Introduction
The tumor necrosis factor receptor-associated factor
(TRAF) family, including seven protein members (TRAF1-7), has
emerged as the major signal transducers for the TNF receptor and
interleukin-1 receptor/Toll-like receptor (IL-1R/TLR)
superfamilies. TRAFs collectively play a pivotal role in diverse
biological processes, including immunity, inflammation and
apoptosis (1,2). TRAF2 is unique as an adaptor protein
among the TRAF family members. Numerous studies have shown that
TRAF2 is a critical mediator of NF-κB (3–8), JNK
and p38 pathway activation (6,9,10), and
overexpression of the native TRAF2 gene can activate JNK, p38 and
NF-κB in the absence of extracellular stimuli (7,9,11,12).
microRNAs (miRNAs) are a class of small non-coding
RNAs, 18–25 nucleotides in length, which play important roles in
post-transcriptional gene expression. By binding to complementary
sequences predominantly found in the 3′-UTR of target mRNAs, miRNAs
block the translation or decrease the stability of mRNAs (13–15).
More than 60% of human protein-coding genes are under selective
pressure to maintain pairing to miRNAs, suggesting that most
mammalian mRNAs are conserved targets of miRNAs (16). miRNAs are involved in a variety of
biological processes including tumor cell proliferation,
differentiation and apoptosis (17–20).
They act as either oncogenes or tumor suppressors in various types
of human cancers including breast cancer (21,22).
In our previous study, we found that TRAF2 is
upregulated in breast cancer (23)
and regulates cell apoptosis and proliferation in breast cancer
cell lines MCF-7 and MDA-MB-231 (24). We speculated that aberrant
expression of certain miRNAs contributes to overexpression of the
TRAF2 gene in breast cancer.
In the present study, we identified miR-502-5p as a
direct regulator of TRAF2 in breast cancer. We also confirmed that
miR-502-5p plays a suppressive role in breast cancer through
targeting oncogenic TRAF2, suggesting that miR-502-5p may be a
useful biomarker for the diagnosis and therapy of human breast
cancer.
Materials and methods
Patient tissues and cell lines
Breast cancer and paired normal tissues were
obtained from 30 breast cancer patients at the First Affiliated
Hospital of China Medical University between January 2010 and July
2012 with informed consent. The study was approved by the First
Affiliated Hospital Ethics Review Committee. Verification of the
specimens was performed by a pathologist, and the samples were
immediately frozen at −80°C after being removed from the patients.
The human normal breast cell line MCF-10A and two human breast
cancer cell lines MCF-7 and MDA-MB-231 were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
HEK293 (embryonic kidney) cell line was purchased from the Cell
Biology Institute of Shanghai, Chinese Academy of Science
(Shanghai, China).
Cell culture
MCF-10A cells were cultured in DMEM/F12 (1:1)
(HyClone, Logan, UT, USA) supplemented with 5% equine serum
(HyClone), 10 μg/ml insulin and 20 ng/ml EGF. MDA-MB-231 cells were
cultured in L15 (HyClone) supplemented with 10% FBS (HyClone) and
100 units of penicillin-streptomycin. MCF-7 and HEK293 cells were
routinely cultured in DMEM supplemented with 10% FBS and 100 units
of penicillin-streptomycin. All of the cell lines were maintained
at 37°C with 5% CO2 in a humidified incubator.
Gene transfection
Small RNAs including a mimic negative control,
miR-502-5p mimic, inhibitor negative control and miR-502-5p
inhibitor were from Shanghai Genepharma Co., Ltd. (Shanghai,
China). MCF-7 and MDA-MB-231 cells were transfected with the miRNA
duplex at a final concentration of 20 nM using Lipofectamine™ 2000
in accordance with the manufacturer’s procedure. In addition, a
TRAF2 small interfering RNA (siRNA) designed and synthesized by
Shanghai Genepharma and the hTRAF2pLPCX-HA-Flag/P874 plasmid
(Addgene plasmid 20229) were purchased from Addgene (Cambridge, CA,
USA) and were transfected into MCF-7 cells. The sequences of the
TRAF2 siRNA were 5′-GGA CCA AGA CAA GAU UGA ATT-3′ (sense) and
5′-UUC AAU CUU GUC UUG GUC CTT-3′ (antisense).
