Introduction
Studies using molecular biology techniques confirmed
that breast cancer (BC) is a complex disease whose heterogeneity
and clinical-therapeutic implications must be determined by new
prognostic and predictive markers to personalize therapy to
individual patients. Identification of BC subgroups with a
different clinical course and different response to systemic
treatment is crucial for the improvement of therapy results and
will help introduce new, molecularly targeted treatment options to
the standard regimen. In line with the St. Gallen expert consensus
(1), the evaluation of ER, PgR,
HER-2 status and proliferation index measured by Ki-67
immunoreactivity is key as regards prognostic value, in addition to
TNM classification.
PARP-1 was identified by Chambon et al
(2) in 1963 as a protein whose
enzymatic activity allows it to generate ADP-ribose polymers. Gene
encoding PARP-1, one of the housekeeping genes, sits on the long
arm of chromosome 1 and spans 23 exons (3). PARP-1 protein comprises three main
domains: i) DNA binding domain (DBD), located at the N-terminal end
of the protein; ii) automodification domain (AMD) and iii)
catalytic domain (CD), which spans the C-terminus of PARP-1
molecule (4–7). DBD is composed of three zinc finger
motifs (FI-III/Zn1–3) that direct PARP-1 binding to DNA (FI/Zn1 and
FII/Zn2) and are involved in interdomain and protein-protein
interactions, which is crucial for DNA-dependent enzyme activation
(FIII/Zn3) (8,9). AMD is important for the proper
biological functioning, and encompasses approximately 15 fragments
(mainly glutamic acid) which are sites of PAR chains attachment. CD
is situated at the C-terminus of PARP-1 molecule and is responsible
for NAD+ binding and subsequent elongation and branching
of PAR (3,10).
Studies conducted to date suggest an important role
of PARP-1 in repair of single-strand breaks (SSBs) by base excision
repair (BER). The role of PARP-1 in other repair mechanisms
requires further analysis due to contradictory reports (7,11). The
so-called damage sensor model is proposed in which activated PARP-1
identifies DNA breaks (by DBD) and temporarily binds to the ends of
the damaged DNA. With the use of electron microscopy, studies
showed that PARP-1 in the form of homodimer interacts
electrostatically with DNA covering 7 nucleotides up and down the
damage site and is a specific sensor that leads the recruitment
machinery to the site of damage (6,12).
PARP-1’s enzymatic activity can increase 500-fold on its binding to
the damaged DNA, which results in quick elongation of PAR chain and
additional automodification of PARP-1 with ADP-ribose, inhibiting
enzymatic activity (negative feedback) and detachment of PARP-1
from DNA chain, which ensures further repair of the damage by
certain proteins (13).
It was also shown that ADP-ribosylation of histones
(mainly H1 and H2B) ensures loosening of densely packed chromatin
structure and recruitment of repair machinery, which is necessary
for the adequate correction of DNA changes (14,15).
It is also worth noting that most enzymatic activity (~85%) is
attributed to PARP-1; however, this polymerase is supported in its
function of damage signaller by PARP-2, which is necessary to
ensure effective BER (16).
Inhibition of PARP-1 enzymatic activity by specific
inhibitors results in genome instability, and promotes the
occurrence of various DNA disorders, which eventually results in
cell death. The other area of research into PARP inhibitors is
related to their synergistic activity with genotoxic cytostatic
agents and radiotherapy (16,17).
To date, no predictive factor has been identified
that would be a reliable qualifier for potential inclusion of PARP
inhibitor therapy in BC treatment. Immunohistochemical assessment
of PARP-1 reactivity in BC cells and the subsequent decision on
starting the therapy or exclusion of the patient may be an
alternative, as in the case of HER-2. The above hypothesis needs to
be confirmed in extensive, multicentre research studies.
The significance of PARP-1 immunoreactivity in
breast carcinogenesis and the impact on long-term survival remains
thus far unclear. In the present study, we investigated the
prognostic value of PARP-1 protein expression by
immunohistochemical analyses in 83 patients with stage II ductal
BC. We assessed the relationship between the subcellular
localization of these proteins in conjunction with the pathological
and clinical characteristics of the patients studied. Considering
the favorable prognosis of patients diagnosed with BC (5-year
survival rate of almost 90%) and recurrence that is often observed
after as many as 20 years after the original diagnosis and
treatment, we sought to study a group of patients observed for a
period of over 5 years. A unique, clinically and therapeutically
homogeneous group was enrolled in the study, with accurately
documented 15-year observations.
Patient clinical history was analyzed for a period
of 15 years which is an exceptionally long observation period and
adds considerable value to the research, but that, however, gives
rise to problems relating to the evaluation of predictive value of
PARP-1. Fifteen years ago different therapeutic methods were used,
namely, more radical surgeries, different radiotherapy schedules
and hormonal therapy was applied without the knowledge of steroid
receptor expression. The above makes it impossible to apply
predictive test results to the currently treated patients; however,
the analysis of such a long clinical course provides important
information on the prognostic role of PARP-1 expression in BC. It
must be stressed that prognostic value of PARP-1 expression in such
a homogeneous group or in the group with such long clinical
observations has not previously been described.
Materials and methods
Patients
Tissue samples were obtained from 83 patients
treated radically for stage II ductal BC, diagnosed between 1993
and 1994 in the Lower Silesian Oncology Centre in Wroclaw, Poland.
The mean age of the patients was 55.2 years. The patients were
selected based on the availability of tissues. All patients
underwent surgery (Madden mastectomy) with or without adjuvant
treatment. Following the applied treatment, the patients were
subjected to continuous monitoring in the Lower Silesia Oncology
Centre. Data related to relapse and mortality were accumulated
using medical documentation available in the Lower Silesia Oncology
Centre. Overall survival (OS), cancer-specific overall survival
(CSOS) and disease-free survival (DFS) rates were established for
all patients. The total number of patients included was stipulated
by a single series performed by our institution, the follow-up
period of 15 years, and the highly homogeneous characteristics of
the tumors selected (ductal invasive BC, G2 and G3, clinical stage
II according to UICC, Madden mastectomy). Detailed characteristics
of the patient cohort are listed in Table I. This study was approved by the
Institutional Review Board of the Wroclaw Medical University,
Poland.
 | Table IPatient and tumor characteristics and
their correlation with enhanced immunoreactivity (IRS ≥6) and
nuclear-cytoplasmic expression (NCE) of PARP-1. |
Table I
Patient and tumor characteristics and
their correlation with enhanced immunoreactivity (IRS ≥6) and
nuclear-cytoplasmic expression (NCE) of PARP-1.
| Patient
characteristics | No. (%)a | PARP-1 high
expression | PARP-1 NCE |
|---|
| All patients | 83
(100) | | |
| Age (mean
55.2±10.3; median, 55)d | | 0.025 | 0.209 |
| Menopaused | | 0.244 | 0.339 |
| Premenopausal | 27 (32.5) | | |
|
Postmenopausal | 56 (67.5) | | |
| Histology: invasive
ductal BC | 83 (100) | | |
| TNM stage according
to UICCd | | 0.013 | 0.236 |
| II A | 33 (39.8) | | |
| II B | 50 (60.2) | | |
| Tumor size in mm
(pT)c: mean, 31.0±12.3; median,
30 | | 0.481 | 0.656 |
| Nodal metastases
(N)d | | 0.221 | 0.149 |
| N(−) | 47 (56.6) | | |
| N(+) | 36 (43.4) | | |
| Gradingd | | 0.003 | 0.099 |
| G2 | 59 (71.1) | | |
| G3 | 24 (28.9) | | |
| Therapyb,d | | | |
| Tamoxifen | 49 (59.0) | 0.169 | 0.353 |
|
Cyclophosphamide/methotrexate/5-fluorouracil | 23 (27.7) | 0.112 | 0.257 |
|
Anthracyclines | 1 (1.2) | | |
| Without adjuvant
chemotherapy | 59 (71.1) | | |
| Radiotherapy | 37 (44.6) | 0.081 | 0.098 |
| ER statusc | | 0.009 | 0.106 |
| Negative | 22 (26.5) | | |
| Positive | 61 (73.5) | | |
| PgR statusc | | 0.221 | 0.230 |
| Negative | 22 (26.5) | | |
| Positive | 61 (73.5) | | |
| HER-2
statusc | | 0.807 | 0.284 |
| Negative (0, 1+,
2+) | 64 (77.1) | | |
| Positive (3+) | 19 (22.9) | | |
| Recurrenced | | 0.057 | 0.011 |
| Yes | 32 (38.6) | | |
| No | 51 (61.4) | | |
Tumor samples
Tumor specimens were fixed in 10% buffered formalin
and embedded in paraffin. All hematoxylin and eosin (H&E)
stained sections were examined by two pathologists. Due to the
absence of a population-based BC screening at the time the present
study was initiated, the size of the primary tumors was different
from the detriment of the value of other, including European
countries. The median tumor size was also determined by the
inclusion of a homogeneous group of clinical stage II BC (see
above). Tumor stages were assessed according to the TNM
classification system (18). The
tumor grades were estimated according to the Bloom-Richardson
protocol, with the Elston and Ellis (19) modification (Table I).
