Introduction
Cancer is a major cause of human mortality
worldwide. Many anticancer therapies, including chemotherapy and
anticancer drugs, are known to cause adverse side-effects (1,2).
Metastatic melanoma accounts for ~80% of melanoma deaths owing to
its aggressiveness and resistance to existing therapies (3,4).
Metastasis is a characteristic of highly malignant cancers with
poor clinical outcomes. Excess extracellular matrix (ECM)
degradation is a characteristic of tumor invasion and metastasis
(5–7). Matrix metalloproteinases (MMPs) aid
tumor cells in MMP degradation. MMPs are a group of zinc-dependent
ECM degrading enzymes that facilitate the proteolysis of ECM
proteins such as collagen, proteoglycan, fibronectin, elastin and
laminin (8). The expression of MMP
genes is primarily regulated at the transcriptional level [through
AP-1 or nuclear factor κB (NF-κB) via mitogen-activated protein
kinase (MAPK) or phosphoinositide 3-kinase (PI3K)/Akt pathways] and
post-transcriptional level, as well as at the protein level via
their activators or inhibitors and by their cell surface
localization (9–12). Recent reports on cyclooxygenase
(COX)-2 expression in cancer indicated that this enzyme stimulates
tumor growth, invasion and metastasis in association with MMPs
(13). COX-2 is an inducible
isoform that participates in pro-inflammatory responses to certain
stimuli such as mitogens, cytokines and growth factors (14). These studies revealed that MMPs and
their regulatory pathways may be promising targets for
anti-metastatic and chemotherapeutic therapy.
Mushrooms have been used to treat various diseases
including tumors. Inonotus obliquus, a traditional medicinal
mushroom, has been widely used to promote health and longevity.
Many studies reported that I. obliquus has many biological
activities including antitumor, antimutagenic, anti-oxidative,
antimitotic, antihyperglycemic, anti-inflammatory and
immunostimulating activities (15–20).
However, the anti-metastatic effect and signaling pathway mechanism
of polysaccharides from I. obliquus (PIO) remain unknown.
Therefore, in the present study, we investigated the
anti-metastatic effects and potential signaling pathways of PIO in
the highly metastatic B16-F10 mouse melanoma cells in
vitro.
Materials and methods
Preparation of polysaccharides from I.
obliquus (PIO)
Dried fruiting bodies of I. obliquus were
purchased from a local market and ground in a blender. Milled
mushroom (20 g) was extracted with distilled water (600 ml) at
121°C for 2 h. Extracts were centrifuged at 5,000 rpm for 20 min,
filtered through 0.45-μm Whatman filter paper (#4; Whatman, UK) to
remove insoluble matter and then freeze-dried. Polysaccharides were
precipitated from resuspended extracts using 75% ethanol, collected
by filtration through 0.45-μm Whatman filter paper, resuspended,
and dialyzed against distilled water for 5 days to remove
low-molecular-weight compounds (21,22).
Materials
Fetal bovine serum (FBS), penicillin G, and
streptomycin were obtained from Gibco (Grand Island, NY, USA).
Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Lonza
(Walkersville, MD, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and isopropyl alcohol were purchased from Sigma Chemical Co. (St.
Louis, MO, USA). β-actin monoclonal antibody (mAb), extracellular
signal-regulated kinase (ERK) MAPK Ab, phospho-ERK MAPK Ab,
stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK)
Ab, phospho-SAPK/JNK Ab, p38 MAPK Ab, phospho-p38 MAPK Ab, COX-2
Ab, MMP-2 Ab, MMP-9 Ab, and NF-κB p65 mAb were purchased from Cell
Signaling Technology (Beverly, MA, USA), Santa Cruz Biotechnology
(Santa Cruz, CA, USA), or BD Biosciences (San Jose, CA, USA),
respectively. All other chemicals were of analytical grade.
Cell culture
The B16-F10 murine melanoma cell line was obtained
from the Korea Cell Line Bank (Seoul, Korea). B16-F10 cells were
cultured in DMEM supplemented with 10% heat inactivated FBS, 100
U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained
at 37°C in a humidified 5% CO2 incubator.
Cell viability
The effect of PIO on the viability of B16-F10 cells
was measured using the MTT assay, which is based on the reduction
of a tetrazolium salt by mitochondrial dehydrogenase in viable
cells. Cells were pre-incubated in 12-well plates for 24 h at 37°C
humidified atmosphere of 5% CO2 incubator. Then, cells
were incubated with PIO (1–1,000 μg/ml) for 24 h. After incubation,
cells were washed with 1X PBS to remove dead cells, and then 50 μl
of MTT stock solution (2 mg/ml) was added to each well, which was
then incubated for 2 h. After incubation, the MTT assay was
performed to quantitate cellular viability. Finally, isopropyl
alcohol was added to solubilize the formazan salt formed, and the
amount of formazan salt was determined by measuring the absorbance
at 595 nm using an enzyme-linked immunosorbent assay microplate
reader.
Flow cytometry
The apoptotic death of tumor cells was examined
using a fluorescein isothiocyanate (FITC)-labeled Annexin
V/propidium iodide (PI) apoptosis detection kit (Molecular Probes,
Eugene, OR, USA) according to the manufacturer’s instructions.
Briefly, cells were harvested by trypsinization, washed with PBS
and centrifuged to collect the cell pellet. The number of cells was
adjusted to 1×106 cells/ml. The cells were then
resuspended in binding buffer (10 mM HEPES, 140 mM NaCl, and 2.5 mM
CaCl2, at pH 7.4) and stained with FITC-labeled Annexin
V and PI at room temperature for 15 min in the dark. Flow
cytometric analysis was performed using a FACSCalibur flow
cytometer (Becton-Dickinson, Mountain View, CA, USA) within 1 h
after supravital staining. FITC-labeled Annexin V was analyzed
using excitation and emission settings of 488 and 535 nm,
respectively. PI was analyzed using excitation and emission
settings of 488 and 575 nm, respectively. For each flow cytometer
run, 10,000 cells were required. The percentages of cells were
calculated using the CellQuest software (Becton-Dickinson). The
cells in the early stages of apoptosis were Annexin V-positive and
PI-negative; however, the cells in the late stages of apoptosis
were both Annexin V- and PI-positive. The apoptotic index (%) was
calculated as the sum of late apoptotic cells divided for the total
number of events.
Wound healing assay
The wound healing assay was performed as previously
described with some modifications (23). Briefly, B16-F10 cells were grown to
confluence in 6-well dishes for 24 h in serum-free medium (three
dishes per group). The medium was replaced with serum-containing
medium followed by the addition of PIO at various concentrations
(0, 25, 50 and 100 μg/ml), and the cells in monolayer were
disrupted (i.e., wounded) by scraping them with a 100-μl
micropipette tip. At the indicated times (0, 24 and 48 h) after
scraping, the cells were washed twice with PBS (pH 7.4). Finally,
the cells were gently washed three times with PBS and photographed
with an optical microscope at ×40.
In vitro migration and invasion
assay
The migration of B16-F10 cells was also measured by
chemotactic directional migration using a 6-well Transwell insert.