Detection of transcriptional
expression
Total RNA was extracted from the specimens and cell
lines using TRIzol (Takara Bio, Dalian, China) according to the
manufacturer’s instructions. microRNA was separated using a miRcute
miRNA isolation kit (Tiangen Biotech, Bejing, China) and then
measured by reading the absorbance at OD260/280 nm. qRT-PCR was
carried out using the ABI 7500 Real-Time PCR system (Applied
Biosystems, Foster City, CA, USA) to test the expression of
miR-502-5p in the breast cancer tissues and cell lines. For the
detection of mature miR-502-5p, reverse transcription and
quantitative PCR were performed using the One Step PrimeScript
miRNA cDNA Synthesis kit (Takara) and SYBR® Premix Ex
Taq™ II (Takara). U6 small nuclear RNA (snRNA) expression was
assayed for normalization. A miR-502-5p specific primer and a
universal reverse primer RTQ-UNIr were used for the amplification.
Primer sequences for miR-502-5p and RTQ-UNIr were: 5′-ATC CTT GCT
ATC TGG GTG CTA-3′ and 5′-CGA ATT CTA GAG CTC GAG GCA GGC GAC ATG
GCT GGC TAG TTA AGC TTG GTA CCG AGC TCG GAT CCA CTA GTC C(T)-3′,
respectively. Primer sequences for U6 were: F-5′-CTC GCT TCG GCA
GCA CA-3′ and R-5′-AAC GCT TC A CGA ATT TGC GT-3′. The PCR
conditions for miR-502-5p and U6 snRNA consisted of 95°C for 30
sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec.
ΔCt was calculated by subtracting the Ct of U6 mRNA from the Ct of
the RNAs of interest. ΔΔCt was then calculated by subtracting the
ΔCt of the negative control from the ΔCt of the samples. The
fold-change in microRNA was calculated according to the equation RQ
= 2−ΔΔCt.
Flow cytometry-based apoptosis assay
Cells were grown in 6-well plates to ~60% confluency
and transiently transfected with miRNAs, TRAF2 siRNA and the
hTRAF2pLPCX-HA-Flag/P874 plasmid, respectively. The cells were
digested and collected at 48 h post-transfection, and then washed
with 1X PBS twice. For detection of apoptosis, the cells were
treated by Annexin V-EGFP apoptosis detection kit according to the
manufacturer’s instructions (Nanjing KeyGen Biotech. Co., Ltd.,
Nanjing, China) and then analyzed with a flow cytometer
(FACScalibur; Becton-Dickinson, Franklin Lakes, NJ, USA).
In vitro MTT and colony formation
assays
For the MTT assay, 4–5×103 of the MCF-7
and MDA-MB-231 cells at 24 h post-transfection were plated into
96-well plates. Cells were then cultured for 1, 2, 3, 4 and 5 days,
respectively. The absorbance at 570 nm was measured after
incubation of the cells with 100 μl sterile MTT dye (0.5 mg/ml;
Sigma) for 4 h at 37°C and 150 μl DMSO for 15 min. The cell growth
curve was then constructed using OD570 nm as the ordinate axis. In
the colony formation assay, 3–5×103 of the MCF-7 and
MDA-MB-231 cells at 24 h post-transfection were seeded in 60-mm
Petri dishes and allowed to grow until visible colonies formed.
After 10–12 days, the colonies were fixed with methanol, stained
with hematoxylin and scored using a microscope.
Luciferase reporter assay
Prediction of TRAF2 as a target of miR-502-5p was
made with TargetScan (http://www.targetscan.org/), miRanda (http://cbio.mskcc.org/cgi-bin/mirnaviewer/mirnaviewer.pl?type=miRanda)
and miRDB (http://mirdb.org/miRDB/),
respectively. Wild-type pGL3-TRAF2-3′UTR (wt-pGL3-TRAF2-3′UTR) and
mutant pGL3-TRAF2-3′UTR (mut-pGL3-TRAF2-3′UTR) plasmids were
obtained from Shanghai Genechem Co., Ltd. (Shanghai, China). The
HEK293 and MCF-7 cells seeded in 96-well plates in triplicate were
cotransfected with wt-pGL3-TRAF2-3′UTR or mut-pGL3-TRAF2-3′UTR and
miRNA-502-5p mimic or mimic negative control using Lipofectamine™
2000 in accordance with the manufacturer’s procedure. The pRL-TK
(Promega Corp., Madison, WI, USA) was transfected as a
normalization control. Cells were collected at 24 h
post-transfection, and luciferase activity was then measured using
a dual-luciferase reporter assay kit (Promega) and recorded by a
chemiluminescence meter (Promega).
