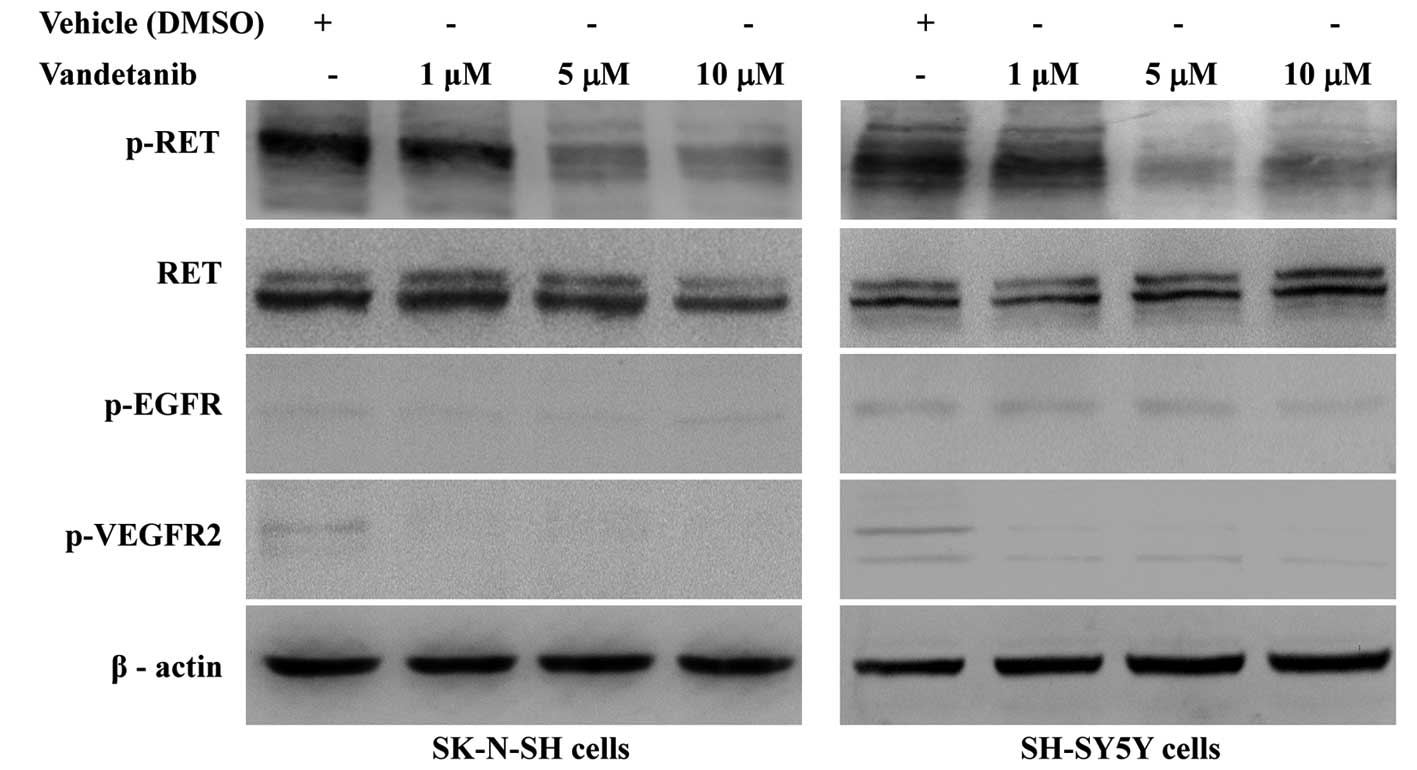
Vandetanib inhibits RET phosphorylation
in human NB cell lines
To evaluate the inhibitory effect of vandetanib on
RET activation, SK-N-SH and SH-SY5Y cells were treated with
vandetanib (1, 5 or 10 μM) for 48 h. Whole-cell lysates from the
two NB cell lines were evaluated by western blot analysis. As shown
in Fig. 4, phosphorylated RET was
detected in the two cell lines treated with vehicle (DMSO), and the
protein level was markedly reduced in cells treated with 5 μM
vandetanib. There were no significant differences in phosphorylated
RET levels in cells treated with 5 and 10 μM vandetanib. However,
extremely low levels of the phosphorylated EGFR and VEGFR2 were
detectable in cells treated with vehicle, with minimal to no levels
detectable in cells treated with vandetanib.
