Introduction
Gastric cancer consists of primary malignant lesions
in any part of the stomach and causes ~870,000 deaths worldwide per
year (1). The highest incidences
are in eastern Asia (China, Japan, Korea), South America and
Eastern Europe (2). Most stomach
cancer is caused by H. pylori infection (3). The mechanism by which H. pylori
induces stomach cancer potentially involves chronic inflammation or
the action virulence factors such as CagA (4). Dietary factors including some foods
with high sodium such as smoked foods, salted fish and pickled
vegetables are associated with higher risk (5). Smoking increases the risk of
developing gastric cancer significantly (6). Other factors associated with increased
risk are autoimmune atrophic gastritis, intestinal metaplasia and
genetic factors (7). Gastric
cancers are overwhelmingly adenocarcinoma (8) and gastric adenocarcinoma (GAC) is a
malignant epithelial tumor which originates from glandular
epithelium of the gastric mucosa. According to Lauren
classification, it is divided as two major types, intestinal-type
and diffuse-type.
MCM7 is one of the highly conserved mini-chromosome
maintenance proteins (9) that are
essential for the initiation of genomic replication. The hexametric
protein complex formed by the MCM proteins is a key component of
the pre-replication complex (pre-RC) and involved in the formation
of replication forks and in the recruitment of other DNA
replication related proteins (10,11).
Some studies have suggested that MCM4, 6 and 7 complexes contain
DNA helicase activity (12,13) and act as a DNA unwinding enzyme.
MCM7 amplification and overexpression in several human malignancies
has been identified (14–16), and its function in cancer
development is gradually revealed.
However, in GAC, MCM7 has not been functionally
investigated and evaluated on its clinical significance. In the
present study, we provide the first evidence that MCM7
overexpression is correlated with diffuse-type GAC patient survival
and siRNA mediated downregulation of MCM7 in gastric cancer cell
lines has anti-oncogenic effect.
Materials and methods
Cell lines and primary GAC samples
The 9 GAC cell lines (MKN28, KATOIII, MKN45, SNU16,
SNU1, MKN7, MKN1, NCI-N87 and AGS) (17), were cultured in RPMI-1640 (Gibco)
supplemented with 10% fetal bovine serum (FBS; EU Gibco), 100 U/ml
penicillin and 10 μg/ml streptomycin in a humidified atmosphere of
5% CO2 at 37°C. Archival tissue blocks from 123 patients
with diffuse-type GAC were retrieved from the Department of
Anatomical and Cellular Pathology, Prince of Wales Hospital (PWH),
Hong Kong. Frozen tissues from another 6 pairs of primary GACs and
corresponding non-tumorous gastric mucosa were collected from
patients who underwent gastectomy before any therapeutic
intervention. The AJCC (7th edition) was used for cancer
staging.
RNA extraction and qRT-PCR
Total RNA was extracted using RNeasy Mini kit
according to the manufacturer’s instruction (Qiagen). High-Capacity
cDNA reverse transcription kits (Applied Biosystems) were applied
to achieve cDNAs. For qRT-PCR, SYBR-Green PCR Master Mix (Applied
Biosystems) was applied for MCM7 expression (sense, ACTT
GTGGAATGGCAGGAAG and antisense, CTAGGCATCC CACTCAAAGG). The normal
gastric mRNA was from Ambion (AM 7996). The relative expression
level was normalized by glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) and calculated using the 2−ΔΔCt method.
Quantitative PCR was performed in triplicate reaction wells
including positive and negative controls.
Immunohistochemical staining and
scoring
Immunohistochemical staining of MCM7 was performed
in 4 μm-thick sections. After de-waxing in xylene, sections were
rehydrated, rinsed in distilled water, and incubated in 3%
H2O2 solution for 25 min to block endogenous
peroxidase activities. Antigen retrieval was done by using pressure
cooker with 10 nM citrate buffer (pH 6.0) for a total of 30 min
(high power 10 min followed by low power 20 min). The primary
antibody MCM7 (1:1,000, CDC47 Ab-2; NeoMarkers, Fremont, CA, USA)
was applied at 4°C overnight and chromogen development was
performed using the standard avidin-biotin method (Dako). The
nuclear expression of MCM7 was scored by estimating proportion of
tumor cells with positive nuclear staining into 4 different groups
(negative, none; weak, ≤10%; moderate, 10 to ≤25%; strong,
>25%).
Western blot analysis
Protein was extracted from the GAC cell lines,
normal gastric epithelium tissues together with 6 paired primary
samples using RIPA lysis buffer with proteinase inhibitor. Protein
mixed (20 μg) with 5X SDS loading buffer was loaded per lane,
separated by 12% SDS-polyacrylamide gel electrophoresis. MCM7
protein was detected with MCM7 antibody (1:50, CDC47 Ab-2;
NeoMarkers). Cleaved-PARP (Asp214) (1:1,000, #9541; Cell Signaling
Technology, Danvers, MA, USA) was also evaluated to check late
apoptosis.
