Introduction
Colorectal cancer (CRC) is one of the most common
malignancies and is the leading cause of cancer-related mortality,
posing a major public health concern worldwide (1). In Asia, the overall CRC cure rate has
not improved dramatically in the last decade with the 5-year
survival rate at ~60% (2). Over the
past decades, a number of the critical mutations in key signaling
pathways underlying the initiation and development of CRC have been
shown to be associated with the regulation of cellular metabolism,
proliferation, differentiation and survival (4,5).
However, despite the fact that much progress has been made in the
therapeutic management of CRC, patient prognosis still remains poor
due to disease recurrence and metastasis. Therefore, elucidation of
mechanisms underlying CRC occurrence and development is needed in
order to develop novel therapeutic approaches to CRC. Over the
years, genetic changes responsible for CRC recurrence and
metastasis have also been extensively explored. Although a large
number of genetic changes have been documented, there are still
many molecular aspects of colorectal tumorigenesis that must be
elucidated (3–5).
The deleted in liver cancer-1 (DLC-1) gene,
also called StAR-related lipid transfer protein 12 (STARD12), is a
novel candidate tumor-suppressor gene located on human chromosome
8p21–22 (6). This region harbors a
number of tumor-suppressor genes, and loss of heterozygosity (LOH)
in this region is frequently detected in a variety of human
cancers, including CRC (5,7–11). It
has been suggested that DLC-1 may inhibit cell proliferation
and induce cell apoptosis in human cancer via regulation of cyclin
D1 expression which acts downstream of β-catenin (12). It is well known that β-catenin is a
key factor in the Wnt/β-catenin signaling pathway (13). Furthermore, it has been shown that
cyclin D1 expression is associated with β-catenin expression and
correlates with favorable prognosis in CRC (14). Moreover, the initiating event in
tumorigenesis of most CRCs is the aberrant activation of the
Wnt/β-catenin signaling pathway (15). Mutations in the components of the
Wnt signaling pathway, such as the adenomatous polyposis coli
(APC) gene and the β-catenin gene (CTNNB1), play
important roles in initiation and development of CRC (16). Since both DLC-1 and the Wnt
pathway are associated with CRC, DLC-1 may interact with the
Wnt pathway in colorectal carcinogenesis. In the present study, we
aimed to investigate the role of DLC-1 in tumor growth and
invasion as well as to explore its possible interactions with the
Wnt signaling pathway. The DLC-1 effects on cell growth and
invasion were examined in a DLC-1-transfected colon cancer
cell line SW480. In addition, in CRC cells overexpressing
DLC-1, the role of the Wnt/β-catenin pathway components
(β-catenin, GSK-3β and c-myc) in cell growth and invasion was
investigated to further establish the relationship between DLC-1
and the Wnt/β-catenin pathway.
Materials and methods
Cell culture and transfection
reagents
The pcDNA3.1(+)-GFP plasmid, oligo DNA, Pfu DNA
polymerase, packaging plasmids (pHelper1.0/pHelper2.0), lentiviral
vectors and other routine molecular reagents were purchased from
Gemma Co., Ltd. (Shanghai, China). Plasmid and DNA
isolation/purification kits were purchased from Qiagen (Shanghai,
China). T4 DNA ligase and restriction enzymes were purchased from
Fermentas Co. (Thermo Fisher Scientific, Waltham, MA, USA).
Lipofectamine™ 2000 and TRIzol were purchased from Invitrogen
(Carlsbad, CA, USA). Culture medium Dulbecco’s modified Eagle’s
medium (DMEM) was purchased from HyClone (Logan, UT, USA). Fetal
bovine serum (FBS) and other culture-related materials were
purchased from Gibco (Carlsbad, CA, USA). The human colon cancer
SW480 cell line, packaging cell lines Top10 and 293T were provided
by The Key Laboratory of Surgery, Chongqing Medical University
(Chongqing, China) and were cultured in DMEM supplemented with 15%
FBS and maintained in a humidified incubator at 37°C with 5%
CO2.
Construction of the expression plasmid
and the lentiviral vector
A full-length DLC-1 cDNA was subcloned into the
pcDNA3.1(+)-GFP plasmid by using T4 DNA ligase, constructing the
recombinant plasmid pcDNA3.1(+)-GFP-DLC-1. The sequence and
orientation of the vector inserts were confirmed by restriction
enzyme digestion and DNA sequencing. Next, competent cell line
Top10 was transfected with the recombinant plasmid using
Lipofectamine 2000, according to the manufacturer’s instructions.
The transfected Top10 cells were cultured in ampicillin plates for
16 h. Positive cell clones were selected and cultured overnight.
The recombinant plasmid was amplified in the Top10 competent cells.
Restriction analysis with BamHI and NotI enzymes and
DNA sequencing were used to confirm the true cloning of
DLC-1.
Recombinant plasmids pcDNA3.1(+)-GFP-DLC-1 and the
packaging vectors (15 μg of pHelper1.0 and 10 μg of pHelper2.0)
were extracted respectively by highly purified and non-toxic
extraction. The 293T cells in the exponential phase of growth were
seeded into 6-well plates containing DMEM supplemented with 10% FBS
at 6×105/ml. Recombinant lentiviruses were packaged by
transfecting the 293T cell line with 20 μg of
pcDNA3.1(+)-GFP-DLC-1, 15 μg pHelper1.0 and 10 μg pHelper2.0 using
100 μl of Lipofectamine 2000, according to the manufacturer’s
instructions. A negative control was constructed by transfecting
the 293T cell line with the pcDNA3.1(+)-GFP plasmid. The medium was
replaced with 25 ml of complete medium 8 h after transfection and
cultured at 37°C in a humidified incubator with 5% CO2.
Forty-eight hours after transfection, cells were collected, and
centrifuged at 12,000 × g for 3 h. Supernatant cells were filtered
through a PVDF membrane and kept in an ice bath overnight. The
transient expression of green fluorescent protein (GFP) in the
transfected cells was detected by fluorescence microscopy. Virus
titers of the lentiviral vectors were determined by fluorescence
microscopy and flow cytometry (TU/ml).
Colon cancer cell line transfection
SW480 cells were seeded in 6-well plates at a
concentration of 1×105 cells/well, and maintained at
37°C in a humidified 5% CO2 incubator overnight. One
hundred microliters of lentivirus fluid was diluted 10-fold with
DMEM containing 15% FBS, and 5 μg/ml polybrene was added. The
medium in 6-well plates was extracted and 1 ml of lentivirus fluid
was added to each well, cultured at 37°C in a humidified 5%
CO2 incubator for 24 h. Next, the lentivirus fluid in
the 6-well plates was removed, and 1 ml of fresh DMEM was added to
each well for cultivation at 37°C in a humidified 5% CO2
incubator for 48 h. The cells with positive GFP were screened,
selected and amplified in culture bottles to construct stable cell
lines. The infection rate was determined using fluorescence
microscopy and flow cytometry. Negative controls were performed by
transfecting the SW480 cell line with the pcDNA3.1(+)-GFP
lentiviral vector, and untransfected SW480 cells were used as a
blank control in all experiments.
Real-time PCR
Total RNA from cells in the exponential phase of
growth was extracted by use of TRIzol reagent, according to the
manufacturer’s instructions. After being treated with DNase-I, 1 mg
of total RNA was reverse transcribed into complementary DNA (cDNA)
with oligo(dT) using a cDNA synthesis kit (OriGene Technologies,
Inc., Rockville, MD USA). RNA purity and integrity were determined
by the OD value and electrophoresis. Real-time PCR primers
(designed by Gemma Co., Ltd.) were as follows: DLC-1,
5′-CCGCCTGAGCATCTACGA-3′ (forward) and 5′-TTCTCCGACCACTGATTGACTA-3′
(reverse); GSK-3β, 5′-GAACTGTCAAGTAATCCACCTCTG-3′ (forward) and
5′-CCACGGTCTCCAGTATTAGCATC-3′ (reverse); β-catenin,
5′-CTGCCAAGTGGGTGGTATAGAG-3′ (forward) and 5′-CGGGACAAAGGGCAAGAT-3′
(reverse); c-myc, 5′-GCTGCTTAGACGCTGGATTT-3′ (forward) and
5′-CGAGTCGTAGTCGAGGTCATAGTT-3′ (reverse); GAPDH,
5′-CATGAGAAGTATGACAACAGCCT-3′ (forward) and
5′-AGTCCTTCCACGATACCAAAGT-3′ (reverse).
The PCR conditions were as follows: initial
denaturation at 95°C for 3 min, followed by 40 cycles of 95°C for
30 sec, 62°C for 40 sec and 72°C for 30 sec. After 40 cycles of PCR
amplification, dissociation analysis was performed by melting the
products from 60 to 95°C. All reactions were performed using a
real-time PCR kit (DAAN Gene Co., Ltd., Guangzhou, China).