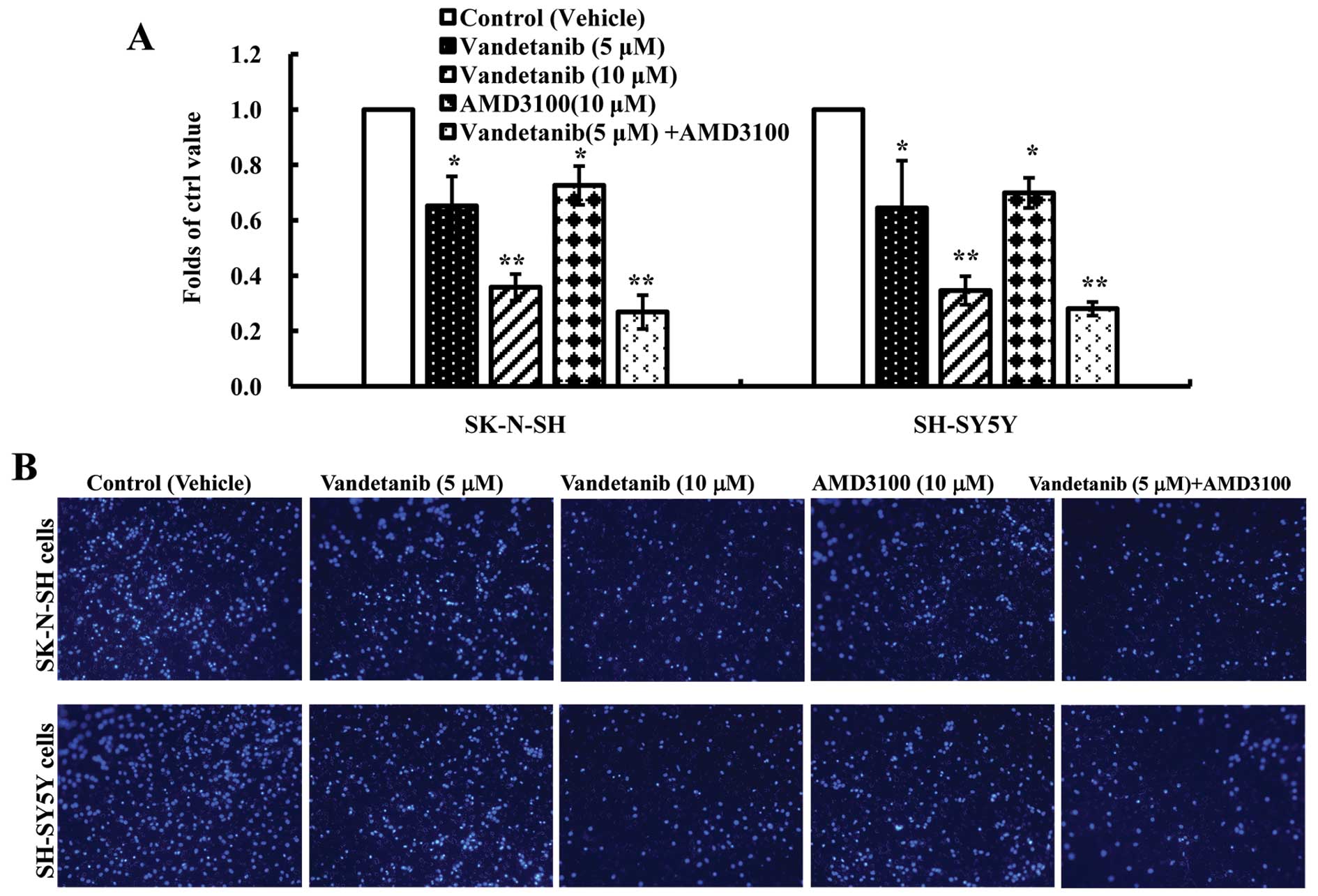
Vandetanib inhibits human NB cell
migration in vitro
To investigate the inhibitory effect of vandetanib
on the migration of NB cells in vitro, the migration
efficiency of NB cells in response to serum and SDF-1α was tested
using a Transwell migration system. The migration efficiency was
calculated as the ratio of migrated cells over control. In the
presence of vandetanib (5 μM) or AMD3100, the number of migrated
cells for the two NB cell lines was significantly decreased
compared with that of the control group (p<0.05). Furthermore,
we found that the migrated cell number in the presence of
vandetanib (10 μM) was less than in the presence of AMD3100 and the
difference was statistically significant (p<0.01). In addition,
the combination of vandetanib and AMD3100 statistically increased
its inhibitory effect on the migration of NB cells compared with
the vandetanib-alone group (p<0.05) (Fig. 5). These data suggested that the
migration of NB cells may be inhibited by vandetanib and the
SDF-1/CXCR4 axis played an important role in NB migration; however,
further studies need to be performed to determine whether
vandetanib inhibits NB cell migration via the SDF-1/CXCR4 axis.
