Introduction
Esophageal cancer is the sixth leading cause of
cancer-related mortality worldwide, and esophageal squamous cell
carcinoma (ESCC) is the main histologic type of esophageal cancer
(1). The incidence of ESCC varies
widely by nearly 16-fold throughout the world, with the highest
rates in Eastern Asia and Southern and Eastern Africa (2). Currently, surgical resection is the
main curative therapeutic option. Although recent advances in
diagnosis and treatment have improved patient prognosis, the 5-year
survival rate is still low at ~30% (3).
Homeobox (HOX) genes encode a group of
transcription factors, which can directly drive the transcription
of target genes (4). However,
further investigation of the detailed molecular mechanisms is
needed. HOX genes play crucial roles in embryogenesis and
tumorigenesis. There are three aberrations noted in HOX
expression in solid tumors: re-expression, new expression,
downregulation or deficiency (4).
Takahashi et al comprehensively evaluated the expression of
all HOX genes in 48 primary ESCC tissues and 7 normal
esophageal tissues by RT-qPCR. Their data suggested that disordered
expression of HOX genes was significantly associated with
the tumorigenesis and development of ESCC (5). In our previous study, homeobox A13
gene (HOXA13) was found to be overexpressed in ESCC tissues
when compared to that in normal tissues (3). Colony formation and nude mouse
tumorigenicity assays revealed that HOXA13 promoted
tumorigenesis in vitro and in vivo. Moreover, the
prognosis of HOXA13-positive patients was significantly worse than
that of HOXA13-negative patients (6).
Considering that HOXA13 acts as a transcription
factor, to identify its potential targets, protein expression
changes after HOXA13 knockdown were detected by 2-dimensional
electrophoresis (7). Among the
proteins downregulated after HOXA13 knockdown, Annexin A2
(ANXA2) and superoxide dismutase 2 (SOD2) were selected for further
study. Consistent expression of HOXA13, ANXA2 and
SOD2 was validated by western blotting. CHIP-DSL also
revealed that SOD2 and ANXA2 were both potential
targets of HOXA13. However, little is known concerning the clinical
significance of HOXA13/ANXA2 or
HOXA13/SOD2 coexpression in ESCC.
ANXA2, a calcium-dependent phospholipid binding
protein, is implicated in apoptosis, calcium signaling, tumor
invasion, metastasis and angiogenesis. Overexpression of
ANXA2, as well as its prognostic value, has been described
in colorectal (8), gastric
(9) and pancreatic cancer (10,11),
hepatocellular carcinoma (12), and
lung (13) and breast cancer
(14). However, little is known
concerning its expression in ESCC.
Superoxide dismutases (SODs) are a family of
antioxidant enzymes that neutralize the reactive free superoxide
radical (O2−) in mitochondrial reactive
oxygen species (ROS) (15,16). SOD2 (also named MnSOD) is
highly expressed in cervical carcinoma (17), and in breast (18), gastric and colorectal cancer
(19). Studies conducted on SOD2 in
cancer focused mainly on its tumor-suppressor role, with a smaller
but mounting number of studies suggesting that SOD2 acts as
an oncogene (15). It was reported
that SOD2 overexpression inhibited POX-induced apoptosis by
avoiding oxidative damage to mitochondria (20–22).
However, little is known concerning its role in ESCC
carcinogenesis.
In the present study, HOXA13, ANXA2
and SOD2 expression was evaluated in normal esophageal
mucosa and ESCC tissues, and the correlation between HOXA13
and ANXA2 and SOD2 expression was examined at both
the transcriptional and translational levels. In addition, the
association of HOXA13, ANXA2 and SOD2
coexpression and prognosis was investigated in ESCC patients.
Materials and methods
Patients and demographic data
The present study cohort consisted of 121 patients
from a prospective database of esophageal cancer patients, and all
of them underwent surgery at the Department of Thoracic Surgery I,
Peking University School of Oncology from July 2000 to November
2009. Our validation cohort consisted of 137 ESCC patients in
addition to the above-mentioned database, which were treated
between February 1996 and June 2003 at the Department of Thoracic
Surgery.
All of the patients underwent radical esophagectomy,
and none of them received adjuvant chemotherapy or radiotherapy
prior to surgery. Resected samples were immediately sent for
histological examination with hematoxylin and eosin staining.
Tumor-node-metastasis (TNM) stage was evaluated according to the
criteria of the UICC, 1987. To clarify the survival conditions,
life-long follow-up was available by regular review (examination
records) after surgery or by direct telephone interview until
recurrence, metastasis or death caused by tumor. The demographic
information (gender and age) and tumor characteristics (histology,
differentiation and TNM stage) were acquired from medical and
pathological records. A total of 18 patients with fresh frozen
cancerous and non-cancerous samples with complete clinical data
were collected from Anyang Cancer Hospital, Henan. Tissues were
collected immediately after surgical removal and snap-frozen in
liquid nitrogen until further use. All participants provided
informed consent, and the study was approved by the Ethics and
Academic Committees of Peking University School of Oncology.
Immunohistochemistry (IHC)
Formalin-fixed and paraffin-embedded 4-μm tissue
sections were routinely immunostained. After deparaffinization in
xylene and rehydration in a graded ethanol series, 3% hydrogen
peroxide solution was put on the slide for 10 min, and antigen
retrieval was carried out in citrate solution (pH 6.0) by
microwave. The sections were blocked with goat serum for 15 min and
then incubated with mouse monoclonal antibody to ANXA2 and SOD2
(Abcam) at 4°C overnight. The mouse monoclonal antibody against
human ANXA2 and SOD2 was used at 2 μg/ml (1:500) and 0.5 mg/ml
(1:400), and the secondary antibody was goat anti-mouse
biotin-conjugated IgG. Diaminobenzidine (DAB) chromogenic reaction
was used for detection. Two experienced pathologists independently
examined the immunohistochemical signals. The scores were evaluated
according to the number of stained cells and staining intensity.