Real-time PCR was carried out in triplicate. The human GAPDH
gene was chosen as the endogenous control according to a
preliminary experiment, and the expression levels of four target
genes (DLC-1, GSK-3β, c-myc and
β-catenin) were examined. The relative gene expression
levels were calculated using the ΔΔCt method.
Western blot analysis
Total cell proteins were isolated using protein
sample extraction reagent. Equal amounts of protein were separated
on SDS-PAGE gels and transferred onto PVDF membranes. Membranes
were blocked with 5% w/v skimmed milk in 0.1% Tween-20 for 1 h and
incubated with primary antibodies (1:1,000) at 4°C overnight before
incubation with the secondary antibodies (1:1,000) for 1 h at room
temperature. The following antibodies were used: DLC-1 (15460-1-AP,
PTG) 1:1,000, GSK-3β (51065-1-AP, PTG) 1:1,000, β-catenin
(51067-2-AP, PTG) 1:2,000, c-myc (10828-1-AP, PTG) 1:500, GAPDH
(Sigma G8795) 1:12,000, HRP-conjugated goat anti-mouse IgG (JIR
115-035-003) 1:5,000 and HRP-conjugated goat anti-rabbit IgG (JIR
111-035-003) 1:5,000. Specific proteins were visualized using the
SuperSignal West Pico Chemiluminent Substrates system (Thermo
Fisher Scientific) and were then exposed with Kodak X-ray film.
Protein band intensities were determined densitometrically using
the Gel-Pro analyzer software (Media Cybernetics, Inc., Rockville,
MD, USA). All experiments were performed in triplicate.
Cell proliferation assay
Transfection was performed when the cells reached
70–90% confluency. Cell proliferation was measured by a
methylthiazol tetrazolium (MTT) assay. Forty-eight hours after
transfection, cells were cultured at a density of 5×103
cells/well in triplicate in 96-well plates with 10% FBS at 37°C and
5% CO2. The MTT assay was performed daily for up to 5
days. Briefly, 10 μl of CCK-8 solution [Cell Counting Kit (CCK-8);
Qcbio S&T Co., Ltd., Shanghai, China] was added to each well
and maintained in an incubator at 37°C with 5% CO2 for 1
h. The absorbance of each well was determined at 450 nm using a
microtiter plate reader (Molecular Devices, Sunnyvale, CA, USA).
The CCK-8 values were detected every 24 h, and the results are
expressed as the mean ± SD of three independent experiments.
Colony formation assay
Cells in the exponential phase of growth were
selected and suspended into single cells by pipetting, and then
inoculated into 10-cm Petri dishes. The cell suspension was further
diluted with a gradient factor. Approximately 500 cells were added
to the Petri dishes which were incubated at 37°C for 2–3 weeks
until visible colonies appeared. Petri dishes were gently washed
twice with PBS. Colonies were fixed with 5 ml methanol for 15 min,
stained with Giemsa for 10–30 min, and then counted. Viable
colonies containing at least 50 cells were counted.
Cell cycle analysis
The cells in the exponential phase of growth were
centrifuged at 1,200 × g for 5 min, washed twice with PBS, and
fixed with 70% ethanol at −20°C for 12 h. Cells were then
centrifuged and collected. Cells were digested with 50 μg/ml of
RNAase A in 100 μl of PBS for 30 min at room temperature and then
stained with 5 μl of propidium iodide (PI) (SunShine Bio,
Guangzhou, China) at room temperature for 30 min in the dark.
Samples were then analyzed by flow cytometry.
Analysis of apoptosis
The cells in the exponential phase of growth were
digested with the pancreatic enzyme, trypsin. After being washed
twice with cold PBS, the cells were collected by centrifugation,
and mixed with 400 μl of 1X binding buffer, and then the 5 μl of
Annexin V-FITC (Sunshine Bio) was added and incubated at 2–8°C in
the dark for 5 min, and subsequently 10 μl of PI was added and
incubated at 2–8°C in the dark for 5 min. Cell apoptosis was
detected by flow cytometry.
Migration assay
Transwell chambers were used to detect the migratory
ability of cells. The cells were detached, counted, and
re-suspended in culture medium containing 2% FBS at a cell density
of 106/ml. One hundred microliters of cell suspension
was added into each well in the upper Transwell chamber. Five
hundred microliters of culture medium containing 10% FBS was added
to the 24-well plate in the lower Transwell chamber, and incubated
for 24 h. The Transwell chamber was taken out and washed twice with
PBS. The cells on the surface of the Transwell chamber were wiped
off with cotton swabs. The cells were then fixed with methanol for
30 min and stained with Giemsa for 10 min. The cells that remained
at the bottom side of the membrane were counted under a microscope
in five random visual fields.
Invasion assay
Transwell chambers were used for detecting the
invasive ability of cells. Fifty microliters of 25% BD Matrigel/75%
serum-free medium mixture was added to the upper chambers and
incubated at 37°C with 5% CO2. SW480 cells (100 ml of
1×106/ml) were added to the upper chamber, and 500 μl of
20% FBS was added to the lower chamber. After a 24-h incubation,
the chamber was washed twice with PBS, and the cells remaining on
the upper surface of the membrane were removed using cotton tips.
Cells that adhered to the underside of the membrane were fixed with
methanol for 30 min and stained with Giemsa for 30 min. Cells in
five random visual fields were counted.
Statistical analysis
All data are representative of at least three
independent experiments. For continuous variables, data are
expressed as mean ± standard error of the mean (SEM). The
difference among groups was determined by analysis of variance
(one-way ANOVA) followed by Newman-Keuls post-hoc study. Comparison
of rates were analyzed using the Chi-squared test. Comparison of
mRNA and protein expression levels among groups was analyzed using
the non-parametric Wilcoxon signed-ranks test. SPSS Statistics 19.0
was used for statistical analysis. Statistical significance was
defined as P<0.05.
Results
Plasmid preparation and
identification
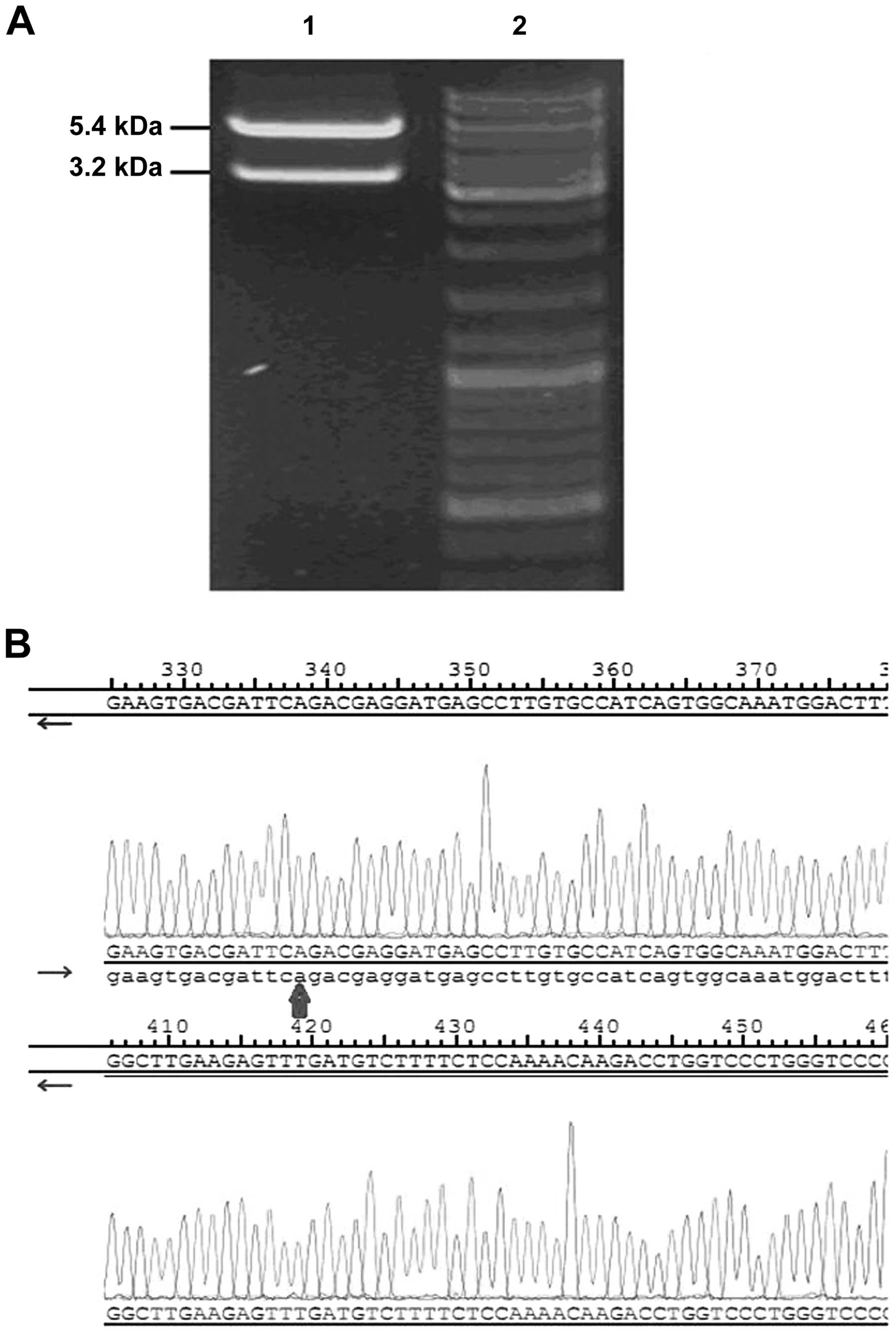
As a confirmatory step to evaluate recombinant
plasmid integrity, the recombinant plasmid pcDNA3.1(+)-GFP-DLC-1
was digested with BamHI and NotI restriction enzymes.
