Introduction
Autophagy, an evolutionarily conserved cell death
process, plays a critical role in maintaining energy homeostasis,
protein and organelle recycling by transferring defective cytoplasm
and organelles into double-membraned vesicles, termed
autophagosomes, to degrade and regenerate materials and ATP
(1). Autophagy can be activated
simultaneously with apoptosis under nutrient starvation, hypoxia or
other therapeutic stress (2).
However, unlike the latter, autophagy can facilitate genetic
stability (3) and cellular
homeostasis and play a protective role against stress (4,5).
Recent research shows that autophagy is activated as a survival
mechanism in cancer under different types of stress (6,7) and
helps cancer cells against environment stress and provides a
temporary survival pathway by promoting energy regeneration and by
maintaining genetic stability and cellular homeostasis. Research
has revealed that autophagy guarantees tumor cell survival when
apoptosis is inactivated (8).
Defective autophagy was found to render mouse mammary epithelial
cells susceptible to metabolic stress, prone to DNA damage and
genomic instability via gene amplification (9,10).
However, another study found that autophagy mainly contributes to
tumor suppression. It can mitigate metabolic stress and genome
damage to inhibit tumorigenesis. Wang et al found that Akt
suppresses autophagy by mTOR-independent phosphorylation of
beclin1, ultimately promoting tumorigenesis (11). Whether autophagy plays a
tumor-suppressor role or a tumor-promoter role is not clear and
needs to be further researched.
Vascularization plays an important role in oxygen
and nutrient supplementation, proliferation, invasion and
metastasis in tumor tissues. The formation of tumor feeding vessels
mainly relies on existing normal endothelial vasculature, which can
grow inside tumors and supply oxygen and nutrients. Vasculogenic
mimicry (VM) is a new type of tumor angiogenesis model (12). Many highly malignant tumors can
simulate vascular channel forming pathways, and differentiate into
vascular channels which supply nutrients, oxygen and provide
invasive channels to interior tumor tissue (13). Because of the independence of the
endothelial vascular, vascular endothelial tumor-suppressor drugs
are not sensitive to this type of vascularization (14). VM has been found in many highly
metastatic malignant tumors (15,16).
What is more, its existence is often closely related to the
response to various types of tumor treatment and patient prognosis
(17,18). Yet, the mechanism of VM is still not
clear. A recent study found that autophagy-specific genes beclin1
and LC3 were both highly expressed in VM-positive melanoma when
compared to expression levels in negative samples. The action of
autophagy was found to be related to VM and the metastasis of
melanoma (19). Therefore, we
hypothesized that autophagy contributes to VM formation, which aids
cancer cell survival and metastasis. In our research, we aimed to
ascertain whether autophagy facilitates VM formation, how it
functions and the possible mechanisms finally elucidating the
effect of autophagy effect on the promotion of cancer.
Materials and methods
Cell culture
Human gastric cancer cell line SGC7901 was grown in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS)
(both from HyClone, Logan, UT, USA), 100 U/ml penicillin and 100
g/ml streptomycin (both from Beyotime Institute of Biotechnology,
China). Cells were maintained at 37°C in a humidified atmosphere of
5% CO2.
Short hairpin RNA (shRNA) construction
for beclin1 knockdown and transfection
shRNA to silence beclin1, an autophagy-specific
gene, was designed, synthesized and subcloned into a vector while a
negative control was also established. The recombinant vector was
then transfected into the SGC7901 cell line and the cells were
screened with G418 and assayed using RT-PCR and western
blotting.
Tube formation assay
One milliliter of viable cells
(2.5×105/ml) was added to each well of 24-well plates
containing 0.2 ml Matrigel matrix (BD Biosciences, Bedford, MA,
USA). Plates were incubated at 37°C in 5% CO2 for 24 h.
The vascular mimicry formation ability was evaluated by counting
the average length of the vascular mimicry, number and intersecting
nods. Each experiment was performed at least three times.
Measurement of cell viability
Cell viability was assessed by the
2-(4,5-dimethyltriazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT;
Amresco Inc., Solon, OH, USA) colorimetric assay. Briefly, 20 μl of
MTT (5 mg/ml) was added to each well. After a 4-h incubation at
37°C, the cell supernatants were discarded, MTT crystals were
dissolved with DMSO and the absorbance was measured at 450 nm. All
experiments were conducted with 4–6 wells per experiment and
repeated at least 3 times.
Cellular immunofluorescence staining
Cells were fixed in 4% paraformaldehyde for 10 min
at room temperature with permeation using 0.1% Triton X-100. Slides
were washed three times with phosphate-buffered saline (PBS),
blocked with 5% bovine serum albumin in PBS for 1 h at 37°C,
incubated with antibodies against LC-3 (1:400; Beyotime) overnight
at 4°C and with PE-Cy3 secondary antibodies (1:500; Beyotime) for 1
h at room temperature. The slides were observed using a
fluorescence microscope.