The percentage of ANXA2 or SOD2-positive tumor cells was evaluated
on a scale of 0–3 (0, no staining; 1+, ≤10%; 2+, 11–30%; 3+,
31–50%; 4+, >50%). Thus, the expression level of ANXA2 and SOD2
were divided into two groups in terms of the score: negative (0,
1+, 2+) and positive (3+, 4+). For the evaluation and scoring of
HOXA13, the same criteria were used as described in our
previous study (6).
Cell culture and RNA isolation
Human esophageal cancer EC109, EC9706, KYSE150,
KYSE410 and KYSE510 cells were obtained from ATCC (Manassas, VA,
USA). These cell lines were cultured in 1640 medium (HyClone,
Logan, UT, USA) supplemented with heat-inactivated fetal bovine
serum (Gibco, Carlsbad, CA, USA) and 100 U/ml penicillin sodium in
a humidified atmosphere with 5% CO2 at 37°C.
Total RNAs of fresh frozen tumor specimens and
sorted cells (EC109, EC9706, KYSE150, KYSE410 and KYSE510) were
extracted by TRIzol (Invitrogen, Carlsbad, CA, USA). RNAs were
reverse transcripted to single strand cDNAs by two-step RT-PCR
(Fermentas Life Sciences).
Real-time RT-qPCR
Quantitative real-time PCR was performed using
SYBR-Green Real-Time PCR Master Mix (Applied Biosystems) to detect
mRNA expression levels of the target genes.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the
endogenic control. Special primers were designed using Oligo Primer
Analysis Software (version 5.0). The sequence of the primers used
are as follows: for HOA13, forward
5′-AGCGCGTGCCTTATACCAAG-3′ and reverse 5′-GCCGCTCAGAGAGATTCGT-3′;
for ANXA2, forward 5′-CTCTACACCCCCAAGTGCAT-3′ and reverse
5′-TCAGTGCTGATGCAAGTTCC-3′; for SOD2, forward
5′-AAGGGAGATGTTACAGCCCAGATA-3′ and reverse
5′-TCCAGAAAATGCTATGATTGATATGAC-3′; for GAPDH, forward
5′-GACCCCTTCATTGACCTCAAC-3′ and reverse 5′-CTTCTCCATGGTGGTGAAGA-3′.
All assays were carried out in triplicate under the 7500 Real-Time
PCR System (Applied Biosystems) and repeated three times according
to the manufacturer’s protocol. Evaluation of relative expression
was calculated by comparative Ct (threshold cycle) method.
2−ΔCt referred to the fold of the mRNA expression of the
target gene compared to GAPDH expression in the same sample.
Statistical analysis
Analysis was performed using SPSS 17.0 software. The
χ2 test or Fisher’s exact test was used to compare the
relationship between HOXA13, ANXA2, SOD2 expression and
clinicopathological characteristics of the ESCC patients.
HOXA13, ANXA2 and SOD2 mRNA expression levels
in cancerous and non-cancerous tissues are presented as the means ±
SD and were compared by paired t-test. Wilcoxon test was employed
to compare the protein levels of HOXA13, ANXA2 and
SOD2 between cancerous and non-cancerous tissues. Pearson’s
correlation coefficient analysis was applied to analyze the
correlation of HOXA13, ANXA2 and SOD2 mRNA
expression. Spearman’s correlation coefficient analysis was applied
to analyze the correlation of the protein levels.
The overall survival measured from the day of
surgery was estimated by Kaplan-Meier curves, and the differences
were analyzed by log-rank test. Cox proportional hazards model was
used for multivariate survival analysis. The variables analyzed in
the model included age, gender, tumor location, histology, tumor
cell differentiation, TNM stage, HOXA13, ANXA2 and
SOD2 expression. Hazard ratios and 95% confidence intervals
were calculated. p<0.05 was considered to indicate a
statistically significant result.
Results