In vitro functional study assays
Transfection was performed using Lipofectamine 2000
transfection reagent (Invitrogen) and siMCM7 (SI05064276 and
SI05064283; Qiagen) at the concentration of 25 nM. Scramble siRNA
was included as control. Cell proliferation was assessed using
CellTiter 96® Non-Radioactive Cell Proliferation Assay
(MTT) (Promega, Madison, WI, USA) according to the manufacturer’s
instructions. The absorbance at 570 nm was measured and documented
for the 5-day MTT proliferation analysis (Victor3; Perken-Elmer,
Waltham, MA, USA). Monolayer colony formation assay was performed
as described in our previous report (18). We used 6-well plates for this assay,
3 for siMCM7 and 3 for siScramble. The time-point was 8 days after
transfection. Colonies were fixed with 70% ethanol for 15 min and
stained with 2% crystal violet. Colonies with cell number of >50
cells/colony were counted. Cell invasion assay was performed in a
24-well invasion chamber (BD BioCoat Matrigel Invasion Chamber; BD
Biosciences). Cells were harvested from the culture dishes 24 h
after transfection, washed three times with culture medium
containing 1% FBS, and resuspended in medium with 1% FBS. Then, 300
μl of the cell suspension (1×105 cells) was added into
the Transwells, with 500 μl of culture medium containing 10% FBS in
the lower chamber. After 24-h incubation at 37°C, cells that
invaded through the Matrigel membrane were fixed with 100% methanol
for 2 min and stained with 1% Toluidine blue for another 2 min.
Cells on the underside of the membrane were counted from 3
microscope fields and the average number recorded.
Statistical analysis
In the functional assays like MTT proliferation,
monolayer colony formation and cell invasion, Student’s t-test was
used to compare the phenotype difference of siMCM7 knockdown cells
and siScramble transfected cells. Correlations between MCM7
expression with other clinicopathologic parameters were evaluated
