Introduction
Lung cancer is a disease characterized by
uncontrolled cell growth in lung tissues. Due to the high incidence
and mortality rate, lung cancer was the leading cause of
cancer-related deaths in men and the second leading cause of
cancer-related deaths in women (1,2). Lung
cancer is responsible for more than one million deaths worldwide
annually. Among all lung cancer types, 75–85% of cases are
non-small-cell lung cancer (NSCLC), including lung adenocarcinoma.
Early diagnosis of NSCLC is difficult, and most patients are
diagnosed at the middle or advanced stage. Thus, most patients have
no opportunity for surgical resection. Therefore, these patients
receive radiotherapy as a sole choice. Tyldesley et al
showed that ~60% of NSCLC cases require radiotherapy, both at
initial treatment and at the later course of the disease (3). Postoperative radiotherapy alone, or
intraoperative brachytherapy combined with preoperative radiation
therapy and surgery were reported as the treatment modalities of
superior sulcus tumors. Radiotherapy combined with chemotherapy is
the best option for patients with stage III NSCLC (4).
Although radiotherapy plays a critical role in the
management of NSCLC (5), tumor
radioresistance, such as intrinsic radioresistance or acquired
radioresistance, is one of the main obstacles for improving the
efficacy of radiotherapy. Accumulating evidence indicates that
radioresistance is related to certain genes, for example, p53
(6), epidermal growth factor
receptor (EGFR) (7), DDB2, LOX and
CDH2 (8). Overexpression of RAS
leads to radioresistance, while ATM increases sensitivity to
ionizing radiation (9).
Investigation of the expression of these genes has increased our
understanding of the molecular mechanisms and pathways involved in
radioresistance, yet, the detailed functional mechanisms remain
largely elusive.
microRNAs (miRNAs) are small non-coding RNAs 20–22
nucleotides long, which were originally characterized in eukaryotes
as non-protein coding RNAs (10).
The expression changes of miRNAs have subsequently been reported in
many types of human tumors (11–13).
As tumor-suppressors (miR-15a and miR-16-1) or oncogenes (miR-155
or members of the miR-17-92 cluster), miRNAs have been proposed to
contribute to oncogenesis. The miRNA let-7 has also been
demonstrated to regulate the radiation response of human cancers
(14). Through suppressing let-7g
processing, LIN28B plays an important role in the radiation
response of lung cancer cells (15). These studies indicate that miRNAs
regulate the radiation responses of various types of human
cancers.
In a recent study, we found that miR-511 regulates
lung adenocarcinoma A549 cell proliferation in vitro and
in vivo (16). To further
identify the role of miR-511 in radioresistant lung adenocarcinoma
cells, we generated a radioresistant A549/R cell line and found
that miR-511 regulated the growth of radioresistant A549/R cells by
increasing BAX. This indicates that miR-511 is an important
molecule for radioresistance therapy.
Materials and methods
Cell culture and transfection
Human lung adenocarcinoma A549 cells (Shanghai
Institute of Cell Biology, China) were cultured in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal calf serum (both from
HyClone, USA) and 100 U/ml penicillin-streptomycin (Sigma, USA) at
37°C in 5% CO2.
All transfection experiments were carried out with
Lipofectamine 2000 (Invitrogen, USA) according to the
manufacturer’s instructions. In brief, 5×105 cells were
treated with 1 μg miRNA and 2.5 μl of Lipofectamine 2000. All
transfections were carried out in triplicates.
Establishment of the radioresistant lung
adenocarcinoma A549 cell line
A radioresistant lung adenocarcinoma A549 cell line
was obtained after prolonged exposure to X-rays for 68 Gy (2
Gy/day, 5 days/week). Briefly, when cells grew to ~60% confluence
in 25 cm2 culture flasks, the A549 cells were irradiated
with 2 Gy of X-ray irradiation with a linear accelerator (6-MV
X-ray) at a rate of 3 Gy/min. One-centimeter thick
tissue-equivalent bolus was placed on the top of the plate to
ensure homogeneity. The fractionated irradiations were continued
until the total concentration reached 68 Gy. The radioresistant
A549/R cell line was established.
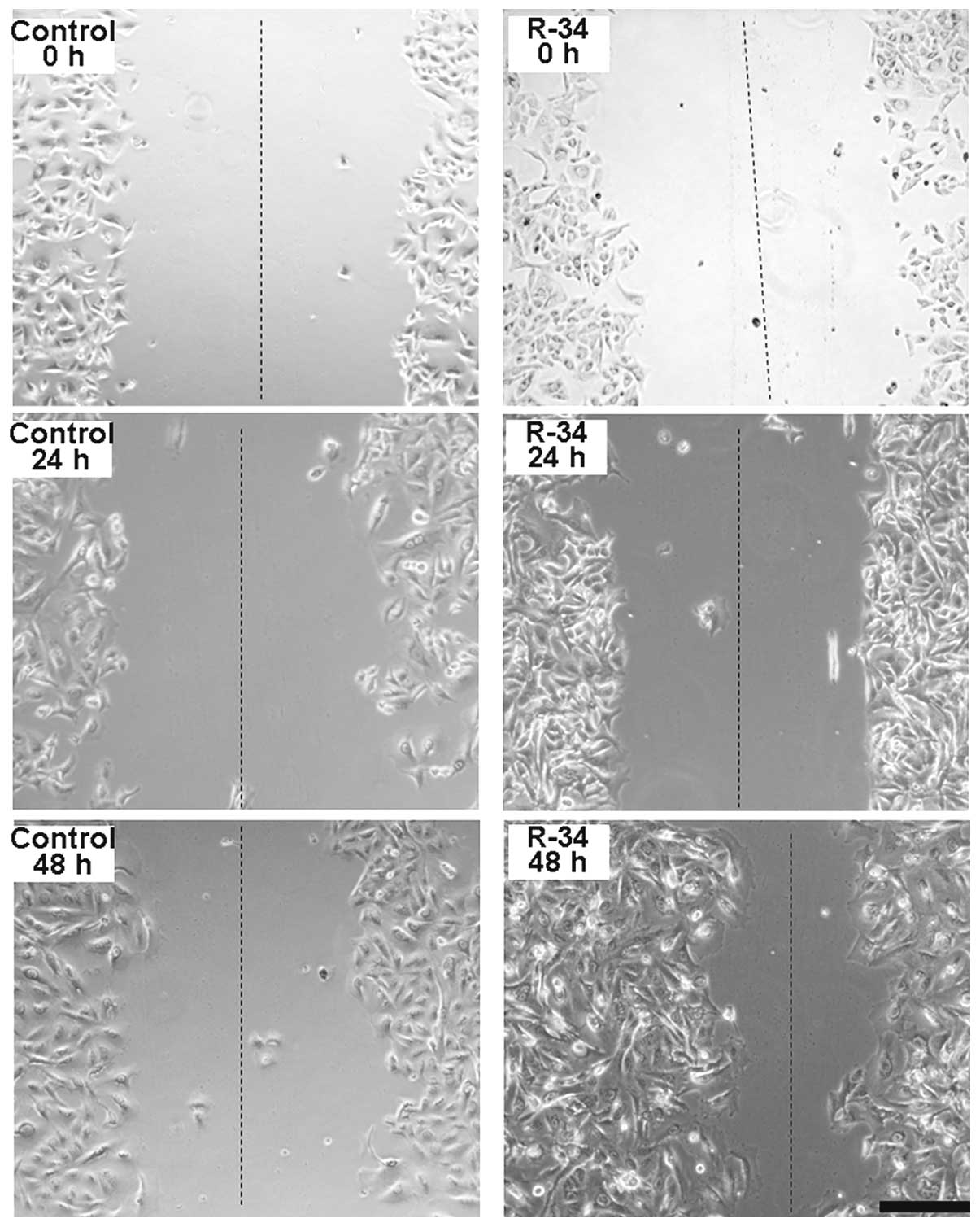
Wound healing assay
Radioresistant A549/R cells were subjected to an
in vitro wound healing assay with images captured at 0, 24
and 48 h after incubation using a microscope. The rate of migration
was measured by quantifying the distance that the A549/R cells
moved from the edge of the scratch toward the center of the scratch
(marked by imaginary dotted lines).
