Introduction
Hepatocellular carcinoma (HCC) is one of the leading
causes of cancer-related deaths worldwide (1). Although surgical treatment and
non-surgical therapeutic modalities such as radiotherapy,
chemotherapy and interventional therapy have been employed, the
prognosis of HCC patients remains discouraging due to the high
recurrence rate and frequent incidence of intrahepatic metastasis
(2,3). Angiogenesis is essential for
continuous tumor growth and provides an avenue for hematogenous
metastasis (4–9). Tumor angiogenesis is strongly
regulated by multiple intracellular signaling transduction cascades
such as the Notch pathway (10,11).
The activation of Notch signaling is initiated by the binding of
transmembrane Notch proteins (Notch1, Notch2, Notch3 and Notch4) to
their specific membrane-bound ligands (Jagged1, Jagged2 and
Delta-likes Dll1, Dll3 and Dll4). Mounting evidence shows that
perturbation of the Notch pathway often leads to tumorigenesis
(12–16), and the potential role of Notch
signaling in the development of HCC designates Notch and its
ligands as promising targets for HCC therapy (17–20).
Natural products, including traditional Chinese
medicines (TCMs), have been used clinically for thousands of years
as important alternative remedies for a variety of diseases
including cancer (21–23). Therefore, interest in the use of
natural products as therapeutic agents for cancer has recently
increased. Livistona chinensis, belonging to the
monocotyledonous Palmaceae family, is a medicinal herb widely
distributed in Eastern Asia. The seeds of Livistona
chinensis have long been used in China to clinically treat
various types of cancer (24–27).
Our previous findings suggest that Livistona chinensis seeds
may be effective in cancer treatment via promoting
mitochondrial-dependent apoptosis in vivo and in
vitro (28). To further
elucidate the molecular mechanisms of its antitumor activity, in
the present study, we used a HCC xenograft mouse model to evaluate
the effect of an ethanol extract of Livistonae chinensis
seeds (EELC) on tumor angiogenesis and on the activation of the
Notch pathway.
Materials and methods
Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA and
TRIzol reagent were purchased from Invitrogen (Carlsbad, CA, USA).
SuperScript II reverse transcriptase was obtained from Promega
(Madison, WI, USA). CD31, vascular endothelial growth factor A
(VEGF-A), VEGFR-2, Notch, Dll4 and Jagged1 antibodies and secondary
antibodies were obtained from Cell Signaling (Beverly, MA, USA).
All other chemicals, unless otherwise stated, were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Preparation of ethanol extract from
Livistona chinensis seeds
Livistona chinensis (500 g) seeds were
extracted with 5,000 ml of 85% ethanol using a refluxing method and
were filtered. The resultant solution was concentrated to a
relative density of 1.05, and the dried powder of EELC was obtained
by spraying desiccation method using a spray dryer (Model B-290;
Buchi, Switzerland). For animal experiments, the powder of EELC was
dissolved in saline to a working concentration of 300 mg/ml.
Cell culture
Human HCC HepG2 cells were obtained from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). The
cells were cultured in DMEM containing 10% (v/v) FBS, and 100 U/ml
penicillin and 100 μg/ml streptomycin in a 37°C humidified
incubator with 5% CO2. The cells were subcultured at
80–90% confluency.
Animals
Male BALB/c athymic (nude) mice (with an initial
body weight of 20–22 g) were obtained from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China) and housed under pathogen-free
conditions with controlled temperature (22°C), humidity, and a 12 h
light/dark cycle. Food and water were given ad libitum
throughout the experiment. All animal treatments were strictly
performed in accordance with international ethical guidelines and
the National Institutes of Health Guide Concerning the Care and Use
of Laboratory Animals. The experiments were approved by the
Institutional Animal Care and Use Committee of Fujian University of
Traditional Chinese Medicine.
In vivo nude mouse xenograft study
HepG2 (4×106) cells mixed with Matrigel
(1:1) were subcutaneously injected into the right flank of mice to
initiate tumor growth. After 7 days of xenograft implantation when
tumor size reached ~3 mm in diameter, mice were randomized into two
groups (n=10) and given an intragastric administration of 3 g/kg of
EELC or saline daily, 5 days a week for 21 days. At the end of the
experiment, the animals were anaesthetized with pelltobarbitalum
natricum, and the tumor tissue was removed. A portion of each tumor
was fixed in 10% buffered formalin, and the remaining tissue was
snap-frozen in liquid nitrogen and stored at −80°C.