The 8-μm pore filters (Corning Incorporated, Corning, NY, USA) were
coated with gelatin (Sigma) and B16-F10 cells (1×106
cells/ml) were placed in the upper chamber with or without PIO (50
or 100 μg/ml) and allowed to undergo migration for 24 h. The
non-migrated cells in the upper chamber were removed with a cotton
swab. The filters were stained with 2% crystal violet. Migrated
cells adherent to the underside of the filter were counted and
photographed using an optical microscope at ×40. The invasion of
B16-F10 cells was measured using Matrigel-coated Transwell cell
culture chambers (8-μm pore size) as previously described. After
the cells were cultured for 24 h in serum-free DMEM, they were
collected, resuspended in serum-free medium, placed in the upper
chamber of the Transwell insert (1×106 cells/ml), and
incubated with or without PIO (50 or 100 μg/ml). DMEM containing
10% FBS was placed in the lower chamber. All the cells in each
treatment group were incubated for 24 h at 37°C in a humidified
atmosphere with 95% air and 5% CO2. The non-invasive
cells that remained in the upper chamber were removed by wiping
with a cotton swab, and the invasive cells were fixed with 4%
formaldehyde in PBS and stained with 2% crystal violet in 2%
ethanol. The invasive cells in the lower surface of the filter that
penetrated through the Matrigel were counted and photographed using
an optical microscope at ×40 (24).
Zymography analysis
Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) substrate-embedded enzymography
(zymography) was used to identify collagenase and gelatinase
activities (25,26). Briefly, the supernatant collected
from the cell culture was resolved in 10% SDS-PAGE gels, which were
prepared by the incorporation of gelatin (1 mg/ml) before casting.
After electrophoresis, the gels were washed twice for 30 min in
2.5% Triton X-100 with shaking. The gels were then incubated at
37°C for 24–48 h in reaction buffer containing 50 mM Tris-HCl (pH
7.6), 10 mM CaCl2, 150 mM NaCl, and 20% sodium azide,
followed by staining with 0.25% Coomassie brilliant blue G-250 in
50% methanol and 10% acetic acid for 1–2 h. The completely stained
gels were appropriately destained with 40% methanol and 10% acetic
acid. The enzyme activities were evident as clear (unstained)
regions against the dark background.
Western blot analysis
After treatment, the cells were washed in 1X PBS and
lysed in lysis buffer [10 mM Tris-HCl (pH 7.5), 10 mM
NaH2PO4/NaHPO4 (pH 7.5), 130 mM
NaCl, 1% Triton X-100, 10 mM NaPPi, 1 mM phenylmethylsulfonyl
fluoride, 2 μg/ml pepstatin A] for 30 min on ice. Lysates were
centrifuged at 12,000 × g for 20 min at 4°C. The supernatant was
collected, and its protein content was measured using a Bio-Rad
protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA) before
analysis. The cytosolic or nuclear protein samples were loaded at
10 μg of protein/lane, separated by SDS-PAGE in 10–15% gel, and
transferred to NC membranes (Immun-Blot NC membrane, 0.2 μm;
Bio-Rad Laboratories). Membranes were blocked with 1.5% skim milk
in 1X Tris-buffered saline (TBS) containing 0.1% Tween-20 for 1 h,
and they were incubated with primary antibodies at 4°C overnight.
Finally, the membranes were treated with horseradish
peroxidase-coupled secondary antibodies for 1 h at 4°C. The
membranes were washed with TBS after each antibody binding
reaction. The detection of each protein was performed using an
enhanced chemiluminescence kit (Millipore Co., Billerica, MA,
USA).
Nuclear protein extraction
Nuclear extracts were prepared by lysing nuclei in
high-salt buffer supplemented with protease and phosphatase
inhibitors using a nuclear extraction kit (Panomics Inc., Fremont,
CA, USA) according to the manufacturer’s protocol. Protein
concentrations were quantified using the Bio-Rad protein assay.
Statistical analysis
Data are expressed as mean ± standard error (SE)
values, and the results were obtained from at least three
independent experiments performed in triplicate. The data were
analyzed using Student’s t-test to evaluate significant
differences. A P<0.05 was regarded as statistically
significant.
Results
Effect of PIO on viability in B16-F10
cells
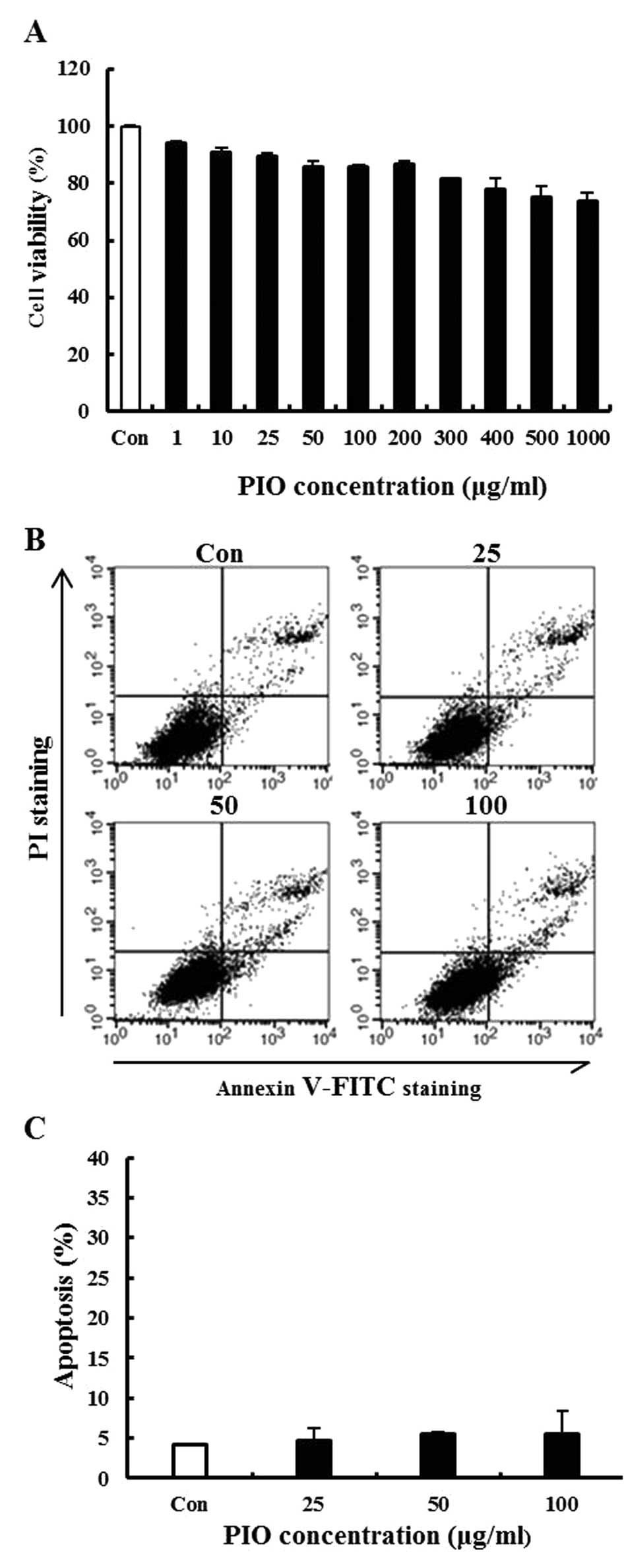
To investigate the cytotoxicity of PIO in B16-F10
melanoma cells, cells were treated with PIO at various
concentrations ranging from 0 to 1,000 μg/ml for 24 h and cell
viability was determined by the MTT assay. We found that PIO did
not significantly affect the growth of B16-F10 cells (Fig. 1A). To evaluate whether the
growth-inhibitory of PIO was associated with apoptosis, a
double-staining method using FITC-labeled Annexin V and PI was
performed. During the early stage of apoptosis, cells display
phosphatidylserine on their outer cell membranes, which is readily
detectable using Annexin V. During the later stages of apoptosis,
as the plasma membrane becomes increasingly permeable, PI can move
across the cell membrane to bind to cellular DNA. Double staining
the cells with Annexin V and PI allowed us to detect apoptotic
cells by flow cytometry. Low concentrations of PIO (25, 50 or 100
μg/ml) did not have an apoptotic effect on the cells (Fig. 1B and C). Therefore, these results
demonstrated that PIO at concentrations ranging from 0 to 100 μg/ml
did not induce cell death and apoptosis in the highly metastatic
B16-F10 melanoma cell line. This concentration range was then
applied in all subsequent experiments.
