Introduction
The generation of stably-transfected cell lines is a
common and very important technology in the life sciences (1). It is commonly practiced in order to
understand a specific gene’s function in the cells or organisms
(2). In contrast to transient
expression, stable expression allows long-term and defined
investigation of the gene of interest (3). To date, considerable achievements have
been made using this technology (4–6);
researchers in the life sciences have gained significant insight
through the modification of the genetic code.
It is well known that phenotypic changes are a
result of multiple genetic interactions and are often complex.
Exogenous DNA could be inserted into genome through transfection
technology and exert its function after it is introduced into a
cell under selection stress, to finally generate the stable
modified cell lines with the desired characteristics (7). However, the technique has its
limitation due to the randomness of incorporation of exogenous gene
and the possibility of affecting other gene expression patterns
(1), especially for introducing
exogenous DNA into cancer cells that already have high genetic
variance (8). Although the desired
expression pattern is obtained in the stably transformed cancer
cell line, the characteristics of the stable cell line may not be
in line with the function of exogenous DNA.
In the present study, we provided evidence of the
effect of exogenous DNA stress on the required function of the
transformed cells. Overexpression plasmid containing exogenous
S100P gene (S100P) and knockdown plasmid containing shRNA targeted
to S100P sequence (shS100P) were transfected into a human salivary
adenoid cystic carcinoma (SACC) cell line SACC-83. Then, the cell
mobility and invasion ability were detected and the results showed
that the characteristics of these SACC-83-derived stably
transfected cell lines were not governed by S100P expression.
Therefore, we suspected that genomic instability caused by
exogenous DNA stress could determine the required characteristics
in SACC-83 based cell lines.
Materials and methods
Construction of S100P overexpression
plasmid and S100P knockdown plasmid
Human S100P gene was generated from SACC-LM cDNA by
reverse transcription-polymerase chain reaction using the following
primers: forward primer containing EcoRI restriction
endonuclease site in the 5′ end (5′-TTGAATTCATGACGGAACTAGAGACAGCCATG-3′)
and reverse primer with BamHI restriction endonuclease site
in the 5′ end (5′-TTGGATCCTCATTTGAGTCCTGCCTT
CTCAAAG-3′). Restriction enzyme sites are underlined. The PCR
fragment was cut by EcoRI and BamHI, and then
subcloned into pCDNA3.1 vector to generate an expression construct,
pCDNA-S100P. The identity of the coding sequence for S100P in the
pCDNA vector was confirmed by DNA sequencing. The S100P
gene-specific shRNA expression pRI-GFP/Neo-shS100P plasmid was
constructed using synthetic oligonucleotides cloned into
pRI-GFP/Neo plasmid. The sequence AATGGAGATGCCC AGGTGGAC is
designed for specific targeting of human S100P gene.
Cell cultures and establishment of stable
cell lines
Human SACC cell line SACC-83 was used for the
establishment of stably transfected SACC-83-derived cell lines
through transfection of pCDNA-S100P, pRI-GFP/Neo-shS100P, pCDNA3.1
plasmids, respectively. Briefly, S100P overexpression and S100P
knockdown plasmids were respectively transfected into SACC-83 with
the Lipofectamine 2000 according to the manufacturer’s instructions
(Invitrogen, Carlsbad, CA, USA). Stable cell lines derived from
single colony were established following selection with 1,000 μg/ml
G418 (Sigma-Aldrich, St. Louis, MO, USA), then evaluated by testing
S100P expression via real-time PCR and western blot analysis. Since
empty vectors pCDNA3.1 and pRI-GFP/Neo generated stably transfected
cell lines had the similar characteristics of migration and
invasion (data not shown), hereafter, only the pCDNA3.1 stably
transfected cell line, named SACC-83-MOCK was used as the mock
control. SACC-83 and SACC-83-derived cell lines (SACC-83-S100P,
SACC-83-shS100P and SACC-83-MOCK) were cultured in RPMI-1640
(Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine
serum (FBS; Thermo Fisher Scientific Inc., Waltham, MA, USA), 100
U/ml penicillin and 100 μg/ml streptomycin (Gibco) and maintained
in a humidified incubator at 37°C with 5% CO2 for the
following experiments.