MTT assay
Forty-eight hours after transfection, the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed to detect the growth inhibition rate of A549/R
cells. Cells (1×104) were cultured into each well of
96-well flat plates, and then 10 μl MTT (5 mg/ml) was added to each
well. After 4 h the supernatant was removed and 100 μl dimethyl
sulfoxide (DMSO) (Sigma) was added. The OD value was measured with
an enzyme-linked immunosorbent assay reader (ELx800; USA) at 570
nm. Cell growth inhibition rate = (ODcontrol -
ODsample )/ODcontrol × 100 (%) (16,17).
Detection of apoptosis
The Annexin V-FITC/PI apoptosis detection kit
(KeyGen Biotech, Nanjing, China) was used to stain the apoptotic
cells according to the manufacturer’s instructions. The
radioresistant A549/R cells were gently washed with
phosphate-buffered saline (PBS) twice. Then, the samples
(1×105 cells) were centrifuged for 5 min at 2000 rpm,
and the supernatant was discarded. Binding buffer (500 μl) was
added for resuspension. Then, 5 μl Annexin V-FITC and 5 μl
propidium iodide were added to incubate the cells. The cells were
then analyzed using a flow cytometer (FACS; Beckman Coulter,
USA).
Analysis of cell cycle distribution
The radioresistant A549/R cells were harvested and
washed with 1X PBS buffer. Then, the cells were incubated in a
15-ml V-bottomed tube on ice, and 3 ml cold (−20°C) absolute
ethanol was added to fix the cells overnight. Propidium iodide
staining solution (1 ml) (50 μg/ml) was added to the cell pellets.
Finally, 50 μl of RNase A stock solution was added and the cell
mixture was incubated for 3 h at 4°C. The cell cycle distribution
was detected by flow cytometry (Beckman Coulter).
Real-time PCR
miRNA from radioresistant A549/R cells was isolated
by RNAiso (Takara, Shiga, Japan). Poly(A) was added using poly(A)
polymerase (Ambion, Foster City, CA, USA). cDNA was synthesized and
real-time PCR was performed to detect miR-511 levels as in our
previous report (17). The forward
primer used to detect miR-511 was 5′-GTGTCTTTTGCTCTGCAGTC-3′ and
the reverse primer was 5′-AACATGTACAGTCCATGGATG-3′. Human 5S rRNA
served as the control. Forward primer of 5S rRNA was
5′-GCCATACCACCCTGAACG-3′ and the reverse primer was
5′-AACATGTACAGTCCATGGATG-3′. SYBR® Premix Ex Taq™ kit
(Takara, Shiga, Japan) was used according to the manufacturer’s
instructions. The expression of miR-511 was assessed using the
RG3000 system (Corbett Research, Sydney, Australia) as follows:
initiation with 3 min of denaturation at 95°C, followed by 40
cycles of amplification with 20 sec of denaturation at 95°C, 20 sec
at 56°C for annealing, and 20 sec of extension at 72°C.
Fluorescence was detected at 585 nm.
Western blotting
Cells were lysed in cold lysis buffer with 1 mM
phenylmethanesulfonyl fluoride for 30 min on ice. Protein
concentrations were detected using Coomassie Blue Fast staining
solution (Beyotime, China), and each 35 μg protein sample was
subjected to SDS-PAGE, and transferred to polyvinylidene difluoride
membranes. After incubation in a blocking buffer (containing 10%
non-fat milk and 0.1% Tween-20) for 1 h, the membranes were
immunoblotted with antibodies against TRIB2 (1:500; Santa Cruz
Biotechnology, USA) and BAX (1:500; BioWorld, USA). The membranes
were stripped and re-blotted with actin antibody (1:500; Beijing
Zhongshan Golden Bridge Technology Co., Ltd., China) as a control.
Secondary antibodies conjugated to horseradish peroxidase were
incubated for detection. Images were captured by the FluorChem FC2
gel imaging system (Alpha Innotech, USA).
Statistical analysis
The SAS software was used to analyze the
significance of the results. The Student’s t-test (or one-way
ANOVA) was used for intergroup comparisons. A P<0.05 was
considered to indicate a statistically significant result.
Results
Generation of radioresistant A549/R
cells
Radioresistance is one of the main reasons for the
failure of radiotherapy in lung cancer (18). To study the mechanism of the
radioresistance of lung adenocarcinoma, we established
radioresistant A549/R cells after prolonged exposure to X-rays for
68 Gy (2 Gy/day, 5 days/week). The radioresistance of the A549/R
cells was confirmed by a wound healing assay, which indicated that
a higher number of cells were alive in the radioresistant A549/R
cultures than those in the control A549 cells after exposure with 4
Gy of X-ray. Forty-eight hours later, more cells were found to move
from the edge of the scratch toward the center of the scratch in
the radioresistant A549/R cultures than the number in the control
A549 cells (Fig. 1). MTT assay
showed that a higher number of cells were alive in the
radioresistant A549/R cultures than those in the control A549 cells
48 h after exposure to 4 Gy of X-rays (data not shown).