Immunohistochemistry
Immunohistochemical analyses were performed
retrospectively on tissue samples collected for routine diagnostic
purposes. Formalin-fixed, paraffin-embedded tissue sections were
freshly prepared (4 μm). Immunohistochemistry was performed as
previously described (20–22). For the detection of PARP-1, a
polyclonal rabbit antibody (clone ab6079; Abcam, Cambridge, UK) was
diluted 1:150 in the Antibody Diluent, Background Reducing
(DakoCytomation, Warsaw, Poland). For the detection of the estrogen
receptor, an optimally pre-diluted monoclonal mouse antibody was
used (clone 1D5; DakoCytomation, Glostrup, Denmark) and for the
detection of the progesterone receptor, an optimally pre-diluted
monoclonal antibody (clone PgR636, DakoCytomation) was used. For
HER-2 detection, a semi-quantitative diagnostic immunohistochemical
test was used (HercepTest™ kit, K5207; DakoCytomation). Tissue
sections were incubated with antibodies for 1 h at room
temperature. Subsequent incubations involved biotinylated
antibodies (15 min, room temperature) and a
streptavidin-biotinylated peroxidase complex (15 min, room
temperature) (LSAB+, HRP; DakoCytomation, Warsaw, Poland). NovaRed
(Vector Laboratories, Cambridgeshire, UK) was used as a chromogen
(10 min, room temperature). All sections were counterstained with
Mayer’s hematoxylin. In each case, control reactions were included,
in which the specific antibody was substituted by a Primary Mouse
Negative Control (DakoCytomation).
Evaluation of immunohistochemical
reaction intensity
The intensity of the immunohistochemical reaction
was estimated independently by two pathologists. In doubtful cases,
a re-evaluation was performed using a double-headed microscope and
staining was discussed until a consensus was reached.
The expression of PARP-1 was evaluated using the
semi-quantitative scale of the ImmunoReactive Score (IRS) according
to Remmele and Stegner (23)
modified by the authors (22),
which took into account the percentage of reactive cells (no
staining, 0; <25%, 1; 25–50%, 2; 51–75%, 3; >75%, 4) and the
intensity (no staining, 0; weak, 1; intermediate, 2; strong, 3) of
the color reaction, with the final result being the product of both
variables. Consequently, nine possible scores (0, 1, 2, 3, 4, 6, 8,
9 and 12) were obtained.
PARP-1 expression was only observed in the tumor
compartment of BC specimens. We described two patterns of its
intracellular localizations in BC cells: i) nuclear-cytoplasmic
expression (NCE) and ii) cytoplasmic expression (CE).
Additionally, we observed that normal breast tissue,
which was included in some slides, was characterized by weak to
moderate nuclear-cytoplasmic PARP-1 immunoreactivity. In stromal
cells and lymphocytes, nuclear and cytoplasmic PARP-1 staining was
also detected.
Nevertheless, at the stage of subsequent statistical
analyses, a two-grade scale system was applied, allocating 0 points
for expression of PARP-1 <6 (low level of PARP-1
immunoreactivity) and 1 for expression of PARP-1 ≥6 (high PARP-1
immunoreactivity). Definition of these two groups and determination
of the cut-off point is a specific consensus of histopathological
observations and statistical analyses and of the review of
literature concerning PARP-1 expression evaluation.
The evaluation of estrogen and progesterone receptor
expression was performed using standard methods. The staining
intensity (0–3 scale) and proportion of positive cells (0–5 scale)
were reported, and the Allred score that combines the two was
calculated. The HER-2 status was evaluated using an FDA-approved
scoring system of 0, 1+, 2+ and 3+ (0, no immunostaining; 1+, weak
immunostaining, <30% of the tumor cells; 2+, complete membranous
reactivity, either uniform or weak in at least 10% of the tumor
cells; 3+, uniform intense membranous staining in at least 30% of
the tumor cells).
Cell lines
In the present study, we used 4 human BC cell lines:
MCF-7, CAMA-1, SK-BR-3 and R-103 and 3 models of drug-resistant
cell lines. Characteristics and culture of the human gastric
carcinoma cell line EPG85-257P (257P), human pancreatic cell line
EPP85-181P (181P) and human BC cell line (MCF-7), its classical MDR
variants EPG85-257RDB (257RDB) and EPP85-181RDB (181RDB)
overexpressing MDR1/P-gp, and its atypical MDR variants
EPG85-257RNOV (257RNOV), EPP85-181RNOV (181RNOV), MCF-7/ADR were
previously described in detail (Table
II) (24–33).
 | Table IIImmunocytochemical distribution of
PARP-1 expression in cancer cell lines and drug-resistant
sublines. |
Table II
Immunocytochemical distribution of
PARP-1 expression in cancer cell lines and drug-resistant
sublines.
| Cell line | Cytoplasmic PARP-1
(IRS score) | Nuclear PARP-1 (IRS
score) | Origin cancer | Selection
agent | Supposed resistance
mechanisms | Refs. |
|---|
| EPP85-181P | 3 | 0 | Pancreatic | | | (24) |
| EPP85-181RNOV | 6 | 6 | | Mitoxantrone | Topo II | (24) |
| EPP85-181RDB | 9 | 6 | | Daunorubicin | MDR1/P-gp | (24) |
| EPG85-257P | 12 | 4 | Gastric | | | (25 |
| EPG85-257RNOV | 4 | 9 | | Mitoxantrone | BCRP, GPC3, Topo
II, TAP | (25–28) |
| EPG85-257RDB | 4 | 4 | | Daunorubicin | MDR1/P-gp | (29) |
| MCF-7 | 8 | 0 | Breast | | | (30) |
| MCF-7/ADR | 3 | 6 | | Adriamycin,
Antiestrogens | LAMP-1 | (31–33) |
Cell culture
Cells were grown in Leibovitz L-15 medium
(BioWhittaker Inc., Walkersville, MD, USA) supplemented with 10%
fetal calf serum (FCS; Gibco/BRL, Grand Island, NY, USA), 1 mM
L-glutamine, 6.25 mg/l fetuin, 80 IE/l insulin, 2.5 mg/ml
transferrin, 0.5 g/l glucose, 1.1 g/l NaHCO3, 1% minimal
essential vitamins and 20,000 kIE/l trasylol in a humidified
atmosphere of 5% CO2 at 37°C. Prior to resistance
testing, Mycoplasma tests were performed using the Venor Mp kit,
according to the manufacturer’s instructions (Minerva Biolabs GmbH,
Berlin, Germany).
Resistance tests
Drugs were used in their commercially available form
(except cyclophosphamide, which was used in its activated form).