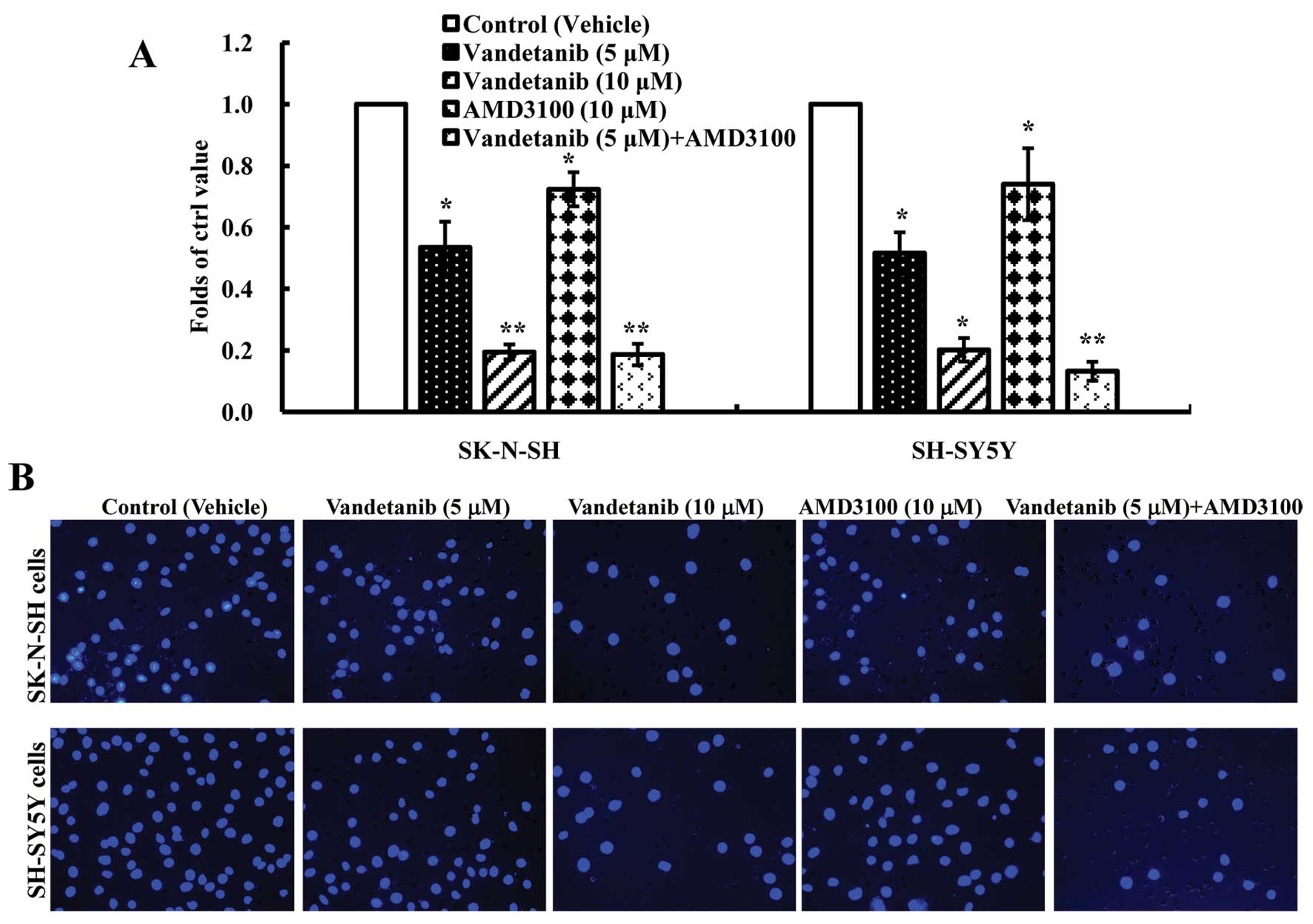
Vandetanib inhibits human NB cell
invasion in vitro
Invasion is another important part of metastasis. To
investigate the inhibitory effect of vandetanib on NB cell
invasion, a Matrigel BioCoat invasion chamber with 8-μm pores was
used to assess the effect on cell invasion. We found that when
treated with vandetanib, the number of both SK-N-SH and SH-SY5Y
cells that travelled through the Matrigel BioCoat micropore
membrane was significantly decreased compared with the control
group (p<0.01) (Fig. 6) and we
also found that AMD3100 blocked reduced cell invasion (p<0.05).
