Introduction
Prostate cancer is one of the most common malignant
diseases among men, with a high rate of mortality (1). Tumor invasion and metastasis account
for the poor survival of prostate cancer patients. During tumor
progression, multiple regulatory molecules are released into the
tumor microenvironment and play crucial roles in the regulation of
tumor invasion and metastasis (2,3). Thus,
investigation of these molecules may provide useful clues for the
underlying mechanisms of prostate cancer invasion and
metastasis.
As a member of the CC chemokine family, eotaxin-1
(CCL11) was initially regarded as an eosinophil chemoattractant,
and is involved in the recruitment of inflammatory cells such as
eosinophils and neutrophils (4).
Overexpression of eotaxin-1 has been found in various inflammatory
diseases such as allergic asthma and atopic dermatitis (5,6).
Further studies have shown that eotaxin-1 is elevated in many human
cancers, and correlates with tumor progression (7). Eotaxin-1 acts mostly via the CC
chemokine receptor-3 (CCR3) (8).
CCL11-CCR3 interactions have been proven to promote cell survival
and growth of anaplastic large cell lymphoma cells (9). In addition to CCR3, a recent study
found that CCR2 and CCR5 are also activated by eotaxin-1 (10). Serum eotaxin-1 has been confirmed as
a diagnostic marker for prostate cancer (11). Yet, the role of eotaxin-1 in
prostate cancer remains elusive. In the present study, we found
that eotaxin-1 promotes prostate cancer cell invasion and
migration. We also determined the involvement of CC chemokine
receptor(s), the ERK pathway and MMP-3 expression in
eotaxin-1-mediated prostate cancer cell invasion.
Materials and methods
Chemicals and antibodies
Eotaxin-1 was purchased from R&D Systems
(Minneapolis, MN, USA). U0126, a MEK inhibitor, was purchased from
Sigma (St. Louis, MO, USA). The antibodies against total-ERK1/2 and
β-actin were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The antibody against phosho-ERK1/2 was obtained
from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Cell culture
The DU-145 human prostate carcinoma cell line was
purchased from the Cell Resource Center of the Chinese Academy of
Medical Sciences (Beijing, China). Cells were cultured in RPMI-1640
medium containing 10% fetal bovine serum (FBS) and incubated in a
CO2 incubator at 37°C.
Real-time PCR
Total mRNA of DU-145 cells was extracted by TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). Then cDNA was obtained
using the Quantscript cDNA kit (Tiangen, Beijing, China), following
the manufacturer’s instructions. The cDNA was subjected to
real-time PCR with the primers as listed in Table I. The real-time PCR protocol was 10
min at 95°C, and 40 cycles of 15 sec at 95°C and 1 min at 60°C. All
expression levels of the examined genes were normalized to the
expression of β-actin and analyzed by the 2−ΔΔCt
method.
 | Table IReal-time PCR primers. |
Table I
Real-time PCR primers.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) | Length (bp) |
|---|
| MMP-3 |
CTGGACTCCGACACTCTGGA |
CAGGAAAGGTTCTGAAGTGACC | 79 |
| CCR2 |
CCACATCTCGTTCTCGGTTTATC |
CAGGGAGCACCGTAATCATAATC | 88 |
| CCR3 |
GTCATCATGGCGGTGTTTTTC |
CAGTGGGAGTAGGCGATCAC | 155 |
| CCR5 |
GTTGGACCAAGCTATGCAGGT |
GCAGAAGCGTTTGGCAATGT | 142 |
| β-actin |
GGATGCAGAAGGAGATCACTG |
CGATCCACACGGAGTACTTG | 90 |
Enzyme-linked immunosorbent assay
(ELISA)
After treatment with or without eotaxin-1 for 12 h,
the cell supernatant was collected and centrifuged at 12,000 rpm
for 15 min. Then the supernatant was subjected to an ELISA kit for
human MMP-3 (Boster, Wuhan, China), according to the manufacturer’s
instructions. Cells were lysed with RIPA buffer, and total protein
of the cells was determined by bicinchoninic acid (BCA) assay. Then
the concentration of MMP-3 was normalized to the total protein of
cells.
Invasion assay and migration assay
Transwell cell culture chambers (Costar, San Diego,
CA, USA) were used in the invasion and migration assays. For the
invasion assay, the top chambers were coated with Matrigel (Sigma)
before use. Next, 1×105 cells in 100 μl RPMI-1640 were
added to the top chambers, while 600 μl RPMI-1640 with 20% FBS was
added to the low chambers. After incubated at 37°C for 12 h, the
invaded cells were fixed with methanol and stained with crystal
violet. The number of cells in 7 random visual fields was observed
and counted under a microscope at ×200 magnification. Data are
presented as a percentage compared to the control group for which
the number of invaded cells was defined as 100%. For the migration
assay, all procedures were the same as described above except the
top chambers were not coated with Matrigel.