Each drug was applied to the cells in 3 concentrations (C1, C2 and
C3). C1 = 10−1 × C2 and C3 = 10 × C2. Concentration C2
(the clinically available drug in the tumor) was deduced from
levels assessed to be clinically achievable in tumor tissue, as
previously discussed (Table III)
(30).
 | Table IIIDrugs used to establish resistance
patterns and their C2 concentrations (the clinically available drug
in the tumor). |
Table III
Drugs used to establish resistance
patterns and their C2 concentrations (the clinically available drug
in the tumor).
| Drug | C2 (μM/ml) | Supplier |
|---|
| 5-Fluorouracil |
38.43×10−5 | Gry-Pharma |
| Cisplatin |
16.66×10−5 | Gry-Pharma |
| Cyclophosphamide
(hydroxylated) |
50.16×10−5 | Asta Werke |
| Doxorubicin |
0.86×10−5 | Cell-Pharma |
| Etoposide |
2.37×10−5 | Gry-Pharma |
| Methotrexate |
0.3×10−5 | Wyeth-Lederle |
| Mitomycin C |
1.49×10−5 | Hexal |
| Mitoxantrone |
0.38×10−5 | Wyeth-Lederle |
| Paclitaxel |
0.29×10−5 | Bristol |
| Topotecan |
×10−5 |
GlaxoSmithKline |
| Vinblastine |
0.1×10−5 | Gry-Pharma |
In each experiment, 500 cells/microtiter dish were
seeded onto 96-well plates. After 2 days, precontrol cells were
fixed and stained using sulforhodamine B (SRB). At the same time,
triplicate cultures were prepared with all 11 studied drugs at C1,
C2 and C3 concentrations. After 4 days, incubation was terminated
by replacing the medium with 10% trichloroacetic acid, followed by
incubation at 40°C for 1 h. Subsequently, the plates were washed 5
times with water and stained by adding 100 μl 0.4% SRB (Sigma, St.
Louis, MO, USA) in 1% acetic acid for 10 min at room temperature.
Washing the plates 5 times with 1% acetic acid eliminated unbound
dye. After air-drying and resolubilization of the protein-bound dye
in 10 mM Tris-HCl (pH 8.0), absorbance was read at 562 nm in an
Elisa-Reader (EL 340 Microplate Bio Kinetics Reader; Bio-Tek
Instruments, Winooski, VT, USA). The measurements were performed in
triplicate in 3 independent experiments. For the calculation of the
RI values, the averages of all 9 measurements were used. The
resistance index (RI) was estimated by the formula: RI =
(npost/npre) ×
[(n2-npre)/(npost-npre)
× 100] where npre is the medium absorbance value of
precontrol at the C2 concentration, npost is the medium
absorbance value of control and n2 is the medium
absorbance value of stained cells tested with the chosen
concentration of the studied drug.
Immunocytochemistry
Immunostaining of PARP-1 was performed using the
studied panel of BC cell lines. Cells were grown on microscopic
slides and fixed in ice-cold methanol-acetone mixture (1:1) for 10
min. Immunostaining reaction was performed in triplicate as
previously described.
Statistical analysis
Statistical analysis was performed using the
Statistica 9.1 software package (StatSoft Inc., Tulsa, OK, USA). OS
was defined as the time between the primary surgical treatment and
mortality, and OS was censored at last follow up for those who were
alive. DFS was defined as the time between the primary surgical
treatment and date of relapse or mortality, whichever occurred
first. DFS was censored at the last follow-up for patients who
survived without disease recurrence. CSOS was defined as the time
between the primary surgical treatment and cancer-associated
mortality, and was censored at the last follow-up for surviving
patients.
Due to the important role of nodal metastatic tumors
as negative prognostic factors, an additional analysis of the role
of PARP-1 expression and its subcellular localization in patients
with and without regional lymph node metastasis (N+ and N−) was
performed.
The χ2 test, exact Fisher test in case of
2×2 tables and Kendall rank correlation were used to analyze
associations between PARP-1 protein expression parameters and
clinicopathological parameters. Differences between two groups were
tested with the Mann-Whitney U test, the log-rank test was used to
compare survival in two groups, the OS rate was estimated by the
Kaplan-Meier method and the influence of explanatory variables on
mortality risk was analyzed by means of the Cox proportional hazard
regression and logistic regression in case of binary survival.
P-values <0.05 were considered to indicate statistically
significant differences.
Results
PARP-1 immunostaining in BC
specimens
PARP-1 expression defined as IRS >0 was found in
the entire group of 83 patients subjected to investigation. The
average IRS was 6.48±2.5 and the median was 8. For the purposes of
statistical analysis, enhanced immunoreactivity of PARP-1 was
defined as IRS ≥6 (55 patients, 66.27%), while low immunoreactivity
was assigned IRS values between 0 and 4 (28 patients, 33.73%)
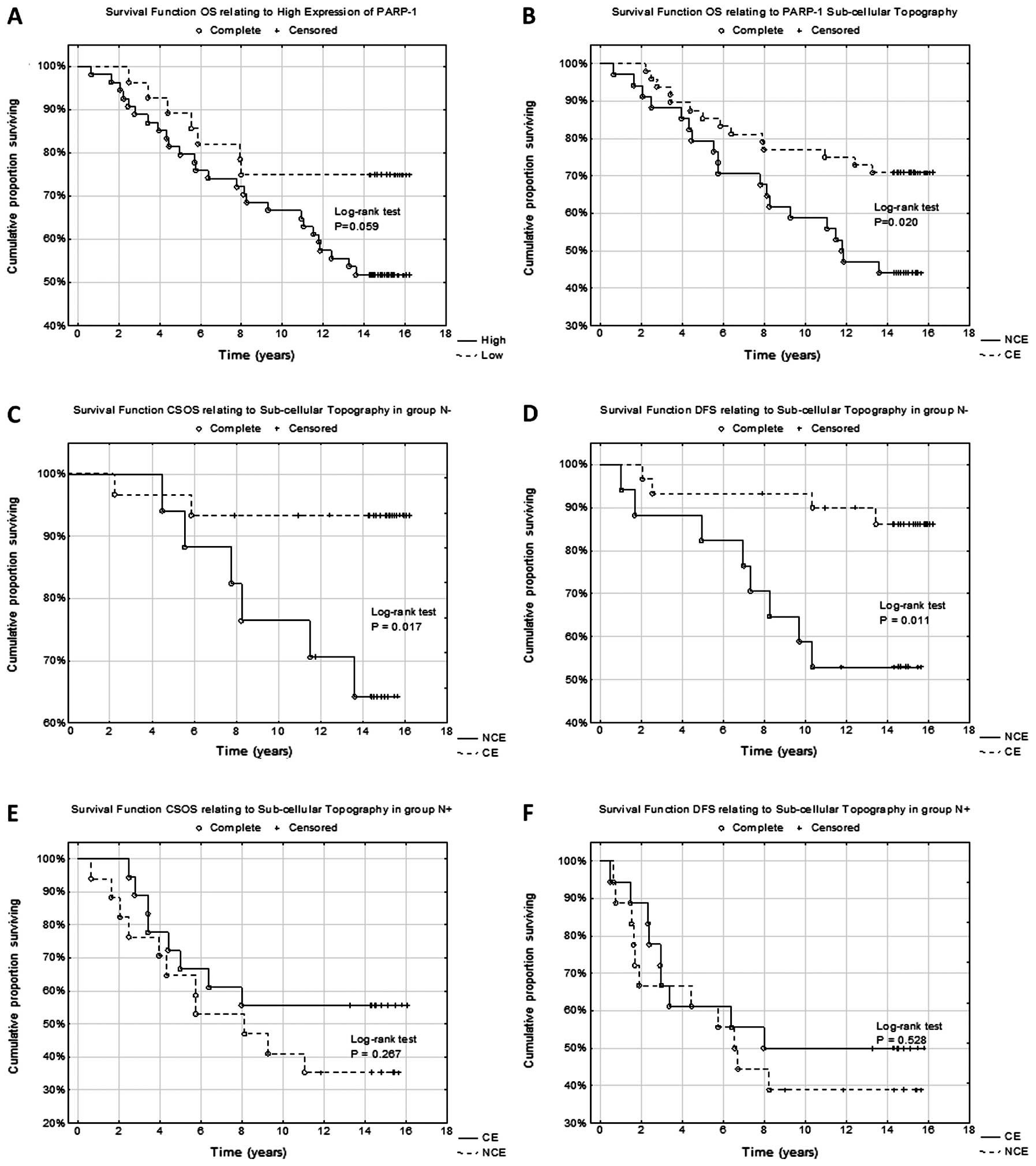
(Fig. 1A and B). Histopathological
evaluation of the specimens revealed two patterns of PARP-1’s
subcellular localizations. Cytoplasmic localization alone was
observed in 48 cases (57.83%) (Fig.