The combination of vandetanib and AMD3100 statistically decreased
cell invasion compared with the control group (p<0.01).
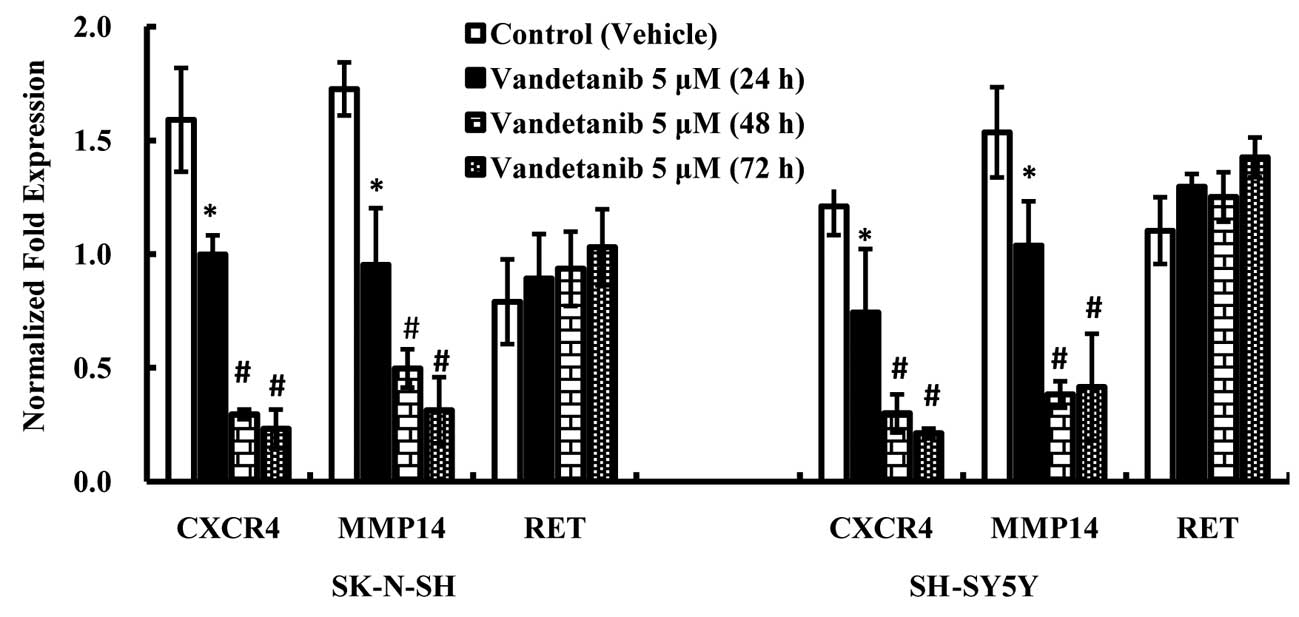
Vandetanib suppresses the expression of
CXCR4 and MMP14 mRNA in human NB cell lines
RT-qPCR was performed to examine the expression of
CXCR4, MMP14 and RET mRNA in NB cells treated with vandetanib. The
results demonstrated that the CXCR4 and MMP14 mRNA expression
levels in NB cell lines treated with vandetanib for 48 h were
significantly lower than in cells treated with vehicle (DMSO) for
48 h (p<0.01) (Fig. 7), and
there was no significant decrease in expression in cells treated
with vandetanib for 72 h compared with those treated with
vandetanib for 48 h. There were no statistically significant
differences in the RET mRNA expression levels among the treatment
groups, although increasing expression of RET was observed as the
concentration of vandetanib increased.