Immunocytochemical staining
The S100P protein was detected using a labeled
streptavidin-biotin method after antigen retrieval. The cells were
cultured on the glass coverslips at 37°C in a humidified
CO2 incubator until they were 50–70% confluent. They
were then fixed with 4% paraformaldehyde for 10 min and subjected
to immunocytochemistry. The glass coverlids were rinsed twice with
PBS, and endogenous peroxidase was blocked by the use of 3%
hydrogen peroxide for 10 min. The samples were blocked with normal
goat serum for 30 min, incubated with anti-human anti-S100P
polyclonal antibody (Epitomics, Burlingame, CA, USA) overnight at
4°C, followed by peroxidase-conjugated immunoglobulin for 30 min,
developed for color with peroxidase substrate 3,3′-diaminobenzidine
(DAB), counterstained with hematoxylin, and recorded using an
Olympus DP Controller.
Quantitative PCR (qPCR) analysis
Total RNA was extracted from SACC-83 or its derived
cell lines using the TRIzol reagent according to the manufacturer’s
instructions (Invitrogen). Complementary DNA was reverse
transcribed by the use of 2.5 μg of RNA as template. qPCR was
performed using the ABI 7500 Real-Time PCR machine (Applied
Biosystems, Carlsbad, CA, USA) coupled with SYBR-Green chemistry
(Roche Diagnostics, Indianapolis, IN, USA). All PCR reactions were
in 20 μl of total volume containing 10 μl of SYBR-Green PCR Master
Mix, 50 ng cDNA, 200 nM of the following primer sets: S100P
(5′-ATGACGGAACTAGAGAC AGCC-3′ and 5′-AGGAAGCCTGGTAGCTCCTT-3′). The
housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
was used as an internal control (5′-ATGGGG AAGGTGAAGGTCG-3′ and
3′-GGGGTCATTGATGGCAA CAATA-5′). All amplifications were carried out
in triplicate for each sample and repeated three times. The thermal
cycling was 10 min at 95°C, followed by 40 cycles at 95°C for 15
sec, at 60°C for 60 sec. The specificity of amplification was
monitored using the dissociation curve of the amplified products.
Relative expression of the target genes was calculated using the
2−ΔΔCt method.
Western blot analysis
Cells were harvested and lysed in RIPA buffer with
protease inhibitors (Roche Diagnostics). Protein concentration was
determined using the BCA Protein Assay (Thermo Fisher Scientific)
and 40 μg of protein was loaded for each sample. Proteins were
separated on an SDS-polyacrylamide gel and transferred to a
polyvinylidene difluoride membrane. The membranes were blocked in
5% non-fat dry milk for 1 h and probed with antibodies against
S100P, matrix metalloproteinase MMP1, MMP2, MMP3, MMP9, MMP11,
MMP13 and MMP14 and β-actin separately at 4°C overnight. After
incubation with peroxidase-linked secondary antibodies,
immunoreactive proteins were visualized by ECL reagent (Applygen
Technology, Inc., Beijing, China).
Wound healing assay
Cell migration was assessed by wound healing assay.
Briefly, ~5×105 cells were cultured as confluent
monolayer, and wounded by scratching across the well with a 200 μl
pipette tip. Then, the dishes were washed by D-Hanks to remove the
deciduous cells. Wounded monolayer was photographed with a ×10
objective lens at 0 and 24 h after wounding. Wound healing was
quantified by measurement of the average linear speed of movement
of the wound edges at the indicated time points.
Transwell invasion assay
Cell invasion assays were performed using Transwell
chambers with a polycarbonate membrane (Millipore, Bedford, MA,
USA) coated with 40 μl diluted matrix gel (BD Biosciences, San
Jose, CA, USA). Cells were trypsinized and seeded at
1×105 cells/well/0.1 ml serum-free RPMI-1640 medium in
the upper chambers; 0.5 ml of RPMI-1640 medium supplemented with
20% FBS was added into each lower chamber. At 24 h after
incubation, cells on the surface of the membrane were wiped off,
and the membranes were fixed with 95% ethanol and stained with 1%
crystal violet (Sigma-Aldrich). The invaded cells clinging to the
bottom of the membrane were photographed by light microscopy at ×20
magnification (Olympus, Tokyo, Japan). The invaded cells were
calculated for the evaluation of invasion ability in each cell
line. All experiments were performed in triplicate and similar
results were obtained from three independent experiments.