1C and D), whereas nuclear-cytoplasmic localization was
identified in 35 cases (42.17%) (Fig.
1E and F).
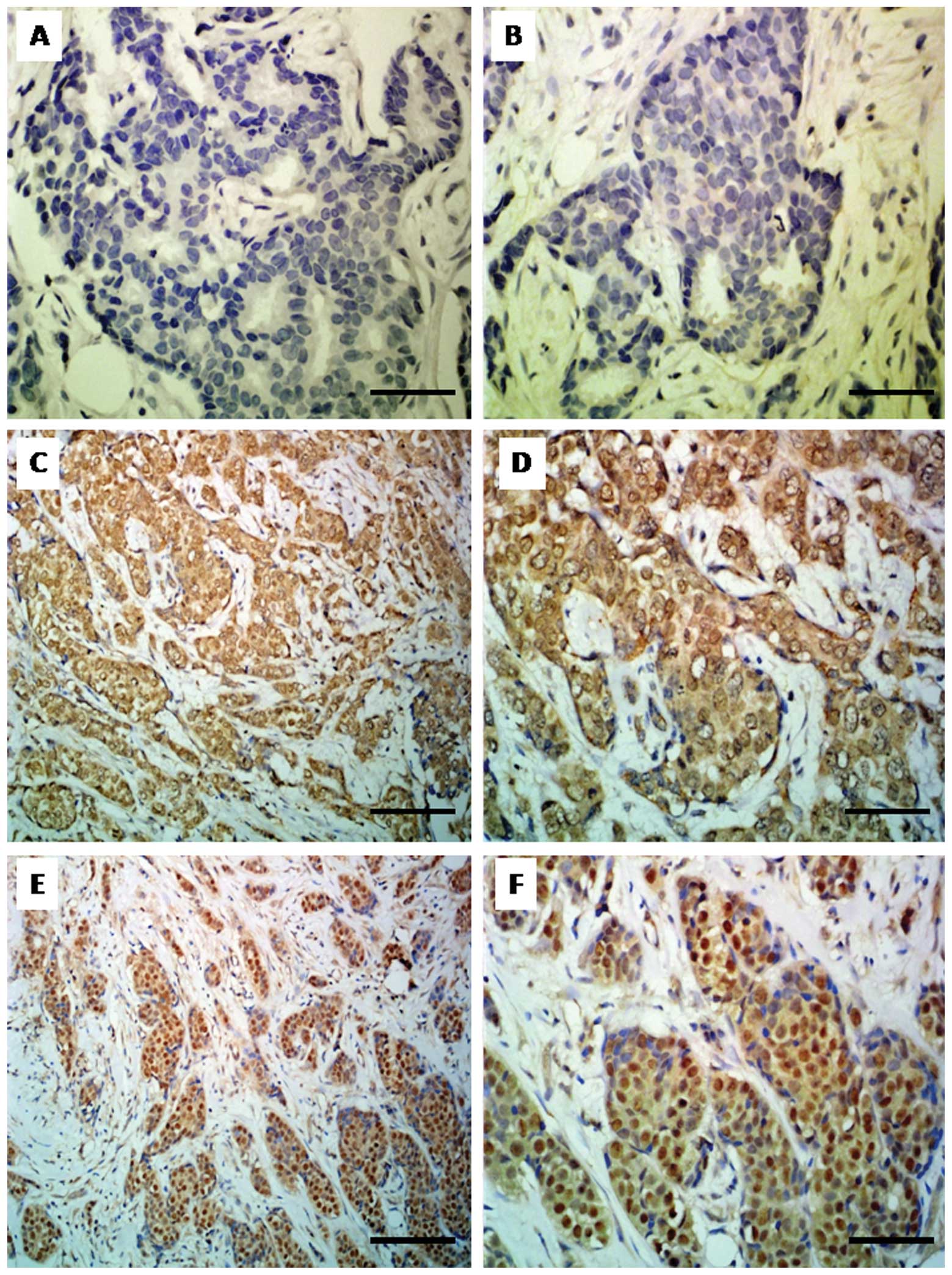 | Figure 1Immunohistochemical analysis of
PARP-1 expression. (A and B) Lack of PARP-1 expression in breast
cancer cells [ImmunoReactive Score (IRS) 0, ×400, hematoxylin]. (C)
Cytoplasmic expression of PARP-1 in breast cancer cells (IRS 8;
magnification, ×200; hematoxylin). (D) Cytoplasmic expression of
PARP-1 (IRS 8; magnification, ×400; hematoxylin). (E)
Nuclear-cytoplasmic expression (NCE) of PARP-1 (IRS 12;
magnification, ×200; hematoxylin). (F) NCE of PARP-1 (IRS 12;
magnification, ×400; hematoxylin). Bars: A, B, D and F, 50 μm; C
and E, 100 μm. |
Relationship between PARP-1 expression
and status of steroid receptors and HER-2 reactivity
There was a statistically significant correlation
between overexpression of PARP-1 (IRS ≥6) and positive ER status
(P=0.009). No significant correlations were identified for PgR or
HER-2 status (Table I).
Intracellular localization of PARP-1 [nuclear-cytoplasmic (NCE);
cytoplasmic (CE)] showed no statistically significant correlations
with ER, PgR or HER-2 expression parameters.
Relationship between PARP-1 expression
and clinicopathological parameters
Higher tumor grading (G) showed statistically
significant correlation with low expression of PARP-1, defined as
IRS 0–4 (P=0.003). Paradoxically, PARP-1 overexpression was closely
related to higher stage according to UICC (II B vs. II A)
(P=0.013). Furthermore, PARP-1 overexpression was significantly
more frequent in patients who were older at the time of diagnosis
(P=0.025). Additionally, higher probability of cancer recurrence
was noted in a group of patients with PARP-1 overexpression, albeit
at the limit of statistical significance (PARP-1 overexpression was
identified in 78.13% patients with cancer; P=0.057) (Table I).
No statistically significant correlations were
observed between overexpression of PARP-1 and the size of the
tumor, the presence of lymph node metastases, menopausal status or
the type of adjuvant therapy (Table
I).
Additionally it was shown that subcellular
localization of PARP-1 is key for the recurrence of BC. In patients
with nuclear-cytoplasmic (NCE) topography, the recurrence of cancer
was highly probable, especially in lung (P=0.011) (Table I).
PARP-1 immunoreactivity and patient
survival; 5-year, 10-year and 15-year observation
No statistically significant correlations were
identified between overexpression of PARP-1 and its localization as
regards 5- and 10- year OS; however 15-year observations presented
notable results. The initial non-significant tendency for higher
mortality risk observed within the first 10 years after diagnosis
augmented within the following 5-year observation period (10th–15th
year) and the analysis of 15-year survival rates showed that
overexpression of PARP-1 (IRS ≥6) was a statistically significant,
unfavorable prognostic factor. Almost 80% of patients with PARP-1
overexpression died during the 15-year observation period (P=0.039)
(Table IV). It is worth noting,
that nuclear-cytoplasmic localization also proved to be an
unfavorable prognostic factor (P=0.015) only during the 15-year
observation period; only 30% of patients with nuclear-cytoplasmic
immunotopography survived the 15-year long observation period.