Western blot assay
Protein was extracted from the cells at 48 h
post-transfection using a protein extraction reagent (Beyotime
Institute of Biotechnology, Shanghai, China), and the protein
concentration was measured using the BCA protein assay kit
(Beyotime, USA). Extracts (50 μg) were separated on 10% SDS-PAGE
and transferred to a PVDF membrane. The membrane was then blocked
with 5% non-fat milk and incubated with the anti-TRAF2 antibody
(1:200 dilution; BD Biosciences, Franklin Lakes, NJ, USA) followed
by horseradish peroxidase-conjugated antibody (1:2,000 dilution;
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China).
Detection was performed by enhanced chemiluminescence (ECL) using a
Western blotting immunological reagent (Santa Cruz Biotechnology)
according to the manufacturer’s instructions. β-actin and GAPDH
were used as internal controls and determined following the same
procedure as above.
Statistical analysis
Data were subjected to statistical analysis using
the SPSS 17.0 software and are shown as means ± SD. Each experiment
was performed for a minimum of three times. For qRT-PCR, the
expression level of miR-502-5p was log2 transformed in breast
cancer and paired normal tissues, respectively. A paired-samples
t-test was used to analyze differences in miR-502-5p expression
between the breast cancer and paired normal tissues. The results of
the cell-based experiments were analyzed by an independent sample
t-test and one-way ANOVA. P<0.05 was considered to indicate a
statistically significant result.
Results
miR-502-5p is lowly expressed in breast
cancer tissues and cell lines
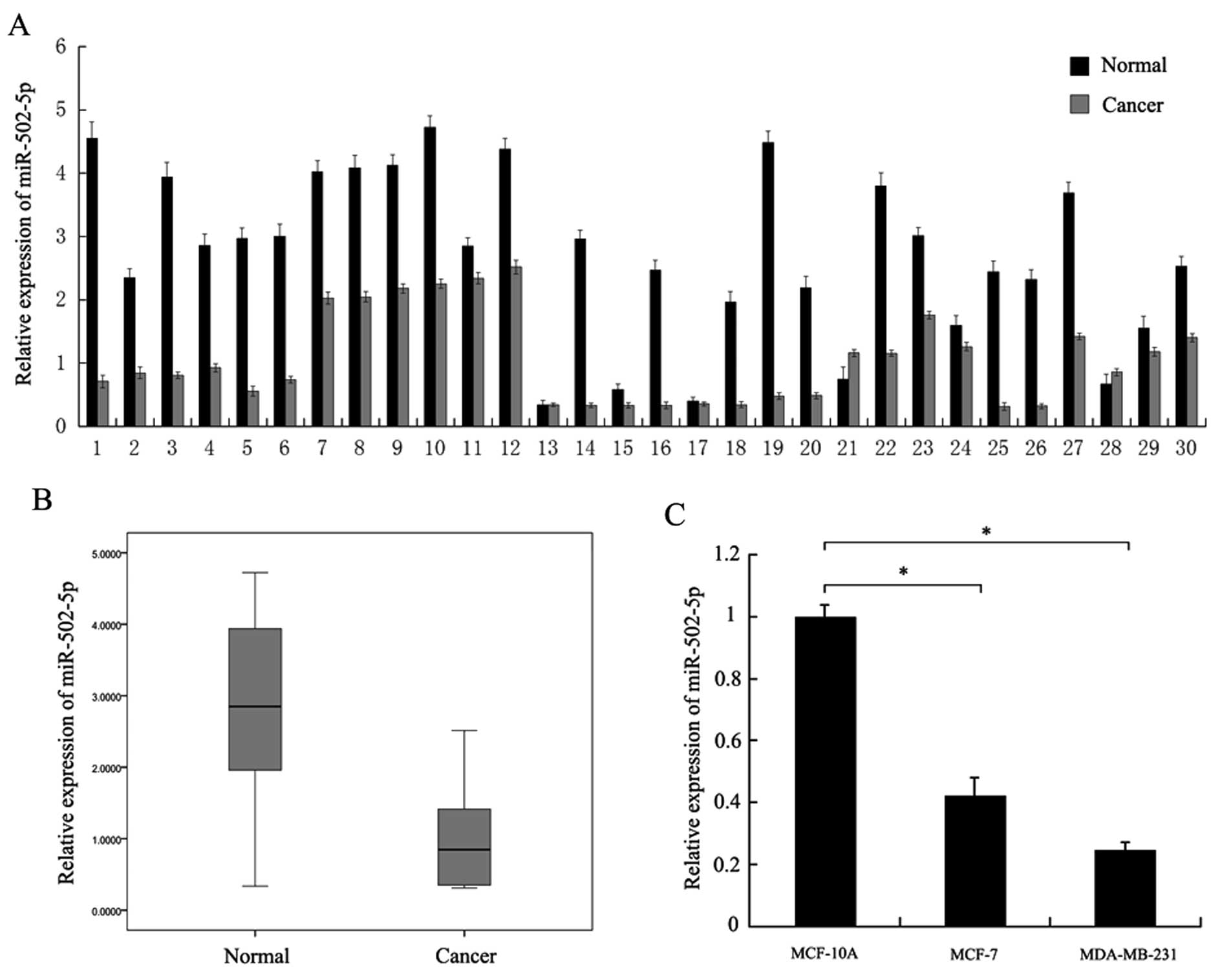
We detected expression of miR-502-5p by qRT-PCR in
30 breast cancer tissue samples with paired normal tissues.
Compared with the normal tissues, the expression of miR-502-5p
(26/30) was significantly downregulated in the breast cancer
tissues (Fig. 1A). Downregulation
of miR-502-5p is also shown in the boxplot (Fig. 1B). We then examined miR-502-5p
expression in the breast cancer cell lines MCF-7 and MDA-MB-231
along with the non-malignant breast epithelial cell line MCF-10A.
miR-502-5p expression was lower in the MCF-7 and MDA-MB-231 cells
than that in the MCF-10A cells (Fig.
1C).
miR-502-5p promotes early apoptosis and
inhibits proliferation in breast cancer cells
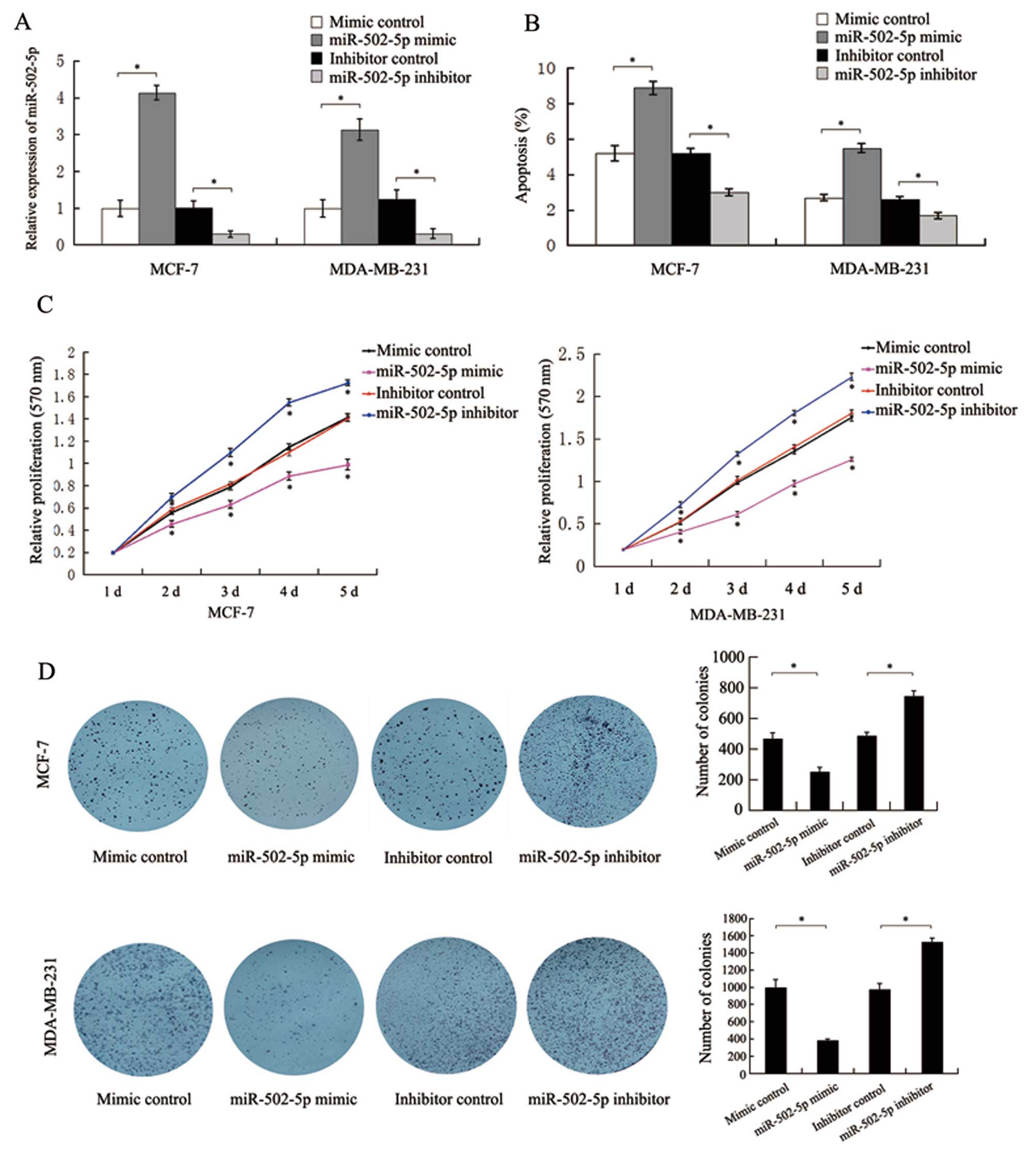
To investigate the impact of miR-502-5p on the
apoptosis and proliferation of breast cancer cells, we evaluated
the transfection efficiency in MCF-7 and MDA-MB-231 cells by