Chromosome analysis
To investigate why the characteristics of
SACC-83-derived transfected cell lines were not in line with S100P
expression levels, chromosome analysis or karyotyping was performed
to evaluate the number and structure of the chromosomes. Briefly,
SACC-83 cells were cultured in nutrient-enriched media to promote
cell division. The chromosomes were then isolated from the nucleus
of the cells and placed on a slide for chromosome analysis. The
abnormalities of the chromosome were evaluated by a genetics
specialist.
Statistical analysis
The data are expressed as the means ± SD and
compared by the one-way ANOVA test through SPSS software, version
13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Establishment of S100P overexpression or
knockdown SACC-83 cell lines
We successfully established an S100P overexpression
cell line (SACC-83-S100P), an S100P knockdown cell line
(SACC-83-shS100P) and a transfected empty vector cell line
(SACC-83-MOCK) using G418 selection and single cell cloning method.
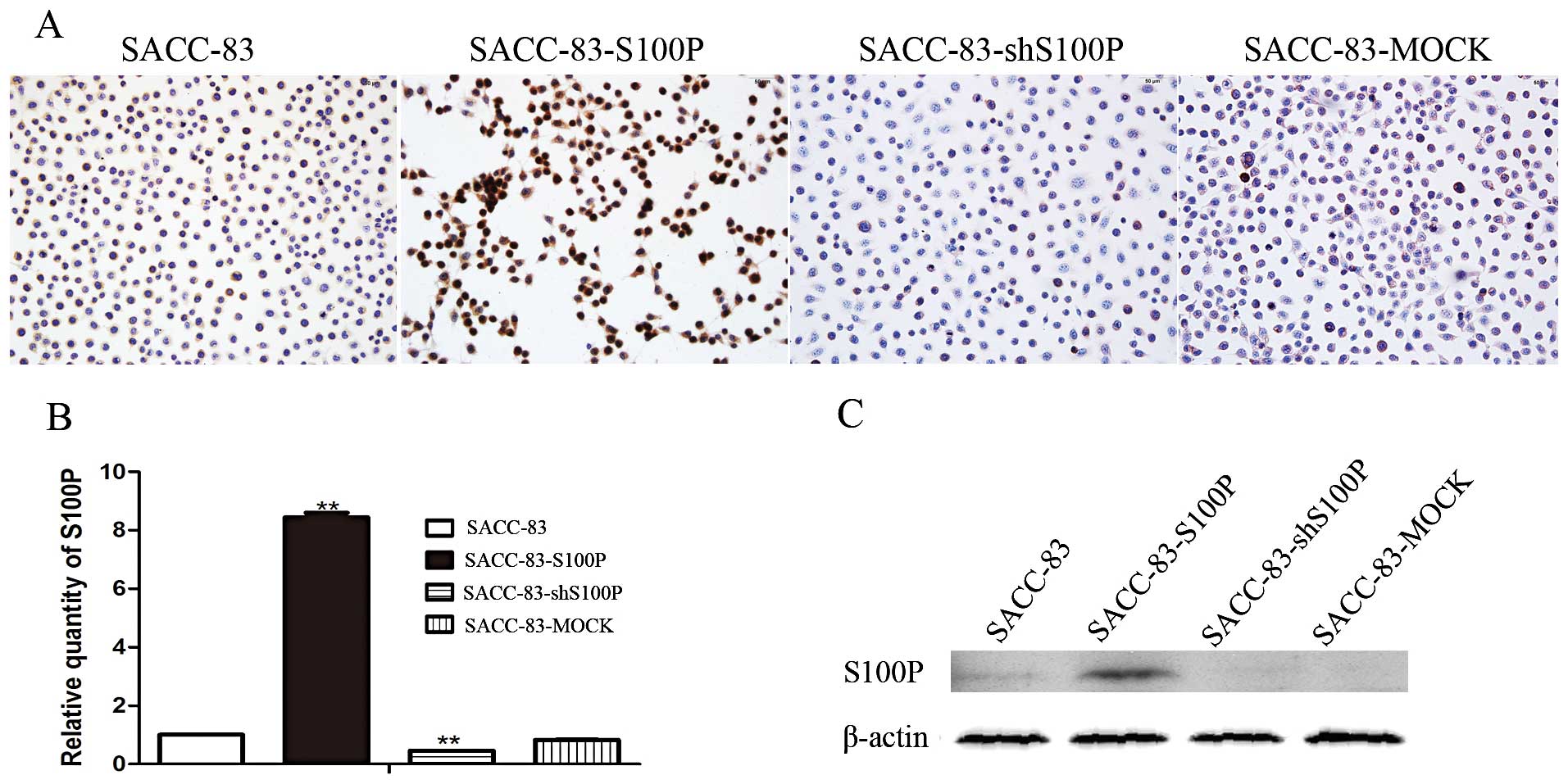
The cell lines were identified by immunostaining, real-time PCR and
western blot assays. Immunostaining results showed that S100P
protein was highly expressed in SACC-83-S100P, low expressed in
SACC-83-shS100P and had a similar expression level with the
parental cell line SACC-83 (Fig.