Real-time quantitative-polymerase chain
reaction (PCR)
Total-RNA was extracted with the use of TRIzol
reagent according to the manufacturer’s instructions (Invitrogen,
Carlsbad, CA, USA). cDNA was generated by reverse transcription
using First Strand cDNA Synthesis kit (Thermo Scientific, Waltham,
MA, USA) and oligo(dT) primers in 20 μl reaction volume containing
5 μg of total RNA pretreated with RNase-free DNase I. PCR was
performed with 25 μl reactions containing 0.5 μl diluted cDNA,
Taq DNA polymerase, and the primers are listed in Table I. The PCR consisted of an initial
denaturation at 94°C for 4 min, followed by 25–35 cycles of 94°C
for 30 sec, 58–68°C for 30 sec and 72°C for 1 min. PCR products
were analyzed on 1.5% agarose gels.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Primer sequences |
|---|
| Notch-1 |
5′-GCAGTTGTGCTCCTGAAGAA
3′-CGGGCGGCCAGAAAC |
| Notch-2 |
5′-ACTTCCTGCCAAGCATTCC
3′-GTCCATGTCTTCAGTGAGAAC |
| Notch-3 |
5′-TGACCGTACTGGCGAGACT
3′-CCGCTTGGCTGCATCAG |
| c-Myc |
5′-CTTCTCTCCGTCCTCGGATTCT
3′-GAAGGTGATCCAGACTCTGACCTT |
| Oct-3/4 |
5′-GGAGATATGCAAAGCAGAAACC
3′-CTCAAAATCCTCTCGTTGTGC |
| Sox-2 |
5′-CGGCAACCAGAAAAACAGC
3′-TCTCCGTCTCCGACAAAAGT |
| Beclin-1 |
5′-AGGTTGAGAAAGGCGAGACAC
3′-ATGGGTTTTGATGGAATAGGAG |
| ATG5 |
5′-CTCRGCCTTGGAACATCACA
3′-AGGGTATGCAGCTGTCCATC |
| ATG7 |
5′-CAAAGCCTCCAAAATTCAGC
3′-GAAGCAGAAAGGCAGCATA |
| MMP-2 |
5′-TGATCTTGACCAGAATACCATCGA
3′-GGCTTGCGAGGGAAGAAGTT |
| MMP-9 |
5′-CCTGGAGACCTGAGAACCAATC
3′-CCACCCGAGTGTAACCATAGC |
| E-cadherin |
5′-CAACTTCCCCTTCTTCACCC
3′-TCCAATGCTATGCCTAGCCG |
Western blotting
The whole-cell lysates were resolved by sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene difluoride (PVDF) membranes
(Millipore, Billerica, MA, USA). Blots were blocked and incubated
with the anti-beclin1 (1:1,000), anti-ATG5 (1:1,000), anti-ATG7
(1:1,000) (all from Cell Signaling Technology Inc.), anti-c-myc
(1:1,000), anti-notch-1 (1:1,000) (both from Abcam, Cambridge, MA,
USA), followed by incubation with a secondary antibody (1:2,000;
Santa Cruz Biotechnology, Santa Cruz, CA, USA). Blots were
visualized using enhanced chemiluminescence detection reagents and
exposed to X-ray film. The blots were stripped and re-probed with
the anti-β-actin antibody.
Matrigel invasion assay
The Transwell chamber was used (8-mm, 24-well
format; Corning, Costar, NY, USA). In the invasion assay, the
insert membranes were coated with diluted Matrigel (BD
Biosciences). Cells (1×105) were added to the upper
chamber and cultured for 24 h. Finally, the insert membranes were
cut and stained with crystal violet (0.04% in water; 100 ml;
Beyotime) and the number of invasion cells were counted under an
inverted microscope and photographed. Meanwhile, at least three
independent experiments were performed for all conditions. The data
are shown as means ± SD.
Statistical analysis
Data are shown as means ± SD. The statistical
difference between the two groups was examined by the Student’s
t-test. Multiple comparisons were performed by one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant result.
Results
VM formation by SGC7901 cells in 3D
culture
Three-dimensional (3D) culture mimicks the features
of the in vivo environment, allowing cancer cells to live in
a matrix or other hydrogel and is superior to two-dimensional (2D)
culture in plastic flasks. Because of the absence of complex
vascular systems which perfuse oxygenation and nutrition, 3D
cultures provide similar hypoxia and a low nutrition stress
environment in vivo for cancer cells (20,21).
In the present study, we used Matrigel to establish
a 3D culture, and mimick hypoxia and low nutrition in an in
vivo stress environment. Gastric cancer SGC7901 cells were grow
in the Marigel matrix. After a 24-h 3D culture, a tubular-like
structure was noted (Fig. 1A),
while a 24-h 2D culture did not change the morphology of SGC7901
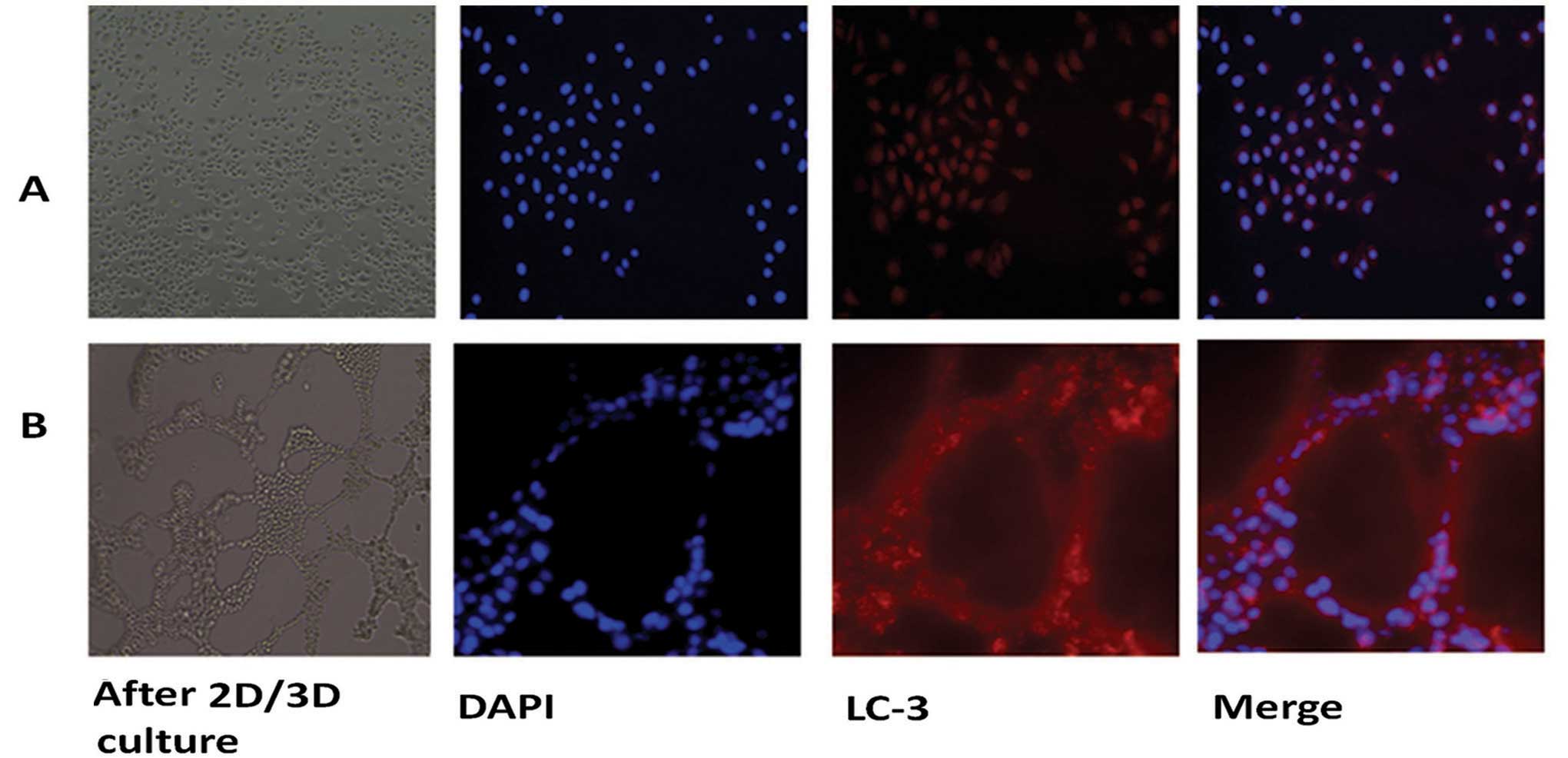
cells as tubular-like structures were not present (Fig. 1B).