by non-parametric Spearman’s rho rank test. The Kaplan-Meier method
was used to estimate the survival rates according to MCM7 nuclear
staining. For the parameters statistically significant in the
univariate survival analysis (P<0.05), the multivariate survival
was achieved by the Cox regression analysis. All statistical
analysis was performed by SPSS software (version 16.0; SPSS Inc.).
A two-tailed P-value of <0.05 was considered to indicate a
statistically significant result.
Results
Upregulation of MCM7 in gastric cell
lines and primary gastric tumors
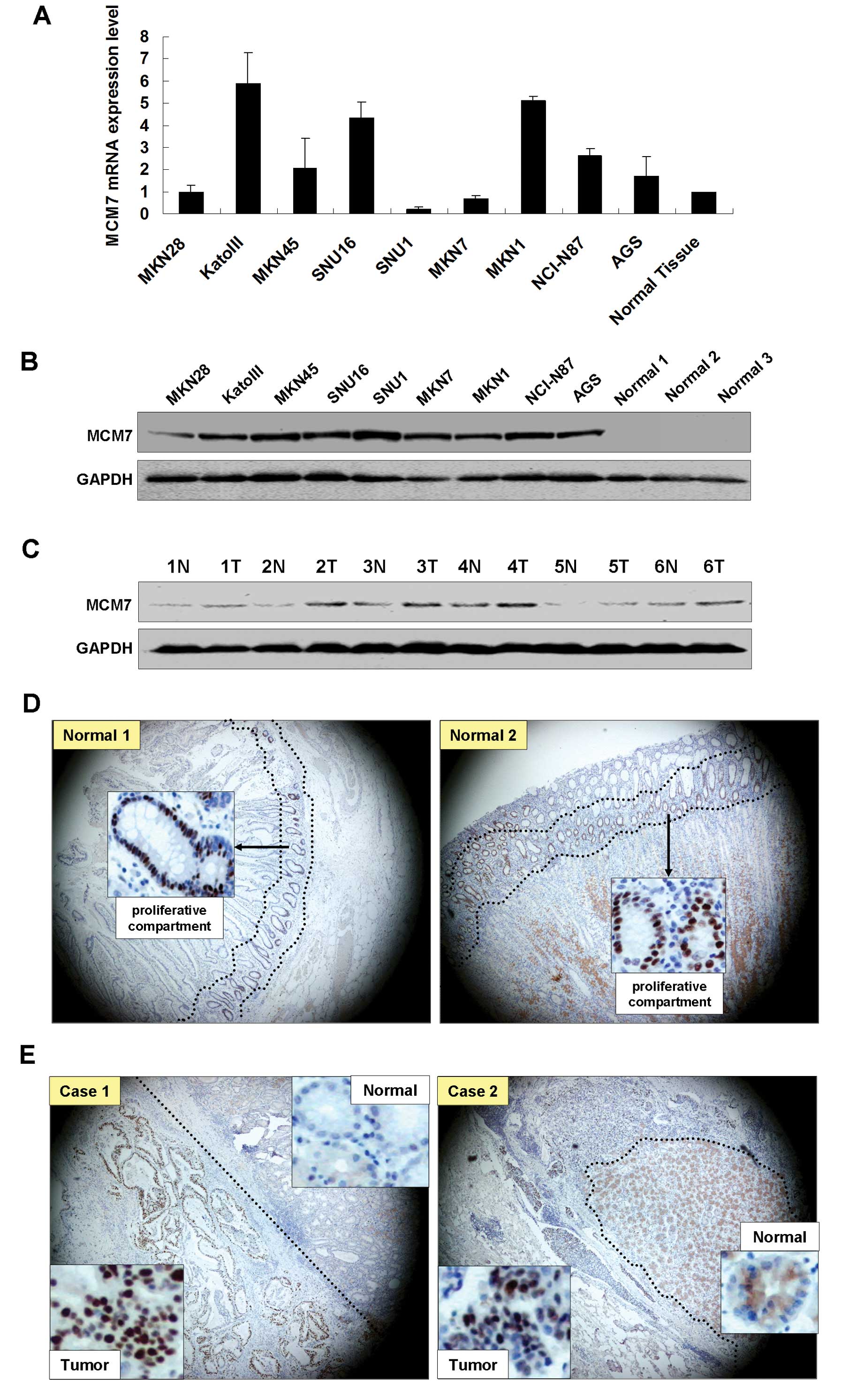
The MCM7 mRNA expression levels were higher in 6 GAC
cell lines (KatoIII, MKN45, SNU16, MKN1, NCI-N87 and AGS) than it
in the normal gastric tissue as shown in Fig. 1A. The upregulation of MCM7 in the
GAC cells was further confirmed by western blot analysis. High MCM7
protein expression was observed in all GAC cell lines compared with
the 3 normal gastric mucosal samples from patients who underwent
weight reduction gastrectomy (Fig.
1B). Comparing tumor with the paired non-tumorous mucosa,
upregulation of MCM7 protein was observed in all 6 tumor samples by
western blot analysis (Fig. 1C). In
normal gastric epithelium tissues, only cells from neck region
(proliferation compartment) showed MCM7 nuclear expression by
immunohistochemistry (Fig. 1D).
Immunohistochemical analysis of 5 paired primary GAC samples showed
positive nuclear staining of MCM7 in the tumor tissues but not in
the non-tumorous gastric glandular epithelium (Fig. 1E).
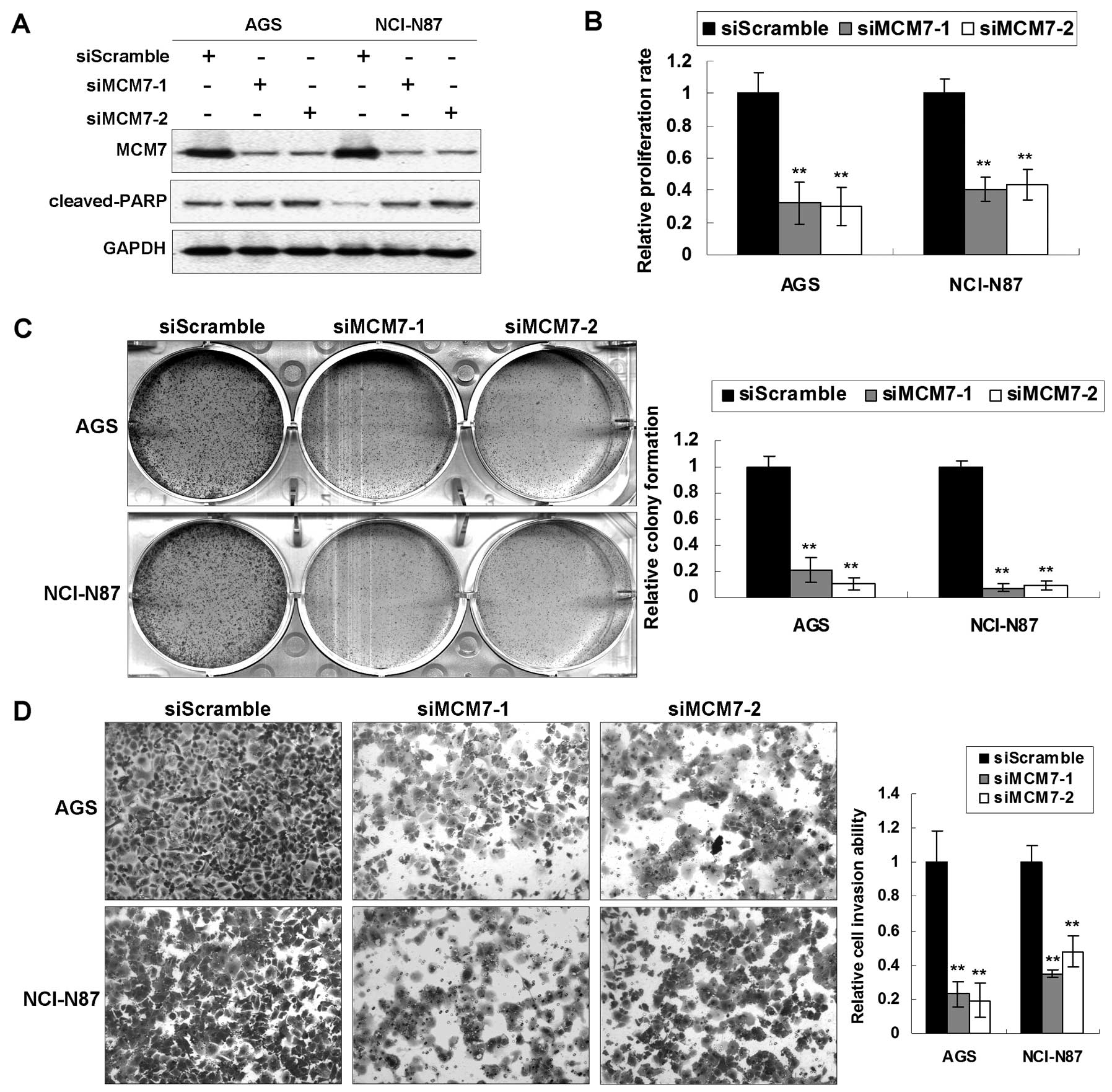
MCM7 knockdown in GAC cells exerts
anti-oncogenic effect in vitro
Upregulation of MCM7 in GACs suggested a potential
tumorigenic role of MCM7 in cancer development. To further
elucidate the functional role it plays, we investigated the effect
of MCM7 knockdown by siRNA in vitro. MCM7 were knocked down
by 2 specific siMCM7s in AGS and NCI-N87 with high transfection
efficiency (Fig. 2A). The 5-day MTT