Effects of PIO on the motility of B16-F10
cells
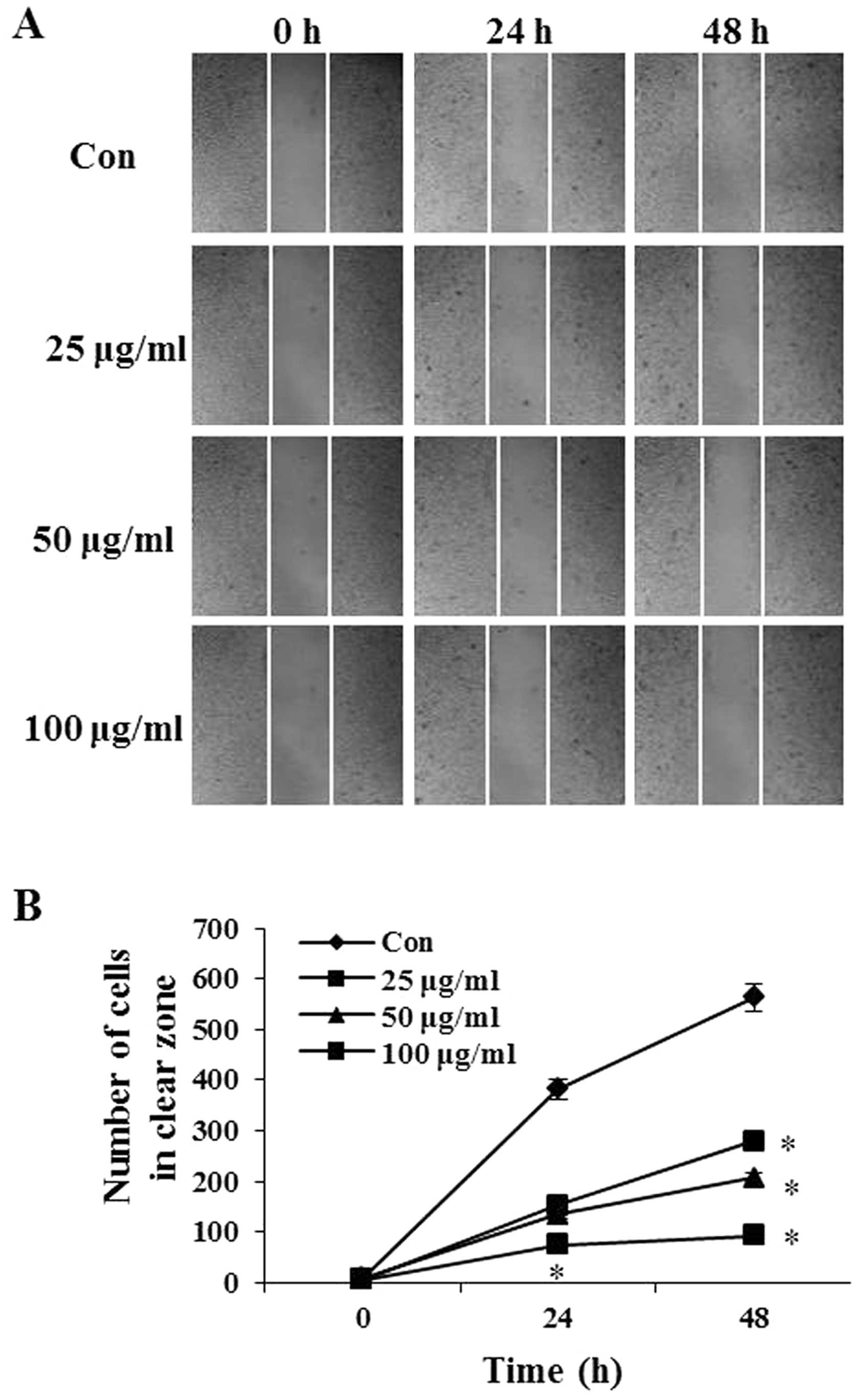
The effect of PIO on B16-F10 cell migration was
determined using the wound-healing assay in which cells were
stimulated to migrate by physical wounding cells. As shown in
Fig. 2A, when cells were treated
with PBS for 24 and 48 h, an apparent and gradual increase of cells
in the denuded zone was observed under light microscopy. B16-F10
cells treated with 25, 50 and 100 μg/ml of PIO displayed a reduced
ability to migrate and fill the wounded area compared with
untreated cells. The quantitative data in Fig. 2B revealed that PIO significantly
inhibited the migration of B16-F10 cells.