Similar to the case of other parameters of expression of the
protein under study, the initially non-significant difference in
its immunotopography became a strong prognostic factor after 10
years of observation (Table
IV).
 | Table IVUnivariate analysis of correlations
between immunohistochemical parameters of PARP-1 expression and 5-,
10- and 15-year overall survival and multivariable Cox regression
analysis of PARP-1 expression and 15-year cancer-specific overall
survival in the group without lymph node metastases, with lymph
node metastases and in the whole cohort of patients. |
Table IV
Univariate analysis of correlations
between immunohistochemical parameters of PARP-1 expression and 5-,
10- and 15-year overall survival and multivariable Cox regression
analysis of PARP-1 expression and 15-year cancer-specific overall
survival in the group without lymph node metastases, with lymph
node metastases and in the whole cohort of patients.
| Parameters of
PARP-1 expression | Univariate logistic
regression |
|---|
|
|---|
| 5-year
survivala | 10-year
survivala | 15-year
survivala | 15-year survival
Odds Ratio (95% CI) |
|---|
| % of positive
cells | 0.285 | 0.129 | 0.037 | 2.00
(0.98–4.06) |
| Intensity | 0.684 | 0.197 | 0.029 | 2.32
(1.05–5.17) |
| IRS | 0.399 | 0.087 | 0.006 | 1.30
(1.06–1.58) |
| High expression
(IRS ≥6) | 0.270 | 0.464 | 0.039 | 2.79
(1.00–7.75) |
| NCE | 0.517 | 0.095 | 0.015 | 3.08
(1.21–7.83) |
|
| Multivariable Cox
regression analysis of 15-year survival |
|
|
| All patients | With lymph node
metastases (N+) | Without nodal
metastases (N−) |
|
|
|
|
| Clinicopathological
parameters | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) |
|
| Tumor size
(pT) | 0.026 | 1.05
(1.01–1.09) | 0.170 | 1.04
(0.98–1.09) | 0.036 | 1.06
(1.00–1.12) |
| NCE | 0.016 | 2.68
(1.21–5.98) | 0.092 | 2.29
(0.87–6.01) | 0.033 | 8.88
(1.19–66.29) |
| % of PARP-1
positive cellsb | 0.028 | 2.81
(1.12–7.03) | 0.033 | 3.68
(1.11–12.20) | 0.756 | 0.77
(0.14–4.13) |
| Nodal
metastases | 0.0003 | 4.79
(2.04–11.23) | - | - | - | - |
The Kaplan-Meier estimators also confirmed the
findings. Nuclear-cytoplasmic localization was closely correlated
with unfavorable prognosis as compared with patients in whom
cytoplasmic of PARP-1 topography (P=0.020) alone was identified
(Fig. 2B). Patients with
overexpression of PARP-1 defined as IRS ≥6 were found to have a
tendency of lower survival rate (Fig.
2A) during the 15-year clinical observation period as compared
with the patients with low expression of PARP-1 (P=0.059).
Prognostic significance of PARP-1
expression in lymph node negative (N−) and lymph node positive (N+)
patients
Nodal metastases identified in histopathological
examination are a strongly unfavorable prognostic factor in the
analyzed group of patients (P<0.001), which justified the
necessity to perform an additional analysis of PARP-1 expression in
both subgroups of patients (N− and N+). It was found that only in
N− patients nuclear-cytoplasmic localization was an unfavorable
prognostic factor exclusively in CSOS and DFS analysis (P=0.017,
P=0.011, respectively) (Fig. 2C and
D). No significant correlation was demonstrated for NCE of PARP-1
during OS analysis (P=0.074). It should be noted that the type of
subcellular distribution of PARP-1 had no prognostic value in N+
patients (Fig. 2E and F). No other
statistically significant correlations related to the prognostic
significance of overexpression or individual parameters of PARP-1
immunoreactivity in N− or N+ patients.
Multivariable Cox regression
analysis
The following four parameters (Table IV), which in the multivariate
proportional hazard regression model with backward stepwise
variables elimination proved to have a statistically significant
effect on the prognosis, were found to be independent factors of
poor prognosis in the group of patients subjected to the study: i)
occurrence of metastasis in lymph nodes (P=0.0003), ii) tumor size
(P=0.026), iii) nuclear-cytoplasmic localization of PARP-1
(P=0.016), and iv) high percentage (0–75 vs. >75% positive
cells) of cells with PARP-1 expression (P=0.028). Other
clinicopathological parameters did not significantly influence the
prognosis in the multivariable Cox regression analysis.
Due to a decisive influence of lymph node metastases
on prognosis, multivariate analysis was conducted separately in N−
and N+ groups of patients (Table
IV). It was demonstrated that for N− patients,
nuclear-cytoplasmic topography of PARP-1 was an independent
unfavorable prognostic factor (P=0.033), which confirmed earlier
findings of univariate analysis. Additionally, in N− patients, it
was found that the larger the tumor the poorer the prognosis was
(P=0.035).
In N+ patients, high percentage of PARP-1-positive
cells had a significant, unfavorable influence on poor outcome in
the 15-year observation (P=0.033). Other clinicopathological
factors did not show statistically significant prognostic value in
the multivariate analysis.
PARP-1 expression in relation to
drug-resistance
The studied BC cell lines demonstrated the following
intensity of reaction for PARP-1: MCF-7, cytoplasmic PARP-1, IRS=4;
nuclear PARP-1, IRS=8; CAMA-1, cytoplasmic PARP-1, IRS=2; nuclear
PARP-1, IRS=4; SK-BR-3, cytoplasmic PARP-1, IRS=3; nuclear PARP-1,
IRS=1; R-103, cytoplasmic PARP-1, IRS=3; nuclear PARP-1, IRS=12.