assay indicated that the MCM7 knockdown reduced cell proliferation
rates to significant levels (AGS, 32.1 and 30.0%; NCI-N87, 40.5 and
43.6%; P<0.001) (Fig. 2B)
compared with siScramble control groups. The viability change
induced by siMCM7 was also confirmed by anchorage-dependent
monolayer colony formation assays. A significant reduction of
colony number and colony size was observed in cells transfected
with siMCM7 compared with the siScramble group (reduced to 21.3 and
10.6% in AGS; 7.6 and 9.2% in NCI-N87; P<0.001) (Fig. 2C). In cell motility assays using BD
BioCoat chamber, we confirmed that the invaded cell number was
significantly lower in the siMCM7 transfected cells than the
control groups (impaired to 23.0 and 19.1% in AGS; 34.9 and 47.6%
in NCI-N87; P<0.001) (Fig. 2D),
suggesting that MCM7 was involved in the invasion of cancer cells.
Moreover, siMCM7 induced late apoptosis in AGS and NCI-N87 cells
which was represented by activation of cleaved-PARP (Fig. 2A).
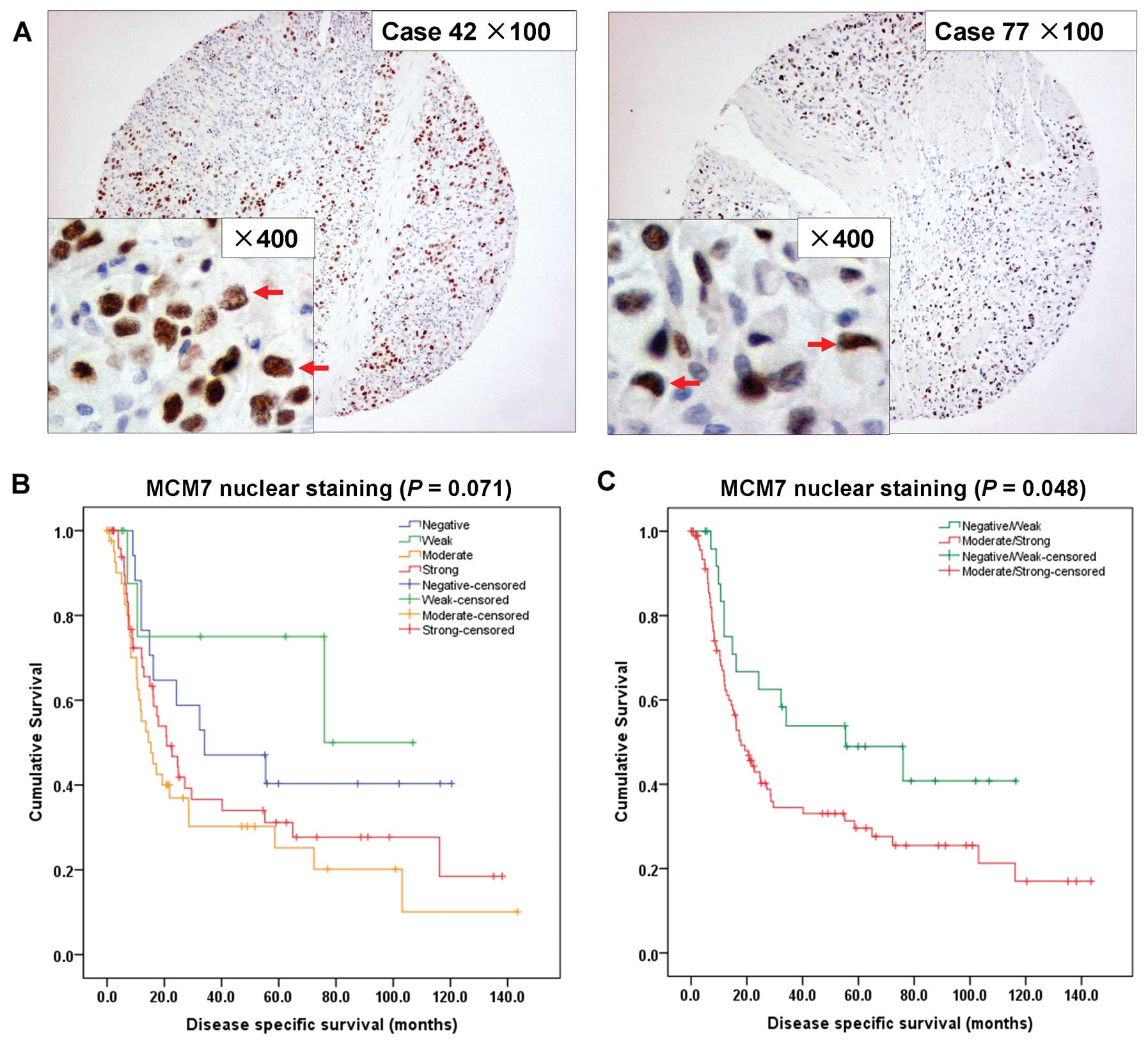
MCM7 overexpression correlates with poor
survival in diffuse-type primary GACs
In total 267 GAC samples, MCM7 expression had no
correlation with survival (P=0.724), thus, we checked its
prognostic correlation according to histological type (intestinal
and diffuse) and stage (early and advanced) separately. We found
only in the diffuse-type GAC, MCM7 correlates with poor
survival.
MCM7 was predominantly expressed in the nuclei of
diffuse-type tumor cells (Fig. 3A).
Of the 123 diffuse-type GAC samples, 16 cases (13.0%) had MCM7
negative nuclear staining, 11 cases (8.9%) had weak staining, 44
cases (35.8%) had moderate staining and 52 cases (42.3%) had strong
staining. No significant survival difference was observed between
the 4-grade MCM7 expression by univariate analysis (P=0.071;
Fig. 3B). Then, we stratified
negative and weak staining cases as negative/weak MCM7 expression
group (27/123, 22.0%), and moderate and strong cases as