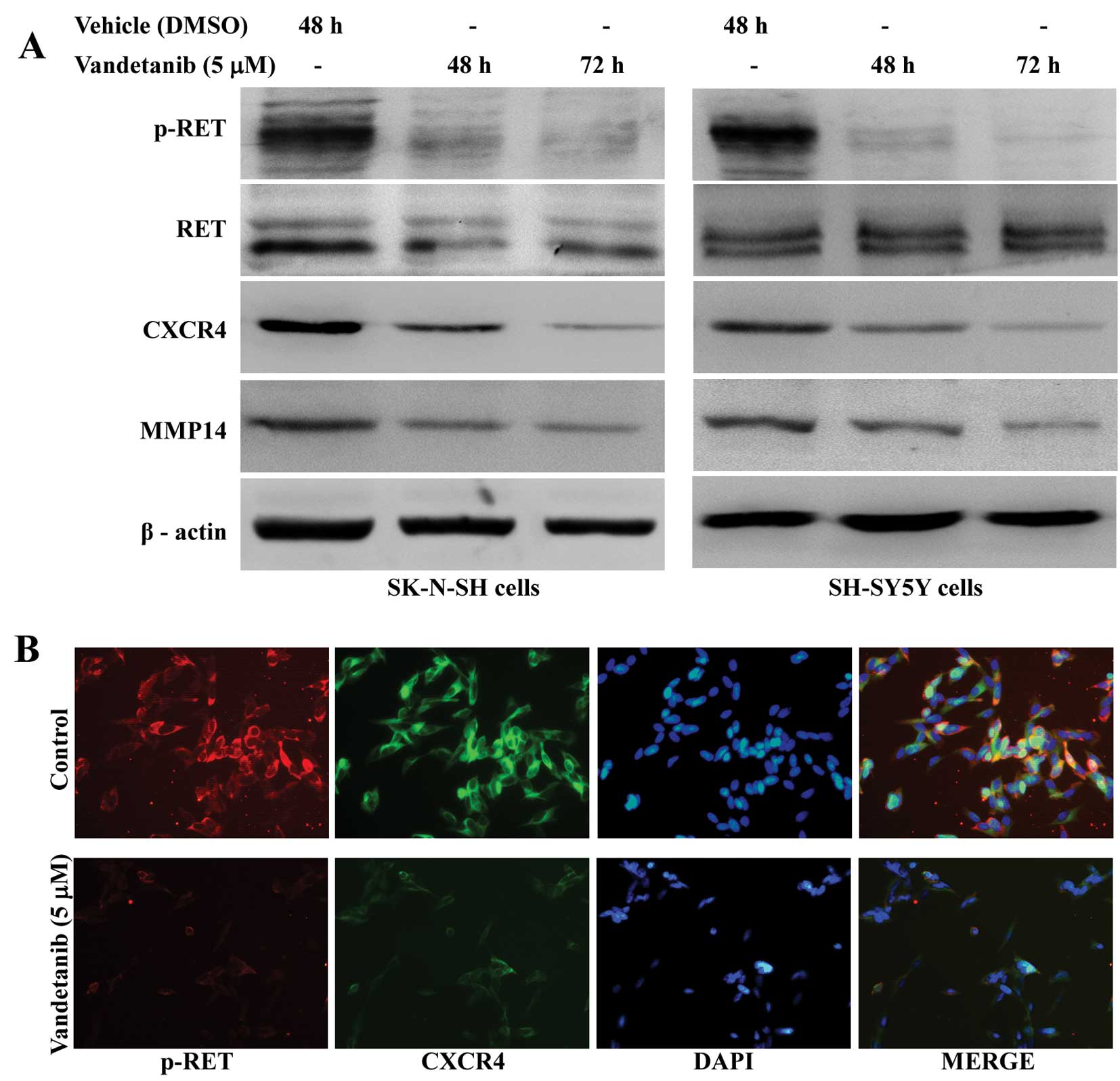
Vandetanib suppresses expression of the
CXCR4 and MMP14 protein in human NB cell lines
The levels of both total expression and cell surface
expression were examined by western blotting and
immunofluorescence. Our results indicated that SK-N-SH and SH-SY5Y
cells exposed to vandetanib for 48 h showed a significant decrease
in total CXCR4 and MMP14 protein levels when compared with the
vehicle control, and these levels remained suppressed with the
extension of treatment time (Fig.