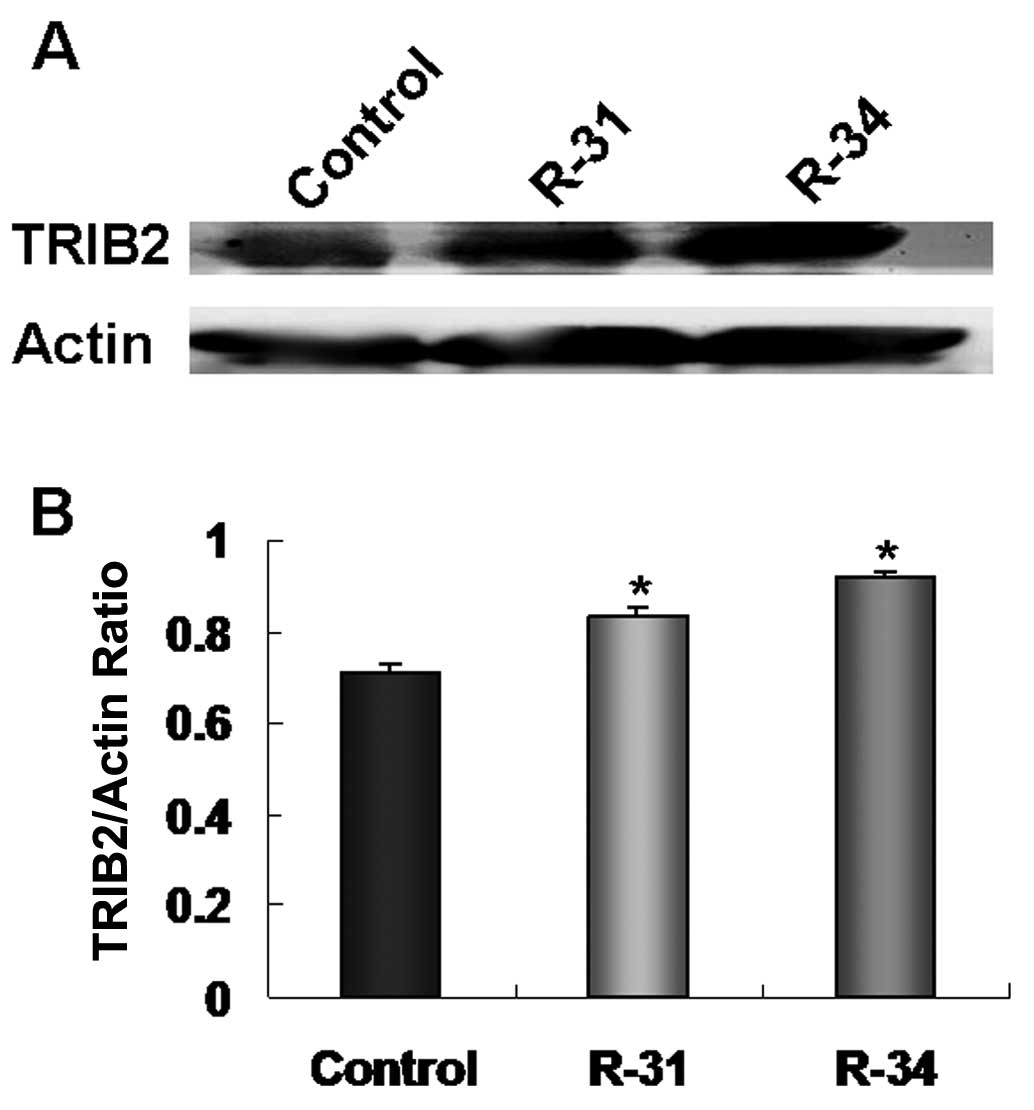
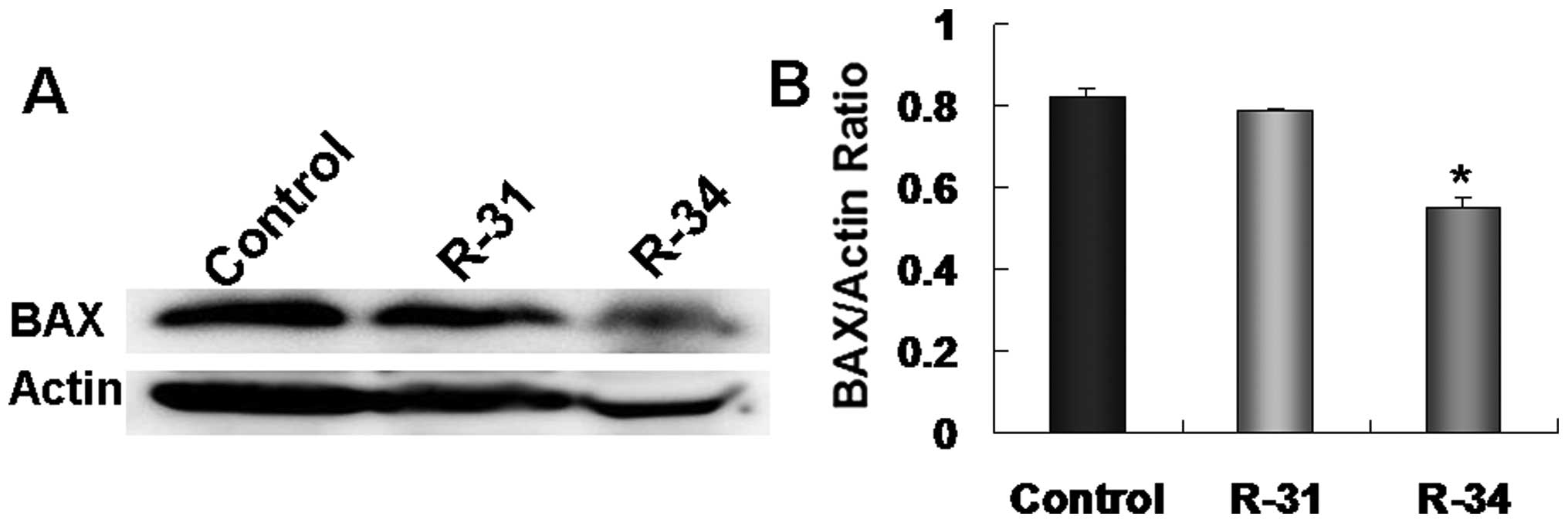
TRIB2 is overexpressed in the
radioresistant A549/R cells
In recent studies (16,19),
we found that TRIB2 plays an oncogenic role in the etiology of lung
adenocarcinoma. To further investigate whether TRIB2 is involved in
the radioresistance of lung adenocarcinoma, the expression of TRIB2
in the radioresistant A549/R cells was detected by western
blotting. We found that TRIB2 was expressed at a high level in the
radioresistant A549/R cells (R-31 and R-34) when compared with this
level in the control A549 cells (Fig.