Western blotting
Cells were lysed in RIPA buffer with protease
inhibitor and phosphatase inhibitor, and the concentration of
protein was determined by BCA assay. Equal amounts of protein were
separated on SDS-PAGE gel, and then transferred onto PVDF membranes
(Bio-Rad, Hercules, CA, USA). The membranes were blocked with 5%
BSA for 1 h, and then incubated with primary antibodies at 4°C
overnight. After being washed with TBST for 3 times, the membranes
were incubated with secondary antibodies for 1 h at room
temperature. Then the bands were visualized and exposed to film by
chemiluminescence (Applygen Technologies Inc., Beijing, China).
Small interfering RNAs (siRNAs) and
transfection
The CCR2 siRNA, CCR3 siRNA and CCR5 siRNA were
obtained from Santa Cruz Biotechnology, Inc. A scramble siRNA that
was also obtained from Santa Cruz Biotechnology was used as the
control siRNA. Cells were transfected with siRNAs using
Lipofectamine 2000 (Invitrogen). After transfection with siRNAs for
48 h, the knockdown efficiency was tested by real-time PCR, and
cells were used in the subsequence experiments.
Statistical analysis
All experiments were repeated three to four times.
The means ± SD was determined and used to represent the results.
Statistical significance was set at p<0.05 and assessed by
Student’s t-test (comparison of two means) or non-parametric ANOVA
(comparison of multiple means).
Results
Eotaxin-1 increases the expression of
MMP-3 in DU-145 cells
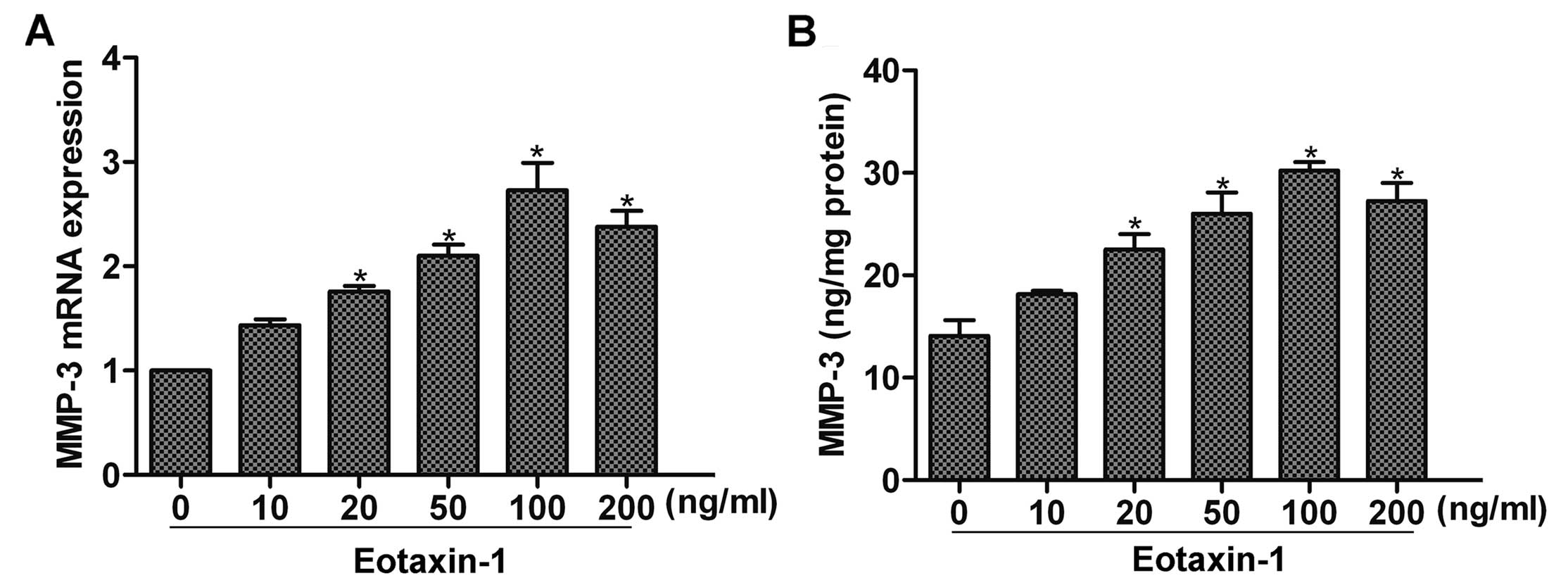
Studies have found that eotaxin-1 can promote MMP-3
expression in inflammatory diseases such as arthritis (12). To determine whether eotaxin-1
affects the expression of MMP-3 in cancer cells, we first
stimulated DU-145 cells with eoxtaxin-1 for 12 h, and then detected
the expression of MMP-3 by real-time PCR and ELISA assay. The
results showed that eotaxin-1 treatment increased the expression of
MMP-3 in a dose-dependent manner, and a peak upregulation was noted
at 100 ng/ml (Fig. 1A and B). Thus,
eotaxin-1 at the concentration of 100 ng/ml was used in the
subsequent experiments.