As expected, a two band pattern appeared after being progressed on
1% agarose gel (Fig. 1A). DNA
sequencing showed that the full length of DLC-1 was
completely and correctly cloned into the recombinant plasmid vector
(Fig. 1B). This confirmed that the
recombinant plasmid pcDNA3.1(+)-GFP-DLC-1 was correctly
constructed.
Identification and quantification of
recombinant lentiviral vectors
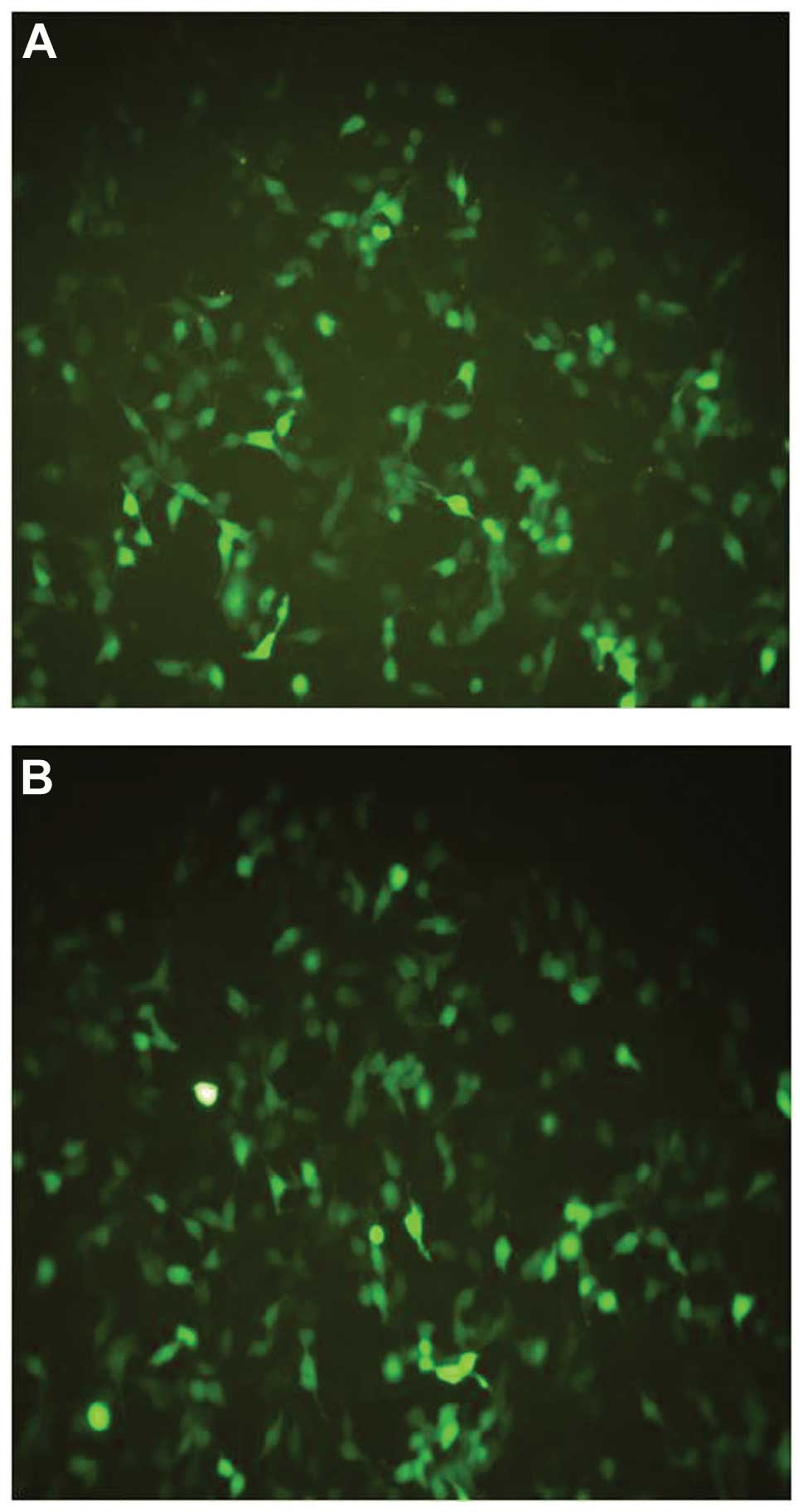
After transfection, the 293T cell line was evaluated
to detect the presence of green fluorescent protein emission under
microscopy. The fluorescent signal from the 4th day to the 6th day
after transfection served as evidence of vector production
(Fig. 2). After the purification of
the virus, the titer of the recombinant viral stocks was evaluated.
The results indicated that the titers of both the recombinant virus
and the negative control were ~109 TU/ml.
Transduction and infectivity
analysis
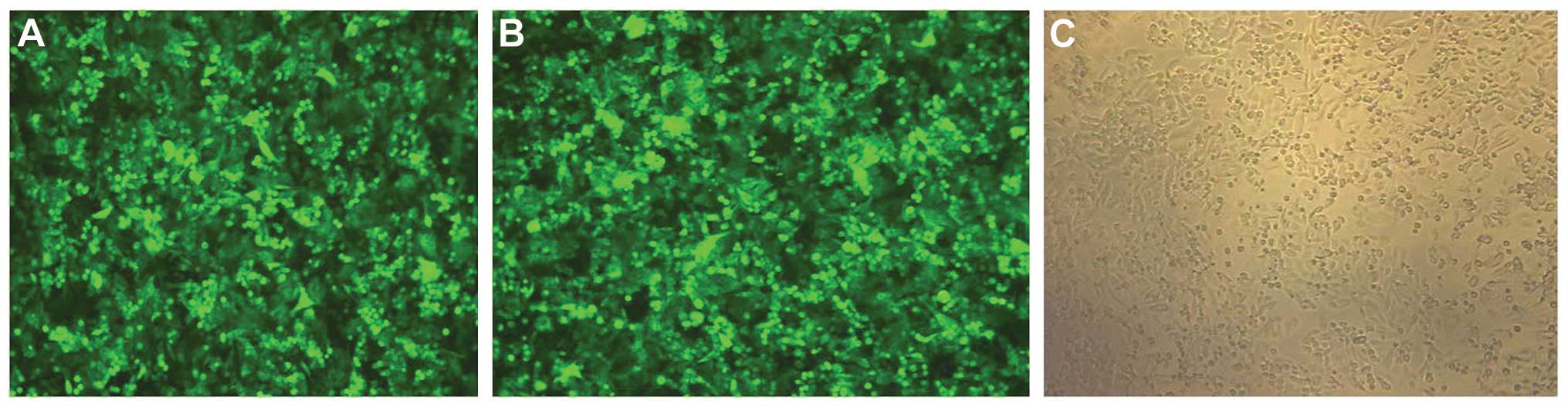
The human colon cancer cell line SW480 was infected
with the recombinant lentiviral vectors and blank lentiviral
vectors, respectively. GFP protein expression was detected by
microscopy 72 h after infection (Fig.
3). Flow cytometric analysis showed expression of GFP in
>90% of live cells in the assessed samples i.e. the infectivity
was 90%.
DLC-1 overexpression in the transfected
SW480 cells
In order to investigate the biological roles of
DLC-1 in colon cancer progression, we constructed the
recombinant lentiviral vector coding the full-length DLC-1,
and introduced it into human colon cancer cell line SW480.
Real-time qPCR and western blotting were used to analyze the
DLC-1 expression levels in the transfected cells. Both
analyses confirmed the DLC-1 overexpression in SW480 cells
transfected with the pcDNA3.1(+)-DLC-1 plasmid.