2A and B), which indicates that TRIB2 plays an important role
in the radioresistance of lung adenocarcinoma.
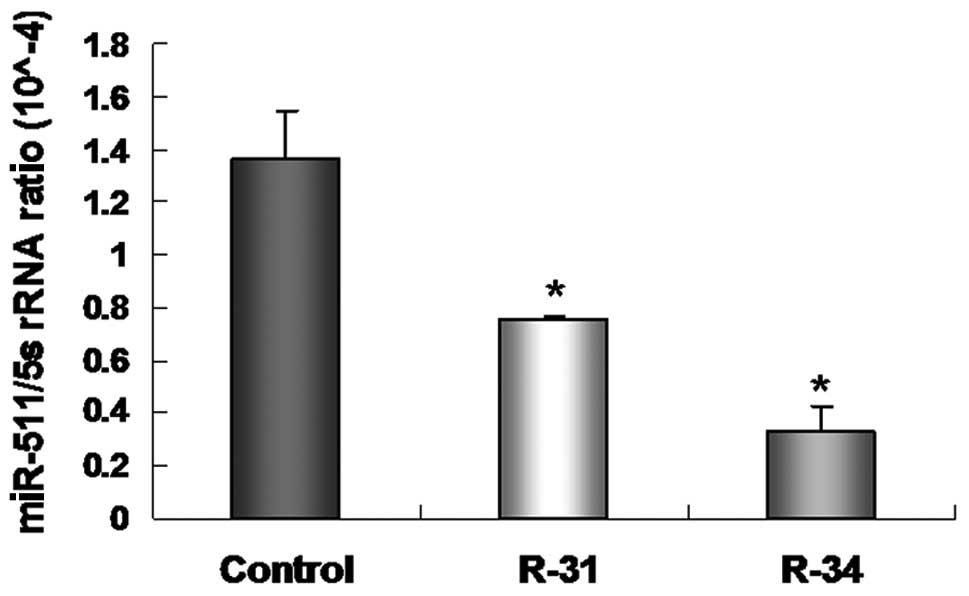
miR-511 inhibits the growth of
radioresistant A549/R cells
Our previous study proved that miR-511, as a
tumor-suppressor gene, suppresses A549 cell proliferation in
vitro and in vivo by regulating TRIB2 (16). In the present study, we analyzed the
levels of miR-511 in radioresistant A549/R cells by real-time PCR.
The level of miR-511 was decreased in the radioresistant A549/R
cells (R-31 and R-34) when compared with this level in the control
A549 cells (Fig. 3).
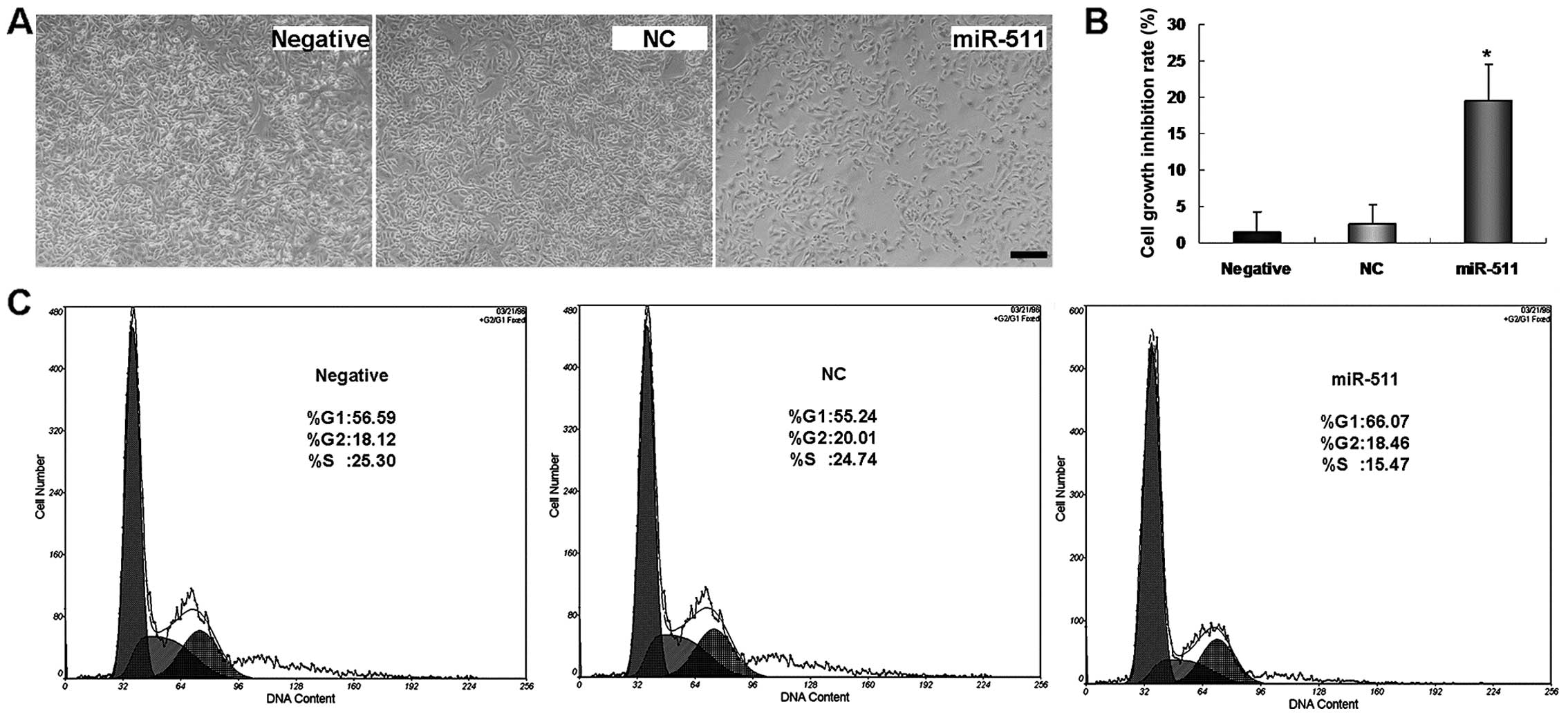
When miR-511 was overexpressed in radioresistant
A549/R cells, we found that miR-511 overexpression inhibited the
growth of the radioresistant A549/R cells. Fewer
miR-511-transfected A549/R cells were found to grow when compared
with the NC or untreated cultures (Fig.