ANXA2 and SOD2 are overexpressed in ESCC
tissues
It has been demonstrated that ANXA2 and
SOD2 are associated with gastric and colorectal cancer. To
investigate whether they are also associated with ESCC, the mRNA
levels of ANXA2 and SOD2 in ESCC and matched
non-cancerous specimens were analyzed by RT-qPCR. When compared
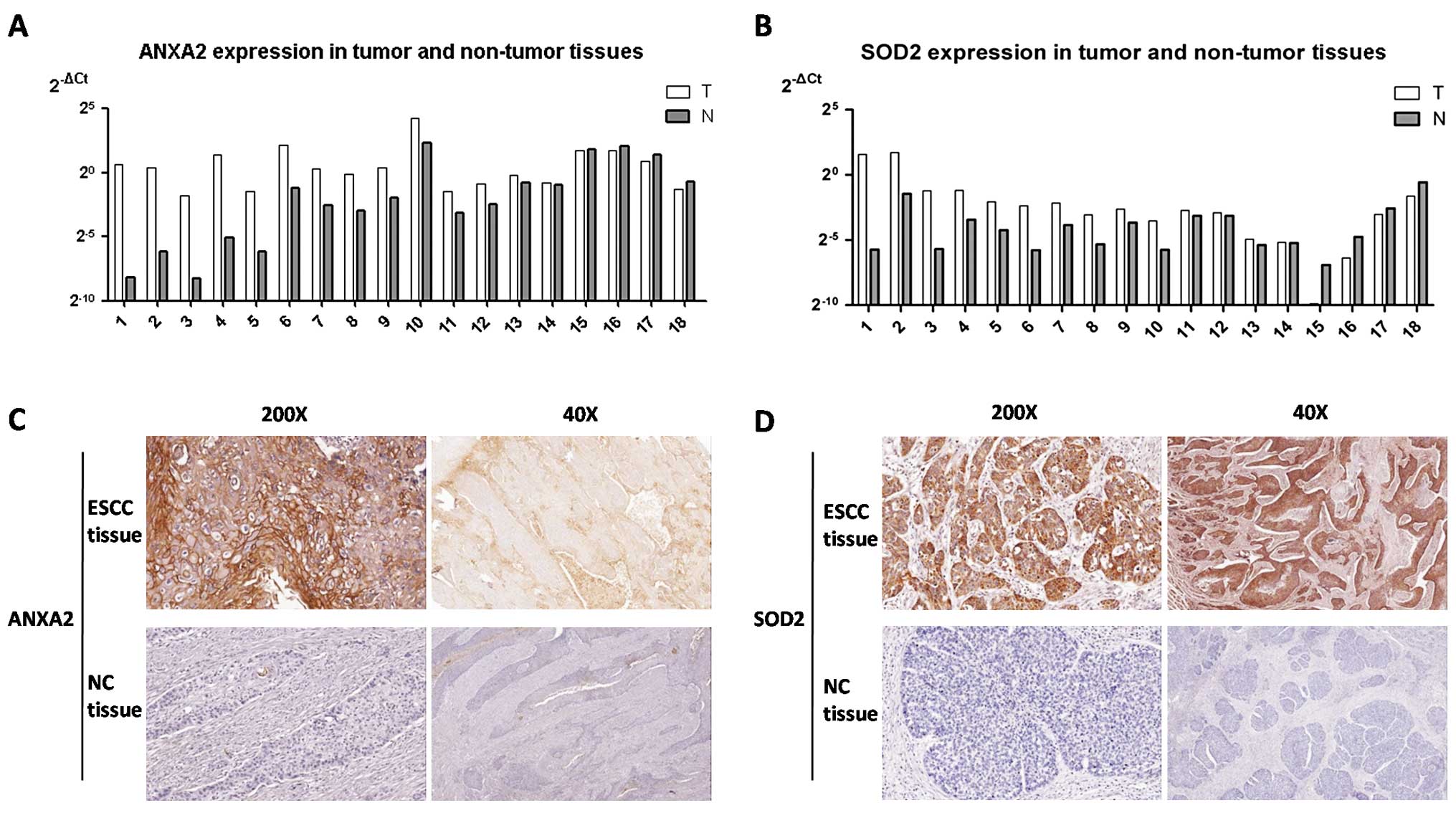
with normal esophageal tissues, ANXA2 and SOD2 showed
higher expression in the ESCC tissues (Fig. 1A and B; Mann-Whitney test,
ANXA2 p=0.012, SOD2 p=0.016). Furthermore, IHC was
employed to assess the protein expression of ANXA2 and SOD2 in 18
ESCC and matched non-cancerous specimens. Positive expression of
ANXA2 was observed in 20% of the ESCC tissues and in 0% of the
non-cancerous tissues (Fig. 1C;
Chi-square test, p=0.023). SOD2 overexpression was detected in 90%
of the ESCC tissues and in 25% of the paired cancer margin tissues
(Fig. 1D; Chi-square test,
p=0.001). In conclusion, expression levels of ANXA2 and
SOD2 were significantly higher in the ESCC tissues than
levels in the non-cancerous specimens.
Expression levels of ANXA2 and SOD2 are
positively correlated with HOXA13
To investigate the correlation between HOXA13 and
its potential target genes, the levels of HOXA13,
ANXA2 and SOD2 in 5 ESCC cell lines (EC109, EC9706,
KYSE150, KYSE410 and KYSE510) were evaluated by RT-qPCR assay. Both
ANXA2 and SOD2 showed a significant positive
correlation with HOXA13 in the 5 ESCC cell lines (data not
shown; Pearson’s correlation, ANXA2 p=0.005, SOD2
p<0.001), particularly SOD2, with a high R2
score of 0.99.
In the 23 pairs of ESCC tissues, a strong positive
correlation was observed between the mRNA expression of
ANXA2 and HOXA13 (Pearson’s correlation r=0.878,
p<0.001). The same correlation was also found between
SOD2 and HOXA13 (Pearson’s correlation r=0.503,
p=0.014). To further verify these potential positive correlations,
IHC was applied in the study and validation cohorts. In the study
cohort, a significant positive correlation was noted between ANXA2
and HOXA13 (Spearman correlation rs=0.200, p=0.028) and
the correlation between SOD2 and HOXA13 also approached
significance (Spearman correlation rs=0.151, p=0.098).
Similar results were observed in the validation cohort. A
significant positive correlation was noted between SOD2 and HOXA13
(Spearman correlation rs=0.148, p=0.084) and the
correlation between ANXA2 and HOXA13 also approached significance
(Spearman correlation rs=0.198, p=0.021).
Collectively, expression of ANXA2 and
SOD2 was positively correlated with HOXA13 in the
ESCC cell lines and tissues.
Overexpression of ANXA2 or SOD2 indicates
poor prognosis of ESCC patients, respectively
Our previous study revealed that high expression of
HOXA13 indicates poor survival; thus, the potential target genes
ANXA2 and SOD2 may also have prognostic value in
ESCC. To study the correlation of ANXA2 and SOD2 with
HOXA13 and their roles in ESCC, we analyzed the expression
of HOXA13, ANXA2 and SOD2 in both the study
and validation cohorts. In the study cohort, HOXA13, ANXA2 and SOD2
expression was significantly correlated with TNM stage (Table I). In the univariate analysis, tumor
invasion (T), lymph node metastasis (N), TNM stage, and expression
of HOXA13, ANXA2 and SOD2 were statistically associated with poor
prognosis, respectively (Table
II). The validation cohort also supported a similar conclusion
(data not shown).