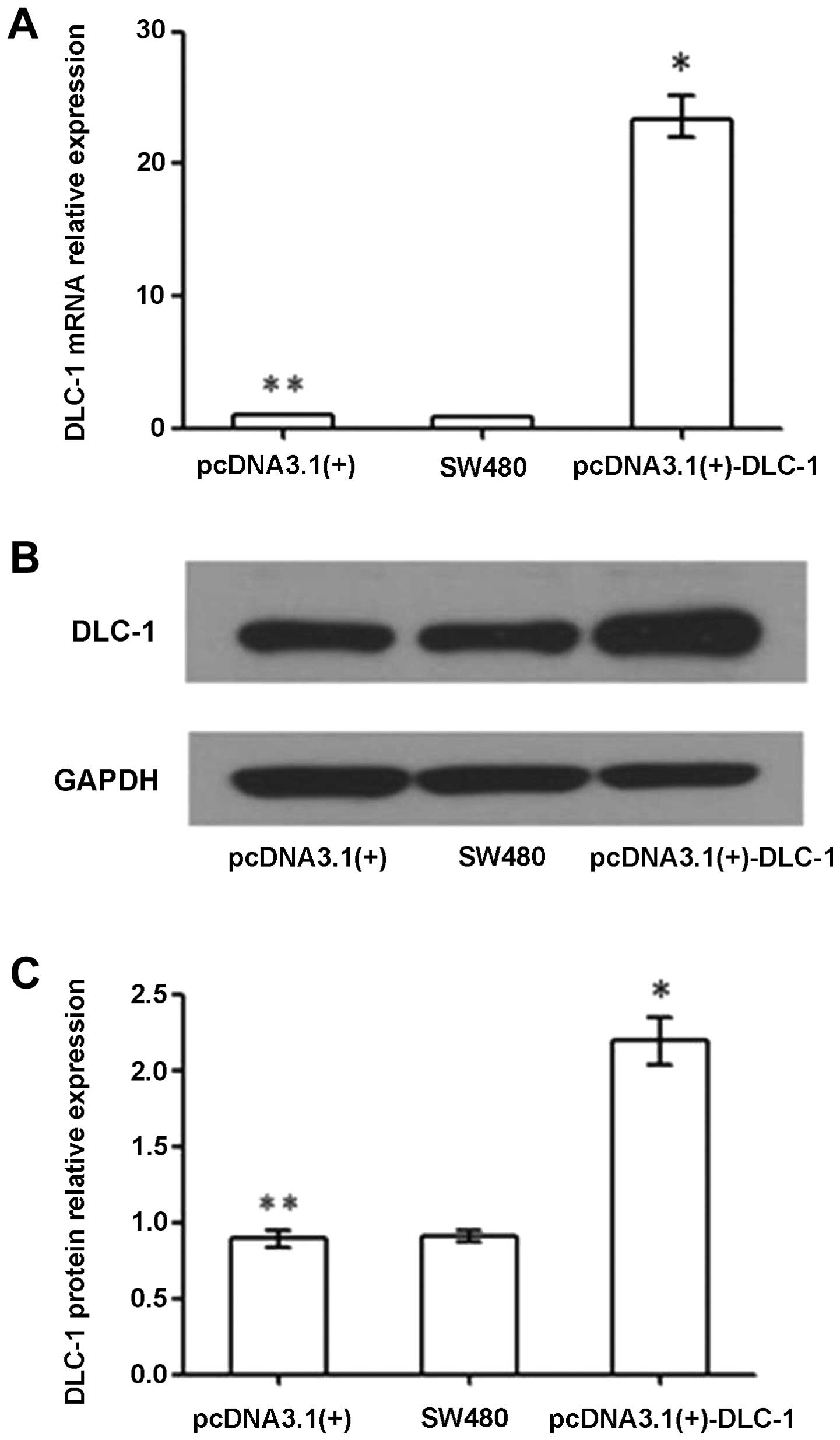
As shown in Fig. 4A,
the relative DLC-1 mRNA expression in the untransfected
SW480 group, the pcDNA3.1(+) group and the pcDNA3.1(+)-DLC-1 group
was 0.80 (0.78–0.81), 1.00 (0.98–1.02) and 23.32 (21.97–24.75),
respectively. DLC-1 mRNA expression in the pcDNA3.1(+)-DLC-1
group was significantly higher than levels in the pcDNA3.1(+) group
and untransfected SW480 group (P<0.01). There was no
statistically significant difference in the DLC-1 mRNA
expression between the pcDNA3.1(+) group and the untransfected
SW480 group (P>0.05).
Western blot analysis was performed 96 h after
infection. Relative DLC-1 protein expression in the
untransfected SW480 group, the pcDNA3.1(+) group and the
pcDNA3.1(+)-DLC-1 group was 0.91±0.04, 0.89±0.06 and 2.19±0.16,
respectively. DLC-1 protein expression in the pcDNA3.1(+)-DLC-1
group was significantly higher than that of the pcDNA3.1(+) group
and the untransfected SW480 group (P<0.01), while there was no
statistically significant difference between the untransfected
SW480 group and the pcDNA3.1(+) group (P>0.05).
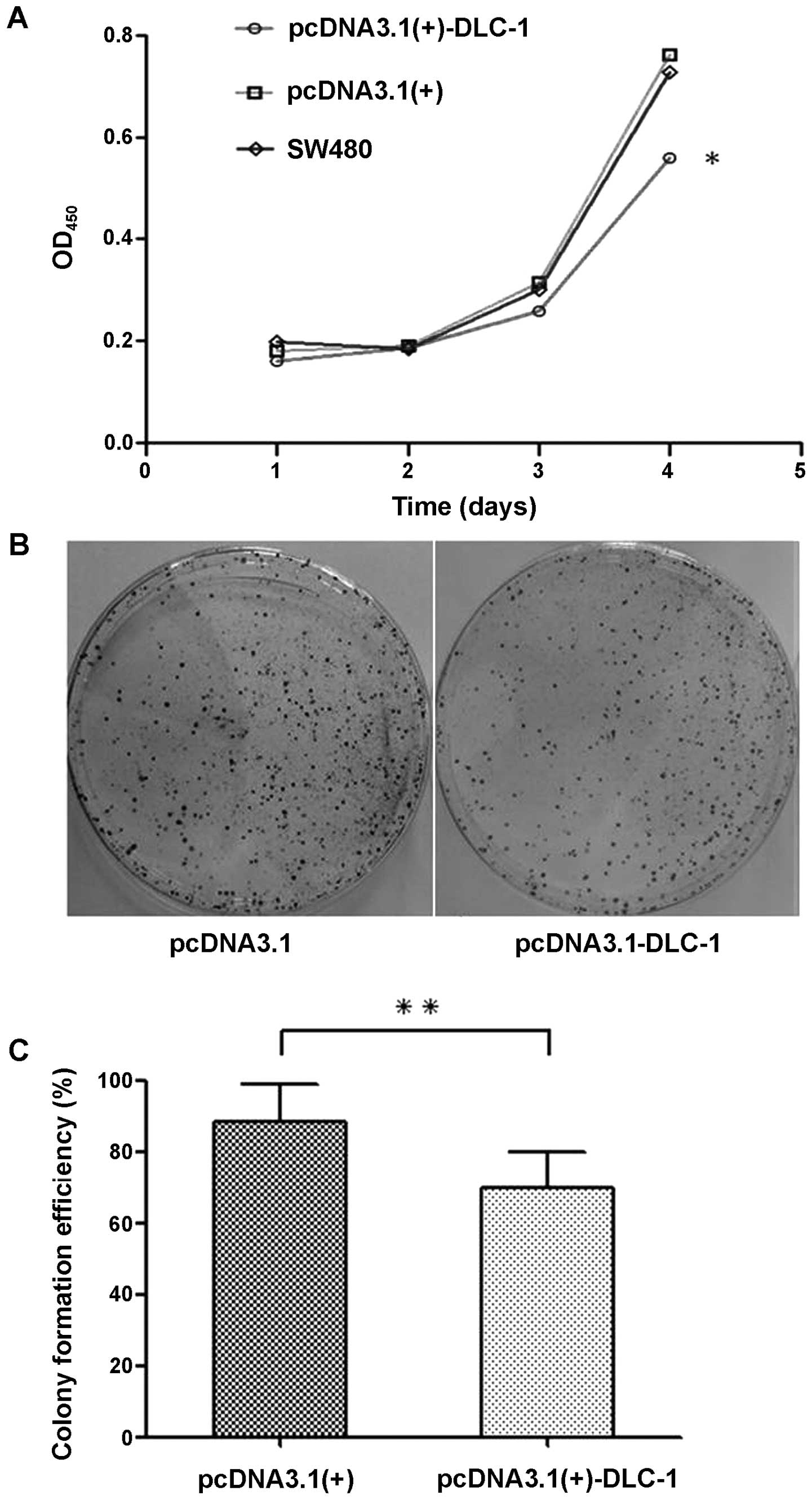
DLC-1 overexpression inhibits
proliferation and colony formation of SW480 cells
Absorbance of transfected cells was detected every
24 h, from 24 to 72 h after transfection (Fig. 5A). Cell proliferation activity of
the pcDNA3.1(+)-DLC-1 group was gradually inhibited from the second
day after transfection, and as time passed, the cell proliferation
activity of this group was significantly decreased compared with
the pcDNA3.1(+) group and the untransfected SW480 group
(P<0.05). In addition, there was no significant difference
between the proliferation of the untransfected SW480 group and the
pcDNA3.1(+) group (P>0.05), which suggested that DLC-1
overexpression may inhibit the proliferation of colon cancer cells.