Autophagy is activated in VM formation of
SGC7901 cells
To determine whether autophagy functions in VM
formation of gastric cancer cells, we examined the expression of
autophagy-specific protein (LC-3) by cellular immunofluorescence
staining and detected the expression of additional
autophagy-specific genes (beclin1, ATG5, ATG7) by RT-PCR and
western blotting in the gastric cancer SGC7901 VM formation
process. Cells (2.5×105) were plated on a 3D gel and
after a 24-h 3D culture, the formation of VM was observed.
Immunofluorescence analysis showed that the expression of
autophagy-specific gene LC3 in the SGC7901 cells was increased
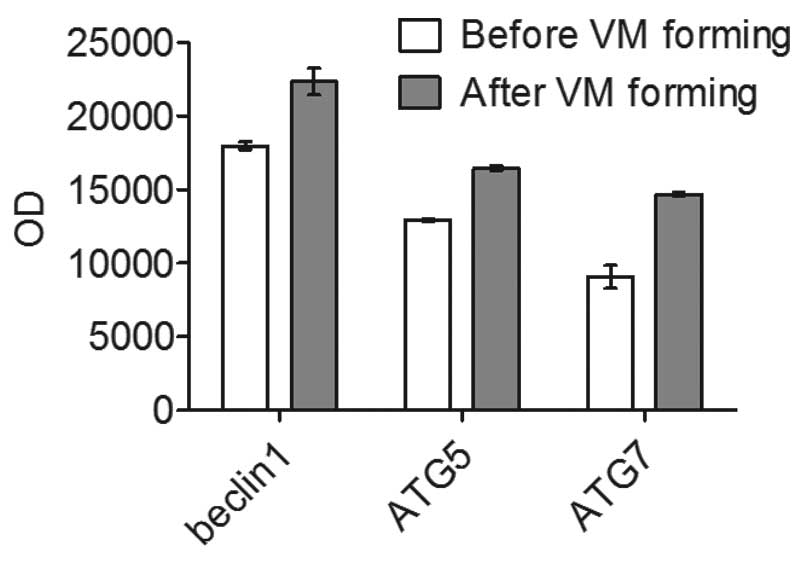
during VM formation after 3D culture (Fig. 1). PCR and western blotting both
showed that the autophagy-specific genes (beclin1, ATG5, ATG7),
accompanied by the formation of VM, were highly expressed after VM
formation in 3D culture. (Fig.
2).
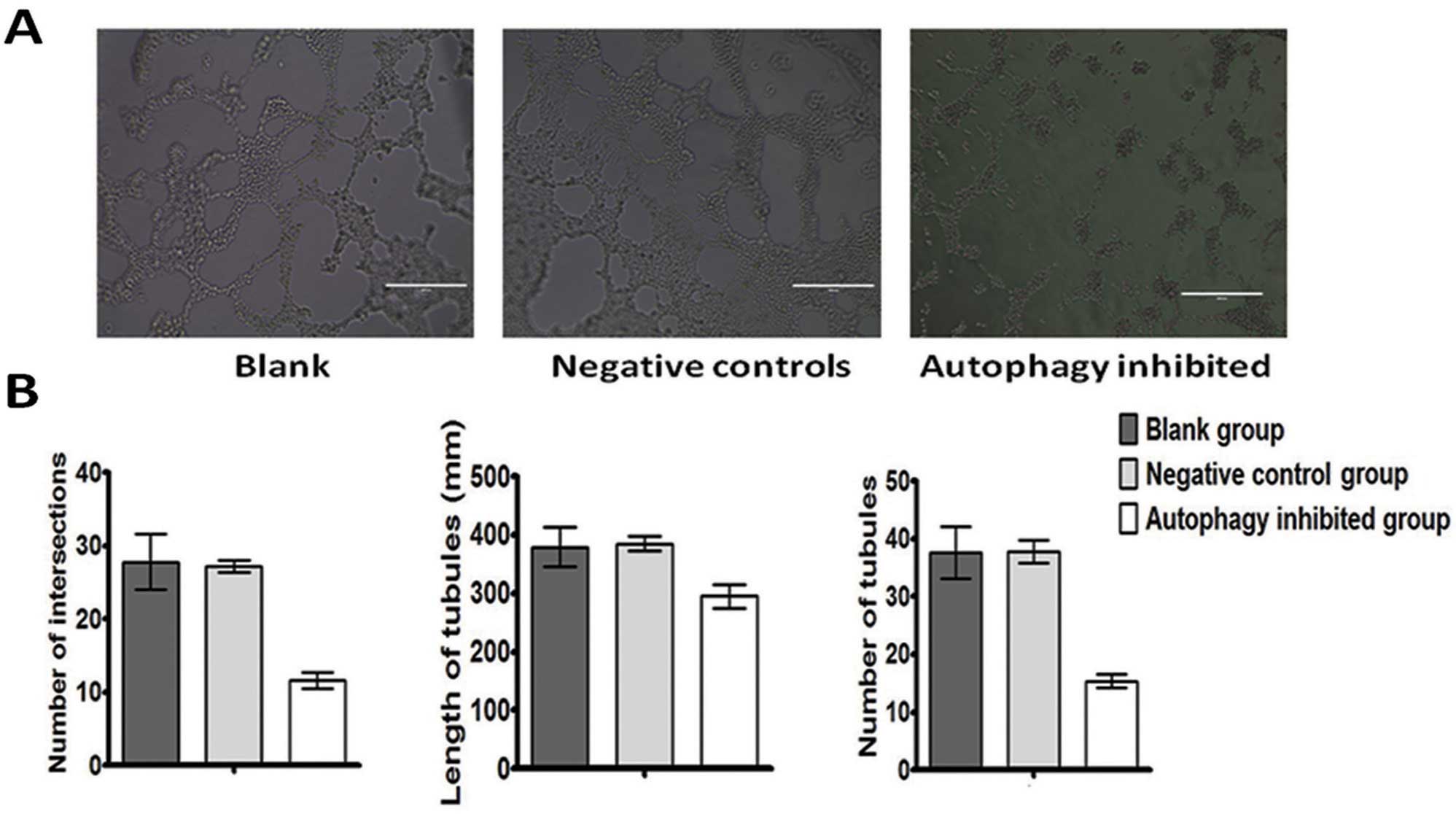
Inhibition of autophagy-specific gene
beclin1 in gastric cancer cells suppresses VM
To explore the function of autophagy in the ability
of gastric cancer cells to form VM, an shRNA to silence beclin1, an
autophagy-specific gene, was designed, synthesized and subcloned
into a vector, while a negative control was also established. Then,
the recombinant vector was transfected into the SGC7901 cell line,
and the cells were screened with G418 and assayed using RT-PCR and
western blotting. Next we plated 2.5×105 cells of the
autophagy-inhibited group, negative control group and the blank
control group on 3D gel, for a 24-h culture. The formation of VM
occured in the negative control group and the blank group while the
inhibition of autophagy suppressed VM formation as shown by tubular
length, number and tubular intersecting nods, compared with the
other groups (Fig. 3).