8A). In addition, immunofluorescence analysis indicated that
phosphorylated RET and CXCR4 protein expression were decreased on
the cell surface of SH-SY5Y cells treated with vandetanib for 48 h
(Fig. 8B).
Discussion
Vandetanib is a novel small molecule inhibitor of
the RET tyrosine kinases, VEGFR and EGFR with the potential to
exert anticancer effects via multiple independent pathways.
Vandetanib has demonstrated significant antitumor activity against
a panel of diverse types of human cancer in clinical trials
(16,17). Our results showed that vandetanib
may inhibit the proliferation of human NB cell lines, consistent
with previous studies (14,15). Our study also revealed an increased
percentage of apoptotic cells and a markedly increased percentage
of G1 phase cells in NB cell lines after treatment with 10 μM
vandetanib. The percentage of apoptotic cells increased
significantly with increasing vandetanib concentrations up to 20
μM. These data suggested that vandetanib inhibits the proliferation
of NB cells and that this inhibition is mediated by the induction
of G1 phase cell cycle arrest at lower concentrations and apoptosis
at higher concentrations.
Previous studies have shown that overexpression or
activation of RET alters cell adhesion and enhances the metastatic
behavior of NB (3,4). Our current results showed vandetanib
concentrations of 5 μM, which do not induce apoptosis, may inhibit
the growth of human NB cell lines and RET phosphorylation in those
cells. A randomized, double-blind phase III trial has demonstrated
the significant antitumor activity of vandetanib in patients with
locally advanced or metastatic medullary thyroid cancer, a
challenging group in which all patients with hereditary MTC and
approximately half of the patients with sporadic MTC suffered
mutations in the RET proto-oncogene (18). In our study, vandetanib proved to be
effective in inhibiting the migration of NB cells in vitro.
Similar results were obtained in an invasion assay as the number of
both SK-N-SH and SH-SY5Y cells treated with vandetanib that
travelled through the Matrigel BioCoat micropore membrane was
significantly decreased. These data suggest that vandetanib may be
effective in the prevention of NB cancer cell migration and
invasion in vitro. A previous study demonstrated that the
migration of NB towards SDF-1 may be suppressed by AMD3100 or
silencing of CXCR4, and also both CXCR4 silencing and AMD3100
inhibition reduced invasion (7).
Our study showed that AMD3100 significantly suppressed the
migration and invasion of NB cells, which is consistent with
previous studies.
The CXCR4/SDF-1 axis has been well established as
one of the most frequently expressed chemokines, which affect
multiple pathways of tumor progression such as tumor cell
proliferation, survival and metastasis in different types of tumors
(19). In addition, a correlation
between high CXCR4 expression in primary tumors and tumor
metastasis was previously observed in NB patients (20,21).
Furthermore, another study demonstrated that functional expression
of the CXCR4 chemokine receptor is induced by RET/PTC oncogenes in
human papillary thyroid carcinomas, and CXCR4 induction depends on
a specific tyrosine residue, tyrosine 1062, that is critical in
mediating downstream signaling (22). In the present study, we showed that
CXCR4 expression is inhibited by vandetanib in SK-N-SH and SH-SY5Y
cells. This result was verified by qPCR, western blotting and
immunofluorescence staining experiments. Based on the results of
our study, we postulate that CXCR4 expression is regulated by
RET/GDNF signaling in NB cells. It is noteworthy that migration in
response to SDF-1α and invasion of NB can be inhibited by both
vandetanib and AMD3100, but it has been demonstrated that the
inhibitory efficiency of vandetanib was stronger than that of
AMD3100. Furthermore, the combination of vandetanib and AMD3100
statistically decreased cell migration and invasion compared with
the vandetanib-alone group. Taken together, these observations
support that vandetanib may inhibit the migration and invasion of
NB and that this is associated with downregulation of the
SDF-1/CXCR4 axis, but the axis is not enough to account for all the
results observed.