Effect of PIO on migration and invasion
of B16-F10 cells
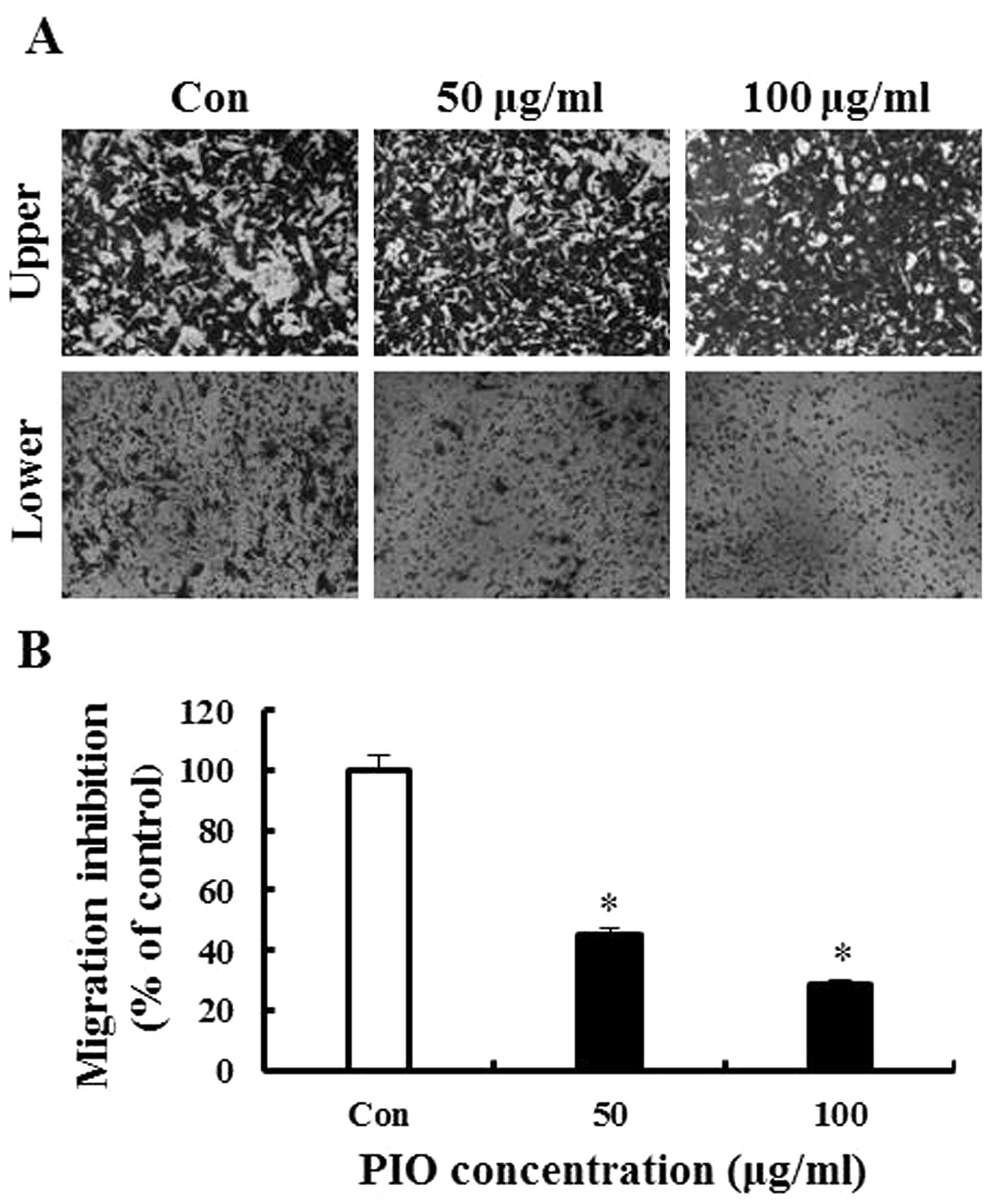
To further evaluate the anti-metastatic activity of
PIO, we assessed the inhibition of B16-F10 melanoma migration and
invasion by PIO using Transwell assay with polycarbonate filters
(pore size, 8 μm) precoated with gelatin or Matrigel. The results
indicated that B16-F10 cells moved from the upper chamber to the
lower chamber in the absence of PIO (control group), suggesting
that B16-F10 melanoma cells can migrate across a Transwell insert
precoated with gelatin. PIO at 50 and 100 μg/ml significantly
inhibited melanoma cell migration by 56 and 72%, respectively
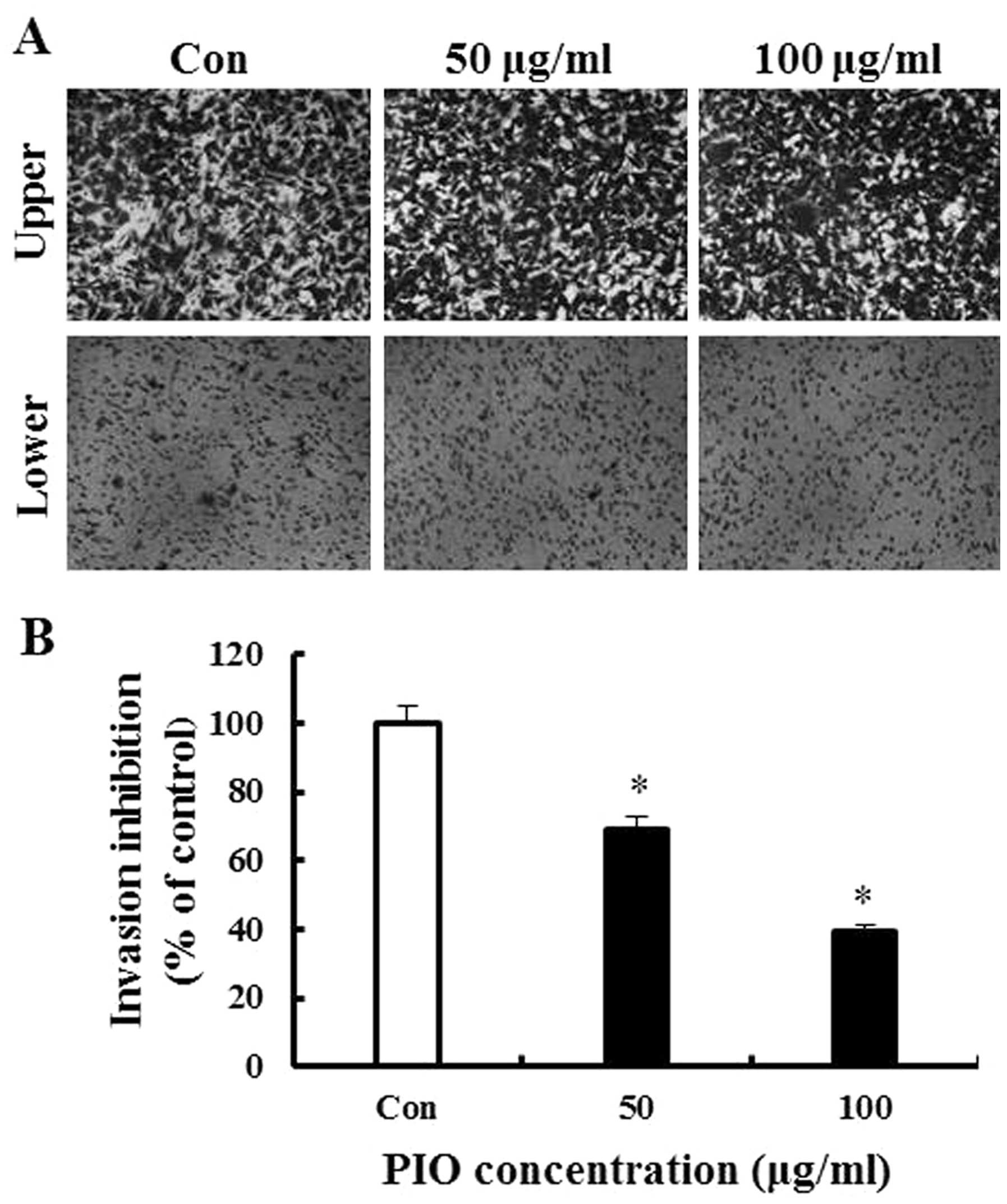
(Fig. 3). As shown in Fig. 4, the results of the invasion assay
illustrated that untreated B16-F10 cells moved from the upper to
the lower chamber, suggesting that B16-F10 cells can invade through
Matrigel-coated Trans-well cell culture chambers. However, the
addition of PIO to the B16-F10 cells resulted in inhibitory effects
on cellular invasion in a concentration-dependent manner. Data in
Fig. 4B indicate that 50 and 100
μg/ml PIO significantly inhibited invasion by 31 and 61%,
respectively. Thus, these results suggested that PIO effectively
reduced melanoma cell migration and invasion.