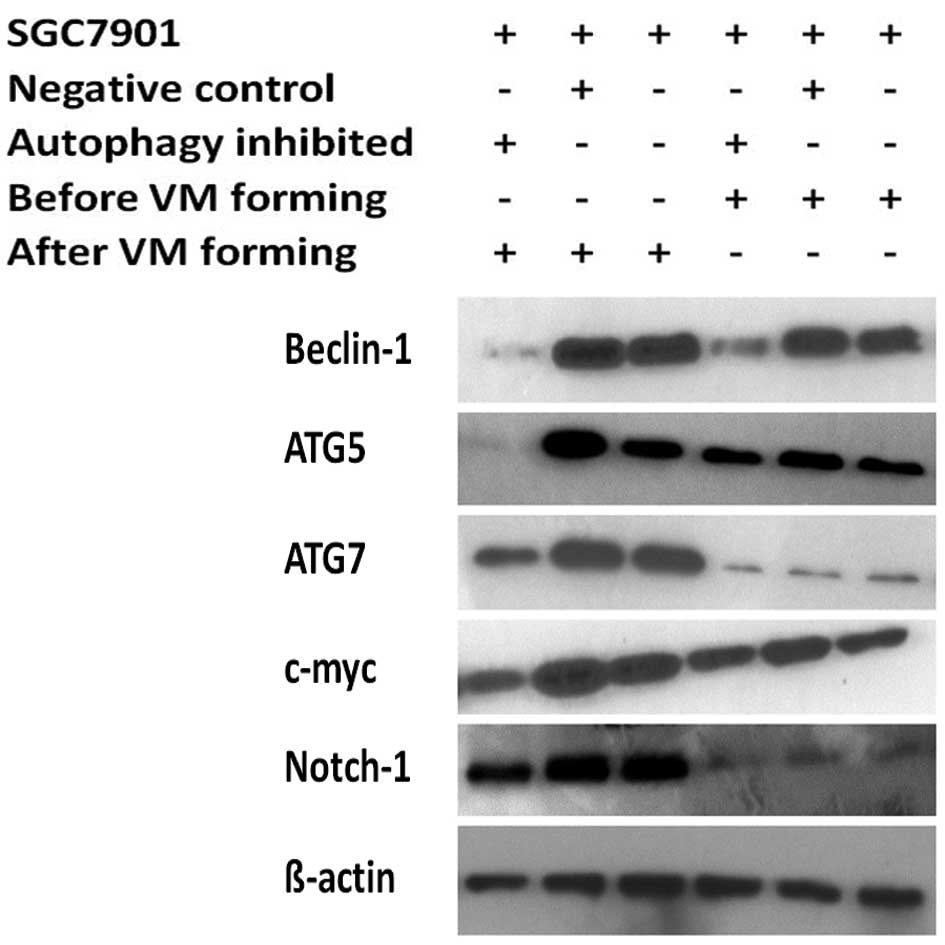
Inhibition of autophagy-specific gene
beclin1 alters the stable expression of genes in the forming of
VM
The above results showed that autophagy suppresses
VM. Yet, it remains unclear as to why autophagy is indispensable
for VM formation. Thus, we detected changes in the expression of
genes between the autophagy inhibited group and the negative
control and blank group before and after VM formation in 3D
culture. We, respectively, detected the expression of pluripotency
genes (c-myc, oct3/4, sox-2, notch1–3) and other important genes
(MMP-2, MMP-9, E-cad) before and after VM formation in 3D culture.
PCR and western blotting showed that during VM formation in 3D
culture, expression levels of all of the genes increased in the
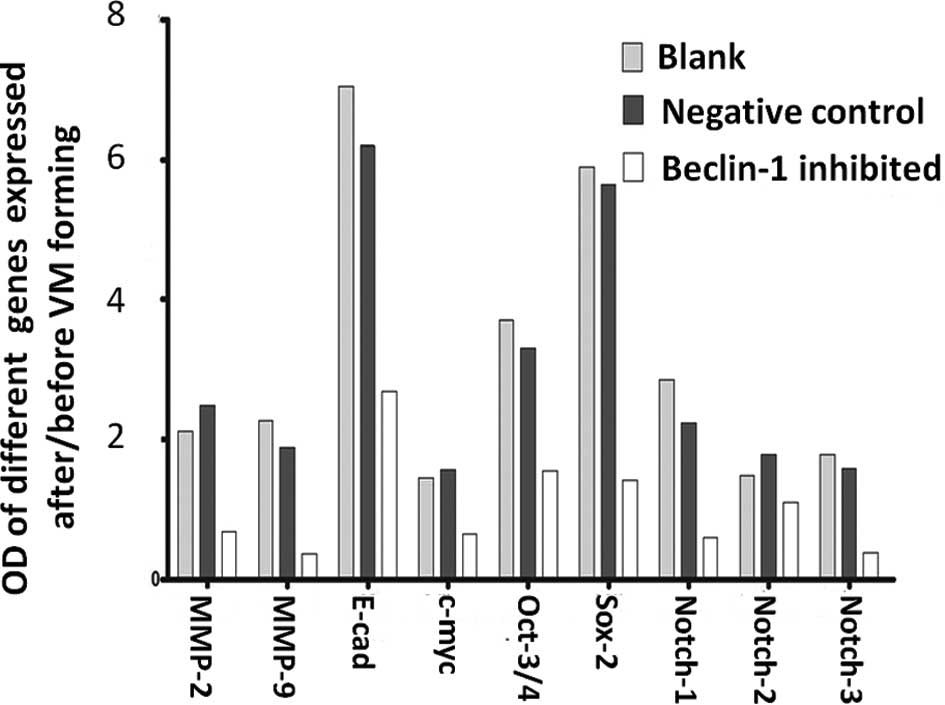
negative control and the blank groups (Figs. 4 and 5), but these expresson levels were
markedly decreased following VM formation when the
autophagy-specific gene beclin1 was inhibited (Fig. 6).