Moreover, a significant decrease was observed in the colony
formation of the pcDNA3.1(+)-DLC-1 group when compared to the
pcDNA3.1(+) group and the untransfected SW480 group (P<0.01)
(Fig. 5B and C).
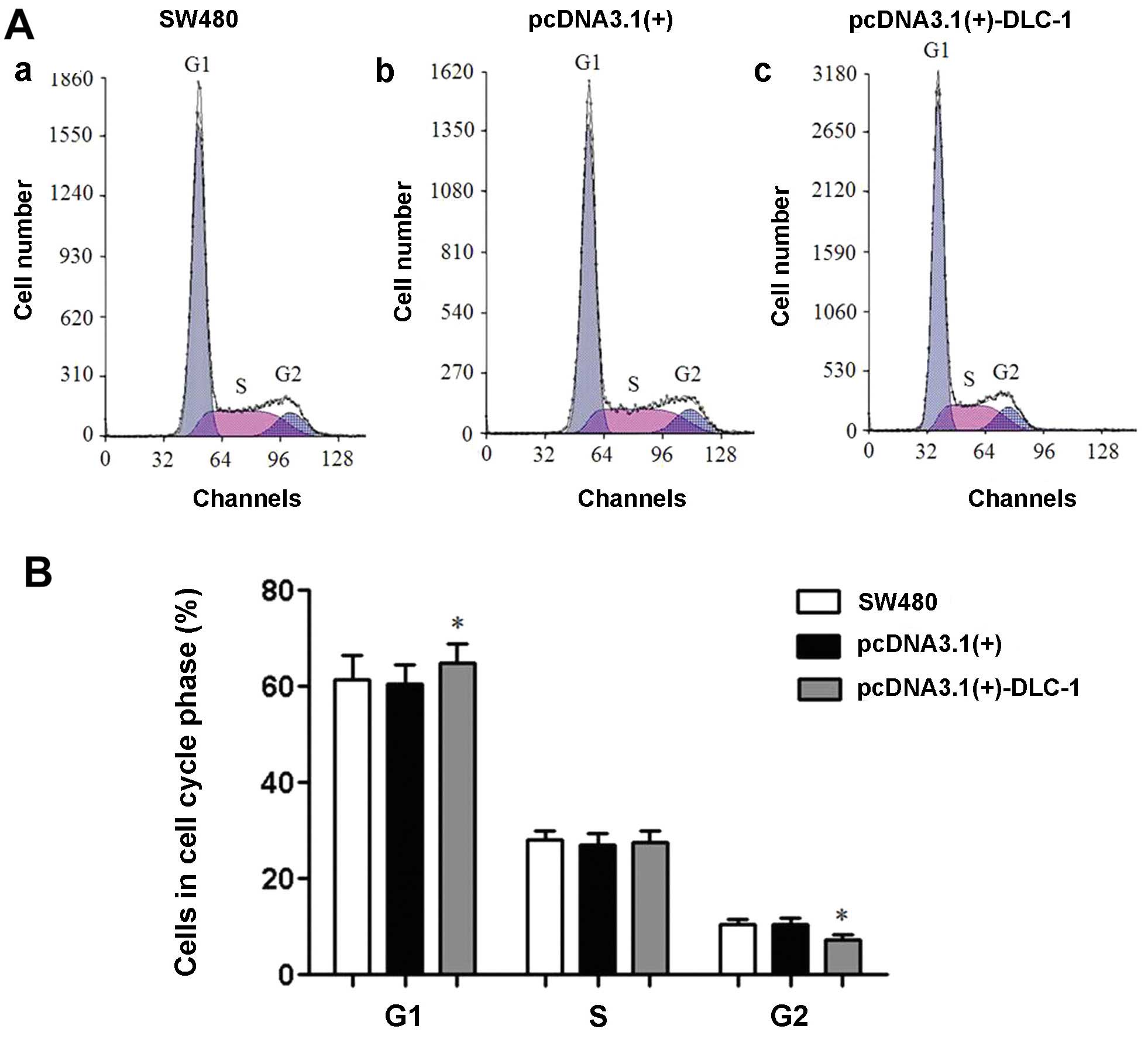
DLC-1 overexpression influences cell
cycle progression
Flow cytometry showed that the pcDNA3.1(+)-DLC-1
cell group was arrested at the G1 phase. Thus, DLC-1 overexpression
influences the progression of colon cancer cells through the cell
cycle (Fig. 6).
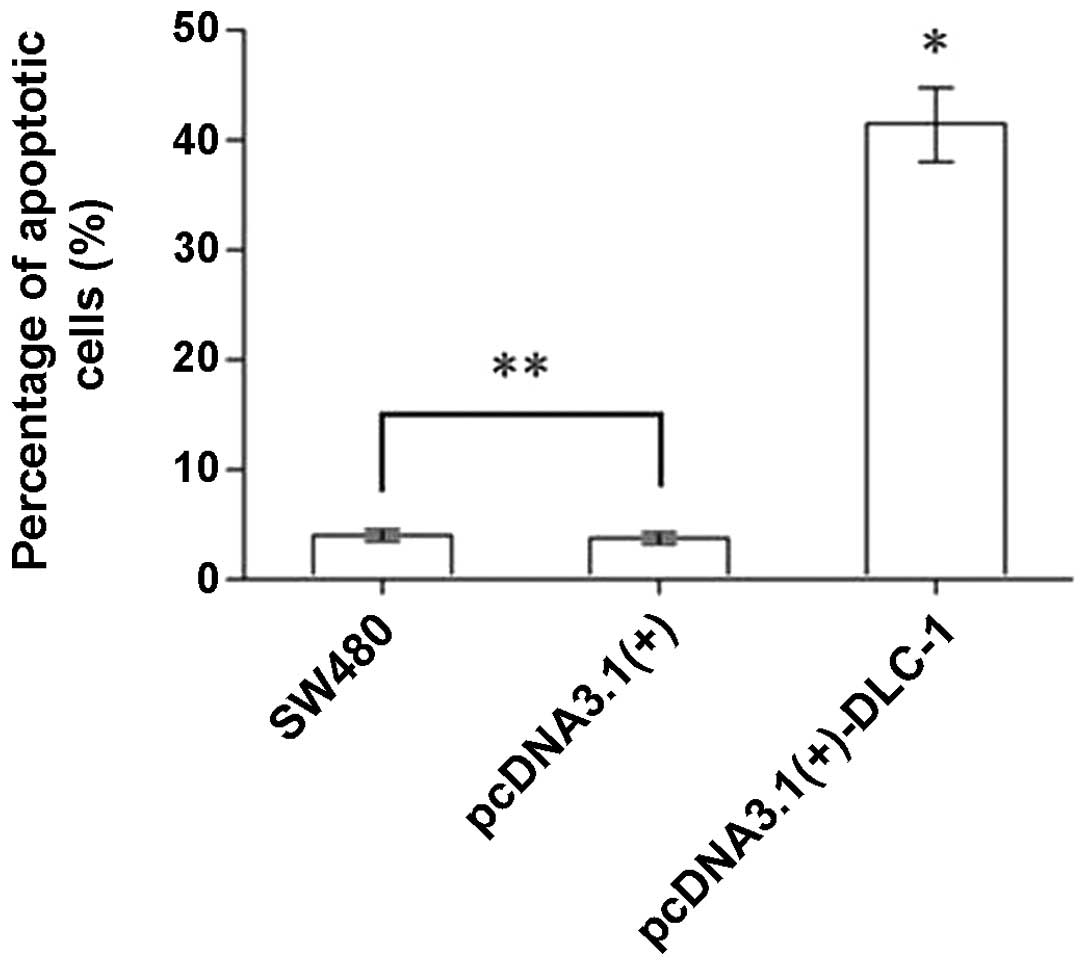
DLC-1 overexpression induces apoptosis in
SW480 cells
The rate of apoptosis of the pcDNA3.1(+)-DLC-1 group
was determined by flow cytometry and was significantly higher than
that of the untransfected SW480 group and the pcDNA3.1(+) group
(P<0.01). No difference was observed between the untransfected
SW480 group and the pcDNA3.1(+) group (P>0.05) (Fig. 7).