4A). MTT assay further proved the inhibitory role of miR-511 in
the growth of radioresistant A549/R cells (Fig. 4B).
The above results indicate that miR-511 suppresses
A549 cell proliferation. To further test whether the suppressive
role of miR-511 is related to the regulation of the cell cycle,
flow cytometric analysis was used to detect the changes in the cell
cycle distribution after radioresistant A549/R cells were
transfected with miR-511. A significant increase in the percentage
of A549/R cells in the G1 phase (Fig.
4C) was found in the miR-511-treated A549/R cells when compared
to this percentage in the control cultures, suggesting that the
suppressive role of miR-511 is related to the regulation of the
cell cycle, most likely due to a block in G1-S phase
transition.
miR-511 induces A549/R cell apoptosis
through downregulation of TRIB2
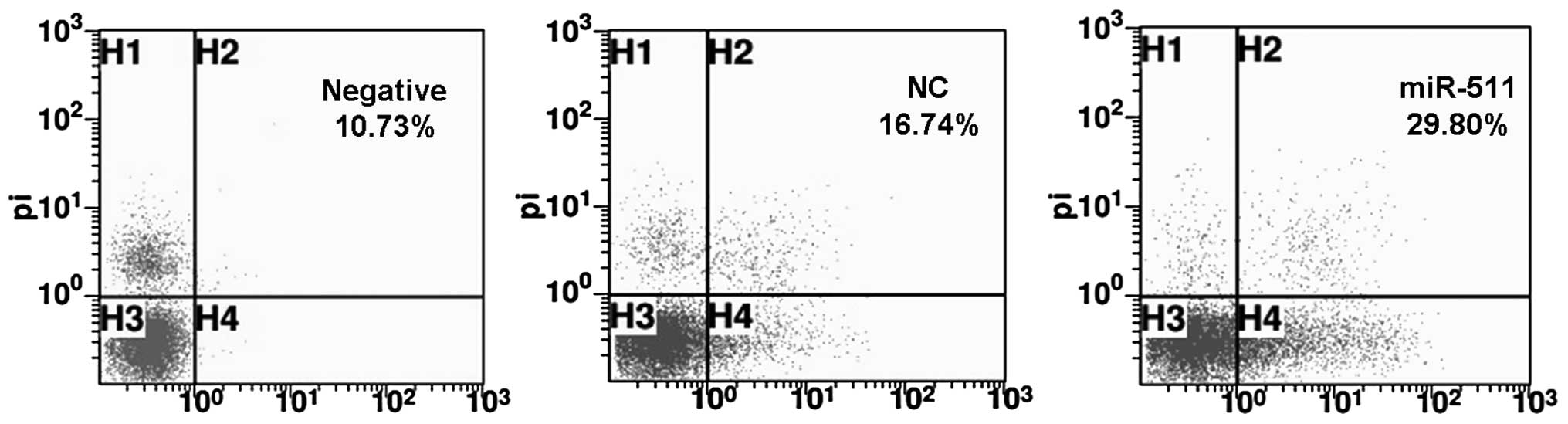
Next, we studied whether miR-511 induces A549/R cell
apoptosis through FACS analysis. We found that the apoptotic rate
was 29.80% in the miR-511-transfected A549/R cells, which was much
higher than that in the control NC-treated (16.74%) or negative
cultures (10.73%). This suggests that miR-511 induces the apoptosis
of radioresistant A549/R cells (Fig.
5).
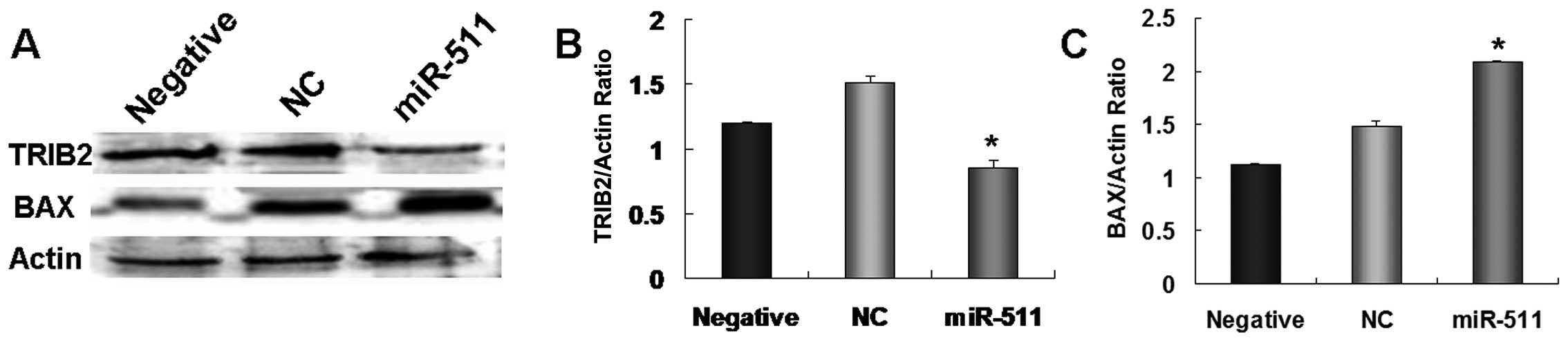
The above results demonstrated that TRIB2 plays an
important role in the radioresistance of lung adenocarcinoma. As an
upstream miRNA, miR-511 may suppress A549 cell proliferation by
regulating TRIB2 (16). Thus, we
tested whether miR-511 regulates TRIB2 expression in radioresistant
A549/R cells. Our results showed that the expression of TRIB2 was
much lower in the miR-511-treated radioresistant A549/R cells than
that in the control cultures (Fig. 6A
and B). These results indicate that miR-511 also induces
apoptosis in radioresistant A549/R cells by downregulation of
TRIB2.
miR-511 increases BAX expression in
apoptotic A549/R cells
TRIBs can regulate mitogen-activated protein kinase
(MAPK) activation (19,20), which further triggers BAX activation
and translocation from the cytosol to the mitochondria (21). Therefore, we aimed to ascertain
whether miR-511 induces radioresistant A549/R cell apoptosis by
triggering BAX activation. We found that the expression of
apoptotic gene Bax was lower in the radioresistant A549/R cells
(R-31 and R-34) when compared with the expression levels in the
control A549 cells (Fig. 7A and B).