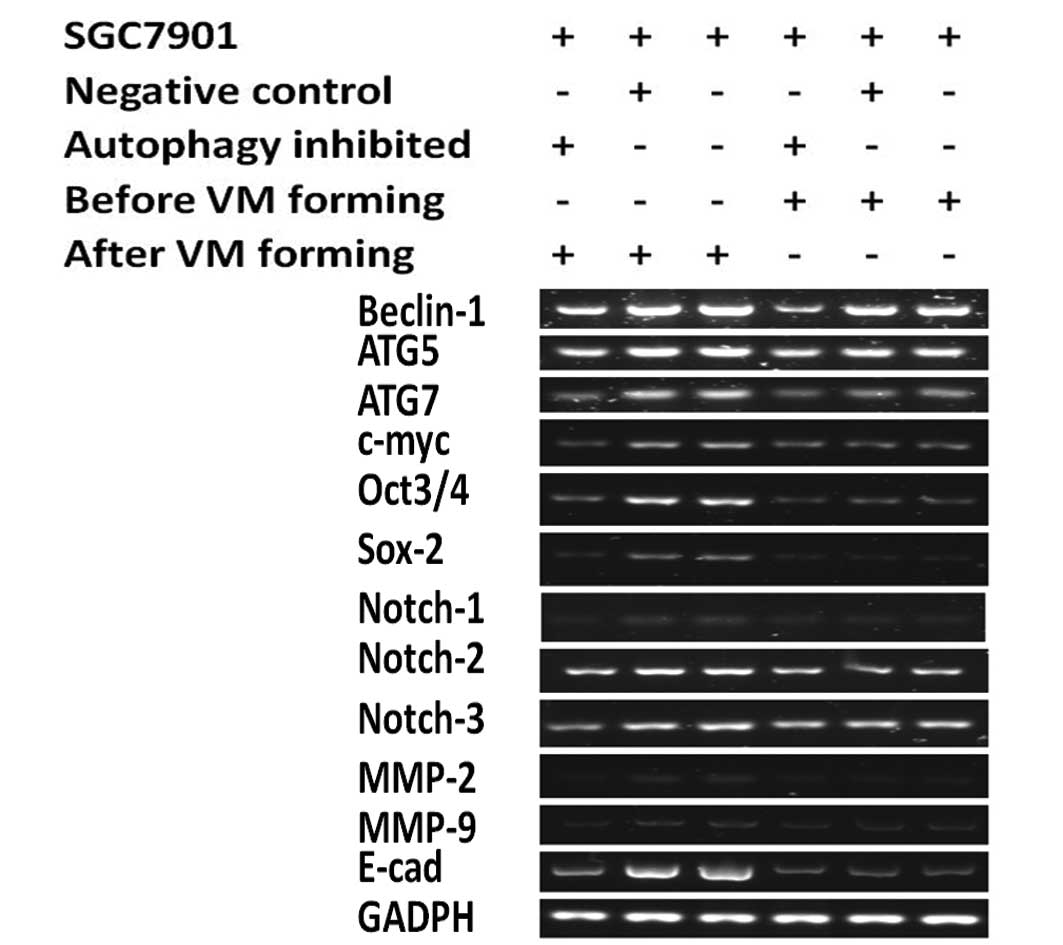 | Figure 4Expression of genes following
vasculogenic mimicry (VM) formation. RT-PCR was used to detect the
expression of autophagy-specific genes (beclin1, ATG5, ATG7),
pluripotency genes (c-myc, oct3/4, sox-2, notch1–3) and other
important VM forming genes (MMP-2, MMP-9, E-cad) in the SGC7901
blank group, negative control group, and autophagy inhibited group,
before and after 3D culture, respectively. Accompanied by the
increased expression of autophagy-specific genes (beclin1, ATG5,
ATG7), pluripotency genes (c-myc, oct3/4, sox-2, notch1–3) and
other VM forming genes were also increased while a decrease in
expression was noted in the beclin1 inhibited group after VM
formation. |
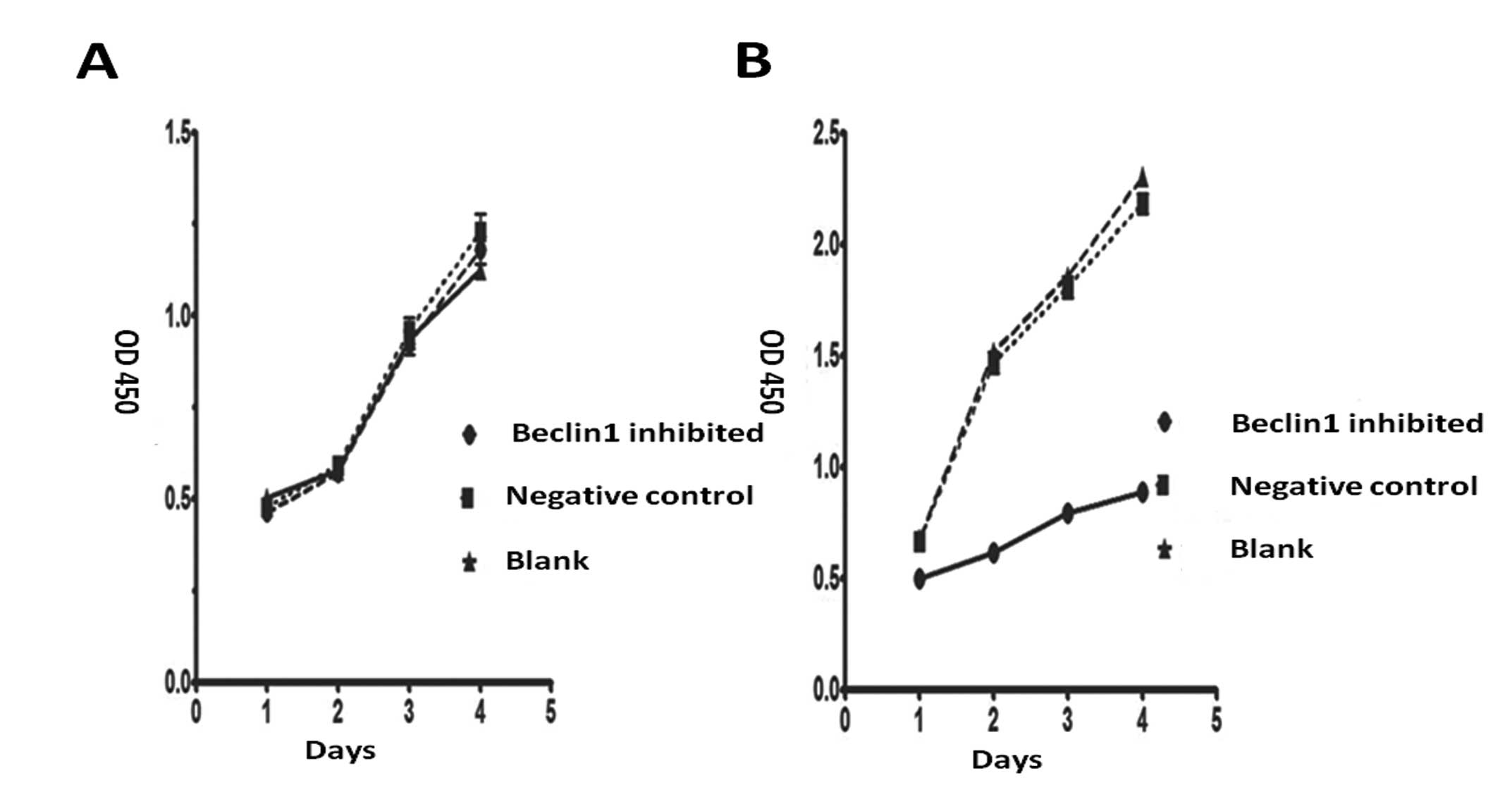
Change in survival, proliferation and
invasion in beclin1-suppressed cells