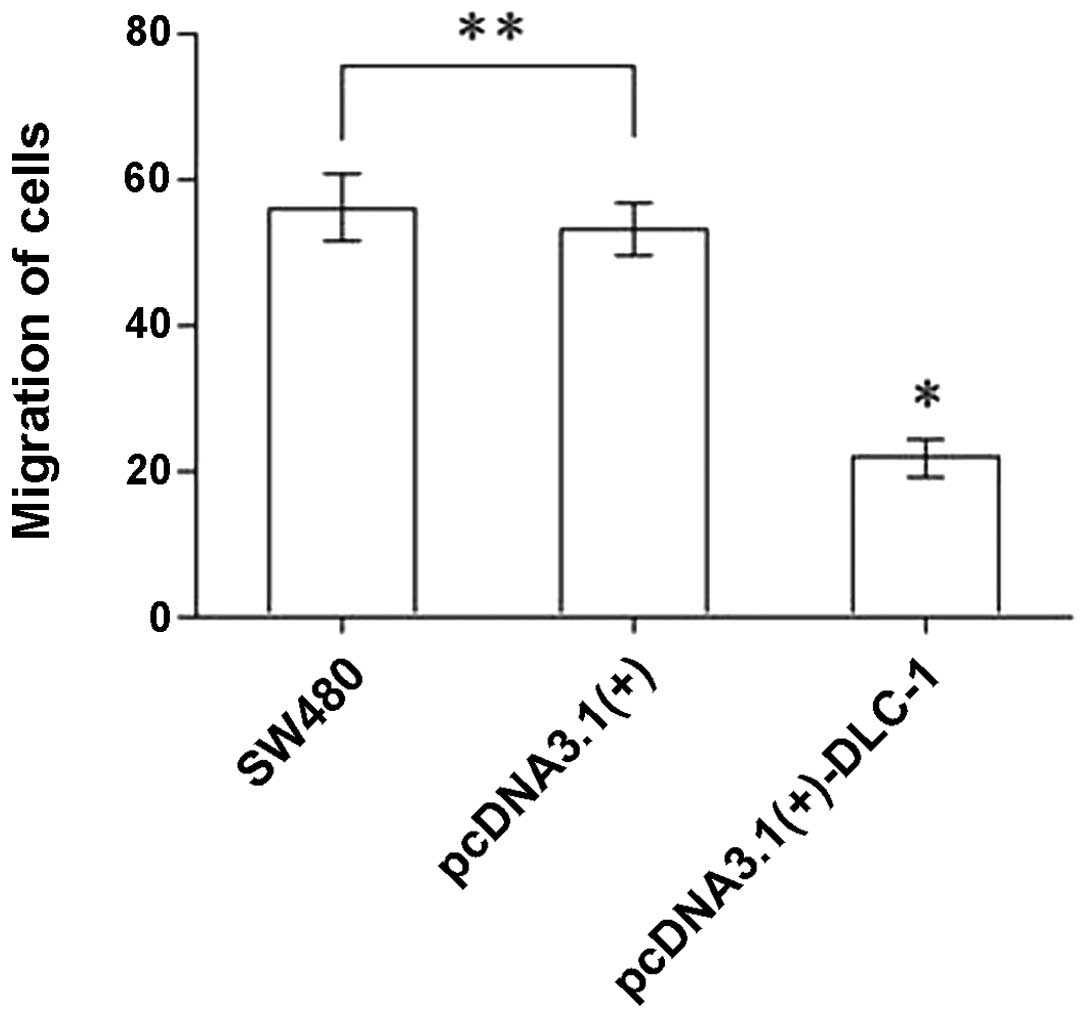
DLC-1 overexpression inhibits migration
of SW480 cells
As shown in Fig. 8,
the number of cells that migrated in the Transwell migration assay
was 56.00±4.53, 53.00±3.54 and 21.80±2.59 in the untransfected
SW480 cells, the pcDNA3.1(+) cells and the pcDNA3.1(+)-DLC-1 cells,
respectively. The number of cells from the pcDNA3.1(+)-DLC-1 group
that passed through the membrane was significantly lower than the
number of cells from the pcDNA3.1(+) group or the untransfected
SW480 group (P<0.01). No difference was observed between the
untransfected SW480 group and the pcDNA3.1(+) group (P>0.05).
These results show that DLC-1 overexpression inhibits the
migratory ability of colon cancer cells.
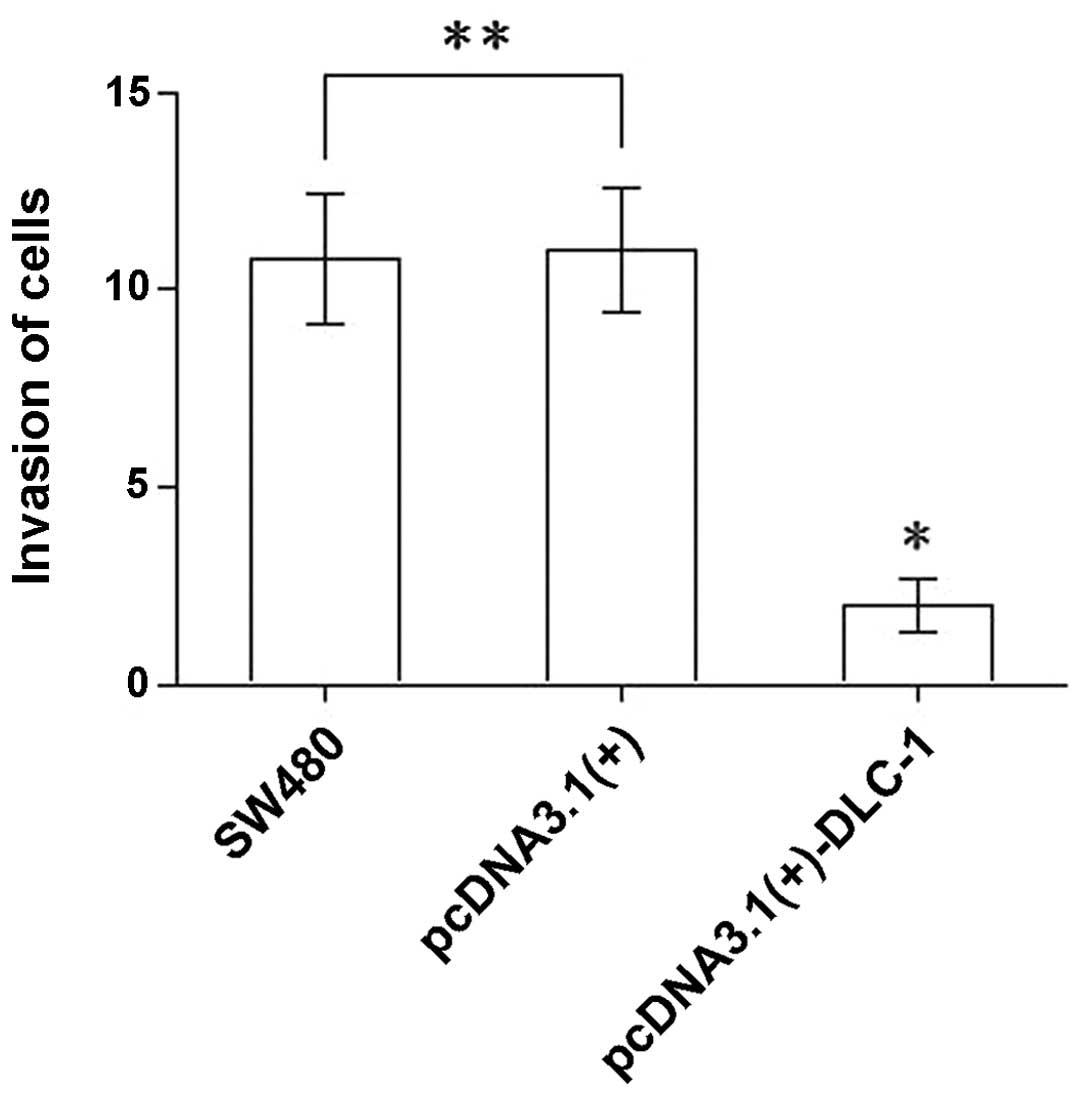
DLC-1 overexpression inhibits the
invasion of SW480 cells
The number of cells that invaded through Matrigel in
the Transwell invasion assay was 10.80±1.64, 11.00±1.58 and
2.00±0.71 for the untransfected SW480 group, the pcDNA3.1(+) group
and the pcDNA3.1(+)-DLC-1 group, respectively (Fig. 9). The number of cells from the
pcDNA3.1(+)-DLC-1 group that passed through the membrane was
significantly lower than that of the pcDNA3.1(+) group and the
untransfected SW480 group (P<0.01). No significant difference
was observed between the untransfected SW480 cells and the
pcDNA3.1(+) group (P>0.05). These results showed that
DLC-1 overexpression inhibits the invasive ability of colon
cancer cells.