1A). Real-time PCR results showed that the mRNA level of S100P
was 1.00±0.001 in SACC-83, 8.44±0.16 in SACC-83-S100P, 0.46±0.001
in SACC-83-shS100P. Compared with SACC-83, SACC-83-S100P cells
produced >8-fold higher levels of S100P mRNA; SACC-83-shS100P
cells yielded 54% lower levels of S100P mRNA, while SACC-83-MOCK
cells expressed comparable levels of S100P (Fig. 1B). Western blot results showed that
S100P protein expression pattern was similar as its mRNA expression
pattern. SACC-83 expressed a very faint band; SACC-S100P expressed
the highest level of S100P protein amongst the four cell lines; and
S100P protein was almost undetectable in SACC-83-shS100P and
SACC-83-MOCK cell lines (Fig.
1C).
Migration and invasion ability of the
SACC-83-derived cell lines
After identification of S100P status in the stably
transfected SACC-83 cell lines, we next examined their biological
behavior. As S100P was reported to be related to cancer metastasis,
we examined their migration and invasion ability. Notably, both
overexpression and knockdown of S100P could stimulate the cell
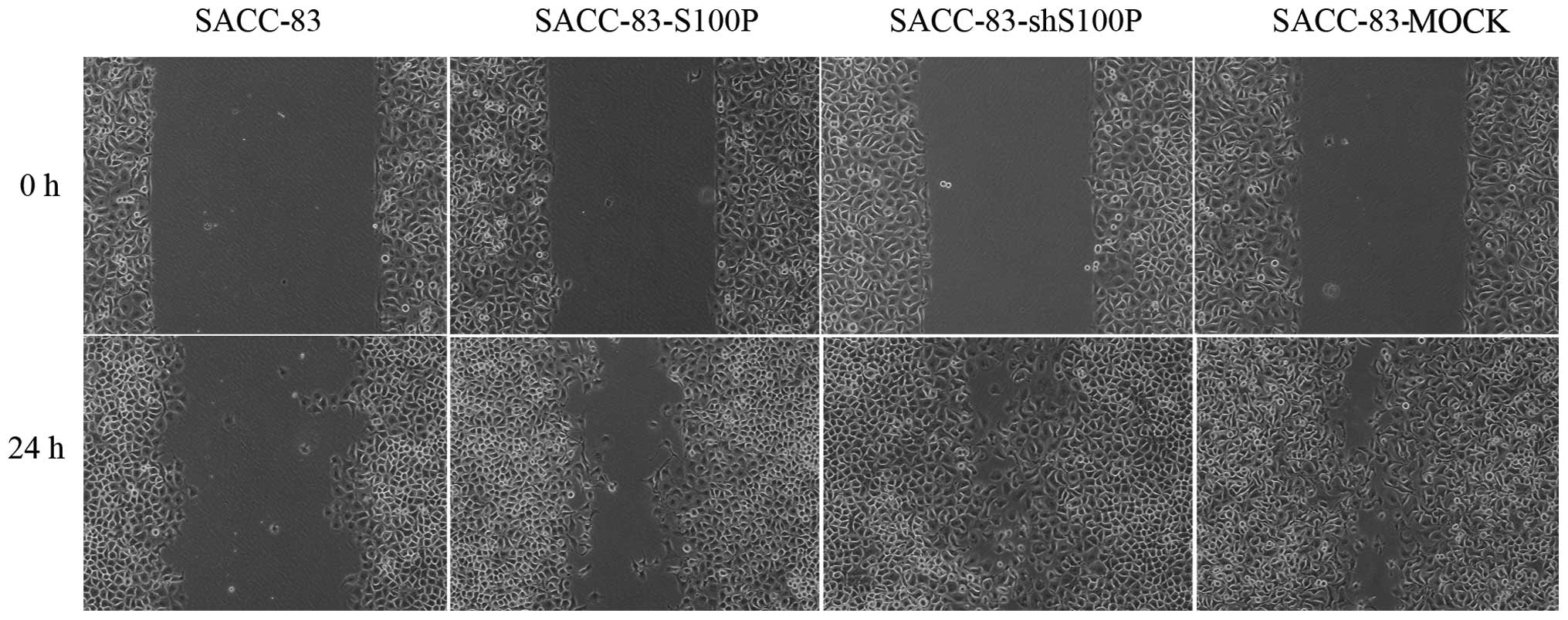
migration and invasion. The migration speeds of SACC-83,
SACC-83-S100P, SACC-83-shS100P and SACC-83-MOCK were 11±1.56,
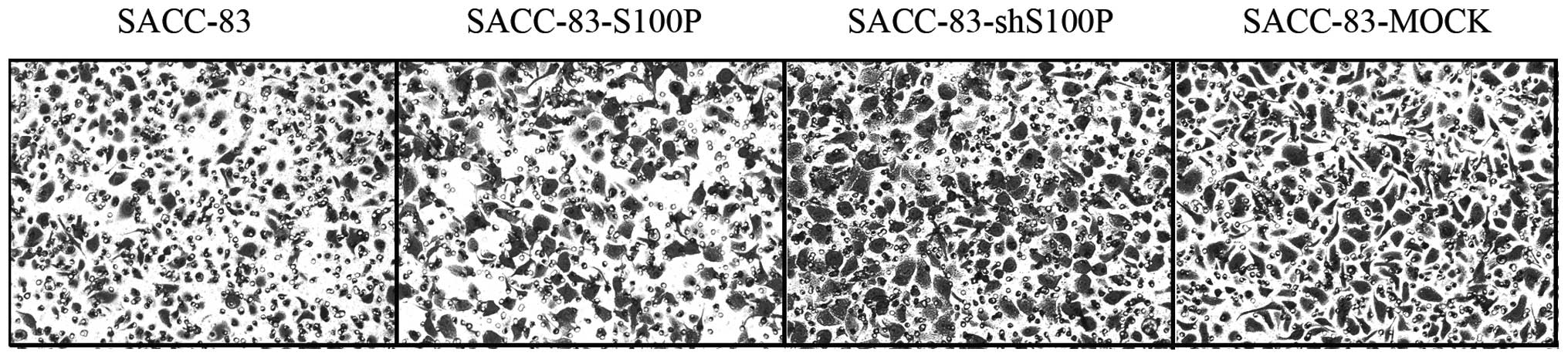
14.81±0.25, 22.93±2.17 and 19.71±1.58 μm/h, respectively (Fig. 2). The relative invaded tumor cells
of SACC-83, SACC-83-S100P, SACC-83-shS100P and SACC-83-MOCK in
Transwell assay were 1±0.30, 2.14±0.30, 2.32±0.27 and 2.92±0.32
(Fig. 3). The migration and
invasion abilities were not related to S100P expression in these
cells. SACC-83-shS100P and SACC-83-MOCK with lower levels of S100P
had higher migration and invasion ability compared to
SACC-83-S100P. Moreover, SACC-83-S100P, SACC-83-shS100P and
SACC-83-MOCK had higher migration and invasion ability compared
with the parental cell line SACC-83. The status of S100P was
neglected in these stably transfected SACC-83 derived cell
lines.