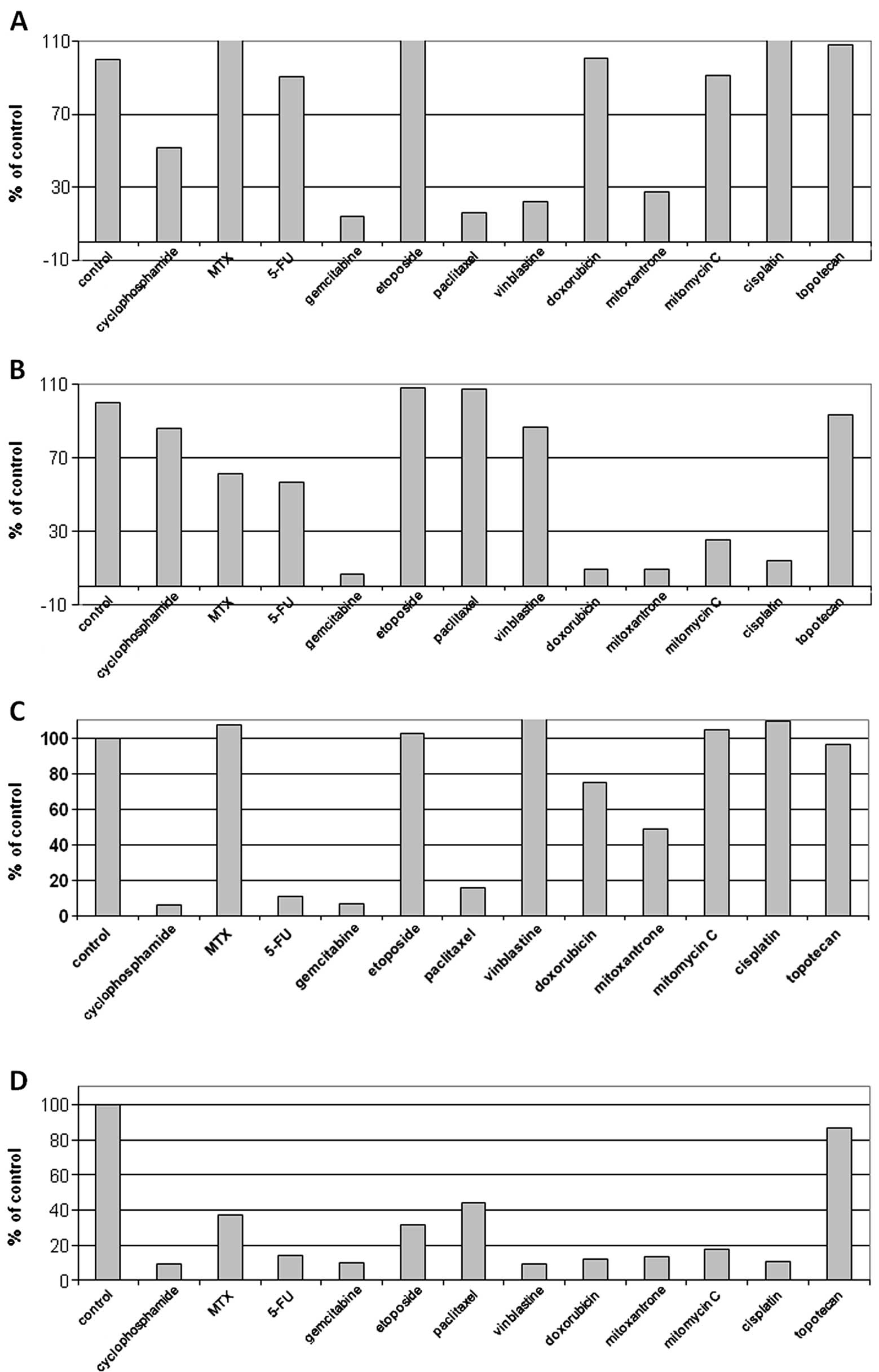
The results of conducted cytotoxicity tests and the results of
immunocytochemical reactions are depicted in Figs. 3A and 4. The investigations demonstrated no
relationship between expression of PARP-1 in BC cells and
sensitivity of the cells to cytostatic drugs.
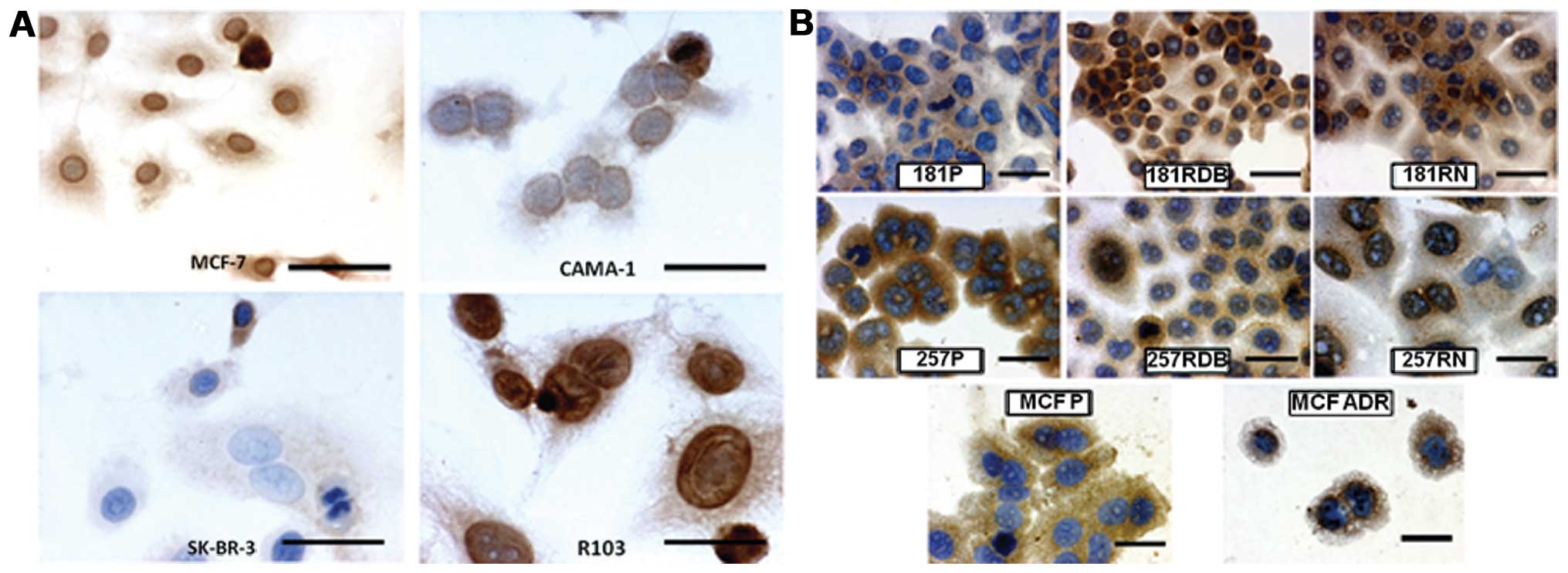 | Figure 3(A) Immunocytochemical localization
of PARP-1 expression in cells of the MCF-7, CAMA-1, SK-BR-3 and
R-103 breast cancer cell lines (x400, hematoxylin). (B)
Immunocytochemical localization of PARP-1 expression in cells of
EPG85-257P (257P), EPG85-257RDB (257RDB), EPG85-257RNOV (257RNOV),
EPP85-181P (181P), EPP85-181RDB (181RDB), EPP85-181RNOV (191RNOV),
MCF-7 and MCF-7/ADR (x600, hematoxylin). Bars: A, 12,5 μm; B, 6.25
μm. |
In the case of cytostatic-resistant cell line
models, we revealed varying positive immunocytochemical reactions.
Cytoplasmic localization of PARP-1 did not correlate with
sensitivity to cytotoxic drugs, but we observed a significantly
higher intensity of PARP-1 immunoreaction in the nucleus of the
cytostatic-resistant cell lines (Table
II, Fig. 3B).
Discussion
In the present study, we investigated the expression
of PARP-1 in a homogeneous group of patients with stage II invasive
ductal BC. In addition, we assessed relationships between the
subcellular localization of this protein, clinicopathological
parameters and patient survival over a 15-year period. In
vitro analysis demonstrated no relationship between expression
of PARP-1 in BC cells and sensitivity of the cells to 11 cytostatic
drugs. Notably, in the case of cytostatic-resistant cell line
models, we revealed that the cytoplasmic localization of PARP-1 did
not correlate with sensitivity to cytotoxic drugs, but we observed
a significantly higher intensity of PARP-1 immunoreaction in the
nucleus of the cytostatic-resistant cell lines. Further extensive
studies are required to describe the part played by a specific
protein in cytotoxic drug resistance. In the present study, we
suggested only that heightened expression of PARP-1 is a
characteristic for cells resistant to cytotoxic drugs. This
phenomenon might only be a phenotype. However, the basis of the
studies on the new predictive markers is to find phenotypes with
characteristic patterns for different drug resistance.