Immunohistochemical analysis
After being fixed with 10% formaldehyde for 12 h,
tumor samples were processed conventionally for paraffin-embedded
tumor slides. The slides were subjected to antigen retrieval and
the endogenous peroxidase activity was quenched with hydrogen
peroxide. After blocking non-specific proteins with normal serum in
phosphate-buffered saline (PBS) (0.1% Tween-20), slides were
incubated with rabbit polyclonal antibodies against CD31, VEGF-A,
VEGFR-2, Notch, Dll4 and Jagged1 (all in a 1:100 dilution). After
washing with PBS, slides were incubated with the biotinylated
secondary antibody followed by conjugated horseradish peroxidase
(HRP)-labeled streptavidin (Dako), and then washed with PBS. The
slides were then incubated with diaminobenzidine (DAB) as the
chromogen, followed by counterstaining with diluted Harris’
hematoxylin (both from Sigma). After staining, five high-power
fields (x400) were randomly selected in each slide, and the average
proportion of positive cells in each field was counted using the
true color multi-functional cell image analysis management system
(Image-Pro Plus; Media Cybernetics, Bethesda, MD, USA). To rule out
any non-specific staining, PBS was used to replace the primary
antibody as a negative control.
RNA extraction and RT-PCR analysis
Briefly, total RNA from tumor tissues was isolated
with TRIzol reagent. Oligo(dT)-primed RNA (1 μg) was
reverse-transcribed with SuperScript II reverse transcriptase
(Promega) according to the manufacturer’s instructions. The
obtained cDNA was used to determine the mRNA amount of VEGF-A,
VEGFR-2, Notch, Dll4 and Jagged1 by PCR. GAPDH was used as an
internal control. The sequences of the primers used for
amplification of VEGF-A, VEGFR-2, Notch, Dll4, Jagged1 and GAPDH
transcripts are as follows: VEGF forward, 5′-GCC TTG CCT TGC TGC
TCT A-3′ and reverse, 5′-GAT GTC CAC CAG GGT CTC G-3′; VEGFR-2
forward, 5′-ACG CCG ATT ATG TGA GA-3′ and reverse, 5′-AGG CAG GAG
TTG AGT ATG-3′; Notch forward, 5′-CCG TCA TCT CCG ACT TCA TC-3′ and
reverse, 5′-GGA CTT GCC CAG GTC ATC TAC-3′; Dll4 forward, 5′-ACA
GTG CCT GAA CCG A-3′ and reverse, 5′-GCC CAC AAA GCC ATA A-3′;
Jagged1 forward, 5′-TCG CTG TAT CTG TCC ACC T-3′ and reverse,
5′-TCC TTT CCA CCC ATT TTT A; GAPDH forward, 5′-GT CAT CCA TGA CAA
CTT TGG-3′ and reverse, 5′-GA GCT TGA CAA AGT GGT CGT-3′.
Statistical analysis
Data are presented as means ± SD for the indicated
number of independently performed experiments, and the data were
analyzed using the SPSS Package for Windows (version 11.5).
Statistical analysis of the data was performed with the Student’s
t-test. Differences with p<0.05 were considered to be
statistically significant.