Eotaxin-1 promotes the invasion and
migration of prostate cancer cells
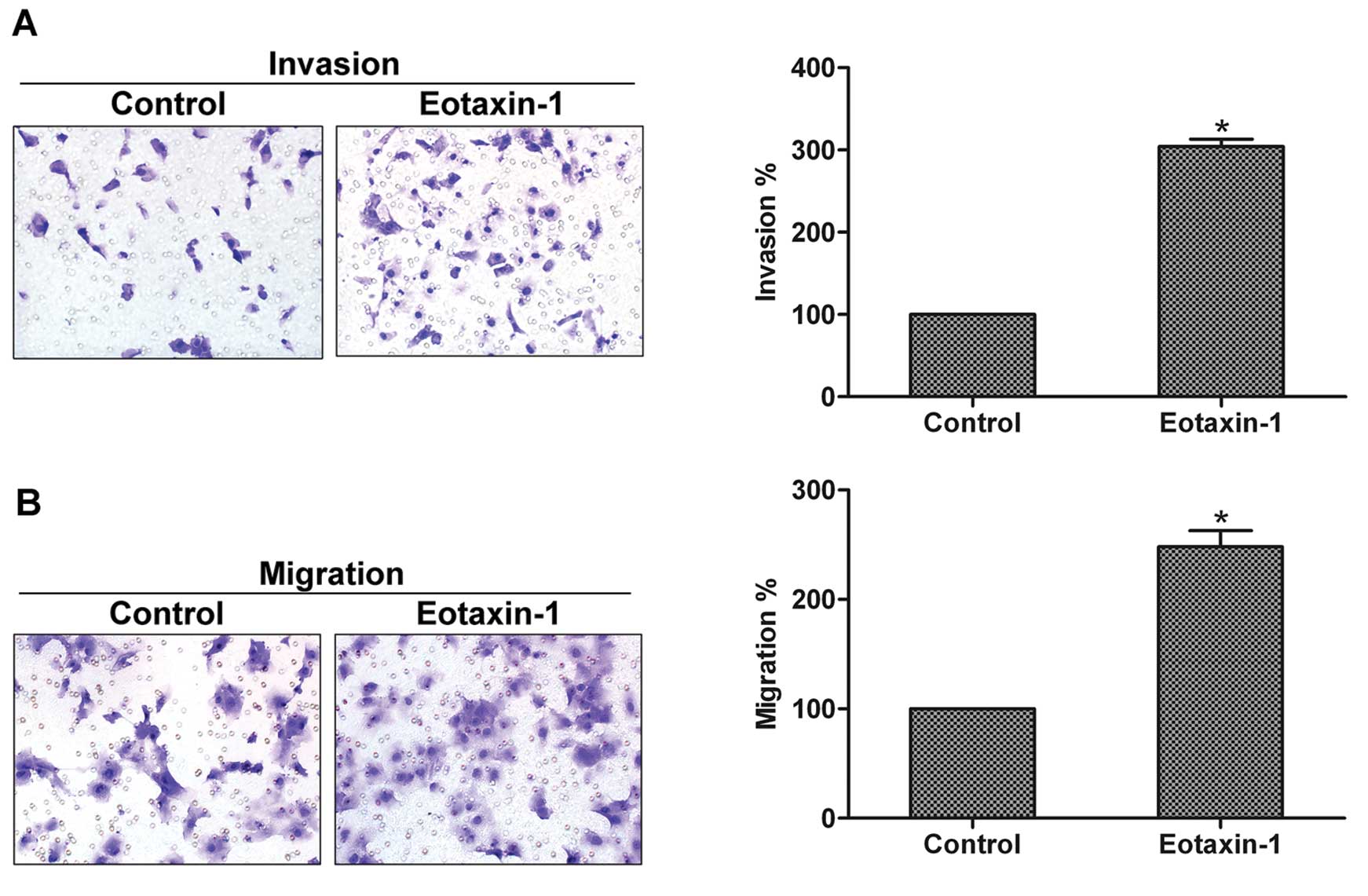
MMP-3 acts as a pivotal player in the invasion of
prostate cancer (13). Therefore,
we investigated the effect of eotaxin-1 on the invasion and
migration of prostate cancer cells. Using invasion and migration
assays, we found that cells incubated with eotaxin-1 exhibited
significantly higher invasion and migration abilities when compared
with the control cells, indicating that eotaxin-1 enhances the
invasion and migration of prostate cancer cells (Fig. 2A and B).