moderate/strong MCM7 expression group (96/123, 78.0%). MCM7 nuclear
expression correlates with poor survival based on these groupings
(P=0.048; Fig. 3C).
We further investigated the clinicopathological
correlation of MCM7 in diffuse-type GACs. Table I summarizes the correlation of MCM7
with clinicopathological parameters in diffuse-type GAC patients.
MCM7 expression only correlated with advanced age (>60 years;
P=0.015). Univariate analysis (Table
II) indicated that MCM7 expression in diffuse-type GACs
associated with poor disease-specific survival (P=0.048). Advanced
age and stage were (T, N and M, respectively) also correlated with
poor survival (P<0.05). By multivariate Cox proportional hazards
regression analysis (Table II),
only stage (T, N and M, respectively) was independently associated
with disease-specific survival (P<0.05).
 | Table ICorrelation of MCM7 nuclear expression
with clinicopathological features. |
Table I
Correlation of MCM7 nuclear expression
with clinicopathological features.
| Diffuse-type GAC
cases (n=123) |
|---|
|
|
|---|
| Negative/weak No.
(%) | Moderate/strong No.
(%) | P-value |
|---|
| Gender |
| Male | 16 (20.5) | 62 (79.5) | 0.655 |
| Female | 11 (24.4) | 34 (75.6) | |
| Age (years) |
| ≤60 | 18 (32.7) | 37 (67.3) |
0.015 |
| >60 | 9 (13.2) | 59 (86.8) | |
| Grade |
| 1 | 0 | 0 | 0.575 |
| 2 | 0 (0.0) | 4 (100.0) | |
| 3 | 27 (22.7) | 92 (77.3) | |
| Stage |
| 1 | 3 (18.8) | 13 (81.3) | 0.879 |
| 2 | 2 (33.3) | 4 (66.7) | |
| 3 | 9 (23.7) | 29 (76.3) | |
| 4 | 13 (20.6) | 50 (79.4) | |
| Stage (T) |
| 1 | 1 (10.0) | 9 (90.0) | 0.770 |
| 2 | 6 (20.7) | 23 (79.3) | |
| 3 | 18 (23.4) | 59 (76.6) | |
| 4 | 2 (28.6) | 5 (71.4) | |
| Stage (N) |
| 0 | 6 (33.3) | 12 (66.7) | 0.521 |
| 1 | 3 (13.6) | 19 (86.4) | |
| 2 | 9 (22.0) | 32 (78.0) | |
| 3 | 9 (21.4) | 33 (78.6) | |
| Stage (M) |
| 0 | 20 (21.3) | 74 (78.7) | 0.799 |
| 1 | 7 (24.1) | 22 (75.9) | |
| Lymph node |
| 0 | 6 (33.3) | 12 (66.7) | 0.224 |
| 1 | 21 (20.0) | 84 (80.0) | |
| H.