Exogenous DNA incorporation into genome
alters the metastasis-related gene expression pattern
To understand why these SACC-83-derived cell lines
represented the undesirable biological abilities, we performed
western blot analysis to detect the changes of MMP family proteins
that were reported to be required in the process of the migration
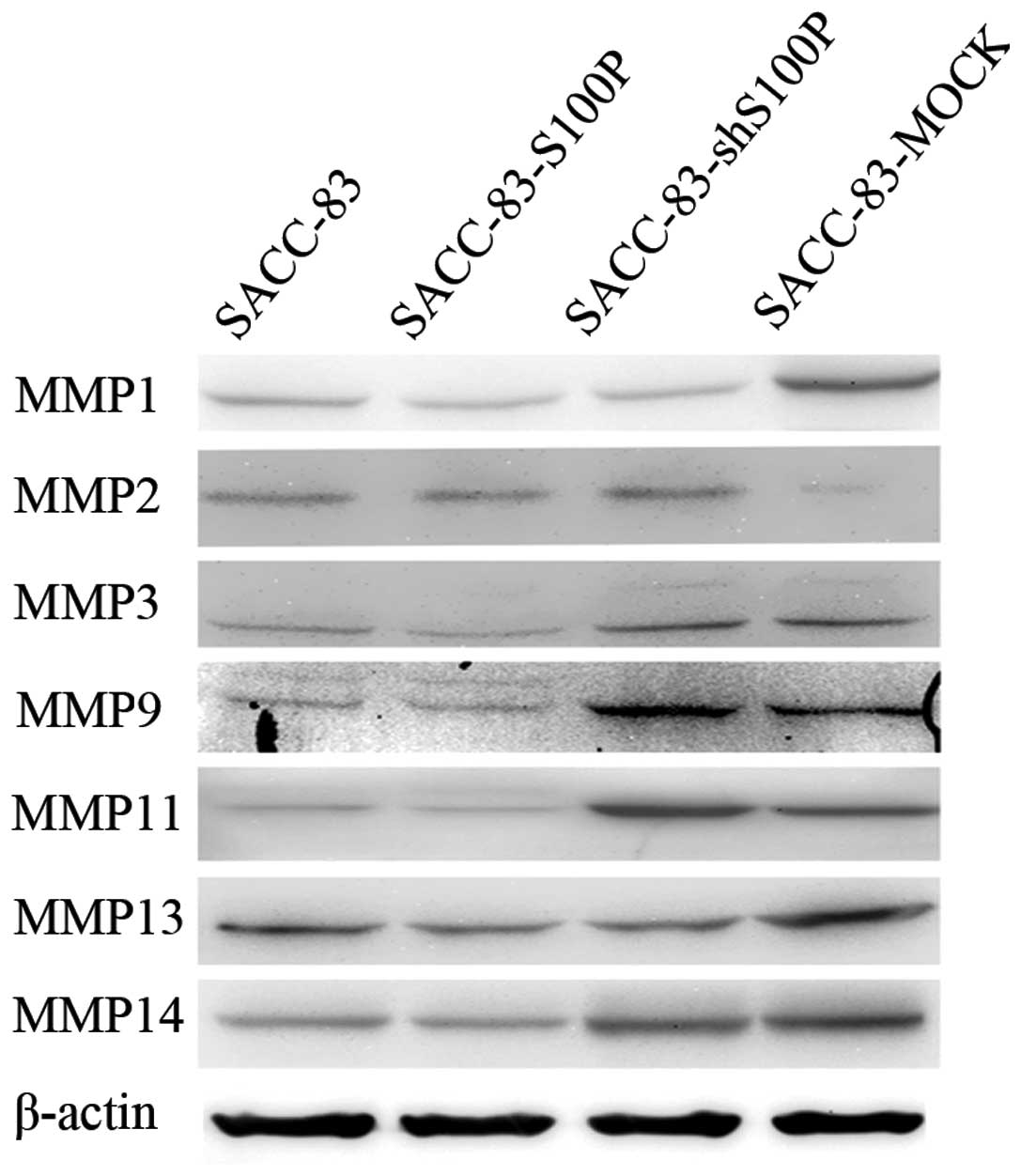
and invasion of SACC. As indicated in Fig. 4, the SACC-83-S100P cell line
expressed the highest MMP2 compared to the other cell lines. The
expression of MMP3, MMP9, MMP11 and MMP14 in the SACC-83-shS100P
cell line was higher than in SACC-83. Compared to the parental cell
line SACC-83, the SACC-83-MOCK cell line expressed higher level of
MMP1, MMP9, MMP11 and MMP14 and lower level of MMP2. The results
indicated that different molecular changes caused by the random
incorporation of exogenous DNA into chromosome led to the same
consequence, that is, the required migration and invasion abilities
were greater in the stably transfected cell lines than in
SACC-83.