Processes such as tumor growth, invasion and
metastasis are strongly influenced by the surrounding
microenvironment of the tumor, and altered extracellular
proteolysis that changes these surroundings represents a major
feature through which tumor cells are able to acquire specific
functions necessary for tumor tissue invasion and metastasis. The
matrix metalloproteinases (MMPs) represent the most prominent
family of proteinases associated with tumorigenesis (23). In particular, matrix
metalloproteinase 14 (MMP14), a well-known membrane type 1-matrix
metalloproteinase (MT1-MMP), is closely associated with these
processes. Mounting evidence supports a dominant role for MMP14 in
the migration and invasion of metastatic tumor cells (24,25). A
report showed that microRNA-9 targets MMP14 to inhibit invasion,
metastasis and angiogenesis of NB cells, which were rescued by
overexpression of MMP14 (26). An
early study demonstrated that MMP14 plays an important role in NB
progression and that its expression is preferentially observed in
tumor specimens from NB patients showing poor clinical outcomes
(27). In light of previous
findings in which the expression of MMP14 was upregulated by
RET/GDNF signaling (6), we
hypothesized that vandetanib may inhibit the expression of MMP14
through its inhibition of the RET tyrosine kinases. In the present
study, we found that the expression of MMP14 mRNA and protein was
significantly decreased in NB cells treated with vandetanib as
compared to the control group. These data suggest that MMP14 also
plays an important role in the inhibition of NB migration and
invasion by vandetanib.
In conclusion, vandetanib may inhibit the
proliferation of NB cells mediated by the induction of G1 phase
cell cycle arrest and apoptosis and vandetanib may suppress the
migration and invasion of NB cell lines in vitro. Therefore,
vandetanib should be effective in the prevention of NB cancer cell
migration and invasion. Furthermore, our data suggest that the
potential mechanisms of vandetanib against migration and invasion
of NB are partly attributed to suppression of the expression of
CXCR4 and MMP14 in human NB cells by vandetanib. Collectively, our
observations suggest that vandetanib may be developed as a new
therapeutic strategy to control tumor growth and prevent NB
metastasis to reduce the progression of relapse and refractoriness
in NB patients. However, further animal experiments and clinical
trials must be performed to determine its precise role in the
treatment of NB metastasis.
Acknowledgements
The authors acknowledge the technical assistance
from Mrs Qing Luo and Mr Xin Li. We appreciate the financial
support provided by the National Natural Science Foundations of
China (30973136).
References
|
1
|
Matthay KK, Reynolds CP, Seeger RC, et al:
Long-term results for children with high-risk neuroblastoma treated
on a randomized trial of myeloablative therapy followed by
13-cis-retinoic acid: a children’s oncology group study. J
Clin Oncol. 27:1007–1013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zage PE, Kletzel M, Murray K, et al:
Outcomes of the POG 9340/9341/9342 trials for children with
high-risk neuroblastoma: a report from the Children’s Oncology
Group. Pediatr Blood Cancer. 51:747–753. 2008.PubMed/NCBI
|
|
3
|
Marshall GM, Peaston AE, Hocker JE, et al:
Expression of multiple endocrine neoplasia 2B RET in neuroblastoma
cells alters cell adhesion in vitro, enhances metastatic behavior
in vivo, and activates Jun kinase. Cancer Res. 57:5399–5405.
1997.PubMed/NCBI
|
|
4
|
Futami H and Sakai R: RET protein promotes
non-adherent growth of NB-39-nu neuroblastoma cell line. Cancer
Sci. 100:1034–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arighi E, Borrello MG and Sariola H: RET
tyrosine kinase signaling in development and cancer. Cytokine
Growth Factor Rev. 16:441–467. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu BC, Cebrian C, Chi X, et al: Etv4 and
Etv5 are required downstream of GDNF and Ret for kidney branching
morphogenesis. Nat Genet. 41:1295–1302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma M, Ye JY, Deng R, Dee CM and Chan GC:
Mesenchymal stromal cells may enhance metastasis of neuroblastoma
via SDF-1/CXCR4 and SDF-1/CXCR7 signaling. Cancer Lett. 312:1–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uygur B and Wu WS: SLUG promotes prostate
cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol
Cancer. 10:1392011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wendel C, Hemping-Bovenkerk A, Krasnyanska
J, et al: CXCR4/CXCL12 participate in extravasation of
metastasizing breast cancer cells within the liver in a rat model.