 | Table IAssociation between HOXA13, ANXA2 and
SOD2 expression and clinical characteristics of the patients with
ESCC in the study cohort (n=121). |
Table I
Association between HOXA13, ANXA2 and
SOD2 expression and clinical characteristics of the patients with
ESCC in the study cohort (n=121).
| HOXA13 | ANXA2 | SOD2 |
|---|
|
|
|
|
|---|
| Clinicopathological
factors | High (n=21) | Low (n=100) | P-value | High (n=22) | Low (n=99) | P-value | High (n=54) | Low (n=67) | P-value |
|---|
| Age (years) | | | 0.219 | | | 0.228 | | | 0.545 |
| ≤50 | 4 | 8 | | 4 | 8 | | 4 | 8 | |
| >50 | 17 | 92 | | 18 | 91 | | 50 | 59 | |
| Gender | | | 0.254 | | | 0.780 | | | 0.827 |
| Male | 18 | 76 | | 18 | 76 | | 41 | 53 | |
| Female | 3 | 24 | | 4 | 23 | | 13 | 14 | |
| Tumor location | | | 0.950 | | | 0.090 | | | 0.117 |
| Upper | 4 | 48 | | 1 | 21 | | 14 | 8 | |
| Middle | 11 | 49 | | 15 | 45 | | 26 | 34 | |
| Lower | 6 | 33 | | 6 | 33 | | 14 | 25 | |
| Tumor cell
differentiation | | | 0.793 | | | 0.533 | | | 0.363 |
| Well | 7 | 35 | | 10 | 32 | | 15 | 27 | |
| Moderate | 6 | 35 | | 6 | 35 | | 20 | 21 | |
| Poor | 8 | 30 | | 6 | 32 | | 19 | 19 | |
| Tumor invasion
(T) | | | 0.075 | | | 0.004 | | | 0.698 |
| T1 | 3 | 25 | | 1 | 27 | | 18 | 10 | |
| T2 | 4 | 30 | | 5 | 29 | | 19 | 15 | |
| T3 | 9 | 39 | | 10 | 38 | | 24 | 24 | |
| T4 | 5 | 6 | | 6 | 5 | | 6 | 5 | |
| Lymph node
metastasis (N) | | | 0.606 | | | 0.316 | | | 0.120 |
| N0 | 13 | 70 | | 13 | 70 | | 33 | 50 | |
| N1,
N2, N3 | 8 | 30 | | 9 | 29 | | 21 | 17 | |
| TNM stage | | | 0.028 | | | 0.003 | | | 0.039 |
| I, Tis | 2 | 32 | | 1 | 33 | | 9 | 25 | |
| IIa, IIb | 8 | 42 | | 9 | 41 | | 25 | 25 | |
| IIIa, IIIb,
IIIc | 11 | 26 | | 12 | 25 | | 20 | 17 | |
 | Table IIClinicopathological features, tumor
markers and patient survival in the study cohort (n=121, univariate
analysis). |
Table II
Clinicopathological features, tumor
markers and patient survival in the study cohort (n=121, univariate
analysis).
| Variables | Hazard ratio (95%
CI) | P-value |
|---|
| Age, years | 1.009
(0.982–1.037) | 0.514 |
| Gender | 1.229
(0.682–2.217) | 0.492 |
| Tumor location
(upper/middle vs. lower) | | 0.561 |
| Middle vs.
upper | 0.895
(0.307–2.610) | 0.337 |
| Lower vs.
upper | 0.825
(0.474–1.437) | 0.497 |
| Tumor cell
differentiation (poor/moderate vs. well) | | 0.534 |
| Moderate vs.
poor | 1.809
(0.675–4.848) | 0.239 |
| Well vs. poor | 1.207
(0.519–2.808) | 0.662 |
| Tumor invasion
(T) | | 0.021 |
| T1 vs.
T4 | 0.338
(0.142–0.806) | 0.014 |
| T2 vs.
T4 | 0.417
(0.184–0.946) | 0.036 |
| T3 vs.
T4 | 0.745
(0.354–1.566) | 0.437 |
| Lymph node
metastasis (N) | 0.447
(0.274–0.728) | 0.001 |
| TNM stage
(I/IIa/IIb vs. III) | | 0.003 |
| I vs. III | 0.194