qRT-PCR. At 48 h post-transfection, the expression levels of
miR-502-5p transfected with miR-502-5p mimic and its inhibitor in
MCF-7 and MDA-MB-231 cells were significantly increased and
decreased respectively, when compared to the level in the controls,
(Fig. 2A). Flow cytometric assay
results indicated that the percentages of early apoptotic MCF-7 and
MDA-MB-231 cells in the miR-502-5p mimic or its inhibitor group
were significantly higher or lower, respectively, than the
percentage in the control groups (Fig.
2B). Meanwhile, no significant difference in the cell cycle was
observed in the cells transfected with the miR-502-5p mimic or its
inhibitor (data not shown), indicating that miR-502-5p does not
affect the cell cycle progression in MCF-7 and MDA-MB-231 cells.
MTT assay results demonstrated that the miR-502-5p mimic or its
inhibitor significantly suppressed or enhanced, respectively, the
cell growth of MCF-7 and MDA-MB-231 when compared to the controls
(Fig. 2C). Colony formation assay
results revealed that the miR-502-5p mimic or its inhibitor
significantly suppressed or promoted, respectively, the colony
formation in the MCF-7 and MDA-MB-231 cells when compared to the
controls (Fig. 2D). The above
results suggest that miR-502-5p plays a suppressive role in breast
cancer cells.