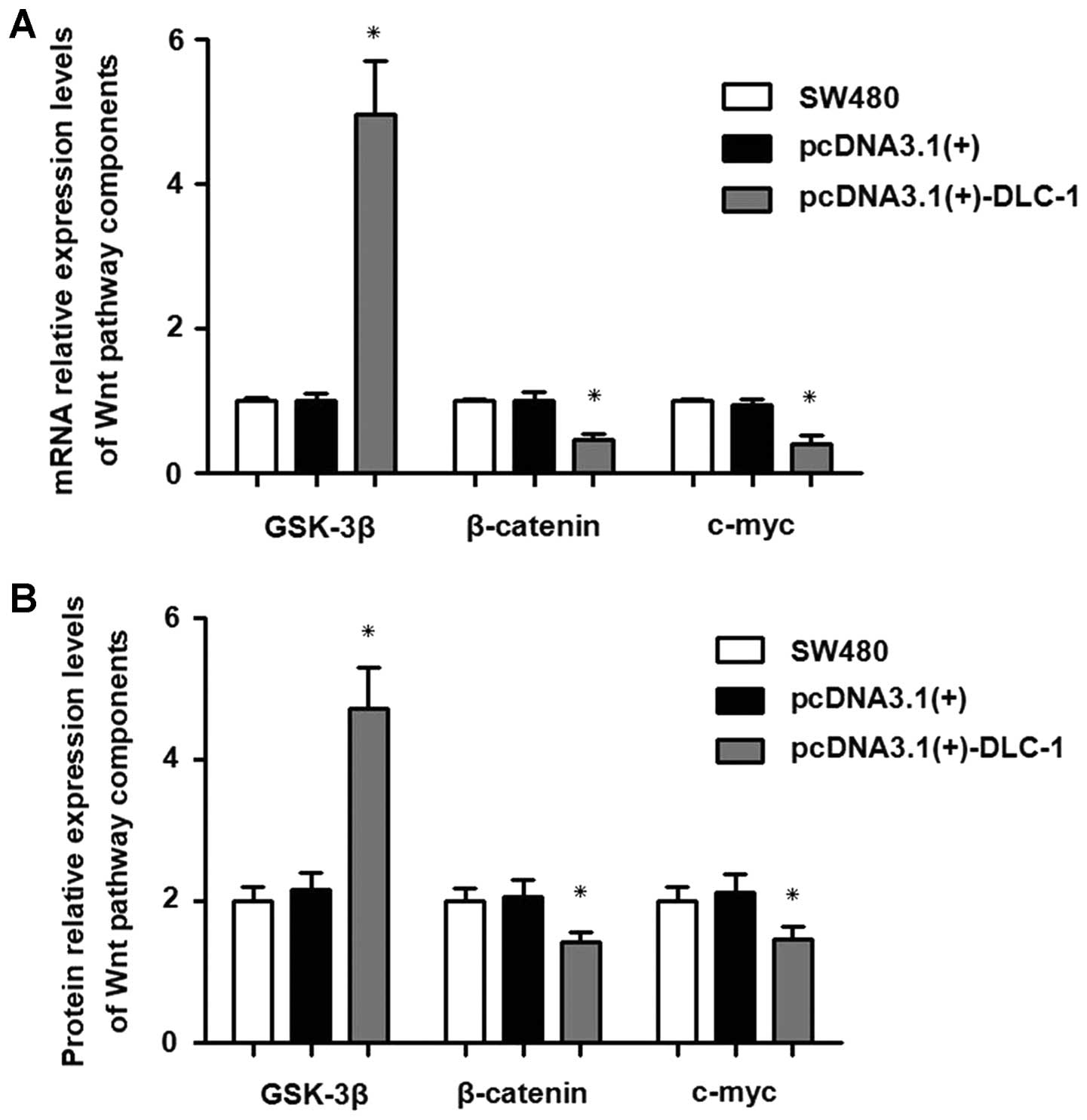
DLC-1 overexpression regulates the
Wnt/β-catenin signaling pathway
Real-time PCR showed that GSK-3β mRNA expression in
the pcDNA3.1(+)-DLC-1 group was significantly higher than that in
the pcDNA3.1(+) group and the untransfected group (P<0.0001).
The β-catenin mRNA expression was significantly lower than that in
the pcDNA3.1(+) group and the SW480 cells (P<0.0001). The c-myc
mRNA expression level was significantly lower than that in the
pcDNA3.1(+) group and the SW480 group (P<0.0001).
The results of the western blot analysis showed that
the expression level of GSK-3β protein was significantly higher
than that in the pcDNA3.1(+) group and the SW480 group
(P<0.0001). The β-catenin protein expression level was
significantly lower than that in the pcDNA3.1(+) group and the
SW480 group (P<0.0001). The c-myc protein expression level was
significantly lower than that in the pcDNA3.1(+) group and the
SW480 group (P<0.0001).
As shown in Fig.
10, GSK-3β mRNA expression was upregulated, while the
expression of both β-catenin and c-myc mRNA was downregulated. The
results of western blot analysis confirmed these findings at the
protein expression level. Therefore, DLC-1 overexpression
upregulates GSK-3β, and downregulates mRNA and protein expression
of β-catenin and c-myc.
Discussion
CRC is the third most common cancer and the fourth
leading cause of cancer-related mortality worldwide (17). Surgical resection alone is
potentially curative in early stages of CRC. Unfortunately, most
CRCs are in advanced stages at the time of diagnosis. Although new
therapies with monoclonal antibodies such as cetuximab and
panitumumab, which target the epidermal growth factor receptor
(EGFR), have had some clinical success (16), metastasis still remains a huge
problem and the prognosis of patients with metastatic CRC still
remains dismal. As accumulating evidence has shown, mutations in a
number of genes and dysregulation of the Wnt/β-catenin signaling
pathways are closely related to colorectal carcinogenesis. Through
dysregulation of this signaling pathway and crosstalk with other
cellular signaling pathways, continuous growth-stimulating signals
induce changes in the pre-cancerous cells themselves as well as in
their microenvironment. In this process, β-catenin plays an
important role (4,5,15,18,19).
Many genetic markers in CRC have promising potential for use in the
treatment selection, prognosis, and early detection of cancer
(16). Therefore, a detailed
understanding of genetic mutations in colorectal carcinogenesis is
essential to develop new approaches to cure CRC and prevent tumor
recurrence.
Recent studies have shown that both the DLC-1
gene and the Wnt/β-catenin signaling pathway play critical roles in
CRC (20–25). DLC-1 is a candidate
tumor-suppressor gene which induces apoptosis and inhibits both
cell growth and tumorigenicity in human hepatocellular carcinoma
cells (26). Another study showed
that DLC-1 suppresses cell growth and invasion in lung
cancer (27). Jin et al
(28) found that decreased
DLC-1 expression in the SW480 cell line promoted cell
proliferation and migration and induced cell cycle arrest at the
G2/M phase, suggesting that the DLC-1 gene is associated
with colon cancer cell proliferation, migration and cell cycle
distribution. A few similar studies on the DLC-1 gene have
confirmed that DLC-1 acts as a tumor-suppressor gene in
several malignant tumors (9–12,20).
However, although the role of DLC-1 during the pathogenesis
of CRC has been suggested, the detailed molecular pathway and
downstream targets have not yet been fully elucidated.
Research suggests that the tumor suppressor effects
of DLC-1 relate to the expression of cyclin D1 (29). Cyclin D1 is the target gene of the
Wnt/β-catenin signaling pathway, and it is tightly related to
β-catenin (30). The Wnt signaling
pathway is highly conserved in various species, controlling cell
proliferation, cell polarity and cell fate among other activities.
In addition, it has been shown that it is often dysregulated in a
variety of diseases including cancer. Extensive studies have shown
that the Wnt/β-catenin signaling pathway plays important roles in
CRC (13,31). The Wnt signaling pathway consists of
several key components such as Wnt proteins, β-catenin, glycogen
synthase kinase-3β (GSK-3β) and APC protein (13,32).
In normal intestinal mucosa, APC expression increases gradually
from the bottom to the top of intestinal crypts (31,34).
When the Wnt signaling pathway is inactive, a destruction complex
consisting of APC, Axin and GSK-3β promotes β-catenin degradation,
which causes a relatively low β-catenin protein level in intestinal
cancer cells (13,32,33).
Mutations in the destruction complex components have been detected
in many diseases including cancer (31–33).
APC mutations lead to inactivation of the destruction
complex, resulting in an increased β-catenin level in the nucleus,
which promotes expression of several target genes (13,31,33,34).
In addition, functional defects in the destruction complex
components inhibit apoptosis, breaking the balance of cell
proliferation and differentiation, which results in formation of
adenomatous polyps (35–37).
In the present study, in order to examine the role
of DLC-1 in CRC cell growth and invasion, we constructed a
recombinant lentiviral vector coding DLC-1 and transfected
the human colon cancer cell line SW480. Both DLC-1 mRNA and
protein levels in the transfected SW480 cells were significantly
upregulated. Next, we showed that DLC-1 overexpression inhibited
cell proliferation, colony formation, migration and invasion but
induced cell apoptosis and arrested the cell cycle at the G1 phase.
Therefore, the results of the present study demonstrate that
DLC-1 may act as a tumor-suppressor gene in colon cancer.