(0.025–1.537) | 0.121 |
| IIa vs. III | 0.265
(0.107–0.659) | 0.004 |
| IIb vs. III | 0.494
(0.210–1.159) | 0.105 |
| HOXA13 expression
(yes vs. no) | 2.020
(1.294–3.145) | 0.002 |
| ANXA2 expression
(yes vs. no) | 2.074
(1.344–3.202) | 0.001 |
| SOD2 expression
(yes vs. no) | 1.764
(1.181–2.634) | 0.006 |
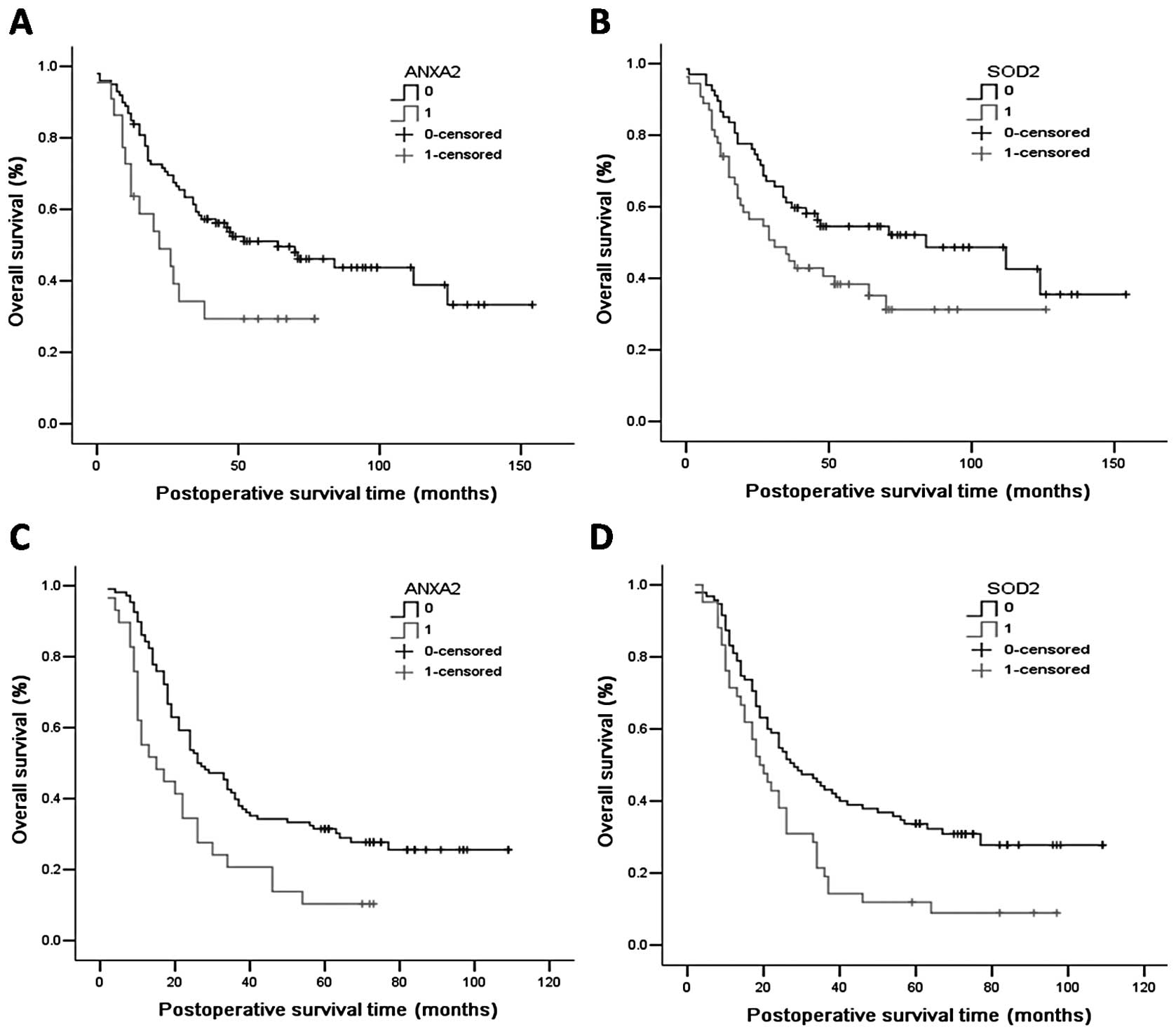
In the study cohort, Kaplan-Meier curve analysis
indicated that the median survival time was 22 months for the
ANXA2-positive patients, which was significantly shorter than the
64 months for ANXA2-negative patients (Fig. 2A, log-rank p=0.026). As for SOD2,
the median survival time was 31 and 84 months for SOD2-positive and
SOD2-negative patients, respectively (Fig. 2B, log-rank p=0.039). In conclusion,
overexpression of ANXA2 or SOD2 indicated poor prognosis of ESCC
patients, respectively. This was similar in the validation cohort.
Kaplan-Meier curve analysis indicated that the median survival time
was 15 months for ANXA2-positive patients, which was significantly
lower than the 26 months for ANXA2-negative patients (Fig. 2C, log-rank p=0.003). As for SOD2,
the median survival time was 19 and 28 months for SOD2-positive and
SOD2-negative patients, respectively (Fig. 2D, log-rank p=0.003).
Combined expression of HOXA13, ANXA2 and
SOD2 has increased prognostic value in ESCC
The coexpression of HOXA13 and its potential targets
ANXA2 and SOD2 were analyzed. On the basis of HOXA13 and ANXA2
expression, in the study cohort, all of the patients were
categorized into three groups: double-positive
(HOXA13+/ANXA2+), single-positive
(HOXA13+/ANXA2− and
HOXA13−/ANXA2+) and double-negative
(HOXA13−/ANXA2−). The median survival time of
double-positive patients was 10 months, significantly lower than
the 64 months for the double-negative patients and 42 months for
the single-positive patients (Fig.
3A, log-rank p=0.002). A similar conclusion was found for the
validation cohort. The median survival time of the double-positive
patients was 13 months, significantly less than the 27 months for
the double-negative patients and 22 months for the single-positive
patients (Fig. 3B, log-rank
p=0.001). For coexpression of HOXA13/SOD2, in the study cohort, the
median survival time was 19, 38 and 84 months for the
double-positive, single-positive and double-negative patients,
respectively (Fig. 3C, log-rank
p=0.010). In the validation cohort, the median survival time was
14, 22 and 29 months for the double-positive, single-positive and
double-negative patients, respectively (Fig. 3D, log-rank p=0.004). For ANXA2 and
SOD2, coexpression of ANXA2 and SOD2 was also predictive of a poor
prognosis in the study cohort (Fig.
3E, log-rank p=0.018) and the validation cohort (Fig. 3F, p=0.001).