We explored the survival and proliferative ability
by MTT, and assessed the invasive ability by Transwell test.
RPMI-1640 medium with and without serum was used to
mimick the normal and low nutrition stress environments. We plated
cells from the negative control group, the blank group and the
autophagy inhibited group in RPMI-1640 medium with and without
serum. Results showed that the level of proliferation in the three
cell groups was similar in the culture with serum. However, the
autophagy inhibited group proliferated much more slowly than the
other two groups in the culture without serum (Fig. 7).
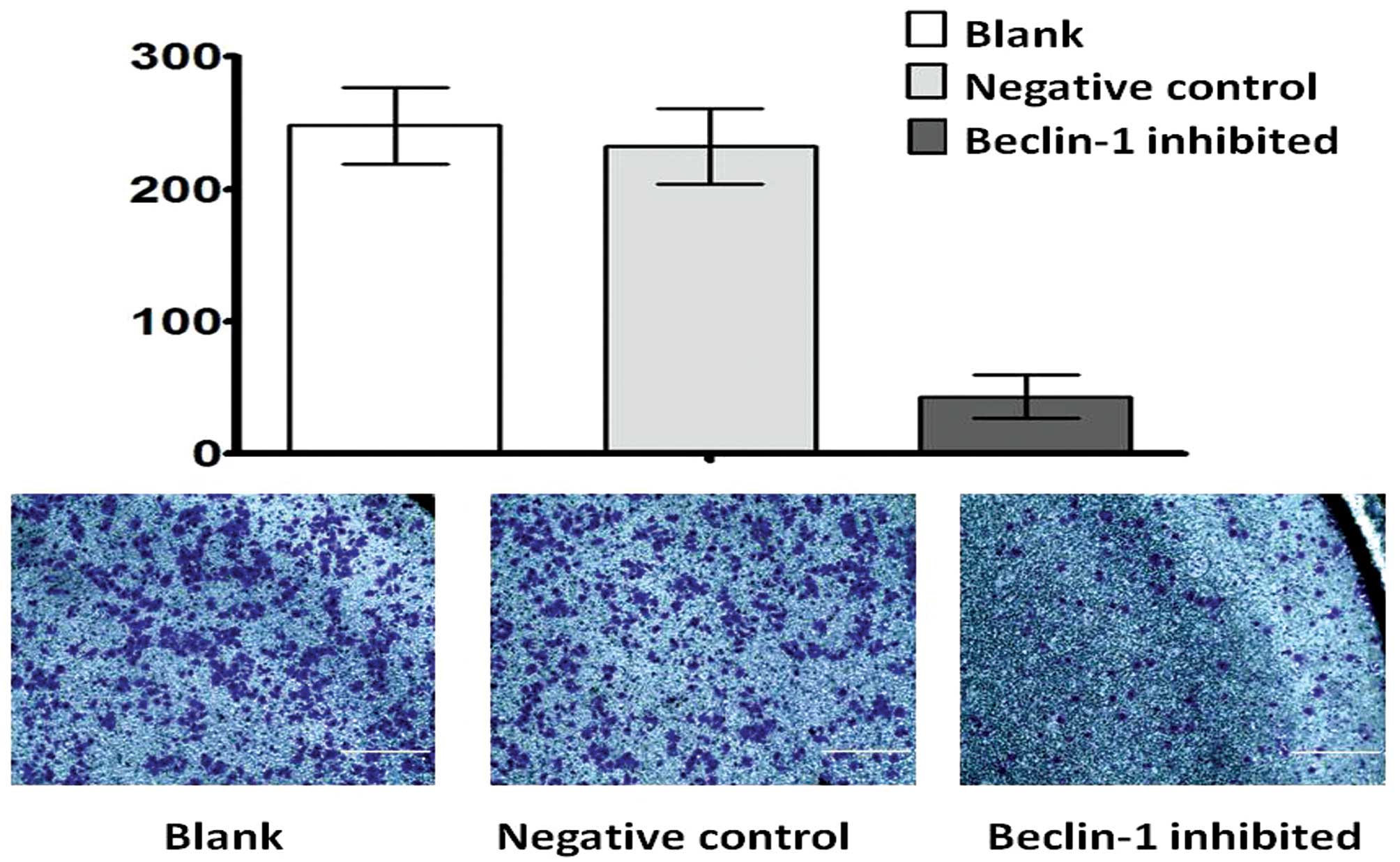
We detected the invasive ability of the cells by
Transwell assay. The migrated cell numbers were significantly
reduced in the autophagy inhibited group, when compared with the
negative control group and the blank group (Fig. 8).