TRAF2 is a direct target of
miR-502-5p
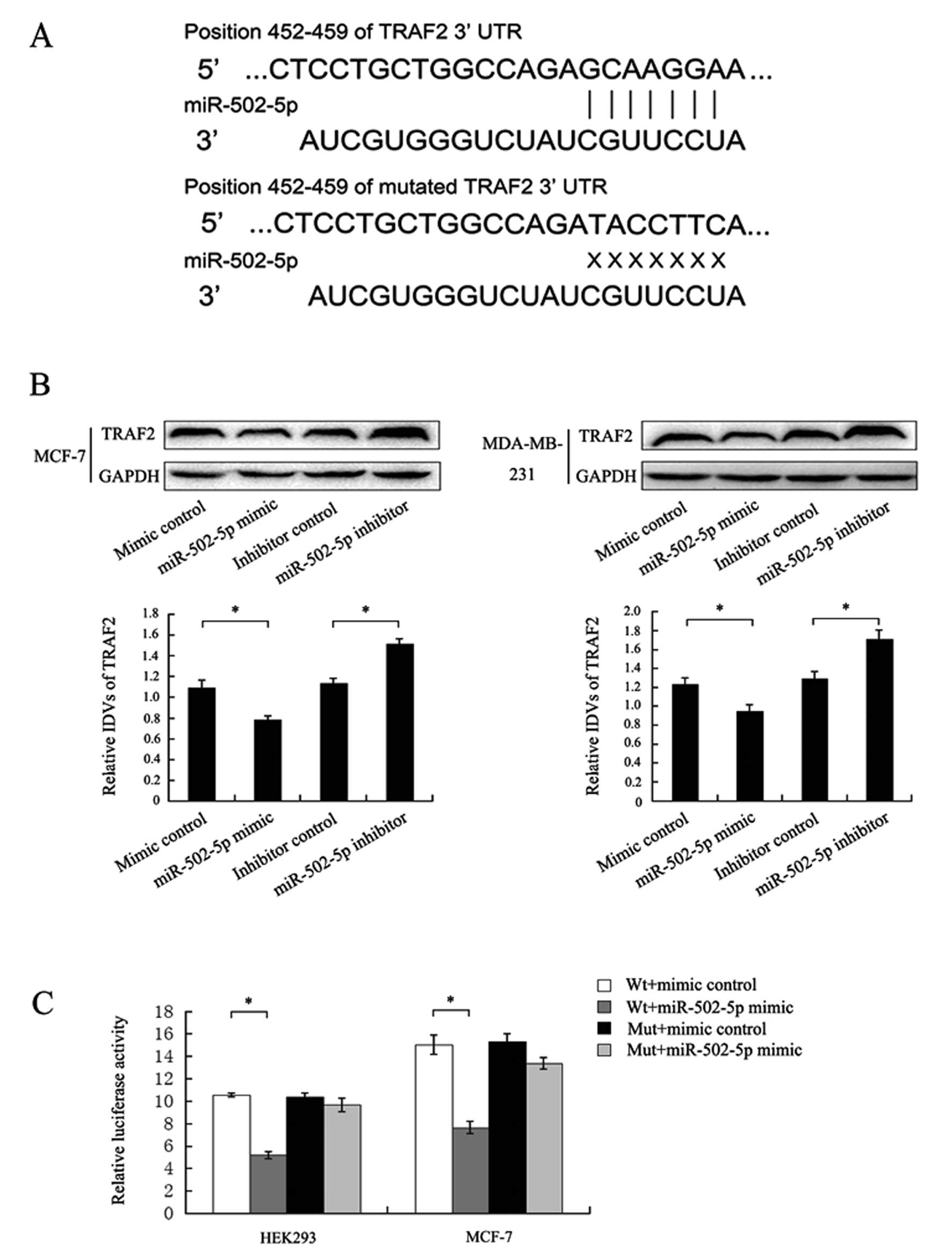
As shown in Fig. 3A,
the 3′-UTR of TRAF2 was found to contain a predicted miR-502-5p
binding site located at 452-459 nt. Western blot assay results
showed that the miR-502-5p mimic or its inhibitor significantly
decreased or increased, respectively, the TRAF2 protein levels when
compared to the levels in the contros MCF-7 and MDA-MB-231 cells
(Fig. 3B), implying that miR-502-5p
regulates the expression of TRAF2. To validate the direct binding
of TRAF2 to miR-502-5p, we constructed wild-type and mutant TRAF2
3′-UTR luciferase reporter vectors. Luciferase reporter assay
results indicated that miR-502-5p significantly inhibited the
luciferase activity when co-transfected with the wild-type vector,
but not when co-transfected with the mutant vector in HEK293 cells
(Fig. 3C). We also obtained the
same results in MCF-7 cells (Fig.