Chromosome analysis
To investigate why random incorporation of exogenous
DNA into genome generated unexpected results, we performed
chromosome analysis. The results showed that SACC-83 contained
hypotetraploid karyotyping with 88±2 chromosomes. Most chromosomes
presented the abnormal morphology with high variance (Fig. 5). This result indicated that we
should be cautious when editing a genome in cancer cells with high
variance.
Discussion
Cancer biology is a major part of the life sciences,
and transfection is very useful technology to investigate the
gene’s function in a cancer cell. In general, we only pick up the
desired stably-transfected cell lines to perform the experiments
and explain the expected outcomes. Everything is investigated under
the pre-set conditions within our knowledge, however, something may
be important, but neglected owing to this orientation. In order to
avoid drawing any biased conclusion derived from the
stably-transfected cancer cell lines, we performed the present
study using stably S100P overexpressed and knocked down SACC-83
cells as our example to demonstrate the effect of exogenous DNA
stress on the required function of the transformed cells already
containing high genome variance. The reason why we selected this
gene is that S100P is found to be differentially expressed in the
homologous but different lung metastatic ability cell lines,
SACC-83 and SACC-LM, in our previous study (9). S100P has been reported to serve many
important functions in diverse origins of cancer, such as
stimulation proliferation, survival, motility and invasiveness
(10–13).
After we established and identified the stable cell
lines, we first analyzed their biological behaviors, and found that
S100P expression level did not govern the mobility and invasion
abilities of the stably transfected SACC-83 cell lines; both
overexpressed and knocked down S100P cell lines had higher mobility
and invasion abilities than the parental cell line SACC-83.
Moreover, the mock-transfected cell line SACC-83-MOCK had the
greatest invasion abilities amongst SACC-83-S100P, SACC-83-shS100P,
SACC-83-MOCK and SACC-83 cell lines. The knockdown cell line
SACC-83-shS100P had the highest mortality ability. The convoluted
results led us to investigate the underlying mechanism. It is well
known that cell behavior is influenced by the interaction of
molecules within the cell, and MMPs play a crucial role in the
extracellular matrix breakdown process of cancer metastasis
(14). MMPs are a family of enzymes
with the ability to degrade all types of extracellular matrix.
Previous studies showed that the function of S100P was associated
with the MMP family (15). Thus, we
investigated some typical molecules in MMP families. Results
analysis showed that different MMP molecules exhibited different
expression abundance in these SACC cell lines, such as MMP1 in
SACC-83-MOCK, MMP2 in SACC-83-S100P and MMP9 in SACC-83-shS100P
were highly expressed, respectively. Moreover, the biological
behaviors of the four SACC cell lines were supported by the
expression pattern of MMPs but not by those of S100P. However,
their invasion and migration abilities were confirmed to be caused
by different molecular mechanism, which may be due to the exogenous
DNA random incorporation.
Next, we examined the karyotyping of the parental
cell line SACC-83 and tried to find the explanation. The result
showed that the genome of SACC-83 had high genetic variance. We
speculated that a cancer cell with high genetic variance plus the
exogenous DNA random incorporation transfection may cause the gene
expression pattern change in the present study, which was supported
by previous studies (16,17). Thus, although the stably transfected
SACC cell lines exhibited the high or low expression of target gene
S100P, the motility and invasiveness of the four SACC cells were
not governed by S100P expression level, and were not consistent
with the previous reports on S100P function during cancer
metastasis (18). The random
insertion of exogenous gene in a cell with high genetic variance
may be the major reason for this unexpected phenomenon. Studies
regarding stable transfection exogenous DNA into a cancer cell
should consider the status of the genetic variance in the targeted
cancer cells in order to avoid any potential bias and unreliable
conclusions derived from the stably transfected cancer lines.