Results
EELC inhibits tumor angiogenesis in the
HCC xenograft mouse tumors
To determine the effect of EELC on tumor
angiogenesis, we performed immunohistochemical (IHC) staining for
the endothelial cell-specific marker CD31 to examine the
intratumoral microvessel density (MVD) of HCC xenograft mouse
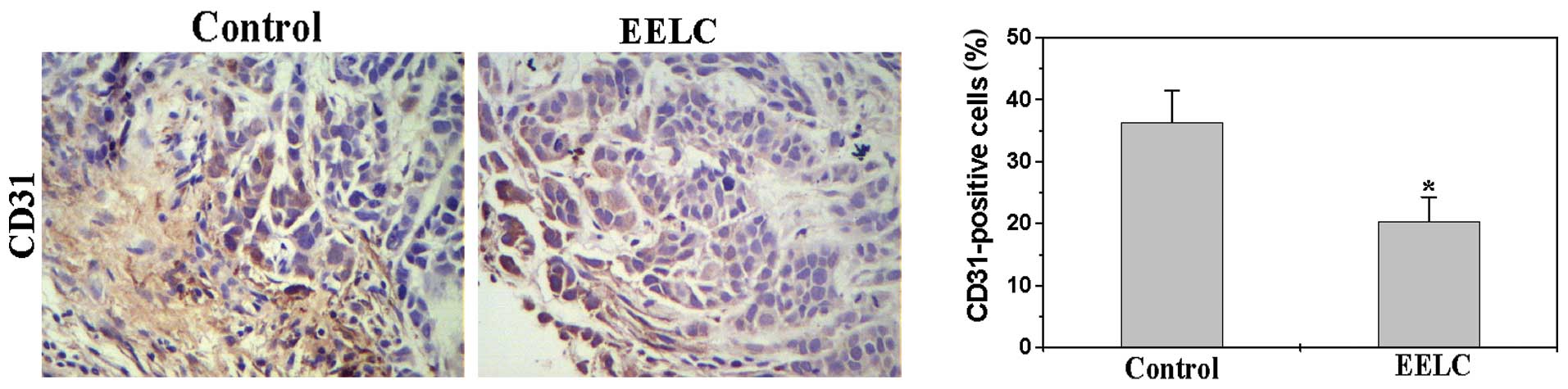
tumors following EELC treatment. As shown in Fig. 1, the percentage of CD31-positive
cells in the control and EELC-treated mice was 36.3±5.21 and
20.41±3.87%, respectively (p<0.05), demonstrating the in
vivo inhibitory effect of EELC on tumor angiogenesis.
EELC inhibits the expression of VEGF-A
and VEGFR-2 in the HCC xenograft mouse tumors
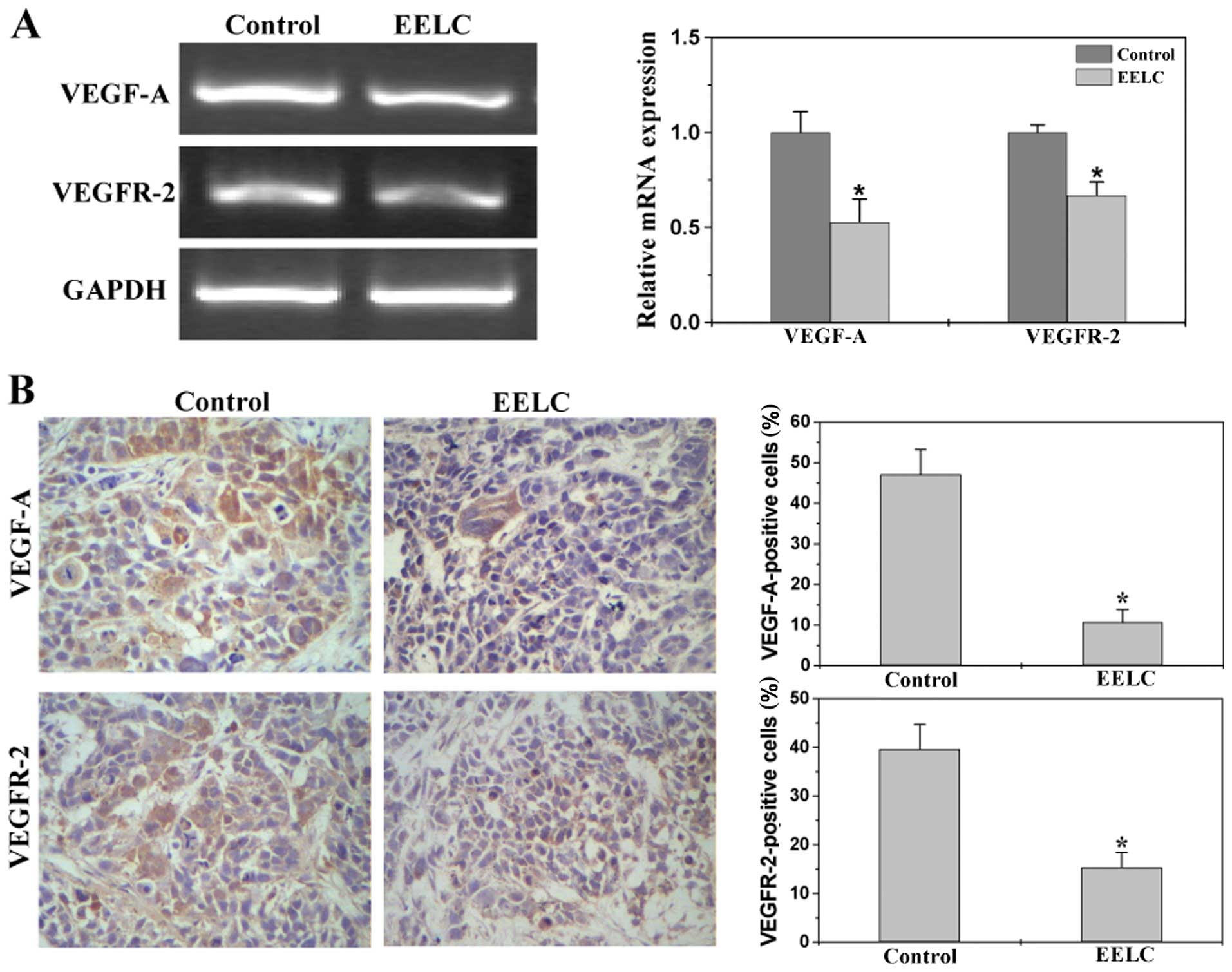
We next determined the effect of EELC on the
expression of VEGF-A and VEGFR-2 in the HCC mouse tumors. Data from
RT-PCR indicated that EELC reduced the mRNA expression of VEGF-A
and VEGFR-2 in tumors (Fig. 2A).
Results of the IHC assay showed that protein expression patterns of
VEGF-A and VEGFR-2 were similar to their respective mRNA levels.
The percentage of VEGF-A- and VEGFR-2-positive cells in the control
group was 47.14±6.14 and 39.63±5.11%, while that in the
EELC-treated mice was 10.78±3.11 and 15.34±3.04% (Fig. 2B).
EELC suppresses the Notch pathway in the
HCC xenograft mouse tumors
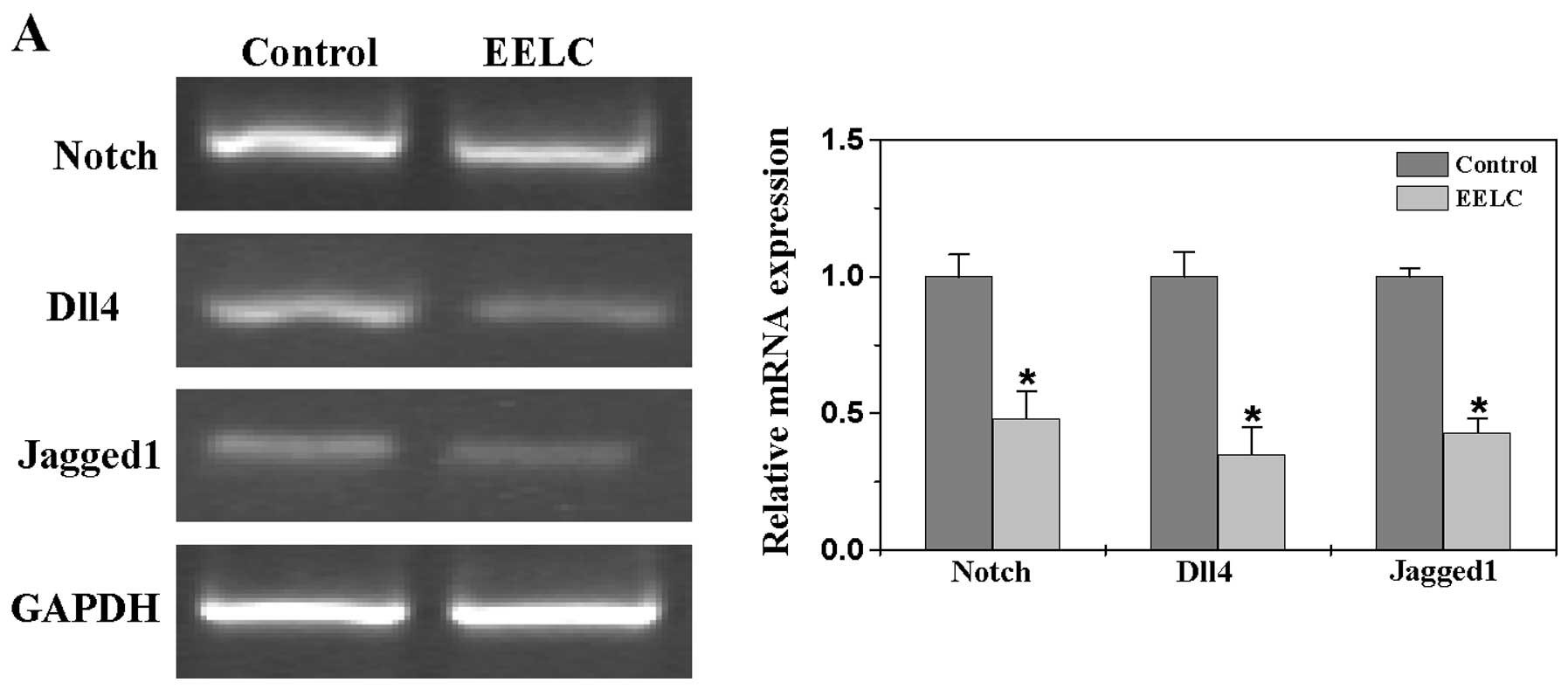
To further investigate the mechanism of
anti-angiogenic activity of EELC, we evaluated its effect on the
Notch pathway by examining the expression of several key mediators
of this signaling. As shown in Fig.