pylori |
| Absence | 14 (20.9) | 53 (79.1) | 0.503 |
| Presence | 13 (27.7) | 34 (72.3) | |
 | Table IIUnivariate and multivariate Cox
regression analysis of clinicopathological factors in patients with
GAC. |
Table II
Univariate and multivariate Cox
regression analysis of clinicopathological factors in patients with
GAC.
| Diffuse-type GAC
cases (n=123) |
|---|
|
|
|---|
| Univariate
(P-value) | Multivariate
(P-value) |
|---|
| Gender | 0.838 | |
| Age |
0.008 | |
| Grade | 0.259 | |
| Stage (T) |
0.002 |
0.020 |
| Stage (N) |
<0.001 |
0.001 |
| Stage (M) |
<0.001 |
0.007 |
| H.
pylori | 0.763 | |
| MCM7 |
0.048 | |
Discussion
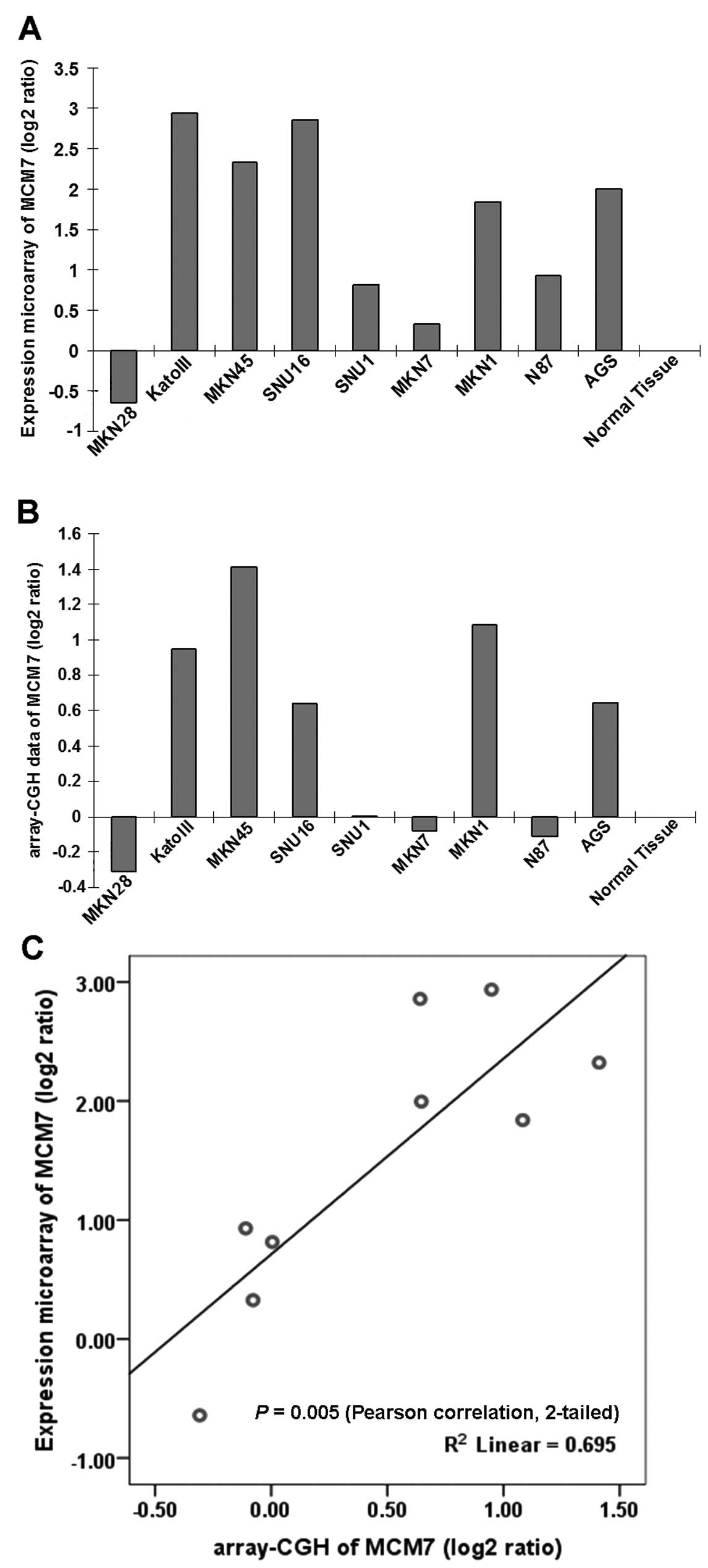
In gastric cancer, MCM7 was first screened from mRNA
expression microarray (Fig. 4A) and
array-CGH (comparative genomic hybridization, Fig. 4B) in 9 GAC cell lines. The MCM7 mRNA
expression showed positive correlation with DNA copy number change
(Fig. 4C), indicating MCM7 DNA copy
number gain was partly responsible for its mRNA upregulation in
GAC, and van Dekken et al (19) screened MCM7 using the same method in
gastroesophageal junction adenocarcinomas due to the DNA
amplification and mRNA upregulation. MCM7 gene on chromosome 7q22
contains an intronic miRNA miR-106b-25 cluster in intron 13
(20). MCM7 oncogenicity may be
linked, at least in part, to overexpression of the hosted miRNAs
which targets TGF-β tumor suppressor pathway in gastric cancer
(21).