In the present study, overexpression of PARP-1
defined as IRS ≥6 was found in 66.3% of patients (55 patients),
while low immunoreactivity of PARP-1 was observed in 33.7% (28
patients). Cytoplasmic localization alone was observed in 48 cases
(57.83%), and nuclear-cytoplasmic localization was observed in 35
cases (42.17%). Domagala et al (34) with the use of tissue microarray,
immunohistochemically determined the expression of PARP-1 in 130
cases of BRCA1-dependent BC and in 594 cases of sporadic BC
(BRCA1-non-related). Using the QS index (QuickScore Method), high
percentage of BC with overexpression of PARP-1 was identified since
enhanced reactivity of PARP-1 was noted in as many as 81.5% of
cases (106/130) of patients with BRCA1-dependent BC and in as many
as 91.2% (542/594) with BRCA1-independent BC (34). Furthermore, in the analyzed
publication, the dominant subcellular localization of PARP-1 was
nuclear expression alone (86.2%, 112/130 BRCA1-dependent BC; 93.8%,
557/594 BRCA1-independent BC). von Minckwitz et al (35) showed overexpression of PARP-1 in
only 23.7% of BC cases (151/638 patients); however, their study
covered significantly wider clinical and histopathological spectrum
of BCs (ductal and lobular histological type; G1-3; cT2-4) as
compared with the present study. Similar to our observations, von
Minckwitz et al (35)
confirmed the presence of PARP-1 within two cellular compartments,
namely cytoplasmic and nuclear ones.
Markedly, nuclear localization was the only one
observed by Rojo et al (36)
conducting research into overexpression of PARP-1 in 330 cases of
BC. The results may be due to a very specific method of measuring
overexpression of PARP-1. Computer assisted microscopic image
analysis was used and the optical density (OD) was calculated. OD
values ranged from 29 to 133.094, and 39.970 proved to be a limit
value over which overexpression of PARP-1 was defined. With this
definition of cut-off point, enhanced reactivity of PARP-1 was
found in 31.2% cases of BC (36).
In the present study, statistical analysis showed
that enhanced PARP-1 expression is closely correlated with positive
ER status (P=0.009). No statistically significant correlations were
identified between PARP-1 expression and PgR or HER-2 status.
Similar results as regards the lack of correlation
of PARP-1 overexpression with PgR or HER-2 status were obtained by
Rojo et al (36), although
it must be stressed that in the study, a strong significant
correlation between positive PARP-1 status and the lack of ER
expression was identified. Results similar to those of Rojo et
al (36) discussed above were
reported by von Minckwitz et al (35) who also showed significant
correlation between PARP-1 overexpression and negative ER and PgR
status, but, similar to our results, found no significant
correlations with HER-2 overexpression. Ozretic et al
(37) investigated the correlation
between increased PARP-1 reactivity in BC cells and negative ER and
PgR status. The researchers showed that PARP-1 overexpression in
nuclear localization is closely correlated with positive ER and PgR
status which corresponds to our findings and rejects the hypothesis
that overexpression of PARP-1 is restricted to triple negative BC
phenotype.
No correlation between PARP-1 overexpression and
lymph node metastases was found. However, von Minckwitz et
al (35) demonstrated that high
reactivity of PARP-1 in cancer tissue is correlated with the
occurrence of metastases in regional lymph nodes. Other researchers
did not show statistically significant correlations between
increased PARP-1 immunoreactivity and the occurrence of nodal
metastases (36).
In the present study, higher tumor grading (G)
showed statistically significant correlation with low PARP-1
expression (P=0.003). It is an unexpected result which confirms
that the role of PARP-1 in BC biology needs to be studied further
as two independent research groups have shown that high tumor grade
is closely correlated with PARP-1 overexpression (35,36).
Dual role of PARP-1 overexpression is confirmed in another
correlation that indicates that PARP-1 overexpression is closely
related to a more advanced clinical stage according to UICC (II B
vs. II A) (P=0.013). Furthermore, PARP-1 overexpression was
significantly more frequent in patients who were older at diagnosis
(P=0.025). No statistically significant correlations were
identified between PARP-1 overexpression and tumor size, which is
consistent with the observation of von Minckwitz et al
(35) and Rojo et al
(36).
The present study showed that subcellular
localization of PARP-1 is of key importance for the recurrence of
BC, especially in lung. Since Rojo et al (36) described only nuclear localization of
PARP-1, all relations associated with negative ER and PgR status,
grade (high G) and adverse prognosis are valid for this
sublocalization. In the study by von Minckwitz et al
(35), nuclear localization was of
no importance both as regards prognosis and analysis with
clinicopathological parameters.
The key stages of the research were analyses of
PARP-1 expression as regards its prognostic value for 5-, 10- and
15-year survival rates. The results were notable as no
statistically significant correlations were found between PARP-1
overexpression and its localization as regards 5- and 10-year
survival; however, the 15-year observation presented marked
results. It was found that PARP-1 overexpression (IRS ≥6) was a
significant, adverse prognostic factor only after 10–15 years of
the initial diagnosis (P=0.039). It should be noted that the
nuclear-cytoplasmic localization also proved to be an adverse
prognostic factor only in the course of the 15-year observation
period (P=0.015).
Nuclear-cytoplasmic localization of PARP-1
expression had an adverse effect on the prognosis for lymph node
negative (N−) patients (P=0.011). No such correlation was found for
lymph node positive (N+) patients. Therefore, NCE became a marker
of distant recurrence in patients with potentially favorable
prognosis.
Von Minckwitz et al (35) showed a significant correlation
between shorter DFS and shorter OS and PARP-1 overexpression.
However, in the present study, cytoplasmic localization was an
unfavorable prognostic and no significant correlations were
observed as regards PARP-1 nuclear expression. Similar to our
results, Rojo et al (36)
showed that PARP-1 overexpression and its nuclear localization are
statistically significant unfavorable prognostic factors that are
closely related to very high risk of cancer recurrence and
cancer-specific mortality in the course of BC disease.
We acknowledge that the small patient population is
a limitation of the present study. However, the number of patients
was strongly influenced by the single series performed by our
institution and highly homogenous characteristic of breast tumors
(ductal invasive BC, G2 and G3, clinical stage II according to
UICC, Madden mastectomy). Indeed, such a long follow-up period is
rare and, in this regard, our research group is unique. Evaluation
of stage II ductal BC patients eliminated part of the clinical
variables that could bias the analysis of prognostic significance.
It should also be emphasized that our patient cohort is well
described and researched. The results of many analyses performed on
this group (originally larger, approximately 100 cases, currently
only 85 due to the usage of tissue material) were previously
published (38–40).
The critical role of the long observation period of
BC patients must be stressed since only 10 years after the initial
diagnosis did the differences in survival of analyzed subgroups of
patients appear and the prognostic significance of PARP-1
overexpression and its subcellular localization could be analyzed.
This finding is of note as no study published until October 2013
described a group of patients subjected to such a long observation.
Considering the continuous improvement of BC therapy, a several
year observation that is significantly longer than the most
frequently reported 5-year observation period is crucial for the
evaluation of the prognostic value of PARP-1 expression.
In conclusion, in non-advanced and N− BC patients
who are classified as having favorable prognosis, a subgroup with a
potentially poorer long-term prognosis (shorter OS, CSOS and DFS)
was identified. Nuclear-cytoplasmic localization of PARP-1
expression was more frequently identified in these patients. This
correlation provides a notable basis for further studies; however,
it remains to be confirmed in larger cohort studies.
Acknowledgements
This study was supported by Wroclaw Medical
University research grant ST-593.
References
|
1
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ; Panel members. Strategies for
subtypes - dealing with the diversity of breast cancer: highlights
of the St Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chambon P, Weill JD and Mandel P:
Nicotinamide mononucleotide activation of new DNA-dependent
polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res
Commun. 11:39–43. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kiliańska ZM, Żołnierczyk J and
Węsierska-Gądek J: Biological activity of poly(ADP-ribose)
polymerase-1. Postepy Hig Med Dosw. 64:344–363. 2010.(In
Polish).
|
|
4
|
Shieh WM, Ame JC, Wilson MV, Wang ZQ, Koh
DW, Jacobson MK and Jacobson EL: Poly(ADP-ribose) polymerase null
mouse cells synthesize ADP-ribose polymers. J Biol Chem.
273:30069–30072. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Otto H, Reche PA, Bazan F, Dittmar K, Haag
F and Koch-Nolte F: In silico characterization of the family
of PARP-like poly(ADP-ribosyl)transferases (pARTs). BMC Genomics.