3A, EELC treatment profoundly reduced the mRNA expression of
Notch, Dll4 and Jagged1 in the tumor tissues. Consistently, their
protein expression was also significantly inhibited following EELC
treatment. The percentage of Notch-, Dll4- and Jagged1-positive
cells in the control group was 28.86±5.35, 32.39±4.27 and
27.33±3.58%, whereas that in the EELC-treated mouse tumors was
15.73±4.42, 13.8±2.24 and 12.15±2.11% (Fig. 3B).
Discussion
Deregulated angiogenesis plays a crucial role in the
development of various diseases including cancer (29–31).
Induction of angiogenesis is mediated by a variety of molecules
released by tumor cells (32).
Vascular endothelial growth factor A (VEGF-A) is one of the most
effective biologic inducers of angiogenesis (33–35).
After secretion VEGF-A, primarily interacts with specific receptors
(VEGFR-2) present on the surface of vascular endothelial cells,
which in turn triggers a tyrosine kinase signaling cascade,
inducing endothelial cell proliferation, migration, survival,
sprouting and consequent tube formation (35,38).
VEGF-A was found to be highly expressed in a wide variety of human
cancers and is associated with cancer progression, invasion and
metastasis and poor patient prognosis (37,38).
Therefore, it is not surprising that VEGF is considered as an
attractive strategic target for inhibiting tumor angiogenesis.
A role for Notch signaling is well documented in
both hematological malignancies and in solid tumors. Notch has been
demonstrated to act either as a tumor suppressor or as an oncogene
in a context-dependent manner (39). In addition, several lines of