Eotaxin-1 induces the activation of
ERK1/2 in prostate cancer cells
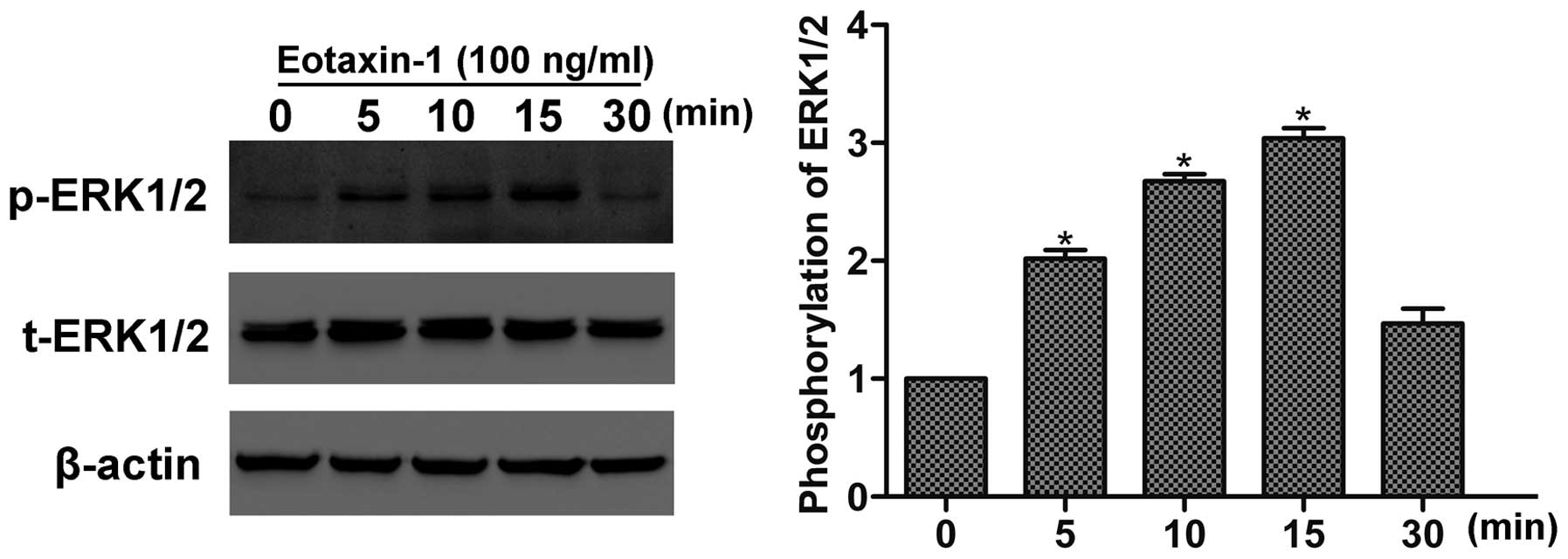
The ERK pathway is essential to the invasion and
metastasis of prostate cancer (14). Here, we detected the phosphorylation
of ERK1/2 following eotaxin-1 treatment by western blotting. As
shown in Fig. 3, eotaxin-1
stimulated the activation of ERK1/2 in a time-dependent manner,
with peak activation at 15 min, suggesting that eotaxin-1 induces
the activation of ERK1/2 in prostate cancer cells.
CCR3 is required for the
eotaxin-1-promoted invasion and migration
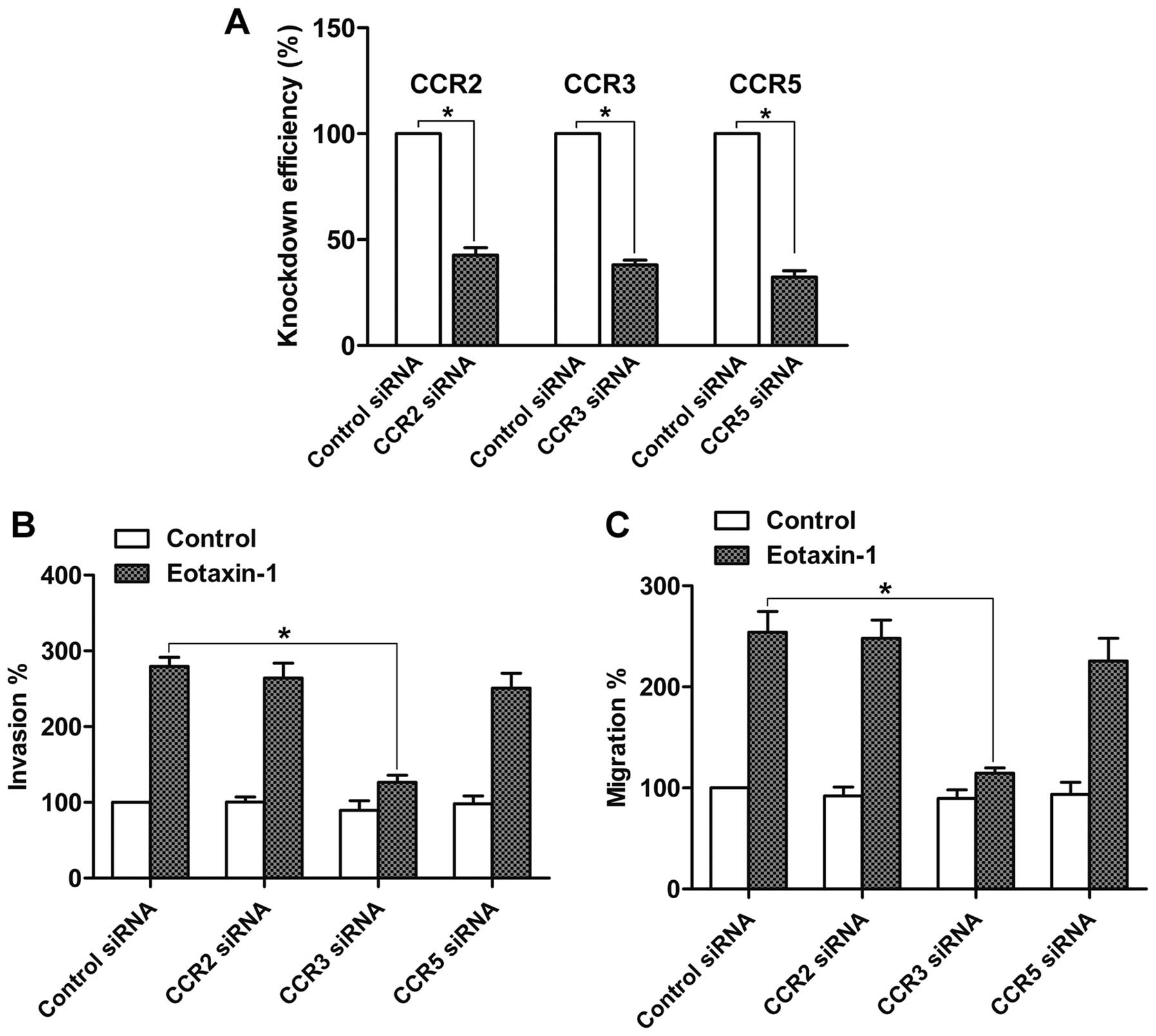
CCR2, CCR3 and CCR5 are the receptors that respond
to eotaxin-1 treatment (10). Thus,
we attempted to ascertain whether these receptors are involved in
the eotaxin-1-promoted invasion and migration by siRNA technology.