Discussion
Autophagy is considered to be a tumor-promoting
process which helps cancer cells sustain life by recycling aged or
damaged organelles and proteins, regenerating ATP and organelle
material in the presence of stress environmental conditions such as
nutrient shortage or hypoxia. However, various studies suggest that
autophagy mainly contributes to tumor suppression during the early
stage of tumorigenesis and deficiency of autophagy leads to genetic
instability and tumorigenesis (22).
In the present study, we found that autophagy
participates in the formation of VM, vascular-like structures
differentiated by cancer cells, which supply nutrients and oxygen
and provide invasive channels to the tumor tissue. In a 3D culture,
following the formation of the tubular like VM structures by
gastric SGC7901 cells, RT-PCR and western blotting revealed that
the expression of autophagy-specific genes (beclin1, ATG5, ATG7)
was markedly increased.
To further explore the indispensable role of
autophagy in the formation of VM, we established an autophagy
inhibited group, negative control group and blank group. After a
24-h 3D culture, only the autophagy inhibited group did not form
VM. Thus, we confirmed that autophagy not only participates in VM
formation but plays an indispensable role in VM formation. In a
hypoxic and low nutrition environment, autophagy is activated to
facilitate VM formation to supply nutrients and oxygen and provide
invasive channels for cancer cells, promting cancer cell survival
and metastasis.
We found that the autophagy inhibited cell group in
the 3D culture did not form VM, and numerous dying or dead cell
were observed. Thus, we used the MTT assay to test the survival
ability of SGC7901 cells in a low nutrition environment when
autophagy was inhibited. The result showed that the survival
ability of the autophagy inhibited cell group, negative control
group, and blank group was not altered in the full nutrition
environment, but in the low nutrition environment, the survival
ability of the autophagy inhibited cell group was reduced when
compared with the other two cell lines in culture without
nutrition. Thus, we confirmed that autophagy guarantees the
survival of SGC7901 cancer cells in an adverse environment,
promotes cancer cell survival and then promotes the formation of VM
in an adverse environment.
Recent research revealed that the formation of VM
relies on the pluripotent ability of a few cancer cells in tumor
tissues, which are called cancer stem cells (CSCs). It is believed
that cancer stem cells, similarly to normal embryonic stem cells,
have the capacity of self-renewal and differentiation that is
responsible for the heterogeneity in cancer (23–26).
Under the control of same pluripotency genes, these pluripotency
cells can differentiate into cells with different capacities, so
they can undergo and adapt to different stress. Previous research
found that when cells were transfected with transcription factors
such as c-myc, oct 4, sox 2 into human and mouse somatic cells, the
cells could become pluripotent stem cells and had directional
differentiation features (27–29),
which help them survive under a stress environment.
In the present study, we found increased expression
of the pluripotency control genes (c-myc, oct-4, sox-2, notch1–3)
following VM formation in 3D culture, which suggests that, under 3D
culture in vitro, with the management of some pluripotency
control genes, CSCs have the capacity of vascular endothelial cells
and form VM to overcome environmental stress. However, the
expression of genes which were increased in the negative control
group and the blank group, were markedly decreased in the autophagy
inhibited cell group. We believe that stem cells protect their
genome from damage to maintain their pool and self-renewal capacity
and during the tumorization of cells, autophagy protects genomic
stability by retarding damage/repair cycle, and protecting cell
homeostasis. Various studies found the significant role of
autophagy in pluripotent stem (iPS) cell generation and
differentiation processes (30,31).
Thus, we can confirm that autophagy contributes to the stable
expression of pluripotency genes which are indispensable to VM
formation. When autophagy is inhibited, cancer cells are unable to
manage pluripotency gene expression in hypoxia, low nutrition or
other adverse environments. Finally, VM cannot form which was
observed in our study.
VE-cadherin, MMP-2 and MMP-9 are invasion and
migration molecules of cancer cells, and have been identified as
important players in melanoma VM. In the absence of VE-cadherin and
MMPs, VM tube formation does not occur (32). Several studies have found that the
invasive and migratory abilities of cells are important in
vascularization (33,34). In the present study, the expression
of VE-cadherin, MMP-2, and MMP-9 was increased after VM formation
in 3D culture, but when autophagy was inhibited expression of these
genes was decreased, and Transwell assay showed that the invasion
of the autophagy inhibited cell group was also markdly decreased
when compared with the negative control group and blank group. This
indicates that autophagy can facilitate the invasive and migratory
abilities of cancer cells by ensuring the stability of expression
of VE-cadherin, MMP-2, and MMP-9, contributing to the formation of
VM in 3D culture.
In conclusion, our data, for the first time,
indicate that autophagy participates in VM formation, and
inhibition of autophagy suppresses VM formation and the relevant
mechanisms. In a stress environment, autophagy, by maintaining the
survival of cancer cells, ensures the stable expression of
pluripotency control genes, managing the invasive and migratory
abilities of cancer cells, and promotes VM formation which supplies
nutrients, oxygen and provide invasive channels to the interior
tumor tissue. In this manner, autophagy promotes the survival and
development of tumors.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81172348) and Science Foundation
of Suzhou (no. SYSD2013090, no. SS0834).
Abbreviations:
|
VM
|
vasculogenic mimicry
|
|
3D
|
three-dimensional
|
|
CSC
|
cancer stem cell
|
|
RT-PCR
|
reverse transcriptase-polymerase chain
reaction
|
|
VE-cadherin
|
vascular endothelial-cadherin
|
|
MMP
|
matrix metalloproteinase
|
References
|
1
|
Singh R and Cuervo AM: Autophagy in the
cellular energetic balance. Cell Metab. 13:495–504. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bursch W: The autophagosomal-lysosomal
compartment in programmed cell death. Cell Death Differ. 8:569–581.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karantza-Wadsworth V, Patel S, Kravchuk O,
Chen G, Mathew R, Jin S and White E: Autophagy mitigates metabolic
stress and genome damage in mammary tumorigenesis. Genes Dev.
21:1621–1635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meléndez A, Tallóczy Z, Seaman M,
Eskelinen EL, Hall DH and Levine B: Autophagy genes are essential
for dauer development and life-span extension in C. elegans.