PLoS One. 7:e300462012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bialek J, Kunanuvat U, Hombach-Klonisch S,
et al: Relaxin enhances the collagenolytic activity and in vitro
invasiveness by upregulating matrix metalloproteinases in human
thyroid carcinoma cells. Mol Cancer Res. 9:673–687. 2011.
View Article : Google Scholar
|
|
11
|
Johnson JL, Pillai S, Pernazza D, Sebti
SM, Lawrence NJ and Chellappan SP: Regulation of matrix
metalloproteinase genes by E2F transcription factors: Rb-Raf-1
interaction as a novel target for metastatic disease. Cancer Res.
72:516–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carlomagno F, Vitagliano D, Guida T, et
al: ZD6474, an orally available inhibitor of KDR tyrosine kinase
activity, efficiently blocks oncogenic RET kinases. Cancer Res.
62:7284–7290. 2002.PubMed/NCBI
|
|
13
|
Wedge SR, Ogilvie DJ, Dukes M, et al:
ZD6474 inhibits vascular endothelial growth factor signaling,
angiogenesis, and tumor growth following oral administration.
Cancer Res. 62:4645–4655. 2002.PubMed/NCBI
|
|
14
|
Beaudry P, Nilsson M, Rioth M, et al:
Potent antitumor effects of ZD6474 on neuroblastoma via dual
targeting of tumor cells and tumor endothelium. Mol Cancer Ther.
7:418–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zage PE, Zeng L, Palla S, et al: A novel
therapeutic combination for neuroblastoma: the vascular endothelial
growth factor receptor/epidermal growth factor receptor/rearranged
during transfection inhibitor vandetanib with
13-cis-retinoic acid. Cancer. 116:2465–2475. 2010.
|
|
16
|
Herbst RS, Sun Y, Eberhardt WE, et al:
Vandetanib plus docetaxel versus docetaxel as second-line treatment
for patients with advanced non-small-cell lung cancer (ZODIAC): a
double-blind, randomised, phase 3 trial. Lancet Oncol. 11:619–626.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meyerhardt JA, Ancukiewicz M, Abrams TA,
et al: Phase I study of cetuximab, irinotecan, and vandetanib
(ZD6474) as therapy for patients with previously treated
metastastic colorectal cancer. PLoS One. 7:e382312012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wells SA Jr, Robinson BG, Gagel RF, et al:
Vandetanib in patients with locally advanced or metastatic
medullary thyroid cancer: a randomized, double-blind phase III
trial. J Clin Oncol. 30:134–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mantovani A, Savino B, Locati M, Zammataro
L, Allavena P and Bonecchi R: The chemokine system in cancer
biology and therapy. Cytokine Growth Factor Rev. 21:27–39. 2010.
View Article : Google Scholar
|
|
20
|
Zhang L, Yeger H, Das B, Irwin MS and
Baruchel S: Tissue microenvironment modulates CXCR4 expression and
tumor metastasis in neuroblastoma. Neoplasia. 9:36–46. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Russell HV, Hicks J, Okcu MF and Nuchtern
JG: CXCR4 expression in neuroblastoma primary tumors is associated
with clinical presentation of bone and bone marrow metastases. J
Pediatr Surg. 39:1506–1511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Castellone MD, Guarino V, De Falco V, et
al: Functional expression of the CXCR4 chemokine receptor is
induced by RET/PTC oncogenes and is a common event in human
papillary thyroid carcinomas. Oncogene. 23:5958–5967. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zarrabi K, Dufour A, Li J, et al:
Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer
cell migration. J Biol Chem. 286:33167–33177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perentes JY, Kirkpatrick ND, Nagano S, et
al: Cancer cell-associated MT1-MMP promotes blood vessel invasion
and distant metastasis in triple-negative mammary tumors. Cancer
Res. 71:4527–4538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Qi M, Li S, et al: microRNA-9
targets matrix metalloproteinase 14 to inhibit invasion,
metastasis, and angiogenesis of neuroblastoma cells. Mol Cancer
Ther. 11:1454–1466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nyalendo C, Sartelet H, Barrette S, Ohta
S, Gingras D and Béliveau R: Identification of membrane-type 1
matrix metalloproteinase tyrosine phosphorylation in association
with neuroblastoma progression. BMC Cancer. 9:4222009. View Article : Google Scholar : PubMed/NCBI
|