evidence suggest that Notch also plays an important role in tumor
angiogenesis. Tumor vasculature and tumor cells have also been
shown to overexpress the Notch ligand Dll4. Genetic studies have
shown that reduction in Dll4 results in hypersprouting phenotypes
and disruption of the vasculature (40,41).
Another Notch ligand Jagged1 expressed in tumor cells has been
shown to stimulate Notch-dependent angiogenesis (42,43).
These findings suggest that Notch-specific blockade may inhibit
tumor growth by disrupting angiogenesis. Therefore, Notch has
become a focus for the development of targeted therapeutics in
cancer.
Similar to the majority of other medicinal herbs,
Livistona chinensis is composed of numerous chemical
components such as flavonoids, diterpenoids, alkaloids, steroides
and polysaccharides. Herbal medicines including Livistona
chinensis are thus considered to be multi-target agents that
exert their therapeutic function in a more holistic manner. Our
previous findings suggest that Livistona chinensis seeds may
be effective in inhibiting HCC xenograft mouse tumor growth, and in
promoting cancer cell apoptosis (28). In the present study, we evaluated
the effect of EELC on tumor angiogenesis using a xenograft mouse
model. Our current data revealed that EELC reduced tumor MVD in HCC
mice. In addition, EELC treatment suppressed the expression of
VEGF-A and VEGFR-2 in tumor tissues, which in turn resulted in the
inhibition of tumor angiogenesis. Furthermore, EELC treatment
inhibited the expression of Notch, Dll4 and Jagged1.