The knockdown efficiency of CCR2, CCR3 and CCR5 siRNAs was examined
by real-time PCR, and each siRNA achieved a prominent knockdown
effect when compared with the control siRNA (Fig. 4A). Then invasion and migration
assays were carried out. The results demonstrated that knockdown of
CCR3 suppressed the eotaxin-1-mediated invasion and migration of
DU-145 cells. However, neither CCR2 nor CCR5 knockdown affected the
eotaxin-1-mediated invasion and migration of DU-145 cells (Fig. 4B and C). These data suggest that
eotaxin-1 promotes prostate cancer cell invasion and migration via
CCR3.
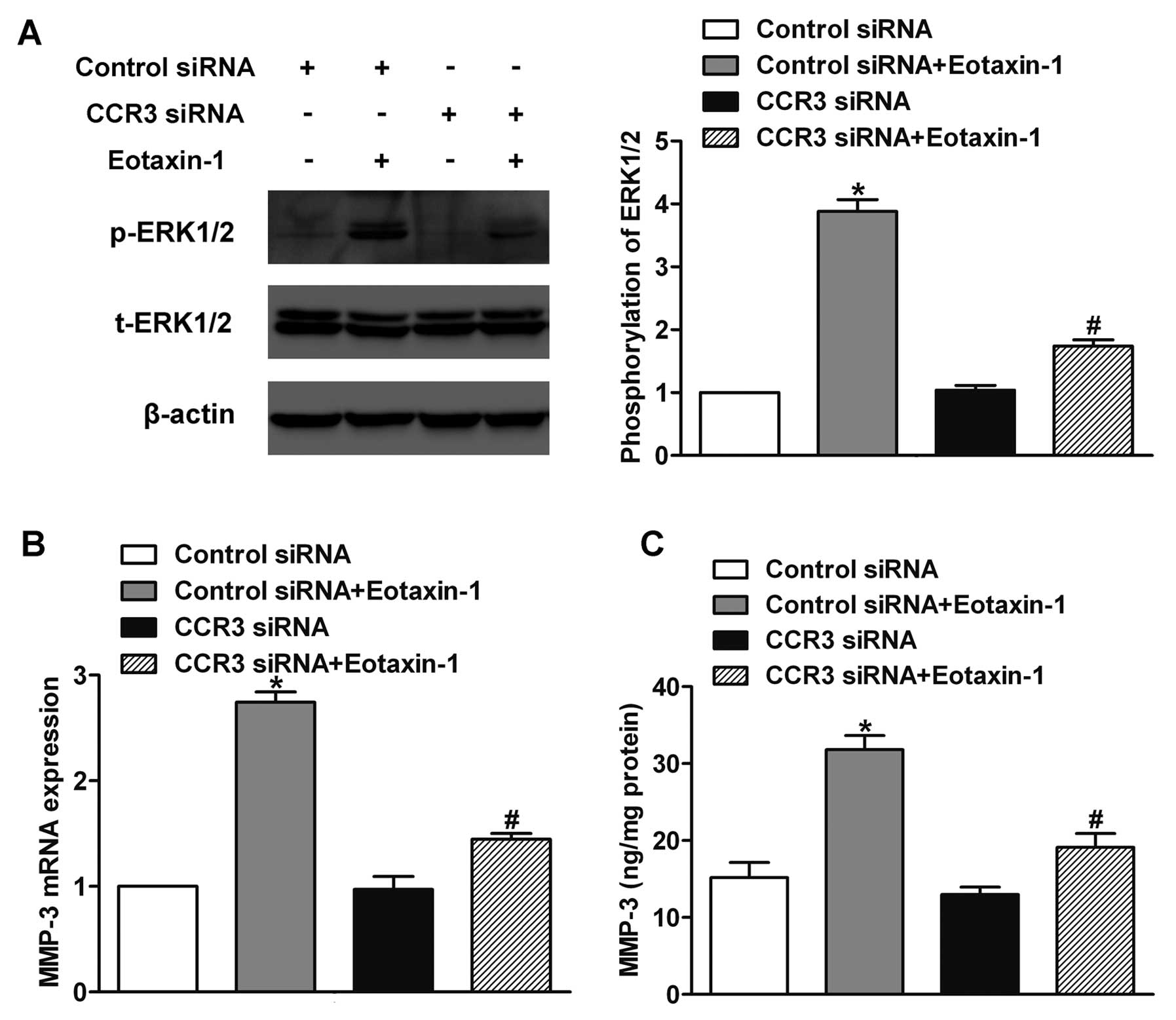
Involvement of CCR3 in
eotaxin-1-regulated ERK1/2 activation and MMP-3 expression
To explore the involvement of CCR3 in
eotaxin-1-induced ERK1/2 activation, we silenced the expression of
CCR3 by siRNA and incubated DU-145 cells with eotaxin-1 for 15 min.