3C). Collectively, these results implied that TRAF2 is one of
the target genes of miR-502-5p and is suppressed by miR-502-5p.
miR-502-5p regulates early apoptosis and
proliferation of breast cancer cells by targeting TRAF2
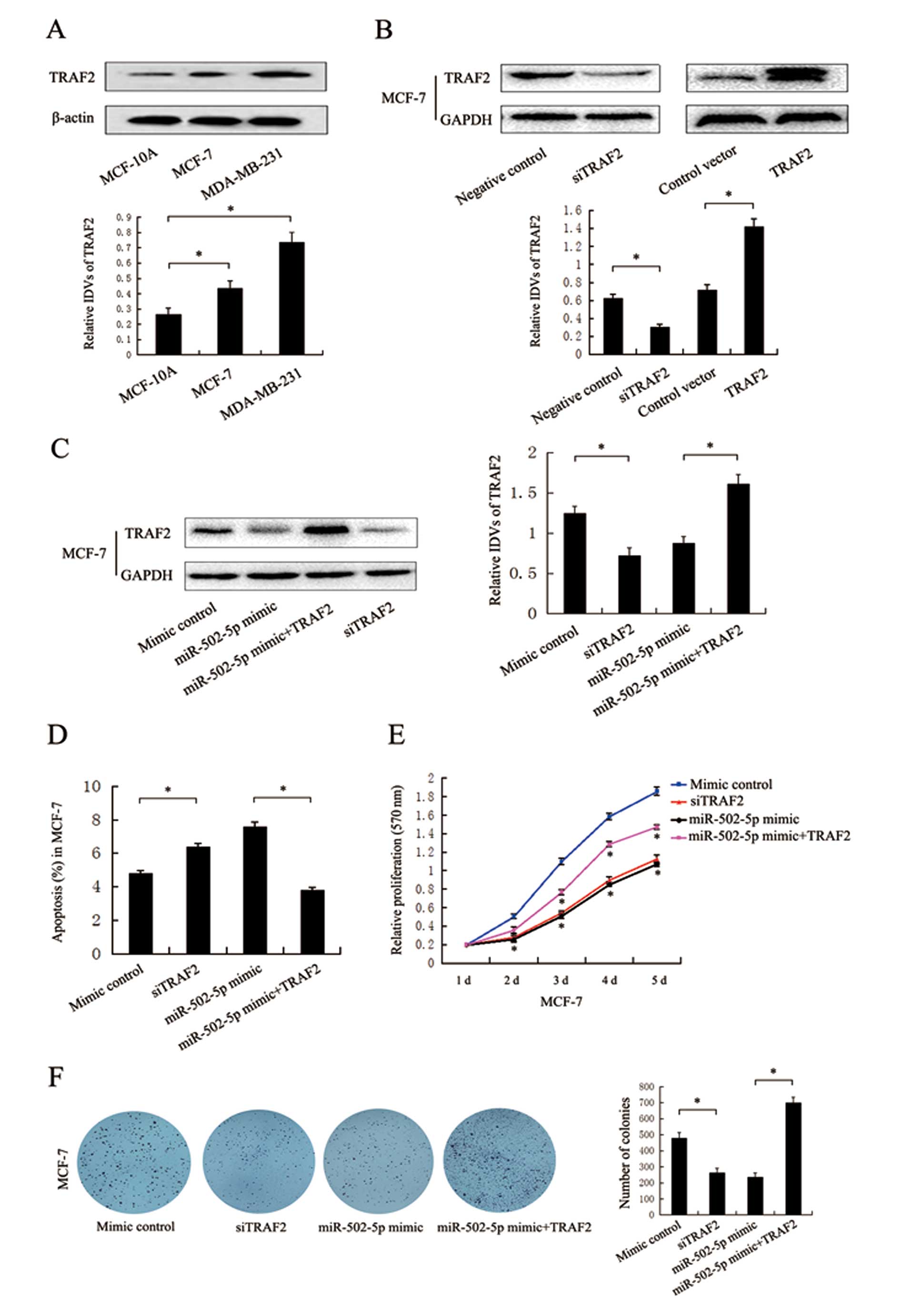
Western blot assay results revealed that TRAF2
protein levels were significantly higher in MCF-7 and MDA-MB-231
cells than that in the MCF-10A cells (Fig. 4A). On the contrary, miR-502-5p
expression levels were significantly lower in MCF-7 and MDA-MB-231
cells than that in the MCF-10A cells (Fig. 1C), suggesting the negative
correlation of miR-502-5p with TRAF2 in breast cancer and normal
cells. To confirm whether miR-502-5p regulates early apoptosis and
proliferation of breast cancer cells by targeting TRAF2, TRAF2
siRNA and the TRAF2 plasmid were used to induce the knockdown and
overexpression, respectively, of TRAF2 expression. Western blot
assay results revealed that TRAF2 siRNA significantly inhibited the
endogenous TRAF2 expression when compared to the expression in the
control group, and the opposite result was found when the TRAF2
plasmid was tranfected into the MCF-7 cells (Fig. 4B). We also found that the TRAF2
plasmid significantly restored the TRAF2 protein level in MCF-7
cells when co-transfected with the miR-502-5p mimic (Fig. 4C). Flow cytometric assay results
indicated that knockdown of TRAF2 significantly increased the early
apoptosis of breast cancer cells when compared to the control group
in the MCF-7 cells (Fig. 4D). MTT
and colony formation assay results showed that knockdown of TRAF2
significantly inhibited cell viability and colony formation when
compared to the control group in the MCF-7 cells (Fig. 4E and F). Overexpression of TRAF2
partly rescued the effects of the miR-502-5p mimic in regard to
early apoptosis, cell viability and colony formation in the MCF-7
cells when co-transfected with the miR-502-5p mimic (Fig. 4D–F). These results suggest that
miR-502-5p may be crucial in the regulation of cell apoptosis and
proliferation through targeting oncogenic TRAF2.
Discussion
Breast cancer is the most commonly diagnosed
malignancy and is the leading cause of cancer-related mortality