In conclusion, our findings suggest that inhibition
of tumor angiogenesis via suppression of the Notch pathway may be
one of the mechanisms by which Livistona chinensis seeds
play an important role in the treatment of cancers.
Acknowledgements
This study was sponsored by the National Natural
Science Foundation of China (81073097 and 81202790).
Abbreviations:
|
EELC
|
ethanol extract of Livistona
chinensis seeds
|
|
MVD
|
microvessel density
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
|
TCM
|
traditional Chinese medicine
|
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Lang H, Sotiropoulos GC, Brokalaki EI,
Schmitz KJ, Bertona C, Meyer G, Frilling A, Paul A, Malagó M and
Broelsch CE: Survival and recurrence rates after resection for
hepatocellular carcinoma in noncirrhotic livers. J Am Coll Surg.
205:27–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gish RG and Baron A: Hepatocellular
carcinoma (HCC): current and evolving therapies. IDrugs.
11:198–203. 2008.PubMed/NCBI
|
|
4
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia L: Cancer complementary and
alternative medicine research at the US National Cancer Institute.
Chin J Integr Med. 18:325–332. 2012. View Article : Google Scholar
|
|
6
|
Carmady B and Smith CA: Use of Chinese
medicine by cancer patients: a review of surveys. Chin Med.
9:222011. View Article : Google Scholar
|
|
7
|
Cheueng S and Tai J: In vitro
studies of the dry fruit of Chinese fan palm Livistona
chinensis. Oncol Rep. 5:1331–1336. 2005.
|
|
8
|
Sartippour MR, Liu C and Shao ZM:
Livistona extract inhibits angiogenesis and cancer growth.
Oncol Rep. 6:1355–1357. 2001.
|
|
9
|
Huang WC, Hsu RM, Chi LM, et al: Selective
downregulation of EGF receptor and downstream MAPK pathway in human
cancer cell lines by active components partially purified from the
seeds of Livistona chinensis R. Brown. Cancer Lett.
248:137–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Li A, Dong XP and Xu XY: Screening
of anti-tumor parts from the seeds of Livistona chinensis
and its anti-angiogenesis effect. Zhong Yao Cai. 31:718–722.
2008.(In Chinese).
|
|
11
|
Lin W, Zhao J, Cao Z, Zhuang Q, Zheng L,
Cai Q, Chen D, Wang L, Hong Z and Peng J: Livistona
chinensis seed suppresses hepatocellular carcinoma growth
through promotion of mitochondrial-dependent apoptosis. Oncol Rep.
29:1859–1866. 2013.
|
|
12
|
Folkman J and Klagsbrun M: Angiogenic
factors. Science. 235:442–447. 1987. View Article : Google Scholar
|
|
13
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schneider BP and Miller KD: Angiogenesis
of breast cancer. J Clin Oncol. 23:1782–1790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uzzan B, Nicolas P, Cucherat M and Perret
GY: Microvessel density as a prognostic factor in women with breast
cancer: a systematic review of the literature and meta-analysis.
Cancer Res. 64:2941–2955. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weidner N: The importance of tumor
angiogenesis: the evidence continues to grow. Am J Clin Pathol.
122:675–677. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kut C, Mac Gabhann F and Popel AS: Where
is VEGF in the body? A meta-analysis of VEGF distribution in
cancer. Br J Cancer. 97:978–985. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeshita K, Satoh M, Ii M, Silver M,
Limbourg FP, Mukai Y, Rikitake Y, Radtke F, Gridley T, Losordo DW
and Liao JK: Critical role of endothelial Notch1 signaling in
postnatal angiogenesis. Circ Res. 100:70–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garcia A and Kandel JJ: Notch: a key
regulator of tumor angiogenesis and metastasis. Histol Histopathol.
27:151–156. 2012.PubMed/NCBI
|
|
20
|
Dufraine J, Funahashi Y and Kitajewski J:
Notch signaling regulates tumor angiogenesis by diverse mechanisms.