Nevertheless, after downregulation of TRIB2 by miR-511 treatment,
BAX expression was obviously increased in the miR-511-transfected
apoptotic A549/R cells when compared to that in the NC-treated and
control cultures (Fig. 6A and C).
Our results indicate that miR-511 induces A549/R cell apoptosis by
increasing the BAX level through regulation of TRIB2.
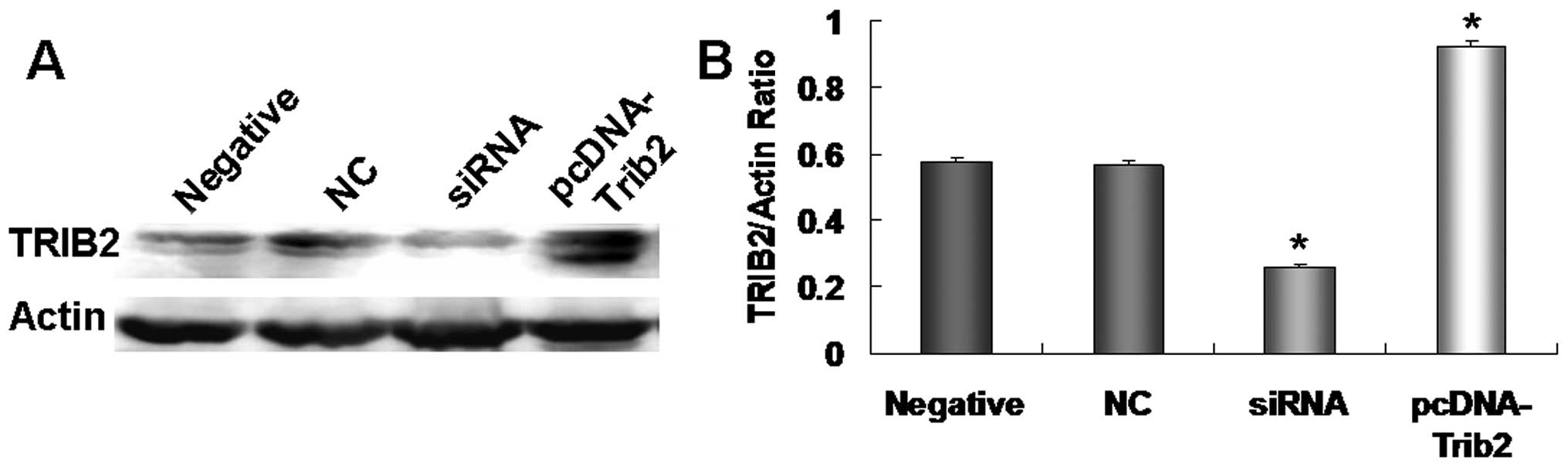
To further test the regulatory role of miR-511 in
increasing the BAX level through TRIB2, we designed an siRNA
specific to TRIB2 to inhibit TRIB2 expression as a negative control
and constructed a pcDNA-TRIB2 vector to increase TRIB2 protein as a
positive control according to our previous studies (16,19).
When radioresistant A549/R cells were treated with the siRNA and
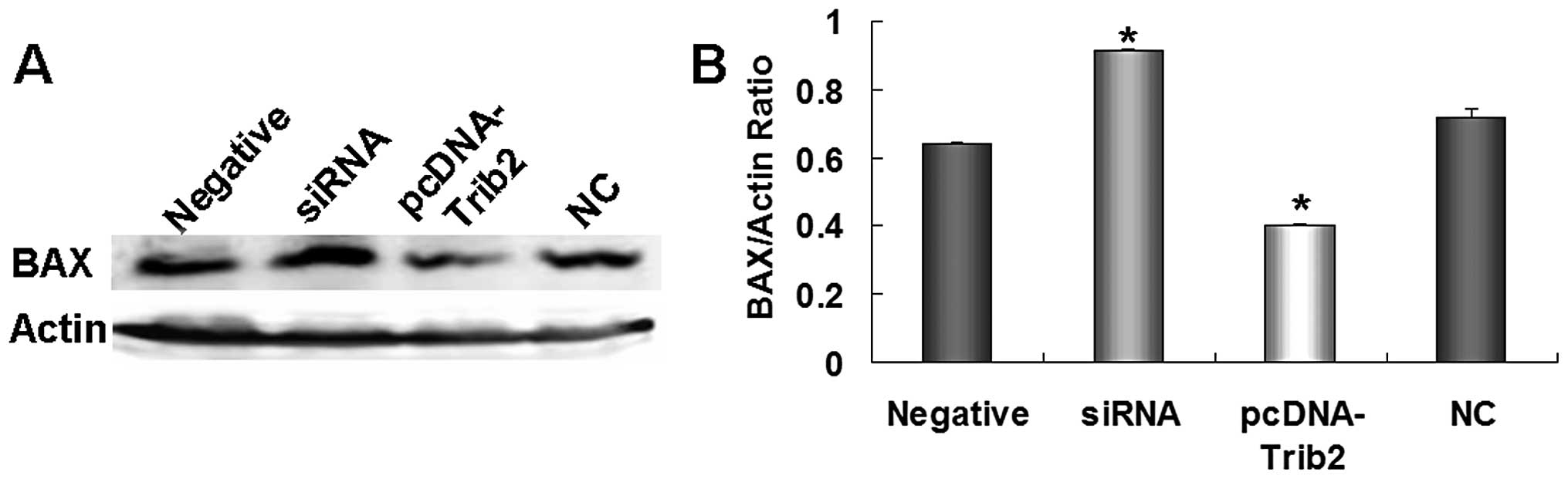
pcDNA-TRIB2 vector, we found that siRNA specific to TRIB2
significantly inhibited TRIB2 expression, and TRIB2 was
overexpressed in the pcDNA-TRIB2-treated cultures (Fig. 8A and B). After TRIB2 expression was
decreased by siRNA, BAX expression was increased 1.5-fold compared
with that in the NC or negative control cultures (Fig. 9A and B). When TRIB2 was upregulated,
BAX expression was reduced obviously in the pcDNA-TRIB2-treated
A549/R cells when compared with those in the NC or negative control
cultures (Fig. 9A and B). Our
results suggest that miR-511 regulates the growth of radioresistant
A549/R cells by overexpression of BAX through TRIB2.