In addition, given that virus infection is a common
inducer for certain types of cancer characteristics of the tumor
cells, it may be a result of an exogenous random insertion to the
chromosome, thus leading to genetic variance and the shift in gene
expression profile of cancer cells.
In conclusion, although the establishment of stably
transfected cancer cell lines is a common method to investigate the
function of a target gene, the status of genetic variance in a
cancer cell should be considered in order to avoid any potential
unreliable conclusion.
Acknowledgements
The present study was supported by a Research Grant
from the National Nature Science Foundation of China (grant no.
81271150).
References
|
1
|
Stuchbury G and Munch G: Optimizing the
generation of stable neuronal cell lines via pre-transfection
restriction enzyme digestion of plasmid DNA. Cytotechnology.
62:189–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glover DJ, Lipps HJ and Jans DA: Towards
safe, non-viral therapeutic gene expression in humans. Nat Rev
Genet. 6:299–310. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim TK and Eberwine JH: Mammalian cell
transfection: the present and the future. Anal Bioanal Chem.
397:3173–3178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kakudo T, Chaki S, Futaki S, et al:
Transferrin-modified liposomes equipped with a pH-sensitive
fusogenic peptide: an artificial viral-like delivery system.
Biochemistry. 43:5618–5628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kursa M, Walker GF, Roessler V, et al:
Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA
complexes for systemic tumor-targeted gene transfer. Bioconjug
Chem. 14:222–231. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zanta MA, Belguise-Valladier P and Behr
JP: Gene delivery: a single nuclear localization signal peptide is
sufficient to carry DNA to the cell nucleus. Proc Natl Acad Sci
USA. 96:91–96. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barrett LE, Sul JY, Takano H, et al:
Region-directed phototransfection reveals the functional
significance of a dendritically synthesized transcription factor.
Nat Methods. 3:455–460. 2006. View
Article : Google Scholar
|
|
8
|
Negrini S, Gorgoulis VG and Halazonetis
TD: Genomic instability - an evolving hallmark of cancer. Nat Rev
Mol Cell Biol. 11:220–228. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Wang Y, Bian H, et al: Molecular
characteristics of homologous salivary adenoid cystic carcinoma
cell lines with different lung metastasis ability. Oncol Rep.
30:207–212. 2013.PubMed/NCBI
|
|
10
|
Cheng YS, Jordan L, Rees T, et al: Levels
of potential oral cancer salivary mRNA biomarkers in oral cancer
patients in remission and oral lichen planus patients. Clin Oral
Investig. Jul 28–2013.(Epub ahead of print).
|
|
11
|
Arumugam T, Simeone DM, Van Golen K, et
al: S100P promotes pancreatic cancer growth, survival, and
invasion. Clin Cancer Res. 11:5356–5364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ge F, Wang C, Wang W, et al: S100P
predicts prognosis and drug resistance in gastric cancer. Int J
Biol Markers. 28:e387–e392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan RH, Chang KT, Chen YL, et al: S100P
expression is a novel prognostic factor in hepatocellular carcinoma
and predicts survival in patients with high tumor stage or early
recurrent tumors. PLoS One. 8:e655012013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
15
|
Namba T, Homan T, Nishimura T, et al:
Up-regulation of S100P expression by non-steroidal
anti-inflammatory drugs and its role in anti-tumorigenic effects. J
Biol Chem. 284:4158–4167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Denko NC, Giaccia AJ, Stringer JR, et al:
The human Ha-ras oncogene induces genomic instability in
murine fibroblasts within one cell cycle. Proc Natl Acad Sci USA.
91:5124–5128. 1994.
|
|
17
|
Gorgoulis VG, Vassiliou LV, Karakaidos P,
et al: Activation of the DNA damage checkpoint and genomic
instability in human precancerous lesions. Nature. 434:907–913.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barry S, Chelala C, Lines K, et al: S100P
is a metastasis-associated gene that facilitates transendothelial
migration of pancreatic cancer cells. Clin Exp Metastasis.
30:251–264. 2013. View Article : Google Scholar : PubMed/NCBI
|