Oncogene. 27:5132–5137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ridgway J, Zhang G, Wu Y, Stawicki S,
Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I,
Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G and Yan
M: Inhibition of Dll4 signalling inhibits tumour growth by
deregulating angiogenesis. Nature. 444:1083–1087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miele L, Golde T and Osborne B: Notch
signaling in cancer. Curr Mol Med. 6:905–918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yanagawa S, Lee JS, Kakimi K, Matsuda Y,
Honjo T and Ishimoto A: Identification of Notch1 as a
frequent target for provirus insertional mutagenesis in T-cell
lymphomas induced by leukemogenic mutants of mouse mammary tumor
virus. J Virol. 74:9786–9791. 2000.
|
|
24
|
Ellisen LW, Bird J, West DC, Soreng AL,
Reynolds TC, Smith SD and Sklar J: TAN-1, the human homolog
of the Drosophila Notch gene, is broken by chromosomal
translocations in T lymphoblastic neoplasms. Cell. 66:649–661.
1991. View Article : Google Scholar
|
|
25
|
Sjölund J, Manetopoulos C, Stockhausen MT
and Axelson H: The Notch pathway in cancer: differentiation gone
awry. Eur J Cancer. 41:2620–2629. 2005.PubMed/NCBI
|
|
26
|
Nijjar SS, Crosby HA, Wallace L, Hubscher
SG and Strain AJ: Notch receptor expression in adult human liver: a
possible role in bile duct formation and hepatic
neovascularization. Hepatology. 34:1184–1192. 2001. View Article : Google Scholar
|
|
27
|
Nijjar SS, Wallace L, Crosby HA, Hubscher
SG and Strain AJ: Altered Notch ligand expression in human liver
disease: further evidence for a role of the Notch signaling pathway
in hepatic neovascularization and biliary ductular defects. Am J
Pathol. 160:1695–1703. 2002. View Article : Google Scholar
|
|
28
|
Dorrell MI, Aguilar E, Scheppke L, Barnett
FH and Friedlander M: Combination angiostatic therapy completely
inhibits ocular and tumor angiogenesis. Proc Natl Acad Sci USA.
104:967–972. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.
|
|
30
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Folkman J: Angiogenesis. Annu Rev Med.
57:1–18. 2006. View Article : Google Scholar
|
|
32
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jain RK: Tumor angiogenesis and
accessibility: role of vascular endothelial growth factor. Semin
Oncol. 29(Suppl 16): 3–9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
therapeutic implications. Semin Oncol. 29(Suppl 16): 10–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maeda K, Chung YS, Ogawa Y, Takatsuka S,
Kang SM, Ogawa M, Sawada T and Sowa M: Prognostic value of vascular
endothelial growth factor expression in gastric carcinoma. Cancer.
77:858–863. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaya M, Wada T, Akatsuka T, Kawaguchi S,
Nagoya S, Shindoh M, Higashino F, Mezawa F, Okada F and Ishii S:
Vascular endothelial growth factor expression in untreated
osteosarcoma is predictive of pulmonary metastasis and poor
prognosis. Clin Cancer Res. 6:572–577. 2000.PubMed/NCBI
|
|
38
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Purow B: Notch inhibition as a promising
new approach to cancer therapy. Adv Exp Med Biol. 727:305–319.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gale NW, Dominguez MG, Noguera I, Pan L,
Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J,
Thurston G and Yancopoulos GD: Haploinsufficiency of delta-like 4
ligand results in embryonic lethality due to major defects in
arterial and vascular development. Proc Natl Acad Sci USA.
101:15949–15954. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Duarte A, Hirashima M, Benedito R,
Trindade A, Diniz P, Bekman E, Costa L, Henrique D and Rossant J:
Dosage-sensitive requirement for mouse Dll4 in artery development.
Genes Dev. 18:2474–2478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Skrtic A, Sokolic L, Borovecki A, Rosa J
and Fenzl V: Immunohistochemical localization of CD31, NOTCH1 and
JAGGED1 proteins in experimentally induced polycystic ovaries of
immature rats. Acta Histochem. 113:262–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Benedito R, Roca C, Sörensen I, Adams S,
Gossler A, Fruttiger M and Adams RH: The notch ligands Dll4 and
Jagged1 have opposing effects on angiogenesis. Cell. 137:1124–1135.
2009. View Article : Google Scholar : PubMed/NCBI
|