Discussion
Radioresistance is one of the main obstacles to
increasing the efficacy of radiotherapy for NSCLC. However, the
nature of acquired radioresistance is still unclear (22). In the present study, we established
a radioresistant A549/R cell line to investigate the role of
miR-511 in suppressing the growth of radioresistant lung
adenocarcinoma cells. Our results showed that overexpression of
miR-511 inhibited the growth of A549/R cells and induced A549/R
cell apoptosis. The suppressive role of miR-511 in regulating the
growth of A549/R cells was most likely due to a block in the G1-S
phase transition of the cell cycle. Our results further
demonstrated that miR-511 regulates the growth of radioresistant
A549/R cells by triggering BAX through TRIB2, which indicates the
potential application of miR-511 for the therapy of radioresistant
lung cancer.
Altered expression of miRNAs is commonly found in
cancer and is associated with the pathogenesis of most
malignancies, including lung cancer. miRNA expression directly
affects cell proliferation, apoptosis and tumorigenesis (23,24).
miRNAs serve as either oncogenes or tumor-suppressor genes,
depending on their targets. High miR-21 expression in tumors was
demonstrated to be associated with poor survival in cancer
patients, and plays a significant role in cancer growth by
regulating stemness in cancer cells (25). In gastric cancer, upregulated
miR-143 was found to be associated with gastric tumor size, stage,
lymph node and distant metastases (26). Certain miRNAs can inhibit tumor
growth. For example, miR-34 is involved in the downstream p53
pathway, and is a potential tumor-suppressor in cancer stem cell
self-renewal and survival (27). As
tumor-suppressor genes, miR-511 and miR-1297 were found to suppress
A549 cell proliferation in vitro and in vivo by
suppressing TRIB2 and further increasing C/EBPα expression
(16). Similarly, in the present
study, we further demonstrated the role of miR-511 in suppressing
the growth of radioresistant A549/R cells.
Radioresistance is one of the main obstacles to
radiotherapy, and a number of miRNAs are involved in the
radioresistance of tumors. For example, miR-21 is reported to
mediate the resistance of glioblastoma cells against radiation via
its target genes (PDCD4 and hMSH2) (28), indicating that miR-21 and its target
genes may be used as potential molecular targets for clinical
radiotherapy. Radiation may also upregulate miR-21 expression in
mouse hippocampal cells through the EGFR/Stat3 pathway, and miR-21
activates the EGFR pathway (29).
Yet, let-7a and let-7b were found to be downregulated following
exposure to ionizing radiation. This decrease in expression was
dependent on p53 and ATM. (30).
Moreover, in the present study, we found that miR-511 expression
was decreased in the radioresistant A549/R cells, but when miR-511
was overexpressed in A549/R cells, a higher number of apoptotic
cells were detected in the miR-511-transfected A549/R cells when
compared to the control cultures. Our results also revealed that
the suppressive effect of miR-511 on the growth of A549/R cells was
related to cell cycle regulation. These studies indicate that
miRNAs play important roles in radioresistance.
TRIB2 is critical for both solid and non-solid
malignancies. TRIB2 expression was found to be higher in acute
lymphoblastic leukemia phenotypes when compared with acute myeloid
leukemias, and is correlated with NOTCH1/FBXW7 mutations (31). TRIB2 was also identified as a
specific Wnt/β-catenin signaling downstream target of liver cancer
and is functionally important for liver cancer cell survival and
transformation (32), indicating
that TRIB2 functions as a signaling nexus to integrate the
Wnt/β-catenin, Hippo/YAP, and C/EBPα pathways in cancer cells. In
our previous studies (16,19), we found that TRIB2, as an oncogene,
was targeted and negatively regulated by let-7c and miR-1297.
Downregulation of TRIB2 expression resulted in lung cancer cell
apoptosis. In the present study, we further demonstrated that TRIB2
was overexpressed in radioresistant lung cancer cells, which was
negatively regulated by miR-511.
TRIB2 can selectively modulate the activity of the
p38 MAPK pathways (19,33), which further triggers BAX expression
(21). BAX expression was reported
to be associated with radioresistance in cancer therapy.
Overexpression of BAX and c-myc ensures the radiosensitivity
of head and neck cancer (34),
while knockdown of pro-apoptotic protein BAX resulted in an
increase in lung cancer radiosensitivity in vitro (35). Similarly, we found that the
expression of apoptotic BAX was decreased in the radioresistant
A549/R cells. Following downregulation of TRIB2 by miR-511
treatment, the BAX expression was obviously increased in the
radioresistant A549/R cells.
In summary, the present study showed that miR-511
regulates A549/R cell proliferation, which was associated with
regulation of the cell cycle. miR-511 may also induce apoptosis of
radioresistant A549/R cells by triggering BAX through TRIB2,
indicating that miR-511 is a potential therapeutic molecule for the
radiotherapy of cancer.
Acknowledgements
This study was supported by the NCET-10-0919,
‘Taishan Scholar’ position, National Natural Science Foundation
(nos. 31371321 and 81200601), the Shandong Science and Technology
Committee (no. ZR2009CL005), and the Foundation of Shandong
Educational Committee of China (nos. J10LC60 and J11LC01).
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
EGFR
|
epidermal growth factor receptor
|
|
miRNA
|
microRNA
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
DMSO
|
dimethyl sulfoxide
|
|
FACS
|
flow cytometry
|
References
|
1
|
Mokdad AH, Marks JS, Stroup DF and
Gerberding JL: Actual causes of death in the United States, 2000.
JAMA. 291:1238–1245. 2004. View Article : Google Scholar
|
|
2
|
Centers for Disease Control and
Prevention. Tobacco use among adults - United States, 2005. MMWR
Morb Mortal Wkly Rep. 55:1145–1148. 2006.
|
|
3
|
Tyldesley S, Boyd C, Schulze K, Walker H
and Mackillop WJ: Estimating the need for radiotherapy for lung
cancer: an evidence-based, epidemiologic approach. Int J Radiat
Oncol Biol Phys. 49:973–985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dillman RO, Herndon J, Seagren SL, Eaton
WL Jr and Green MR: Improved survival in stage III non-small-cell
lung cancer: seven-year follow-up of cancer and leukemia group B
(CALGB) 8433 trial. J Natl Cancer Inst. 88:1210–1215.