6:1392005. View Article : Google Scholar
|
|
6
|
Krishnakumar R and Kraus WL: The PARP side
of the nucleus: molecular actions, physiological outcomes, and
clinical targets. Mol Cell. 39:8–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mangerich A and Bürkle A: How to kill
tumor cells with inhibitors of poly(ADP-ribosyl)ation. Int J
Cancer. 128:251–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Langelier MF, Servent KM, Rogers EE and
Pascal JM: A third zinc-binding domain of human poly(ADP-ribose)
polymerase-1 coordinates DNA-dependent enzyme activation. J Biol
Chem. 283:4105–4114. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Langelier MF, Ruhl DD, Planck JL, Kraus WL
and Pascal JM: The Zn3 domain of human poly(ADP-ribose)
polymerase-1 (PARP-1) functions in both DNA-dependent
poly(ADP-ribose) synthesis activity and chromatin compaction. J
Biol Chem. 285:18877–18887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu JF and Silver DP: Poly ADP-ribose
polymerase inhibitors: science and current clinical development.
Curr Opin Oncol. 22:567–572. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lord CJ and Ashworth A: The DNA damage
response and cancer therapy. Nature. 481:287–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Javle M and Curtin NJ: The role of PARP in
DNA repair and its therapeutic exploitation. Br J Cancer.
105:1114–1122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horton JK and Wilson SH: Hypersensitivity
phenotypes associated with genetic and synthetic inhibitor-induced
base excision repair deficiency. DNA Repair. 6:530–543. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ame JC, Spenlehauer C and de Murcia G: The
PARP superfamily. Bioessays. 26:882–893. 2004. View Article : Google Scholar
|
|
15
|
Plummer ER and Calvert H: Targeting
poly(ADP-ribose) polymerase: a two-armed strategy for cancer
therapy. Clin Cancer Res. 13:6252–6256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Woodhouse BC and Dianov GL: Poly
ADP-ribose polymerase-1: an international molecule of mystery. DNA
Repair. 7:1077–1086. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dębska S, Kubicka J, Czyżkowski R, Habib M
and Potemski P: PARP inhibitors - theoretical basis and clinical
application. Postepy Hig Med Dosw. 66:311–321. 2012.(In
Polish).
|
|
18
|
Sobin LH and Wittekind C: TNM
classification of malignant tumours. 5th edition. Wiley-Liss Inc;
New York: 2002
|
|
19
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I The value of histological
grade in breast cancer: experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar
|
|
20
|
Halon A, Materna V, Drag-Zalesinka M,
Nowak-Markwitz E, Gansukh T, Donizy P, Spaczynski M, Zabel M,
Dietel M, Lage H and Surowiak P: Estrogen receptor alpha expression
in ovarian cancer predicts longer overall survival. Pathol Oncol
Res. 17:511–518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halon A, Nowak-Markwitz E, Maciejczyk A,
Pudelko M, Gansukh T, Györffy B, Donizy P, Murawa D, Matkowski R,
Spaczynski M, Lage H and Surowiak P: Loss of estrogen receptor beta
expression correlates with shorter overall survival and lack of
clinical response to chemotherapy in ovarian cancer patients.
Anticancer Res. 31:711–718. 2011.
|
|
22
|
Szczuraszek K, Halon A, Materna V, Mazur
G, Wrobel T, Kuliczkowski K, et al: Elevated YB-1 expression is a
new unfavorable prognostic factor in non-Hodgkin’s lymphomas.
Anticancer Res. 31:2963–2970. 2011.PubMed/NCBI
|
|
23
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German).
|
|
24
|
Lage H and Dietel M: Multiple mechanisms
confer different drug-resistant phenotypes in pancreatic carcinoma
cells. J Cancer Res Clin Oncol. 128:349–357. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Osheroff N, Corbert AH and Robinson MJ:
Mechanism of action of topoisomerase II-targeted antineoplastic
drugs. Adv Pharmacol. 29B:105–126. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wichert A, Stege A, Midorikawa Y, Holm PS
and Lage H: Glypican-3 is involved in cellular protection against
mitoxantrone in gastric carcinoma cells. Oncogene. 23:945–955.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kellner U, Hutchinson L, Seidel A, Lage H,
Danks MK, Dietel M and Kaufmann SH: Decreased drug accumulation in
a mitoxantrone-resistant gastric carcinoma cell line in the absence
of P-glycoprotein. Int J Cancer. 71:817–824. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lage H, Perlitz C, Abele R, Tampe R,
Dietel M, Schadendorf D and Sinha P: Enhanced expression of human
ABC-transporter tap is associated with cellular resistance to
mitoxantrone. FEBS Lett. 503:179–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lage H: Molecular analysis of therapy
resistance in gastric cancer. Dig Dis. 21:326–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Györffy B, Surowiak P, Kiesslich O,
Denkert C, Schäfer R, Dietel M and Lage H: Resistance prediction
profile for eleven anticancer agents at clinical concentrations
based on the gene expression pattern of thirty cell lines. Int J
Cancer. 118:1699–1712. 2006.
|
|
31
|
Altan N, Chen Y, Schindler M and Simon SM:
Defective acidification in human breast tumor cells and
implications for chemotherapy. J Exp Med. 187:1583–1598. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vickers PJ, Dickson RB, Shoemaker R and
Cowan KH: A multidrug-resistant MCF-7 human breast cancer cell line
which exhibits cross-resistance to antiestrogens and
hormone-independent tumor growth in vivo. Mol Endocrinol.
2:886–892. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ke W, Yu P, Wang J, Wang R, Guo C, Zhou L,
Li C and Li K: MCF-7/ADR cells (re-designated NCI/ADR-RES) are not
derived from MCF-7 breast cancer cells: a loss for breast cancer
multidrug-resistant research. Med Oncol (Suppl). 1:S135–S141. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Domagala P, Huzarski T, Lubinski J, Gugala
K and Domagala W: PARP-1 expression in breast cancer including
BRCA1-associated, triple negative and basal-like tumors: possible
implications for PARP-1 inhibitor therapy. Breast Cancer Res Treat.
127:861–869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
von Minckwitz G, Müller BM, Loibl S,
Budczies J, Hanusch C, Darb-Esfahani S, et al: Cytoplasmic
poly(adenosine diphosphate-ribose) polymerase expression is
predictive and prognostic in patients with breast cancer treated
with neoadjuvant chemotherapy. J Clin Oncol. 29:2150–2157.
2011.
|
|
36
|
Rojo F, Garcia-Parra J, Zazo S, Tusquets
I, Ferrer-Lozano J, Menendez S, et al: Nuclear PARP-1 protein
overexpression is associated with poor overall survival in early
breast cancer. Ann Oncol. 23:1156–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ozretic L, Rhiem K, Huss S, Wappenschmidt
B, Markiefka B, Sinn P, Schmutzler RK and Buettner R: High nuclear
poly(adenosine diphosphate-ribose) polymerase expression is
predictive for BRCA1- and BRCA2-deficient breast cancer. J Clin
Oncol. 29:4586–4588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Surowiak P, Materna V, Matkowski R,
Szczuraszek K, Kornafel J, Wojnar A, Pudelko M, et al: Relationship
between the expression of cyclooxygenase 2 and MDR1/P-glycoprotein
in invasive breast cancers and their prognostic significance.
Breast Cancer Res. 7:R862–R870. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Surowiak P, Materna V, Györffy B,
Matkowski R, Wojnar A, Maciejczyk A, et al: Multivariate analysis
of oestrogen receptor alpha, pS2, metallothionein and CD24
expression in invasive breast cancers. Br J Cancer. 95:339–346.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maciejczyk A, Szelachowska J, Ekiert M,
Matkowski R, Hałoń A, Lage H and Surowiak P: Elevated nuclear YB1
expression is associated with poor survival of patients with early
breast cancer. Anticancer Res. 32:3177–3184. 2012.PubMed/NCBI
|