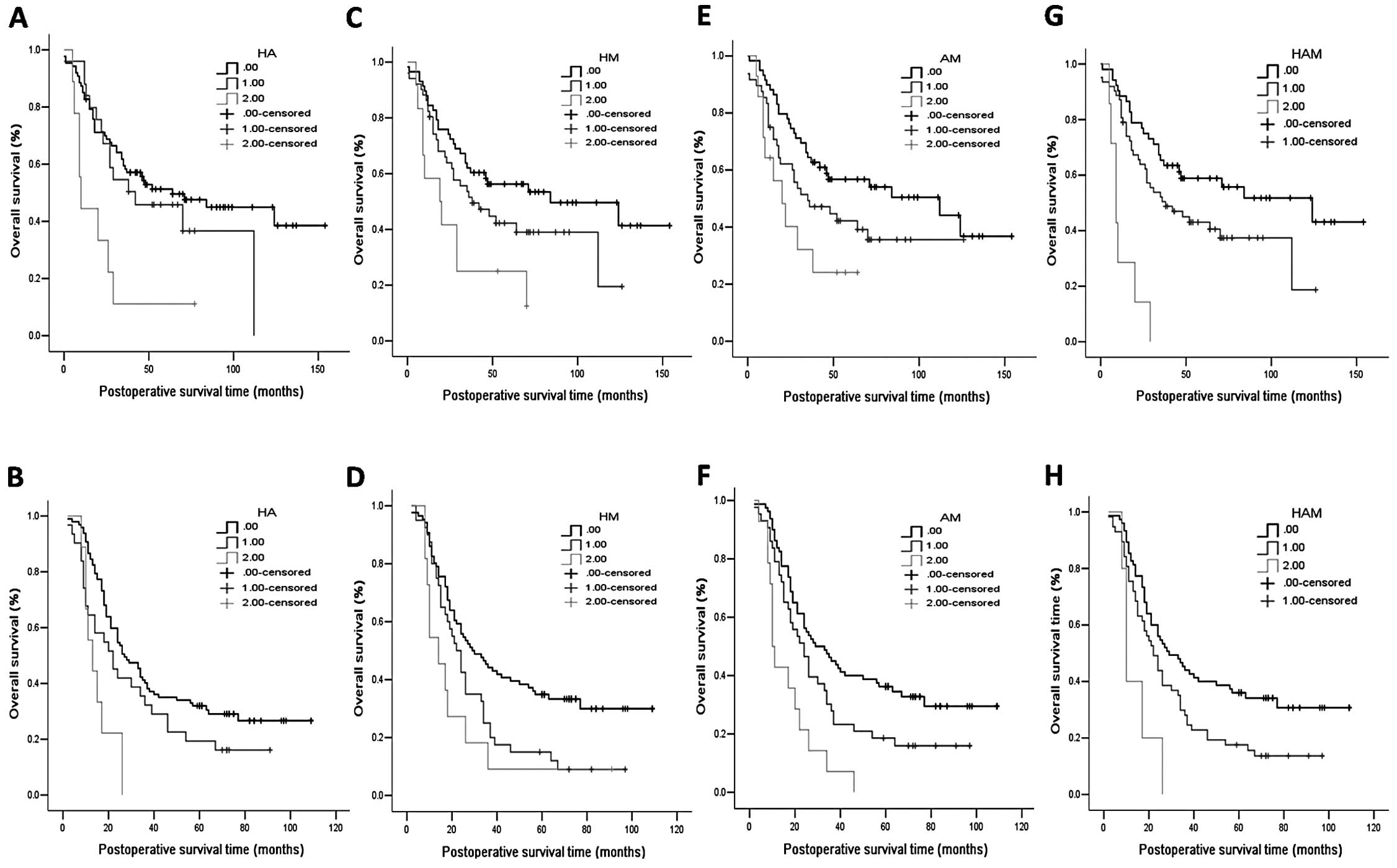 | Figure 3Kaplan-Meier survival curves for ESCC
patients subgrouped according to the combined expression of HOXA13,
ANXA2 and SOD2. The survival of patients with
HOXA13+/ANXA2+ expression was significantly
shorter than that of the patients with
HOXA13−/ANXA2− expression in both the (A)
study and (B) validation cohort. The survival of patients with
HOXA13+/SOD2+ expression was significantly
shorter than that of patients with
HOXA13−/SOD2− expression in both the (C)
study and (D) validation cohort. The survival of patients with
ANXA2+/SOD2+ expression was significantly
shorter than that of patients with
ANXA2−/SOD2− expression in both the (E) study
and (F) validation cohort. The survival of patients with
HOXA13+, ANXA+ and SOD2+
(triple-positive) expression was significantly shorter than that of
the triple-negative patients in both the study cohort (G) and (H)
validation cohort. ESCC, esophageal squamous cell carcinoma; ANXA2,
Annexin A2, SOD2, superoxide dismutase 2. HA, HOXA13+ANXA2
coexpression; HM, HOXA13+SOD2 coexpression; AM, ANXA2+SOD2
coexpression; HAM, HOXA13+ANXA2+SOD2 coexpression. 0, no markers
positive; 1, some markers positive; 2, all markers positive. |
Moreover, when combining the expression of HOXA13,
ANXA2 and SOD2, a better prognostic model was obtained in the two
cohorts. We found that coexpression of HOXA13, ANXA2 and SOD2 was
significantly associated with overall survival in the study cohort
(Fig. 3G, log-rank p<0.001) as
well as in the validation cohort (Fig.
3H, log-rank p=0.001). In the study cohort, TNM stage (p=0.006)
and HOXA13/ANXA2/SOD2 (p=0.002) coexpression are both independent
poor predictors of overall survival time in the multivariate
analysis (Table III). In the
validation cohort, consistent with the above, multivariate analysis
showed that TNM stage (p=0.002) and HOXA13/ANXA2/SOD2 (p=0.017)
coexpression are both independent predictors of poor overall
survival (Table IV).
 | Table IIIIndependent predictors of the overall
survival time in the study cohort (multivariate analysis,
n=121). |
Table III
Independent predictors of the overall
survival time in the study cohort (multivariate analysis,
n=121).
| Variables | Hazard ratio (95%
CI) | P-value |
|---|
| TNM stage
(I/IIa/IIb vs. III) | | 0.006 |
| I vs. III | 0.404
(0.208–0.784) | 0.007 |
| IIa vs. III | 0.358
(0.152–0.840) | 0.018 |
| IIb vs. III | 0.382
(0.206–0.711) | 0.002 |
|
HOXA13/ANXA2/SOD2 | | 0.002 |
| None positive vs.