Then we detected the phosphorylation of ERK1/2 by western blotting.
The results showed that CCR3 knockdown inhibited the
eotaxin-1-induced activation of ERK1/2 (Fig. 5A). To determine the function of CCR3
in eotaxin-1-promoted MMP-3 expression, the expression of CCR3 was
knocked down and the cells were incubated with eotaxin-1 for 12 h.
Next, we examined the expression of MMP-3 by real-time PCR and
ELISA assay. We found that the knockdown of CCR3 decreased the
eotaxin-1-regulated MMP-3 expression at both the mRNA and protein
levels (Fig. 5B and C). These
results indicate that CCR3 is involved in the eotaxin-1-regulated
ERK1/2 activation and MMP-3 expression.
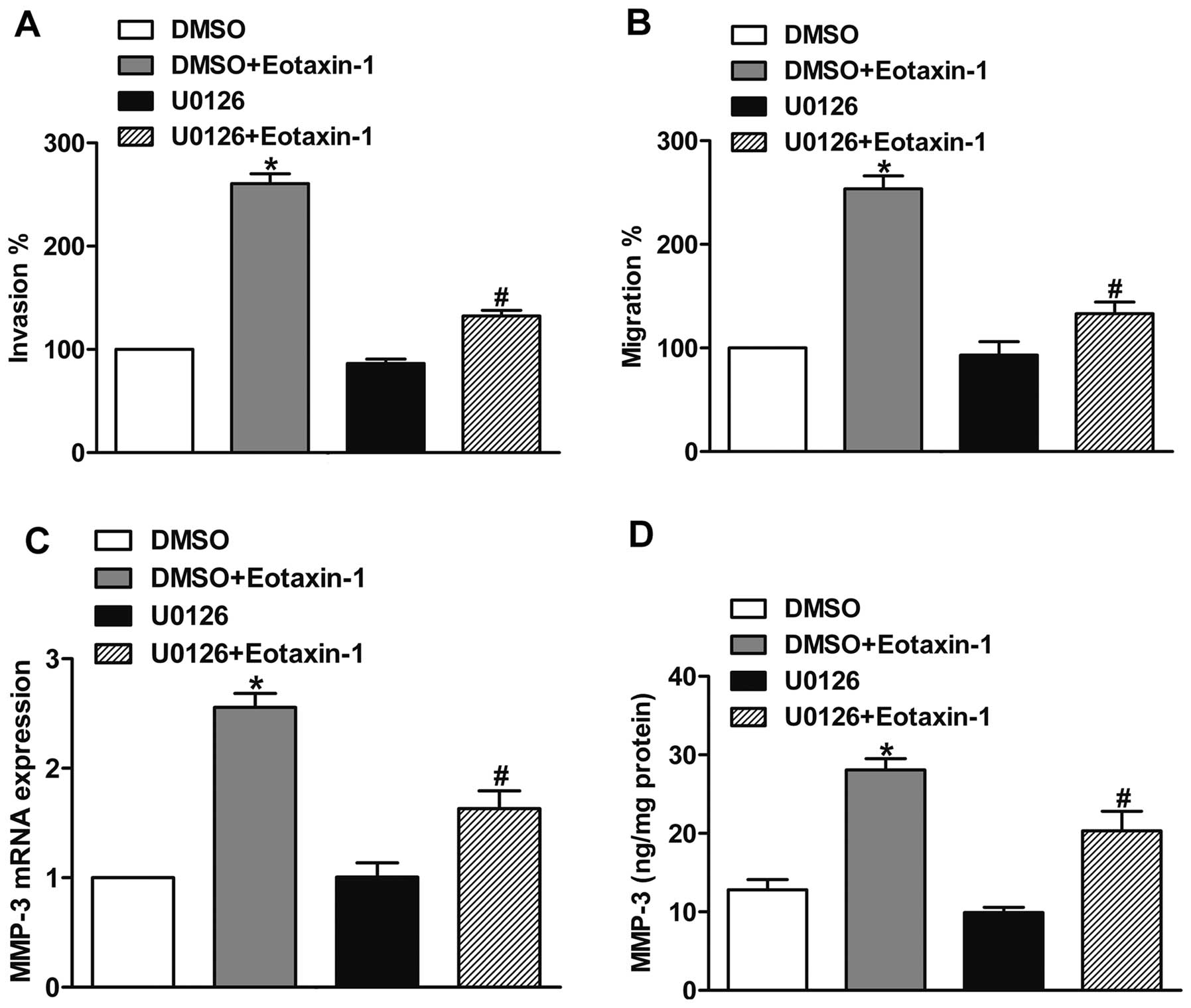
The ERK pathway is required for the
eotaxin-1-promoted invasion and MMP-3 expression
To explore the role of the ERK pathway in
eotaxin-1-promoted invasion, we pretreated DU-145 cells with the
MEK inhibitor U0126 (10 μM) for 30 min to inhibit the activation of
the ERK pathway. Then we subjected the cells to invasion and
migration assays with or without eotaxin-1 treatment. The results
showed that U0126 was able to inhibit the eotaxin-1-promoted
prostate cancer cell invasion and migration (Fig. 6A and B), confirming that the ERK
pathway participates in the eotaxin-1-promoted invasion and
migration of prostate cancer cells. Furthermore, we pretreated
DU-145 cells with U0126 (10 μM) or DMSO (control) for 30 min, and
then incubated the cells with eotaxin-1 for 12 h. Using real-time
PCR and ELISA assay, we found that U0126 suppressed the
eotaxin-1-mediated expression of MMP-3, suggesting that the
eotaxin-1-mediated increase in MMP-3 expression involved the ERK
pathway (Fig. 6C and D).
Discussion
As a member of the CC chemokine family, eotaxin-1 is
able to promote cell migration in vitro, and to induce
angiogenesis in vivo (15–17).
Recently published studies have found that eotaxin-1 is upregulated
in many tumor types such as prostate (11) and breast cancer (18), indicating the involvement of
eotaxin-1 in tumor progression. Here, our results demonstrated that
eotaxin-1 may promote prostate cancer cell invasion via the
CCR3-ERK pathway and MMP-3 expression. These findings strongly
support the notion that eotaxin-1 is a crucial player in the
regulation of prostate cancer cell invasion.
As the major receptor for eotaxin-1, CCR3 was found