Science. 301:1387–1391. 2003.PubMed/NCBI
|
|
5
|
Bergamini E, Cavallini G, Donati A and
Gori Z: The role of macroautophagy in the ageing process,
anti-ageing intervention and age-associated diseases. Int J Biochem
Cell Biol. 36:2392–2404. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartolome A, Guillen C and Benito M:
Autophagy plays a protective role in endoplasmic reticulum
stress-mediated pancreatic β cell death. Autophagy. 8:1757–1768.
2012.
|
|
7
|
Degenhardt K, Mathew R, Beaudoin B, Bray
K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, Nelson
DA, Jin S and White E: Autophagy promotes tumor cell survival and
restricts necrosis, inflammation, and tumorigenesis. Cancer Cell.
10:51–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Degenhardt K and White E: A mouse model
system to genetically dissect the molecular mechanisms regulating
tumorigenesis. Clin Cancer Res. 12:5298–5304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burstein HJ: The distinctive nature of
HER2-positive breast cancers. N Engl J Med. 353:1652–1654. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chin K, DeVries S, Fridlyand J, Spellman
PT, Roydasgupta R, Kuo WL, Lapuk A, Neve RM, Qian Z, Ryder T, Chen
F, Feiler H, Tokuyasu T, Kingsley C, Dairkee S, Meng Z, Chew K,
Pinkel D, Jain A, Ljung BM, Esserman L, Albertson DG, Waldman FM
and Gray JW: Genomic and transcriptional aberrations linked to
breast cancer pathophysiologies. Cancer Cell. 10:529–541. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang RC, Wei Y, An Z, Zou Z, Xiao G,
Bhagat G, White M, Reichelt J and Levine B: Akt-mediated regulation
of autophagy and tumorigenesis through Beclin 1 phosphorylation.
Science. 338:956–959. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe’er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hendrix MJ, Seftor EA, Hess AR and Seftor
RE: Molecular plasticity of human melanoma cells. Oncogene.
22:3070–3075. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guzman G, Cotler SJ, Lin AY, Maniotis AJ
and Folberg R: A pilot study of vasculogenic mimicry
immunohistochemical expression in hepatocellular carcinoma. Arch
Pathol Lab Med. 131:1776–1781. 2007.PubMed/NCBI
|
|
15
|
Folberg R and Maniotis AJ: Vasculogenic
mimicry. APMIS. 112:508–525. 2004. View Article : Google Scholar
|
|
16
|
Sun B, Zhang S, Zhang D, Du J, Guo H, Zhao
X, Zhang W and Hao X: Vasculogenic mimicry is associated with high
tumor grade, invasion and metastasis, and short survival in
patients with hepatocellular carcinoma. Oncol Rep. 16:693–698.
2006.PubMed/NCBI
|
|
17
|
Van der Schaft DW, Seftor RE, Seftor EA,
Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW and
Hendrix MJ: Effects of angiogenesis inhibitors on vascular network
formation by human endothelial and melanoma cells. J Natl Cancer
Inst. 96:1473–1477. 2004.PubMed/NCBI
|
|
18
|
Cai XS, Jia YW, Mei J and Tang RY: Tumor
blood vessels formation in osteosarcoma: vasculogenesis mimicry.
Chin Med. 117:94–98. 2004.PubMed/NCBI
|
|
19
|
Han C, Sun B, Wang W, Cai W, Lou D, Sun Y
and Zhao X: Overexpression of microtubule-associated protein-1
light chain 3 is associated with melanoma metastasis and
vasculogenic mimicry. Tohoku J Exp Med. 223:243–251. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kimlin LC, Casagrande G and Virador VM: In
vitro three-dimensional (3D) models in cancer research: an update.
Mol Carcinog. 52:167–182. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamada KM and Cukierman E: Modeling tissue
morphogenesis and cancer in 3D. Cell. 130:601–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun K, Deng W, Zhang S, Cai N, Jiao S,
Song J and Wei L: Paradoxical roles of autophagy in different
stages of tumorigenesis: protector for normal or cancer cells. Cell
Biosci. 3:352013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clevers H: The cancer stem cell: premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Magee JA, Piskounova E and Morrison SJ:
Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer
Cell. 21:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Visvader JE and Lindeman GJ: Cancer stem
cells: current status and evolving complexities. Cell Stem Cell.
10:717–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vazquez-Martin A, Oliveras-Ferraros C, Del
Barco S, Martin-Castillo B and Menendez JA: The anti-diabetic drug
metformin suppresses self-renewal and proliferation of
trastuzumab-resistant tumor-initiating breast cancer stem cells.
Breast Cancer Res Treat. 126:355–364. 2011. View Article : Google Scholar
|
|
27
|
Yu J, Vodyanik MA, Smuga-Otto K,
Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA,
Ruotti V, Stewart R, Slukvin II and Thomson JA: Induced pluripotent
stem cell lines derived from human somatic cells. Science.
318:1917–1920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lowry WE, Richter L, Yachechko R, Pyle AD,
Tchieu J, Sridharan R, Clark AT and Plath K: Generation of human
induced pluripotent stem cells from dermal fibroblasts. Proc Natl
Acad Sci USA. 105:2883–2888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park IH, Zhao R, West JA, Yabuuchi A, Huo
H, Ince TA, Lerou PH, Lensch MW and Daley GQ: Reprogramming of
human somatic cells to pluripotency with defined factors. Nature.
451:141–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Naka K and Hirao A: Maintenance of genomic
integrity in hematopoietic stem cells. Int J Hematol. 93:434–439.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song YJ, Zhang SS, Guo XL, Sun K, Han ZP,
Li R, Zhao QD, Deng WJ, Xie XQ, Zhang JW, Wu MC and Wei LX:
Autophagy contributes to the survival of CD133+ liver
cancer stem cells in the hypoxic and nutrient-deprived tumor
microenvironment. Cancer Lett. 339:70–81. 2013.PubMed/NCBI
|
|
32
|
Hendrix MJ, Seftor EA, Meltzer PS, Gardner
LM, Hess AR, Kirschmann DA, Schatteman GC and Seftor RE: Expression
and functional significance of VE-cadherin in aggressive human
melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci
USA. 98:8018–8023. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thorns V, Walter GF and Thorns C:
Expression of MMP-2, MMP-7, MMP-9, MMP-10 and MMP-11 in human
astrocytic and oligodendroglial gliomas. Anticancer Res.
23:3937–3944. 2003.PubMed/NCBI
|
|
34
|
Zygmunt M, Munstedt K and Lang U: The role
of vasculo- and angiogenesis in embronic and fetal development. A
short review. Gynakologe. 34:8122001. View Article : Google Scholar
|