all positive | 0.171
(0.068–0.433) | <0.001 |
| Partly positive
vs. all positive | 0.294
(0.124–0.699) | 0.294 |
 | Table IVIndependent predictors of the overall
survival time in the validation cohort (multivariate analysis,
n=137). |
Table IV
Independent predictors of the overall
survival time in the validation cohort (multivariate analysis,
n=137).
| Variables | Hazard ratio (95%
CI) | P-value |
|---|
| TNM stage
(I/IIa/IIb vs. III) | | 0.002 |
| I vs. III | 0.197
(0.048–0.813) | 0.025 |
| IIa vs. III | 0.592
(0.390–0.899) | 0.014 |
| IIb vs. III | 0.363
(0.164–0.806) | 0.013 |
|
HOXA13/ANXA2/SOD2 | | 0.017 |
| None positive vs.
all positive | 0.332
(0.129–0.858) | 0.023 |
| Partly positive
vs. all positive | 0.535
(0.208–1.374) | 0.194 |
Discussion
Homeobox (HOX) genes function as primary
regulators in embryogenesis and tumorgenesis. Transcriptional
factors encoded by HOX, which have been detected as
deregulated in various types of tumors, regulate cell proliferation
and differentiation (4).
Previously, we performed the first comprehensive investigation on
the 39 HOX genes in ESCC; 8 of the 39 HOX genes were
detected in cancerous tissues rather than non-cancerous tissues.
The upregulation of HOXA13 was observed in ESCC cell lines
and cancerous tissues. Colony formation and nude mouse
tumorigenicity assays revealed that HOXA13 promotes tumor
cell proliferation in vitro and in vivo, and
HOXA13 expression is significantly associated with
disease-free survival. Subsequently, a proteomics study and
CHIP-DSL revealed that ANXA2 and SOD2 are potential
targets of HOXA13.
In the present study, we revealed that both
ANXA2 and SOD2 were overexpressed in ESCC tissues
when compared to the levels in the normal esophageal tissues.
Further analysis of ANXA2 and SOD2 expression
combined with HOXA13 expression in the same series of ESCC
tissues indicated a significantly positive correlation between them
at both the protein and mRNA levels. Collectively, ANXA2 and
SOD2 may participate in ESCC tumorigenesis as well as
HOXA13.
However, to date, the molecular pathway linking
HOXA13 and its potential targets is not yet clear. As a
transcriptional factor, the core binding motif of HOXA13 has been
identified: a core sequence of TAA, and TAA-containing sequences
were TAAA (50%), TAAC (30%) and TAAT (20%) (23), which were also found in the promotor
region of both ANXA2 and SOD2 (data not shown).
ANXA2 is a member of the calcium and
phospholipid-dependent proteins. Binding of t-PA and ANXA2 on the
membrane of pancreatic cancer cells was found to activate tumor
cell invasion (10). ANXA2 was
found to facilitate cell cycle and proliferation in non-small cell
lung cancer by inhibiting p53, while the silencing of ANXA2
increased p53 expression, which led to p53-dependent and
-independent G2 arrest (13). The
present study suggests that overexpression of ANXA2 is indicative
of the poor prognosis of ESCC patients, which corroborates the role
of oncogenic ANXA2 revealed by such mechanistic studies.
SOD2 is a member of the manganese superoxide
dismutase family, which encodes a mitochondrial protein. Studies
suggest that SOD2 overexpression is associated with tumor
invasion and metastasis. NF-κB was found to reduce tumor
progression through binding to intronic enhancer element to
activate the expression of SOD2 (18). Our results also indicated that
overexpression of SOD2 is predictive of poor prognosis of ESCC
patients.
Considering the oncogenic role of ANXA2 and
SOD2, and our previous result of their coexpression in ESCC,
we speculate that HOXA13 may act as an oncogene in ESCC by
regulating ANXA2 and SOD2 expression, which still
needs further investigation. Revealing the specific mechanism of
the above association may further our understanding of ESCC
carcinogenesis, with the potential to develop new drug targets of
ESCC and possibly, to establish a more personalized prognosis for
each patient.
Since ANXA2 and SOD2 were found to be
involved in ESCC and were associated with HOXA13,
elucidation of their clinical significance was of great concern. We
revealed that not only ANXA2 or SOD2 expression alone
but also their coexpression with HOXA13 was significantly
correlated with the overall survival of ESCC patients. Kaplan-Meier
survival curve analysis showed that coexpression of
HOXA13/ANXA2/SOD2 was indicative of a poor prognosis of ESCC
patients, while Cox proportional hazards regression model indicated
that coexpression of HOXA13/ANXA2/SOD2, as well as TNM stage, are
both independent prognosis factors of ESCC. To strengthen our
conclusion, all of the results were validated in two independent
cohorts. Collectively, both ANXA2 and SOD2 had a significant
prognostic value for ESCC patient, and their coexpression with
HOXA13 may have added prognostic value, as a complement to the TNM
staging system.
In conclusion, HOXA13 as well as its target genes
ANXA2 and SOD2 are potential negative predictors of overall
survival time of ESCC patients. Thus, combination of their
expression profile and the TNM stage classification may provide a
more accurate prediction of the postoperative outcome of ESCC
patients.
Acknowledgements
This work was supported by the National Natural
Science Foundation for Distinguished Young Scholars (grant no.
81301748), Science Fund for Creative Research Groups of the
National Natural Science Foundation of China (grant no. IRT13003).