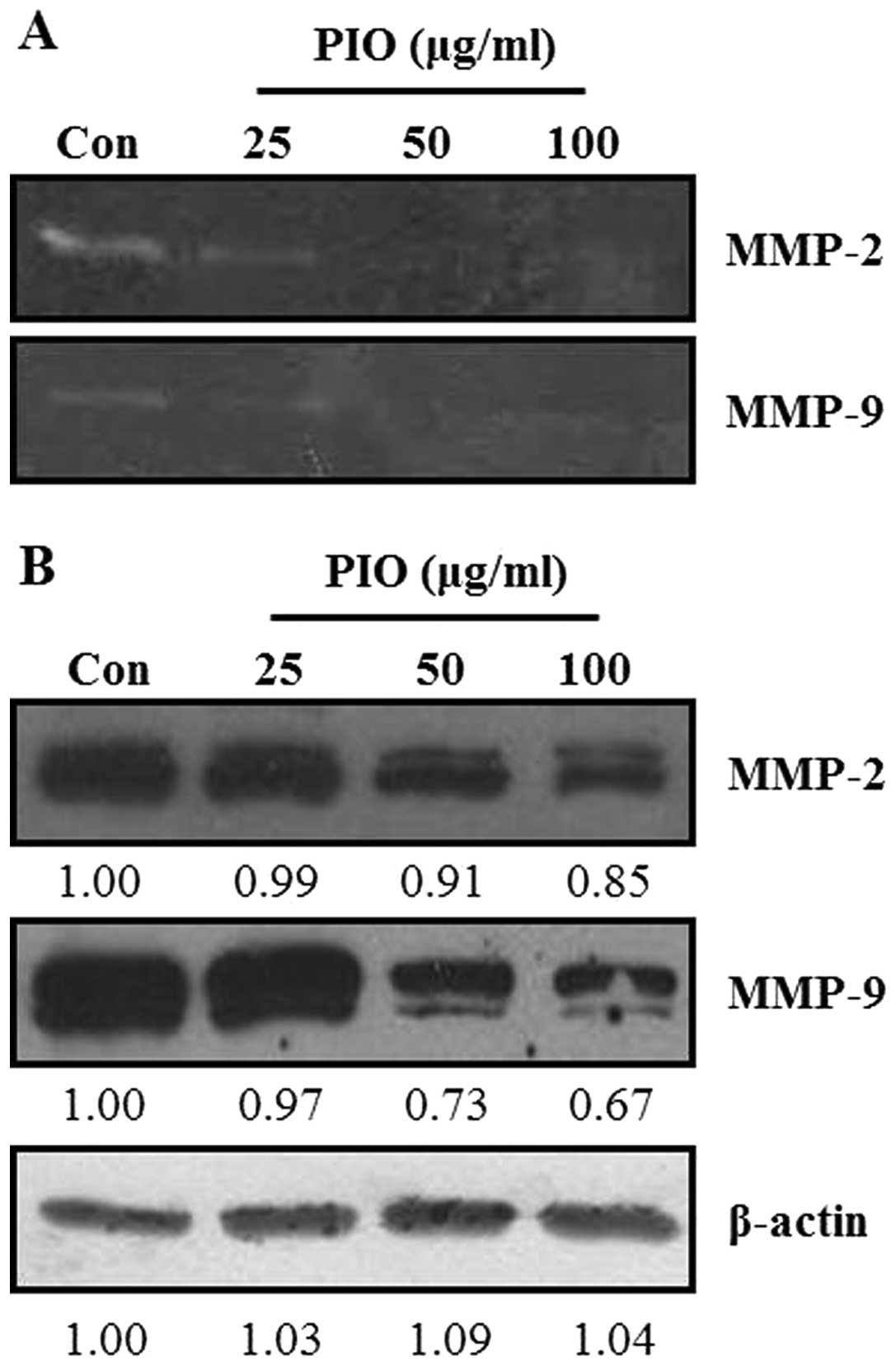
Effect of PIO on the activities and
expression of MMPs of B16-F10 cells
ECM degradation is crucial to cellular invasion,
indicating the inevitable involvement of matrix-degrading
proteinases (27). Therefore, the
effect of PIO on MMP activities was investigated by
gelatin-zymography under a condition of serum starvation to clarify
the contribution of MMPs to the inhibitory effect of PIO on the
invasive ability of cells. As shown in Fig. 5A, gelatin-zymography was used to
analyze the effect of PIO on MMP-2 and MMP-9 activities in B16-F10
melanoma cells. MMP-2 and MMP-9 activities were markedly reduced by
exposure to PIO at concentrations of 25, 50, and 100 μg/ml for 24
h. To further understand the effects of PIO on MMP-2 and MMP-9,
western blot analysis was performed. As shown in Fig. 5B, PIO inhibited the expression of
MMP-2 and MMP-9 in B16-F10 melanoma cells. Therefore, these results
indicated that PIO regulated the expression and activities of MMP-2
and MMP-9.