to be upregulated in human renal cell carcinoma and glioblastoma
(19,20). Activation of CCR3 by eotaxin-1
enhanced the migration of choroidal endothelial cells and smooth
muscle cells (15,21). In the present study, we found that
eotaxin-1 promoted the invasion and migration of prostate cancer
cells via CCR3. CCR3 has been proven to activate the ERK pathway in
smooth muscle cells and large cell lymphoma cells (9,22).
Consistent with that result, we found that CCR3 was involved in the
eotaxin-1-induced activation of ERK1/2 in DU-145 cells. It is well
established that the ERK pathway plays major roles in a series of
tumor progression processes such as proliferation, invasion and
metastasis (23). Here, our results
demonstrated that the ERK pathway is required for the
eotaxin-1-promoted invasion and migration of prostate cancer
cells.
MMP-3 is a member of the matrix metalloproteinases
(MMPs), and is essential to the invasion and metastasis of human
cancers (24). Studies have found
that eotaxin-1 treatment upregulates the expression of MMP-3 in
chondrocytes (25). However,
whether eotaxin-1 treatment affects MMP-3 expression of cancer
cells is still unclear. Here, we found that eotaxin-1 increased the
expression of MMP-3 in DU-145 cells. Since degradation of the
extracellular matrix by MMPs is a prerequisite for tumor invasion
and metastasis (26), our finding
indicates that MMP-3 may participate in the eotaxin-1-promoted
prostate cancer cell invasion. Studies have shown that upregulation
of MMP-3 by eotaxin-1 treatment in human chondrocytes is dependent
on ERK1/2 activation (12). In the
present study, our results showed that eotaxin-1 promoted the
expression of MMP-3 via the CCR3-ERK pathway.
In conclusion, the present study demonstrated that
eotaxin-1 promotes the invasion and migration of prostate cancer
cells. Activation of the CCR3-ERK pathway and upregulation of MMP-3
expression were involved in these processes. In vivo studies
are required to further determine the role of eotaxin-1 and CCR3 in
prostate cancer.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Chung LWK, Baseman A, Assikis V and Zhau
HE: Molecular insights into prostate cancer progression: the
missing link of tumor microenvironment. J Urol. 173:10–20. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harlozinska A: Progress in molecular
mechanisms of tumor metastasis and angiogenesis. Anticancer Res.