MCM7 has been reported to be upregulated in many
types of cancer and functions as oncogene in muscle-invasive
bladder cancer (22), non-small
cell lung cancer (23,24), Hodgkin lymphoma (25), prostate cancer (15), liver cancer (26), meningiomas (27), colorectal cancer (28,29),
medulloblastoma (30) and oral
squamous cell carcinoma (31). By
siRNA-mediated knockdown of MCM7 in GAC cell lines, the cell
proliferation and invasion were inhibited, thus MCM7 knockdown
exerts anti-oncogenic effect in vitro. This result was
concordant with functional studies in other types of cancer. In
medulloblastoma, exogenous overexpression of MCM7 increased tumor
growth and invasiveness, whereas MCM7 knockdown by RNA interference
inhibited cell growth and reduced cell migration and invasion
(30). MCM7 elimination in prostate
cancer and non-small cell lung cancer cell lines also suppressed
cell growth. In a mouse model of PC3 and DU145 xenografts of
prostate cancer, knockdown of MCM7 dramatically reduced the tumor
volumes, decreased tumor metastasis and animal mortality (32).
Some reports indicated that MCM7 was a more reliable
and useful biomarker in assessing tumor proliferation, aggression
and in the prognosis of patients even than the existing markers
such as Ki-67 (33,34). In primary diffuse-type GACs, we
identified that MCM7 was moderately/strongly stained in 96 of 123
GAC (78.0%) and its overexpression was closely correlated with
advanced age (>60 years). However, MCM7 expression was not
detected associated with other clinicopathological factors
including clinical grade, stage and lymph node metastasis. In
endometrial carcinoma (35) and
Hodgkin lymphoma (25), MCM7
overexpression also correlates with advanced age. More importantly,
its upregulation correlates with poor survival and predicts poor
prognosis, indicating MCM7 might have potential as a biomarker and
therapeutic target in diffuse-type GAC.
In conclusion, the present findings indicate that
MCM7 expression may be a useful prognosis indicator in patients
with diffuse-type GACs and its knockdown by RNA interference
quenches its oncogenic property in gastric cancer.
Acknowledgements
The present study is supported by a National Natural
Science Grant of China (81201591).
References
|
1
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith MG, Hold GL, Tahara E and El-Omar
EM: Cellular and molecular aspects of gastric cancer. World J
Gastroenterol. 12:2979–2990. 2006.
|
|
3
|
Shiota S, Suzuki R and Yamaoka Y: The
significance of virulence factors in Helicobacter pylori. J
Dig Dis. 14:341–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sepulveda AR: Helicobacter,
inflammation, and gastric cancer. Curr Pathobiol Rep. 1:9–18. 2013.
View Article : Google Scholar
|
|
5
|
Zhong C, Li KN, Bi JW and Wang BC: Sodium
intake, salt taste and gastric cancer risk according to
Helicobacter pylori infection, smoking, histological type
and tumor site in China. Asian Pac J Cancer Prev. 13:2481–2484.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang MW, Jin MJ, Yu YX, et al:
Associations of lifestyle-related factors, hsa-miR-149 and
hsa-miR-605 gene polymorphisms with gastrointestinal cancer risk.
Mol Carcinog. 51(Suppl 1): E21–E31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gomceli I, Demiriz B and Tez M: Gastric
carcinogenesis. World J Gastroenterol. 18:5164–5170.
2012.PubMed/NCBI
|
|
8
|
Green D, Ponce de Leon S, Leon-Rodriguez E
and Sosa-Sanchez R: Adenocarcinoma of the stomach: univariate and
multivariate analysis of factors associated with survival. Am J
Clin Oncol. 25:84–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kebebew E, Peng M, Reiff E, Duh QY, Clark
OH and McMillan A: Diagnostic and prognostic value of cell-cycle
regulatory genes in malignant thyroid neoplasms. World J Surg.
30:767–774. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo JH: Oncogenic activity of MCM7
transforming cluster. World J Clin Oncol. 2:120–124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei Q, Li J, Liu T, Tong X and Ye X:
Phosphorylation of minichromosome maintenance protein 7 (MCM7) by
cyclin/cyclin-dependent kinase affects its function in cell cycle
regulation. J Biol Chem. 288:19715–19725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishimi Y: A DNA helicase activity is
associated with an MCM4, -6, and -7 protein complex. J Biol Chem.