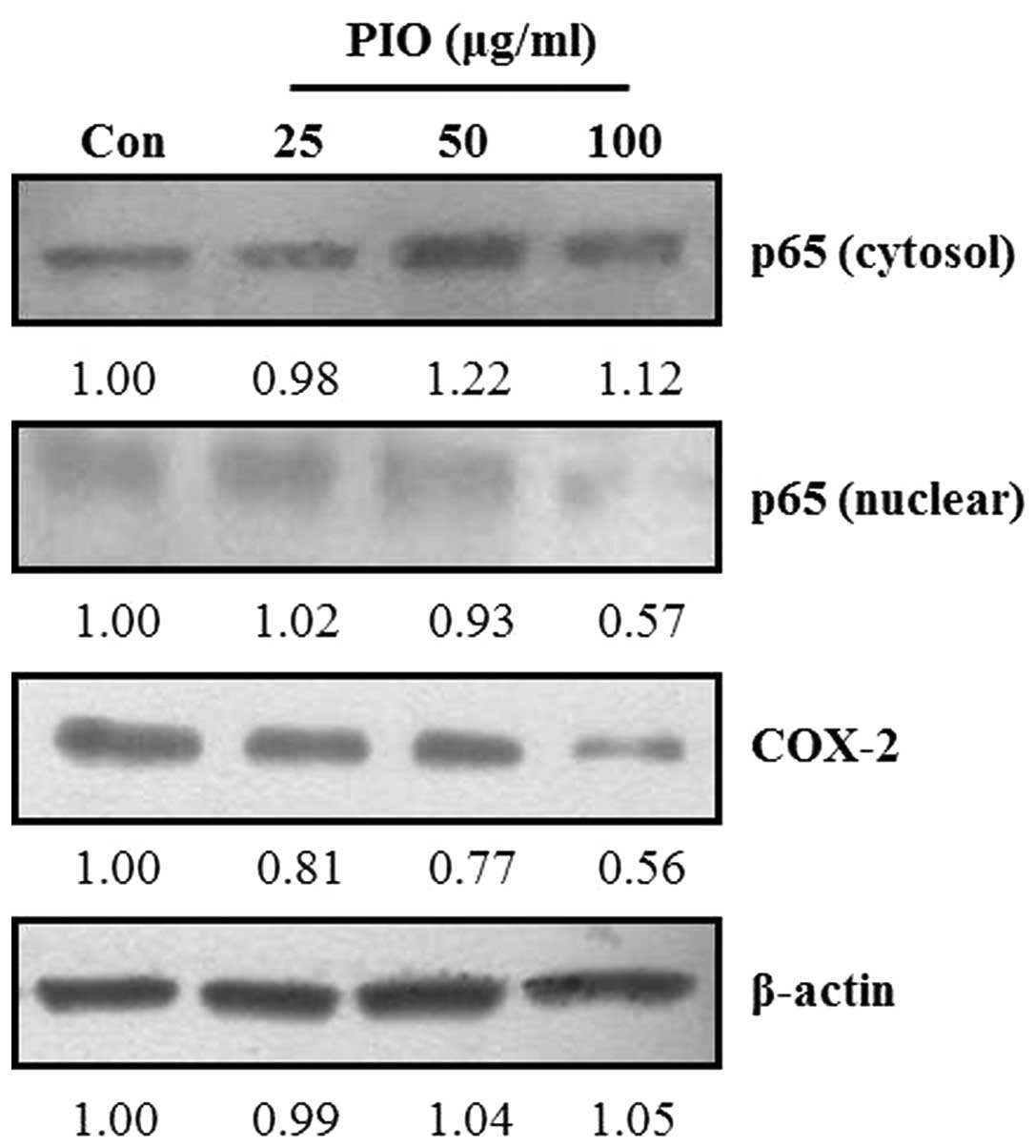
Effect of PIO on NF-κB nuclear
translocation and COX-2 expression levels in B16-F10 cells
Activation of NF-κB in metastatic cancer cells is
involved in the expression of MMP-2 and MMP-9 (28). COX-2 also affects the expression of
MMP-2 and MMP-9 in highly metastatic cancer cells (13). To investigate whether PIO could
regulate the NF-κB signaling pathway in B16-F10 melanoma cells,
cells were treated with the indicated concentrations of PIO. The
translocation of NF-κB and COX-2 expression levels were determined
by western blot analysis. As shown in Fig. 6, the total cytosolic NF-κB protein
levels in B16-F10 cells was increased by PIO treatment compared
with that in untreated cells. By contrast, the protein levels of
NF-κB in the nucleus of B16-F10 cells markedly decreased after PIO
treatment compared with the control levels. Moreover, we found that
the expression levels of COX-2 in PIO-treated B16-F10 cells were
markedly lower than those of the untreated control. These data
indicated that PIO inhibited NF-κB activation and COX-2 expression
in melanoma cancer cells.
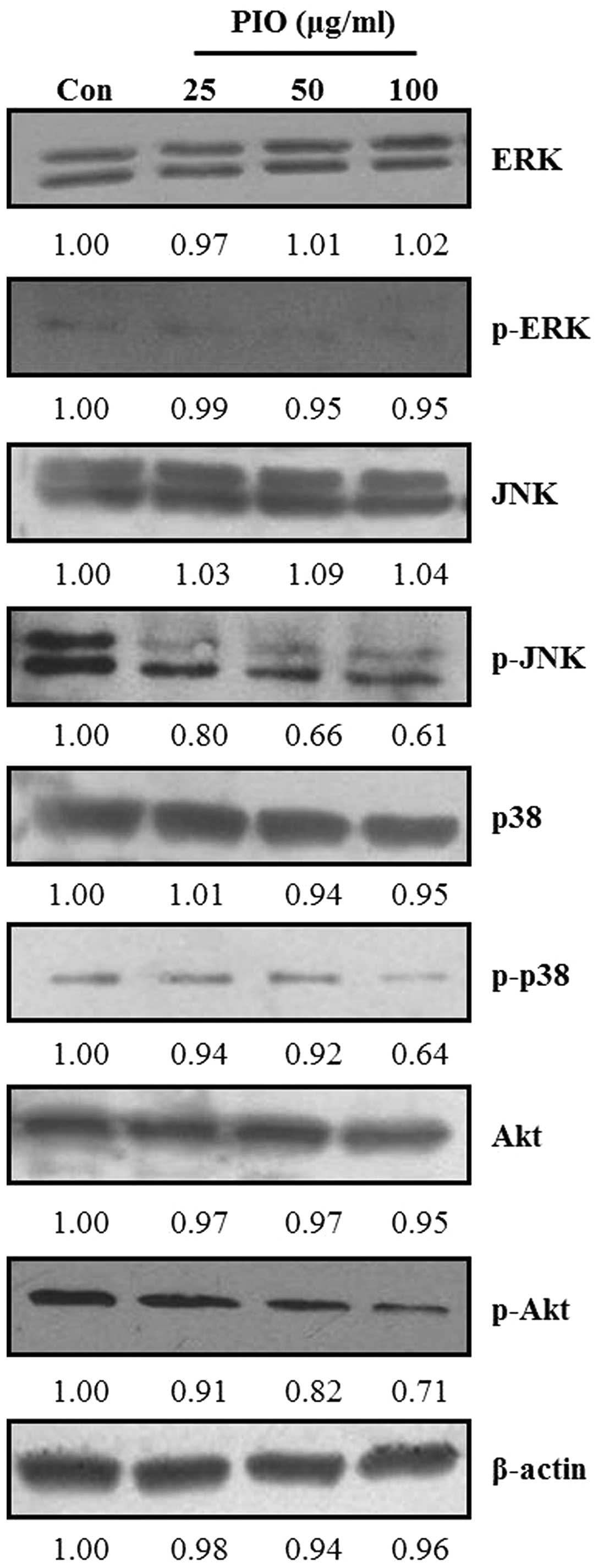
Effect of PIO on MAPK and Akt signaling
pathways in B16-F10 cells
Recent studies reported that MAPK and PI3K/Akt
signaling pathways are involved in cancer cell migration and
invasion (29). MAPKs and Akt have
been demonstrated to be involved in MMP induction in various tumor
types (30). To examine whether PIO
could regulate MAPK and Akt signaling pathways in B16-F10 melanoma
cells, we analyzed the phosphorylation levels of all three MAPKs
(ERK, JNK and p38 MAPK) and Akt by western blot analysis using
respective anti-phospho MAPK and Akt mAbs in B16-F10 cells after
treatment with PIO (25, 50 and 100 μg/ml) for 24 h. As shown in
Fig. 7, PIO did not affect the
expression levels of all three MAPKs and Akt, but PIO suppressed
the phosphorylation levels of ERK, JNK, p38 MAPK and Akt in
comparison with the findings in the untreated control. In
particular, the phosphorylation levels of JNK, p38 MAPK and Akt
were highly inhibited by the addition of PIO at concentrations of
50 and 100 μg/ml. These data indicated that PIO inhibited the
phosphorylation of MAPKs and Akt in melanoma cells.