25:3327–3333. 2005.PubMed/NCBI
|
|
4
|
Menzies-Gow A, Ying S, Sabroe I, et al:
Eotaxin (CCL11) and eotaxin-2 (CCL24) induce recruitment of
eosinophils, basophils, neutrophils, and macrophages as well as
features of early- and late-phase allergic reactions following
cutaneous injection in human atopic and nonatopic volunteers. J
Immunol. 169:2712–2718. 2002. View Article : Google Scholar
|
|
5
|
Campbell EM, Kunkel SL, Strieter RM and
Lukacs NW: Temporal role of chemokines in a murine model of
cockroach allergen-induced airway hyperreactivity and eosinophilia.
J Immunol. 161:7047–7053. 1998.PubMed/NCBI
|
|
6
|
Yawalkar N, Uguccioni M, Schärer J, et al:
Enhanced expression of eotaxin and CCR3 in atopic dermatitis. J
Invest Dermatol. 113:43–48. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nolen BM and Lokshin AE: Targeting CCL11
in the treatment of ovarian cancer. Expert Opin Ther Targets.
14:157–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uguccioni M, Mackay CR, Ochensberger B, et
al: High expression of the chemokine receptor CCR3 in human blood
basophils. Role in activation by eotaxin, MCP-4, and other
chemokines. J Clin Invest. 100:1137–1143. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyagaki T, Sugaya M, Murakami T, et al:
CCL11-CCR3 interactions promote survival of anaplastic large cell
lymphoma cells via ERK1/2 activation. Cancer Res. 71:2056–2065.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levina V, Nolen BM, Marrangoni AM, et al:
Role of eotaxin-1 signaling in ovarian cancer. Clin Cancer Res.
15:2647–2656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agarwal M, He C, Siddiqui J, Wei JT and
Macoska JA: CCL11 (eotaxin-1): a new diagnostic serum marker for
prostate cancer. Prostate. 73:573–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chao PZ, Hsieh MS, Cheng CW, Lin YF and
Chen CH: Regulation of MMP-3 expression and secretion by the
chemokine eotaxin-1 in human chondrocytes. J Biomed Sci. 18:862011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lein M, Nowak L, Jung K, Koenig F, Schnorr
D and Loening SA: Metalloproteinases (MMP-1, MMP-3) and their
inhibitors (TIMP) in blood plasma of patients with prostate
carcinoma. Urologe A. 37:377–381. 1998.(In German).
|
|
14
|
Zhu G, Zhou J, Song W, et al: Role of
GLI-1 in epidermal growth factor-induced invasiveness of ARCaPE
prostate cancer cells. Oncol Rep. 30:904–910. 2013.PubMed/NCBI
|
|
15
|
Kodali RB, Kim WJ, Galaria II, et al:
CCL11 (Eotaxin) induces CCR3-dependent smooth muscle cell
migration. Arterioscler Thromb Vasc Biol. 24:1211–1216. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salcedo R, Young HA, Ponce ML, et al:
Eotaxin (CCL11) induces in vivo angiogenic responses by human
CCR3+ endothelial cells. J Immunol. 166:7571–7578. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muessel MJ, Scott KS, Friedl P, Bradding P
and Wardlaw AJ: CCL11 and GM-CSF differentially use the Rho GTPase
pathway to regulate motility of human eosinophils in a
three-dimensional microenvironment. J Immunol. 180:8354–8360. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Georgiou GK, Igglezou M, Sainis I, et al:
Impact of breast cancer surgery on angiogenesis circulating
biomarkers: a prospective longitudinal study. World J Surg Oncol.
11:2132013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jöhrer KK, Zelle-Rieser C, Perathoner A,
et al: Up-regulation of functional chemokine receptor CCR3 in human
renal cell carcinoma. Clin Cancer Res. 11:2459–2465.
2005.PubMed/NCBI
|
|
20
|
Kouno J, Nagai H, Nagahata T, et al:
Up-regulation of CC chemokine, CCL3L1, and receptors, CCR3, CCR5 in
human glioblastoma that promotes cell growth. J Neurooncol.
70:301–307. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Wittchen ES, Jiang Y, Ambati B,
Grossniklaus HE and Hartnett ME: Upregulation of CCR3 by
age-related stresses promotes choroidal endothelial cell migration
via VEGF-dependent and -independent signaling. Invest Ophthalmol
Vis Sci. 52:8271–8277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Markwick LJ, Clements D, Roberts ME,
Ceresa CC, Knox AJ and Johnson SR: CCR3 induced-p42/44 MAPK
activation protects against staurosporine induced-DNA fragmentation
but not apoptosis in airway smooth muscle cells. Clin Exp Allergy.
42:1040–1050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chakraborti S, Mandal M, Das S, Mandal A
and Chakraborti T: Regulation of matrix metalloproteinases: an
overview. Mol Cell Biochem. 253:269–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsu YH, Hsieh MS, Liang YC, et al:
Production of the chemokine eotaxin-1 in osteoarthritis and its
role in cartilage degradation. J Cell Biochem. 93:929–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bachmeier BE, Nerlich AG, Lichtinghagen R
and Sommerhoff CP: Matrix metalloproteinases (MMPs) in breast
cancer cell lines of different tumorigenicity. Anticancer Res.
21:3821–3828. 2001.PubMed/NCBI
|