272:24508–24513. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You Z, Komamura Y and Ishimi Y:
Biochemical analysis of the intrinsic Mcm4-Mcm6-mcm7 DNA helicase
activity. Mol Cell Biol. 19:8003–8015. 1999.PubMed/NCBI
|
|
14
|
Honeycutt KA, Chen Z, Koster MI, et al:
Deregulated minichromosomal maintenance protein MCM7 contributes to
oncogene driven tumorigenesis. Oncogene. 25:4027–4032. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren B, Yu G, Tseng GC, et al: MCM7
amplification and overexpression are associated with prostate
cancer progression. Oncogene. 25:1090–1098. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kan T, Sato F, Ito T, et al: The
miR-106b-25 polycistron, activated by genomic amplification,
functions as an oncogene by suppressing p21 and Bim.
Gastroenterology. 136:1689–1700. 2009.
|
|
17
|
Kang W, Tong JH, Chan AW, et al: Stathmin1
plays oncogenic role and is a target of microRNA-223 in gastric
cancer. PLoS One. 7:e339192012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang W, Tong JH, Chan AW, et al:
Yes-associated protein 1 exhibits oncogenic property in gastric
cancer and its nuclear accumulation associates with poor prognosis.
Clin Cancer Res. 17:2130–2139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Dekken H, Tilanus HW, Hop WC, et al:
Array comparative genomic hybridization, expression array, and
protein analysis of critical regions on chromosome arms 1q, 7q, and
8p in adenocarcinomas of the gastroesophageal junction. Cancer
Genet Cytogenet. 189:37–42. 2009.
|
|
20
|
Ambs S, Prueitt RL, Yi M, et al: Genomic
profiling of microRNA and messenger RNA reveals deregulated
microRNA expression in prostate cancer. Cancer Res. 68:6162–6170.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Petrocca F, Visone R, Onelli MR, et al:
E2F1-regulated microRNAs impair TGFβ-dependent cell-cycle arrest
and apoptosis in gastric cancer. Cancer Cell. 13:272–286. 2008.
|
|
22
|
Fristrup N, Birkenkamp-Demtroder K,
Reinert T, et al: Multicenter validation of cyclin D1, MCM7,
TRIM29, and UBE2C as prognostic protein markers in
non-muscle-invasive bladder cancer. Am J Pathol. 182:339–349. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu YZ, Jiang YY, Hao JJ, et al:
Prognostic significance of MCM7 expression in the bronchial
brushings of patients with non-small cell lung cancer (NSCLC). Lung
Cancer. 77:176–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toyokawa G, Masuda K, Daigo Y, et al:
Minichromosome Maintenance Protein 7 is a potential therapeutic
target in human cancer and a novel prognostic marker of non-small
cell lung cancer. Mol Cancer. 10:652011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marnerides A, Vassilakopoulos TP,
Boltetsou E, et al: Immunohistochemical expression and prognostic
significance of CCND3, MCM2 and MCM7 in Hodgkin lymhoma. Anticancer
Res. 31:3585–3594. 2011.PubMed/NCBI
|
|
26
|
Zhou YM, Zhang XF, Cao L, et al: MCM7
expression predicts post-operative prognosis for hepatocellular
carcinoma. Liver Int. 32:1505–1509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saydam O, Senol O, Schaaij-Visser TB, et
al: Comparative protein profiling reveals minichromosome
maintenance (MCM) proteins as novel potential tumor markers for
meningiomas. J Proteome Res. 9:485–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pillaire MJ, Selves J, Gordien K, et al: A
‘DNA replication’ signature of progression and negative outcome in
colorectal cancer. Oncogene. 29:876–887. 2010.
|
|
29
|
Nishihara K, Shomori K, Fujioka S, et al:
Minichromosome maintenance protein 7 in colorectal cancer:
implication of prognostic significance. Int J Oncol. 33:245–251.
2008.PubMed/NCBI
|
|
30
|
Lau KM, Chan QK, Pang JC, et al:
Minichromosome maintenance proteins 2, 3 and 7 in medulloblastoma:
overexpression and involvement in regulation of cell migration and
invasion. Oncogene. 29:5475–5489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng CJ, Li HJ, Li JN, Lu YJ and Liao GQ:
Expression of Mcm7 and Cdc6 in oral squamous cell carcinoma and
precancerous lesions. Anticancer Res. 28:3763–3769. 2008.PubMed/NCBI
|
|
32
|
Shi YK, Yu YP, Tseng GC and Luo JH:
Inhibition of prostate cancer growth and metastasis using small
interference RNA specific for minichromosome complex maintenance
component 7. Cancer Gene Ther. 17:694–699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujioka S, Shomori K, Nishihara K, et al:
Expression of minichromosome maintenance 7 (MCM7) in small lung
adenocarcinomas (pT1): Prognostic implication. Lung Cancer.
65:223–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ishino H, Hara Y, Takekoshi S, et al:
Ki-67 and minichromosome maintenance-7 (MCM7) expression in canine
pituitary corticotroph adenomas. Domest Anim Endocrinol.
41:207–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li SS, Xue WC, Khoo US, et al: Replicative
MCM7 protein as a proliferation marker in endometrial carcinoma: a
tissue microarray and clinicopathological analysis. Histopathology.
46:307–313. 2005. View Article : Google Scholar : PubMed/NCBI
|