1996.PubMed/NCBI
|
|
5
|
Bradley JD, Paulus R, Graham MV, et al:
Phase II trial of postoperative adjuvant paclitaxel/carboplatin and
thoracic radiotherapy in resected stage II and IIIA non-small-cell
lung cancer: promising long-term results of the Radiation Therapy
Oncology Group - RTOG 9705. J Clin Oncol. 23:3480–3487. 2005.
View Article : Google Scholar
|
|
6
|
Biard DS, Martin M, Rhun YL, et al:
Concomitant p53 gene mutation and increased radiosensitivity
in rat lung embryo epithelial cells during neoplastic development.
Cancer Res. 54:3361–3364. 1994.PubMed/NCBI
|
|
7
|
Wang H, Yu JM, Yang GR, et al: Further
characterization of the epidermal growth factor receptor ligand
11C-PD153035. Chin Med J. 120:960–964. 2007.PubMed/NCBI
|
|
8
|
Xu QY, Gao Y, Liu Y, Yang WZ and Xu XY:
Identification of differential gene expression profiles of
radioresistant lung cancer cell line established by fractionated
ionizing radiation in vitro. Chin Med J. 121:1830–1837.
2008.PubMed/NCBI
|
|
9
|
Westphal CH, Rowan S, Schmaltz C, Elson A,
Fisher DE and Leder P: atm and p53 cooperate in
apoptosis and suppression of tumorigenesis, but not in resistance
to acute radiation toxicity. Nat Genet. 16:397–401. 1997.
View Article : Google Scholar
|
|
10
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calin GA and Croce CM: MicroRNA-cancer
connection: the beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin GA, Garzon R, Cimmino A, Fabbri M
and Croce CM: MicroRNAs and leukemias: how strong is the
connection? Leuk Res. 30:653–655. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hammond SM: MicroRNAs as oncogenes. Curr
Opin Genet Dev. 16:4–9. 2006. View Article : Google Scholar
|
|
14
|
Weidhaas JB, Babar I, Nallur SM, et al:
MicroRNAs as potential agents to alter resistance to cytotoxic
anticancer therapy. Cancer Res. 67:11111–11116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeong SH, Wu HG and Park WY: LIN28B
confers radio-resistance through the posttranscriptional control of
KRAS. Exp Mol Med. 41:912–918. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang C, Chi YL, Wang PY, et al: miR-511
and miR-1297 inhibit human lung adenocarcinoma cell proliferation
by targeting oncogene TRIB2. PLoS One. 7:e460902012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li YJ, Zhang YX, Wang PY, et al:
Regression of A549 lung cancer tumors by anti-miR-150 vector. Oncol
Rep. 27:129–134. 2012.PubMed/NCBI
|
|
18
|
Yan L, Xu G, Qiao T, Chen W, Yuan S and Li
X: CpG-ODN 7909 increases radiation sensitivity of
radiation-resistant human lung adenocarcinoma cell line by
overexpression of Toll-like receptor 9. Cancer Biother Radiopharm.
28:559–564. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang PY, Sun YX, Zhang S, et al: Let-7c
inhibits A549 cell proliferation through oncogenic TRIB2 related
factors. FEBS Lett. 587:2675–2681. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kiss-Toth E, Bagstaff SM, Sung HY, et al:
Human tribbles, a protein family controlling mitogen-activated
protein kinase cascades. J Biol Chem. 279:42703–42708. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JH, Lee SW, Choi SH, Kim SH, Kim WJ
and Jung JY: p38 MAP kinase and ERK play an important role in
nitric oxide-induced apoptosis of the mouse embryonic stem cells.
Toxicol In Vitro. 27:492–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogawa K, Utsunomiya T, Mimori K, et al:
Differential gene expression profiles of radioresistant pancreatic
cancer cell lines established by fractionated irradiation. Int J
Oncol. 28:705–713. 2006.PubMed/NCBI
|
|
23
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lanza G, Ferracin M, Gafà R, et al:
mRNA/microRNA gene expression profile in microsatellite unstable
colorectal cancer. Mol Cancer. 6:542007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chung WM, Chang WC, Chen L, et al:
MicroRNA-21 promotes the ovarian teratocarcinoma PA1 cell line by
sustaining cancer stem/progenitor populations in vitro. Stem Cell
Res Ther. 4:882013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takagi T, Iio A, Nakagawa Y, Naoe T,
Tanigawa N and Akao Y: Decreased expression of microRNA-143 and
-145 in human gastric cancers. Oncology. 77:12–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tarasov V, Jung P, Verdoodt B, et al:
Differential regulation of microRNAs by p53 revealed by massively
parallel sequencing: miR-34a is a p53 target that induces apoptosis
and G1-arrest. Cell Cycle. 6:1586–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chao TF, Xiong HH, Liu W, Chen Y and Zhang
JX: MiR-21 mediates the radiation resistance of glioblastoma cells
by regulating PDCD4 and hMSH2. J Huazhong Univ Sci Technolog Med
Sci. 33:525–529. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi Y, Zhang X, Tang X, Wang P, Wang H and
Wang Y: MiR-21 is continually elevated long-term in the brain after
exposure to ionizing radiation. Radiat Res. 177:124–128. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saleh AD, Savage JE, Cao L, et al:
Cellular stress induced alterations in microRNA let-7a and let-7b
expression are dependent on p53. PLoS One. 6:e244292011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hannon MM, Lohan F, Erbilgin Y, et al:
Elevated TRIB2 with NOTCH1 activation in
paediatric/adult T-ALL. Br J Haematol. 158:626–634. 2012.
|
|
32
|
Wang J, Park JS, Wei Y, et al: TRIB2 acts
downstream of Wnt/TCF in liver cancer cells to regulate YAP and
C/EBPα function. Mol Cell. 51:211–225. 2013.PubMed/NCBI
|
|
33
|
Wei SC, Rosenberg IM, Cao Z, Huett AS,
Xavier RJ and Podolsky DK: Tribbles 2 (Trib2) is a novel regulator
of toll-like receptor 5 signaling. Inflamm Bowel Dis. 18:877–888.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Csuka O, Remenár E, Koronczay K,
Doleschall Z and Németh G: Predictive value of p53, Bcl2 and bax in
the radiotherapy of head and neck cancer. Pathol Oncol Res.
3:204–210. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim KW, Mutter RW, Cao C, et al: Autophagy
for cancer therapy through inhibition of pro-apoptotic proteins and
mammalian target of rapamycin signaling. J Biol Chem.
281:36883–36890. 2006. View Article : Google Scholar : PubMed/NCBI
|