Discussion
It is well known that tumor metastasis occurs in
many steps including vessel formation, cell attachment, adhesion,
migration, invasion and cell proliferation (31). These events are regulated by an
extremely complex mechanism. Therefore, considerable attention is
focused toward developing agents or drugs that can inhibit
metastasis; however, anti-metastatic agents are still lacking.
Numerous reports have revealed that the inhibition of MMP
expression and/or inhibition of the activities of MMP enzymes can
prevent cancer metastasis (32,33).
MMP-2 (72-kDa gelatinase A) and MMP-9 (92-kDa gelatinase B) are
involved in the invasive metastatic potential of tumor cells. These
MMPs are also associated with COX-2, the activity and expression of
which may modulate the expression and activity of MMPs (34). In the present study, we investigated
the effects of PIO on the migration and invasion of the highly
metastatic B16-F10 mouse melanoma cell line in vitro. Our
results from wound healing, migration and invasion assays also
demonstrated that PIO inhibited the migration and invasion of
B16-F10 cells (Figs. 3 and 4). In addition, the results indicated that
PIO can suppress the expression and activities of MMP-2 and MMP-9,
which facilitate the degradation of ECM and play important roles in
cancer cell migration and invasion (Fig. 5). These findings demonstrated that
the anti-metastatic effects of PIO were associated with the
inhibition of enzymatic degradation processes in metastatic B16-F10
cells and suggest that PIO may be efficacious at preventing the
metastasis of cancer cells. We found that PIO also regulated
potential signaling pathways related to the migration and invasion
of highly metastatic cancer cells (Fig.
7). PIO inhibited the phosphorylation of MAPKs. The MAPK
signaling pathway was found to promote tumor invasion and
metastasis in B16-F10 cells. We also found that PIO inhibited the
phosphorylation of Akt in B16-F10 cells, indicating that PIO could
inhibit the Akt signaling pathway. It was reported that PI3K
activation stimulated the downstream target AKT, which is
associated with cell invasion. It is well documented that the
PI3K-Akt pathway plays important roles in the invasive properties
of cancer cells (35). Previous
reports demonstrated that the MMP-2 and MMP-9 promoter has several
transcription factor-binding motifs including those for NF-κB and
AP-1. Multiple pathways leading to the activation of NF-κB and AP-1
binding factors in tumor cells may contribute to MMP-2 and MMP-9
transcription and enhance invasiveness (36). In the present study, we found that
PIO regulated NF-κB translocation from the cytosol to the nucleus
and the expression and activities of MMP-2 and MMP-9 in B16-F10
cells in vitro. Thus, PIO may suppress the mRNA expression
of MMP-2 and MMP-9 via RNA transcription factors. These findings
indicate that PIO strongly inhibits the metastasis of B16-F10 cells
by inhibiting NF-κB translocation. Then, PIO reduces the
transcription and translation of MMP-2 and MMP-9, thereby
decreasing the activities of MMP-2 and MMP-9. These results suggest
that PIO inhibited the expression and activities of MMP-2 and MMP-9
by inhibiting the NF-κB signaling pathway. Evidence indicates that
COX-2 plays an important role in the carcinogenesis and progression
of cancer, and the induction of MMP-2 and MMP-9 expression may be a
mechanism by which COX-2 promotes the development and metastasis of
cancer (37). The present study
demonstrated that PIO markedly reduced in vitro COX-2, MMP-2
and MMP-9 expression in B16-F10 cells. Furthermore, migration and
invasion data confirmed that PIO significantly inhibited the
migration and invasion of B16-F10 cells. In conclusion, MMPs play
an important role in tumor metastasis. Therefore, it is well
established that MMP gene expression and enzymatic activity are
early targets for preventing cancer metastasis. The present study
suggested that PIO inhibits the migration and invasion of highly
metastatic B16-F10 cells, which may mainly result from the
PIO-mediated inhibition of the expression and activities of MMP-2
and MMP-9 via the suppression of Akt, MAPKs NF-κB and COX-2
signaling pathways.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (NRF-2011-0011522).
Abbreviations:
|
ECM
|
extracellular matrix
|
|
ERK
|
extracellular signal-regulated protein
kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MMP
|
matrix metalloproteinase
|
|
NF-κB
|
nuclear factor κB
|
|
COX
|
cyclooxygenase
|
|
Akt
|
protein kinase B
|
References
|
1
|
Monks NR, Biswas DK and Pardee AB:
Blocking anti-apoptosis as a strategy for cancer chemotherapy:
NF-κB as a target. J Cell Biochem. 92:646–650. 2004.PubMed/NCBI
|
|
2
|
Walsh V and Goodman J: Cancer
chemotherapy, biodiversity, public and private property: the case
of the anti-cancer drug taxol. Soc Sci Med. 49:1215–1225. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosenberg SA and Dudley ME: Cancer
regression in patients with metastatic melanoma after the transfer
of autologous antitumor lymphocytes. Proc Natl Acad Sci USA.
101(Suppl 2): S14639–S14645. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Solari N, Acquati M, Queirolo P, et al:
Primary melanoma of the esophagus with non-metastatic dark lymph
nodes in a female breast cancer patient. Anticancer Res.
27:2849–2853. 2007.PubMed/NCBI
|
|
5
|
Han JY, Kim HS, Lee SH, Park WS, Lee JY
and Yoo NJ: Immunohistochemical expression of integrins and
extracellular matrix proteins in non-small cell lung cancer:
correlation with lymph node metastasis. Lung Cancer. 41:65–70.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ichikawa Y, Ishikawa T, Tanaka K, Togo S
and Shimada H: Extracellular matrix degradation enzymes: important
factors in liver metastasis of colorectal cancer and good targets
for anticancer metastatic therapy. Nihon Geka Gakkai Zasshi.