Moreover, we analyzed the expression levels of the Wnt pathway
components GSK-3β, β-catenin and c-myc gene by real-time PCR and
western blot analysis. Collectively, these results show that DLC-1
overexpression suppresses the Wnt/β-catenin signaling pathway by
upregulating GSK-3β, and downregulating β-catenin and c-myc. In
addition, the results suggest that DLC-1 interacts with the
Wnt/β-catenin signaling pathway. However, the exact details of this
process are still unknown. Therefore, in forthcoming studies, our
aim will be to elucidate the relationship between DLC-1 and
the Wnt/β-catenin signaling pathway in colorectal cancer cells.
In conclusion, the results of the present study
suggest that the tumor-suppressor gene DLC-1 may induce cell
apoptosis, and inhibit both cell growth and invasion through
regulation of the Wnt/β-catenin signaling pathway.
Acknowledgements
The study was supported by the Medical Science and
Technology Research Project Fund (2010-2-048) of Chongqing City
Health Bureau. The authors thank the Chongqing Health Bureau for
the strong support. We thank Medjaden Bioscience Limited for
assisting in the preparation of this manuscript.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Moghimi-Dehkordi B and Safaee A: An
overview of colorectal cancer survival rates and prognosis in Asia.
World J Gastrointest Oncol. 4:71–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arasaradnam RP, Commane DM, Bradburn D and
Mathers JC: A review of dietary factors and its influence on DNA
methylation in colorectal carcinogenesis. Epigenetics. 3:193–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beggs AD and Hodgson SV: The genomics of
colorectal cancer: state of the art. Curr Genomics. 9:1–10. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fearon ER: Molecular genetics of
colorectal cancer. Ann Rev Pathol. 6:479–507. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Worthley DL and Leggett BA: Colorectal
cancer: molecular features and clinical opportunities. Clin Biochem
Rev. 31:31–38. 2010.PubMed/NCBI
|
|
7
|
Yuan BZ, Miller MJ, Keck CL, Zimonjic DB,
Thorgeirsson SS and Popescu NC: Cloning, characterization, and
chromosomal localization of a gene frequently deleted in human
liver cancer (DLC-1) homologous to rat RhoGAP. Cancer
Res. 58:2196–2199. 1998.PubMed/NCBI
|
|
8
|
Nagase T, Kikuno R, Hattori A, Kondo Y,
Okumura K and Ohara O: Prediction of the coding sequences of
unidentified human genes. XIX The complete sequences of 100 new
cDNA clones from brain which code for large proteins in
vitro. DNA Res. 7:347–355. 2012. View Article : Google Scholar
|
|
9
|
Guan M, Zhou X, Soulitzis N, Spandidos DA
and Popescu NC: Aberrant methylation and deacetylation of deleted
in liver cancer-1 gene in prostate cancer: potential clinical
applications. Clin Cancer Res. 12:1412–1419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Durkin ME, Yuan BZ, Zhou X, Zimonjic DB,
Lowy DR, Thorgeirsson SS and Popescu NC: DLC-1: a Rho
GTPase-activating protein and tumour suppressor. J Cell Mol Med.
11:1185–1207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng X, Li C, Liu W, Chen H, Zhou W, Wang
L, Zhu B, Yao K, Jiang X and Ren C: DLC-1, a candidate tumor
suppressor gene, inhibits the proliferation, migration and
tumorigenicity of human nasopharyngeal carcinoma cells. Int J
Oncol. 42:1973–1984. 2013.PubMed/NCBI
|
|
12
|
Zhang T, Zheng J, Jiang N, Wang G, Shi Q,
Liu C and Lu Y: Overexpression of DLC-1 induces cell apoptosis and
proliferation inhibition in the renal cell carcinoma. Cancer Lett.
283:59–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van Amerongen R and Nusse R: Towards an
integrated view of Wnt signaling in development. Development.
136:3205–3214. 2009.PubMed/NCBI
|
|
14
|
Jang KY, Kim YN, Bae JS, Chung MJ, Moon
WS, Kang MJ, Lee DG and Park HS: Expression of cyclin D1 is
associated with β-catenin expression and correlates with good
prognosis in colorectal adenocarcinoma. Transl Oncol. 5:370–378.
2012.
|
|
15
|
Lee HK and Jeong SJ: β-Catenin as a valid
molecular target for the development of therapeutic inhibitors.
JNBT. 2:29–37. 2005.
|
|
16
|
Pritchard CC and Grady WM: Colorectal
cancer molecular biology moves into clinical practice. Gut.
60:116–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
18
|
Sengupta N, Gill KA, MacFie TS, Lai CS,
Suraweera N, Mcdonald S and Silver A: Management of colorectal
cancer: a role for genetics in prevention and treatment? Pathol Res
Pract. 204:469–477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu WK, Wang XJ, Cheng AS, Luo MX, Ng SS,
To KF, Chan FK, Cho CH, Sung JJ and Yu J: Dysregulation and
crosstalk of cellular signaling pathways in colon. Crit Rev Oncol
Hematol. 86:251–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao YC and Lo SH: Deleted in liver
cancer-1 (DLC-1): a tumor suppressor not just for liver. Int J
Biochem Cell Biol. 40:843–847. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu PP, Jin YL, Shang YF, Jin Z, Wu P and
Huang PL: Restoration of DLC1 gene inhibits proliferation and
migration of human colon cancer HT29 cells. Ann Clin Lab Sci.
39:263–269. 2009.PubMed/NCBI
|
|
22
|
Ullmannova V and Popescu NC: Expression
profile of the tumor suppressor genes DLC-1 and DLC-2 in solid
tumors. Int J Oncol. 29:1127–1132. 2006.PubMed/NCBI
|
|
23
|
Wilson PJ, McGlinn E, Marsh A, Evans T,
Arnold J, Wright K, Biden K, Young J, Wainwright B, Wicking C and
Chenevix-Trench G: Sequence variants of DLC1 in colorectal
and ovarian tumours. Hum Mutat. 15:156–165. 2000.
|
|
24
|
Seidler HB, Utsuyama M, Nagaoka S,
Takemura T, Kitagawa M and Hirokawa K: Expression level of Wnt
signaling components possibly influences the biological behavior of
colorectal cancer in different age groups. Exp Mol Pathol.
76:224–233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Doucas H, Garcea G, Neal CP, Manson MM and
Berry DP: Changes in the Wnt signalling pathway in gastrointestinal
cancers and their prognostic significance. Eur J Cancer.
41:365–379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou X, Thorgeirsson SS and Popescu NC:
Restoration of DLC-1 gene expression induces apoptosis and inhibits
both cell growth and tumorigenicity in human hepatocellular
carcinoma cells. Oncogene. 23:1308–1313. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Healy KD, Hodgson L, Kim TY, Shutes A,
Maddileti S, Juliano RL, Hahn KM, Harden TK, Bang YJ and Der CJ:
DLC-1 suppresses non-small cell lung cancer growth and invasion by
RhoGAP-dependent and independent mechanisms. Mol Carcinog.
47:326–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin Y, Tian X, Shang Y and Huang P:
Inhibition of DLC-1 gene expression by RNA interference in the
colon cancer LoVo cell line. Oncol Rep. 19:669–674. 2008.PubMed/NCBI
|
|
29
|
Kim PJ, Plescia J, Clevers H, Fearon ER
and Altieri DC: Survivin and molecular pathogenesis of colorectal
cancer. Lancet. 362:205–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu PP, Su Y, Jin Z, Wu P, Xu JJ and Huang
PL: Effects of DLC1 gene transfection on HT29 cell cycle
distribution: the role of Rho A. Southeast Univ (Med Sci Edi).
28:247–251. 2009.
|
|
31
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.
|
|
32
|
MacDonald BT, Tamai K and He X:
Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev
Cell. 7:9–26. 2009.
|
|
33
|
Kimelman D and Xu W: beta-catenin
destruction complex: insights and questions from a structural
perspective. Oncogene. 25:7482–7491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawasaki Y, Sato R and Akiyama T: Mutated
APC and Asef are involved in the migration of colorectal tumour
cells. Nat Cell Biol. 5:211–215. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyashiro I, Senda T, Matsumine A, Baeg
GH, Kuroda T, Shimano T, Miura S, Noda T, Kobayashi S and Monden M:
Subcellular localization of the APC protein: immunoelectron
microscopic study of the association of the APC protein with
catenin. Oncogene. 11:89–96. 1995.PubMed/NCBI
|
|
36
|
van de Wetering M, Sancho E, Verweij C, de
Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D,
Haramis AP, Tjon-Pon-Fong M, Moerer P, van den Born M, Soete G,
Pals S, Eilers M, Medema R and Clevers H: The β-catenin/TCF-4
complex imposes a crypt progenitor phenotype on colorectal cancer
cells. Cell. 111:241–250. 2002.
|
|
37
|
Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich
SM, Fields JZ and Boman BM: Evidence that APC regulates survivin
expression: a possible mechanism contributing to the stem cell
origin of colon cancer. Cancer Res. 61:8664–8667. 2001.
|