We are grateful to the patients who participated in this study. For
providing patient care and the data base establishment, we would
like to thank Hong-Chao Xiong, MD, Zhen Liang, MD, Qin Bin, MD,
Shao-Hua Ma, MD, Xiao-Zheng Kang, Msc, Yong-Bo Yang, MD, Liang Dai,
MD, Wan-Pu Yan, Msc, He-Li Yang, MD (Thoracic Surgery I). We would
like to thank Bin Dong, MD, Zhong-Wu Li, MD (Pathology Department)
and Yang Ke, MD, PhD, Hong Cai, MD, PhD, Jing-Jing Li, PhD
(Laboratory of Genetics). In addition, we would like to thank the
Ethics and Academic Committees of Peking University School of
Oncology, for approving this study and Zhen-Dong Gu, MD, Meng-Meng
Song, MD, Wen Wang, PhD, Hao Fu, Msc, Ya-Bing Du, Msc, Yun-Fan Ma,
Msc, Hui Wang, Msc, Chuan Huang, Msc (Thoracic Surgery I).
References
|
1
|
Macfarlane GJ and Boyle P: The
epidemiology of oesophageal cancer in the UK and other European
countries. J R Soc Med. 87:334–337. 1994.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3
|
Chen KN, Gu ZD, Ke Y, Li JY, Shi XT and Xu
GW: Expression of 11 HOX genes is deregulated in esophageal
squamous cell carcinoma. Clin Cancer Res. 11:1044–1049.
2005.PubMed/NCBI
|
|
4
|
Shah N and Sukumar S: The Hox genes and
their roles in oncogenesis. Nat Rev Cancer. 10:361–371. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi O, Hamada J, Abe M, et al:
Dysregulated expression of HOX and ParaHOX genes in human
esophageal squamous cell carcinoma. Oncol Rep. 17:753–760.
2007.PubMed/NCBI
|
|
6
|
Gu Z, Shen L, Wang H, et al: HOXA13
promotes cancer cell growth and predicts poor survival of patients
with esophageal squamous cell carcinoma. Cancer Res. 69:4969–4973.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen LY and Chen KN: Exploration of target
genes of HOXA13 in esophageal squamous cell carcinoma cell line.
Cancer Lett. 312:18–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emoto K, Yamada Y, Sawada H, et al:
Annexin II overexpression correlates with stromal tenascin-C
overexpression: a prognostic marker in colorectal carcinoma.
Cancer. 92:1419–1426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Emoto K, Sawada H, Yamada Y, et al:
Annexin II overexpression is correlated with poor prognosis in
human gastric carcinoma. Anticancer Res. 21:1339–1345.
2001.PubMed/NCBI
|
|
10
|
Díaz VM, Hurtado M, Thomson TM, Reventós J
and Paciucci R: Specific interaction of tissue-type plasminogen
activator (t-PA) with annexin II on the membrane of pancreatic
cancer cells activates plasminogen and promotes invasion in vitro.
Gut. 53:993–1000. 2004.PubMed/NCBI
|
|
11
|
Esposito I, Penzel R, Chaib-Harrireche M,
et al: Tenascin C and annexin II expression in the process of
pancreatic carcinogenesis. J Pathol. 208:673–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji NY, Park MY, Kang YH, et al: Evaluation
of annexin II as a potential serum marker for hepatocellular
carcinoma using a developed sandwich ELISA method. Int J Mol Med.
24:765–771. 2009.PubMed/NCBI
|
|
13
|
Wang CY, Chen CL, Tseng YL, et al: Annexin
A2 silencing induces G2 arrest of non-small cell lung
cancer cells through p53-dependent and -independent mechanisms. J
Biol Chem. 287:32512–32524. 2012.PubMed/NCBI
|
|
14
|
Sharma MR, Koltowski L, Ownbey RT,
Tuszynski GP and Sharma MC: Angiogenesis-associated protein annexin
II in breast cancer: selective expression in invasive breast cancer
and contribution to tumor invasion and progression. Exp Mol Pathol.
81:146–156. 2006. View Article : Google Scholar
|
|
15
|
MacMillan-Crow LA and Crow JP: Does more
MnSOD mean more hydrogen peroxide? Anticancer Agents Med Chem.
11:178–180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miriyala S, Holley AK and St Clair DK:
Mitochondrial superoxide dismutase - signals of distinction.
Anticancer Agents Med Chem. 11:181–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakano T, Oka K and Taniguchi N: Manganese
superoxide dismutase expression correlates with p53 status and
local recurrence of cervical carcinoma treated with radiation
therapy. Cancer Res. 56:2771–2775. 1996.
|
|
18
|
Ennen M, Minig V, Grandemange S, et al:
Regulation of the high basal expression of the manganese superoxide
dismutase gene in aggressive breast cancer cells. Free Radic Biol
Med. 50:1771–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toh Y, Kuninaka S, Oshiro T, et al:
Overexpression of manganese superoxide dismutase mRNA may correlate
with aggressiveness in gastric and colorectal adenocarcinomas. Int
J Oncol. 17:107–112. 2000.PubMed/NCBI
|
|
20
|
Venkataraman S, Wagner BA, Jiang X, et al:
Overexpression of manganese superoxide dismutase promotes the
survival of prostate cancer cells exposed to hyperthermia. Free
Radic Res. 38:1119–1132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Borchert GL, Donald SP, et al:
MnSOD inhibits proline oxidase-induced apoptosis in colorectal
cancer cells. Carcinogenesis. 26:1335–1342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu H, Luo ML, Du XL, et al: Up-regulated
manganese superoxide dismutase expression increases apoptosis
resistance in human esophageal squamous cell carcinomas. Chin Med
J. 120:2092–2098. 2007.
|
|
23
|
Salsi V and Zappavigna V: Hoxd13
and Hoxa13 directly control the expression of the
EphA7 Ephrin tyrosine kinase receptor in developing limbs. J
Biol Chem. 281:1992–1999. 2005. View Article : Google Scholar
|