102:376–380. 2001.(In Japanese).
|
|
7
|
Liao J, Schneider A, Datta NS and McCauley
LK: Extracellular calcium as a candidate mediator of prostate
cancer skeletal metastasis. Cancer Res. 66:9065–9073. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santos MC, de Souza AP, Gerlach RF,
Trevilatto PC, Scarel-Caminaga RM and Line SR: Inhibition of human
pulpal gelatinases (MMP-2 and MMP-9) by zinc oxide cements. J Oral
Rehabil. 31:660–664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chien CS, Shen KH, Huang JS, Ko SC and
Shih YW: Antimetastatic potential of fisetin involves inactivation
of the PI3K/Akt and JNK signaling pathways with downregulation of
MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell
Biochem. 333:169–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grimm T, Chovanova Z, Muchova J, et al:
Inhibition of NF-κB activation and MMP-9 secretion by plasma of
human volunteers after ingestion of maritime pine bark extract
(Pycnogenol). J Inflamm. 3:12006.
|
|
11
|
Ispanovic E and Haas TL: JNK and PI3K
differentially regulate MMP-2 and MT1-MMP mRNA and protein in
response to actin cytoskeleton reorganization in endothelial cells.
Am J Physiol Cell Physiol. 291:C579–C588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang HW, Wang X, Zong ZH, Huo X and Zhang
Q: AP-1 inhibits expression of MMP-2/9 and its effects on rat
smooth muscle cells. J Surg Res. 157:e31–e37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui D, Zhang X and Fu Y: Expressions of
COX-2 and MMP-2 in nasopharyngeal carcinoma and the their
relationship with lymph node metastasis. Lin Chung Er Bi Yan Hou
Tou Jing Wai Ke Za Zhi. 22:692–694. 2008.(In Chinese).
|
|
14
|
Yildirim Y, Ozyilkan O, Bilezikci B,
Akcali Z and Haberal M: Lack of influence of cyclooxygenese-2
expression in hepatocellular carcinomas on patient survival. Asian
Pac J Cancer Prev. 9:295–298. 2008.PubMed/NCBI
|
|
15
|
Burczyk J, Gawron A, Slotwinska M,
Smietana B and Terminska K: Antimitotic activity of aqueous
extracts of Inonotus obliquus. Boll Chim Farm. 135:306–309.
1996.PubMed/NCBI
|
|
16
|
Kim YO, Han SB, Lee HW, et al:
Immuno-stimulating effect of the endo-polysaccharide produced by
submerged culture of Inonotus obliquus. Life Sci.
77:2438–2456. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YO, Park HW, Kim JH, Lee JY, Moon SH
and Shin CS: Anti-cancer effect and structural characterization of
endo-polysaccharide from cultivated mycelia of Inonotus
obliquus. Life Sci. 79:72–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun JE, Ao ZH, Lu ZM, et al:
Antihyperglycemic and antilipidperoxidative effects of dry matter
of culture broth of Inonotus obliquus in submerged culture
on normal and alloxan-diabetes mice. J Ethnopharmacol. 118:7–13.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang L, Zhang Z and Wang H: Antioxidant
activities of extracts and subfractions from Inonotus
Obliquus. Int J Food Sci Nutr. 60(Suppl 2): 175–184. 2009.
View Article : Google Scholar
|
|
20
|
Van Q, Nayak BN, Reimer M, Jones PJ,
Fulcher RG and Rempel CB: Anti-inflammatory effect of Inonotus
obliquus, Polygala senega L., and Viburnum
trilobum in a cell screening assay. J Ethnopharmacol.
125:487–493. 2009.
|
|
21
|
Lee JS, Kwon JS, Won DP, et al: Study of
macrophage activation and structural characteristics of purified
polysaccharide from the fruiting body of Cordyceps
militaris. J Microbiol Biotechnol. 20:1053–1060. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Won DP, Lee JS, Kwon DS, Lee KE, Shin WC
and Hong EK: Immunostimulating activity by polysaccharides isolated
from fruiting body of Inonotus obliquus. Mol Cells.
31:165–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodriguez LG, Wu X and Guan JL:
Wound-healing assay. Methods Mol Biol. 294:23–29. 2005.
|
|
24
|
Kramer N, Walzl A, Unger C, et al: In
vitro cell migration and invasion assays. Mutat Res. 752:10–24.
2013. View Article : Google Scholar
|
|
25
|
George SJ and Johnson JL: In situ
zymography. Methods Mol Biol. 151:411–415. 2001.
|
|
26
|
Hawkes SP, Li H and Taniguchi GT:
Zymography and reverse zymography for detecting MMPs, and TIMPs.
Methods Mol Biol. 151:399–410. 2001.PubMed/NCBI
|
|
27
|
Artym VV, Yamada KM and Mueller SC: ECM
degradation assays for analyzing local cell invasion. Methods Mol
Biol. 522:211–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Philip S, Bulbule A and Kundu GC: Matrix
metalloproteinase-2: mechanism and regulation of NF-kappaB-mediated
activation and its role in cell motility and ECM-invasion.
Glycoconj J. 21:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hour MJ, Tsai SC, Wu HC, et al: Antitumor
effects of the novel quinazolinone MJ-33: Inhibition of metastasis
through the MAPK, AKT, NF-κB and AP-1 signaling pathways in DU145
human prostate cancer cells. Int J Oncol. 41:1513–1519.
2012.PubMed/NCBI
|
|
30
|
Cho SJ, Chae MJ, Shin BK, Kim HK and Kim
A: Akt- and MAPK-mediated activation and secretion of MMP-9 into
stroma in breast cancer cells upon heregulin treatment. Mol Med
Rep. 1:83–88. 2008.PubMed/NCBI
|
|
31
|
Bartolome RA, Barderas R, Torres S, et al:
Cadherin-17 interacts with α2β1 integrin to regulate cell
proliferation and adhesion in colorectal cancer cells causing liver
metastasis. Oncogene. April 22–2013.(Epub ahead of print).
|
|
32
|
Cheng Y, Dong Q, Sun LR, Yang CM and Jiang
BX: Correlation between expression of MMP-2, MMP-9, TIMP-2, TIMP-1
and metastasis of neuroblastoma. Zhonghua Zhong Liu Za Zhi.
27:164–166. 2005.(In Chinese).
|
|
33
|
Taras D, Blanc JF, Rullier A, et al:
Pravastatin reduces lung metastasis of rat hepatocellular carcinoma
via a coordinated decrease of MMP expression and activity. J
Hepatol. 46:69–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoon HY, Lee EG, Lee H, et al: Kaempferol
inhibits IL-1β-induced proliferation of rheumatoid arthritis
synovial fibroblasts and the production of COX-2, PGE2 and MMPs.
Int J Mol Med. 32:971–977. 2013.
|
|
35
|
Shukla S, Maclennan GT, Hartman DJ, Fu P,
Resnick MI and Gupta S: Activation of PI3K-Akt signaling pathway
promotes prostate cancer cell invasion. Int J Cancer.
121:1424–1432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park SY, Kim YH, Kim Y and Lee SJ:
Frondoside A has an anti-invasive effect by inhibiting TPA-induced
MMP-9 activation via NF-κB and AP-1 signaling in human breast
cancer cells. Int J Oncol. 41:933–940. 2012.PubMed/NCBI
|
|
37
|
Lipari L, Mauro A, Gallina S, et al:
Expression of gelatinases (MMP-2, MMP-9) and cyclooxygenases
(COX-1, COX-2) in some benign salivary gland tumors. Int J
Immunopathol Pharmacol. 25:107–115. 2012.PubMed/NCBI
|