among women worldwide (25).
Therefore, searching for valuable biomarkers for the early
diagnosis and treatment of breast cancer remains a focus of breast
cancer research. Recent studies indicate that miRNAs are promising
molecular markers for diseases including cancer due to their
stability in human tissues particularly in blood and urine
(26,27).
miRNA-502 was found to be downregulated in colon
cancer tissues and inhibits autophagy, colon cancer cell growth and
cell-cycle progression, suggesting that it may be a novel candidate
for miR-502-based therapeutic strategies (28). However, little is known concerning
the role of miR-502-5p in other types of cancers including breast
cancer. In the present study, we found that the miR-502-5p
expression level was significantly decreased in breast cancer
tissues and cell lines and that exogenous miR-502-5p promoted early
apoptosis and suppressed the growth and colony formation of breast
cancer cells, but had no effect on the breast cancer cell cycle.
Both studies imply that miR-502 plays a suppressive role in colon
and breast cancer.
In the present study, miR-502-5p was predicted to be
a potential upstream regulator of TRAF2. miR-502-5p was negatively
correlated with TRAF2 expression in breast cancer cells, further
implying that TRAF2 is a candidate target of miR-502-5p. The
miR-502-5p mimic significantly decreased the luciferase activity
when it was cotransfected with wt-pGL3-TRAF2-3′UTR compared to the
controls, indicating that miR-502-5p directly binds to TRAF2. In
addition to TRAF2, SET8 was previously confirmed as another direct
target of miR-502-5p (29).
Similar to the role of the miR-502-5p mimic in the
MCF-7 cells, we also found that TRAF2 siRNA significantly enhanced
the early apoptosis and suppressed cell viability and colony
formation. Meanwhile, overexpression of TRAF2 decreased the effects
of the miR-502-5p mimic in the MCF-7 cells to some extent. Based on
the above results, we speculate that miR-502-5p plays a
tumor-suppressive role in breast cancer by directly targeting
TRAF2.
We previously found that TRAF2 regulates cell
proliferation and apoptosis by activating NF-κB nuclear
transcription by directly binding to TRAF4 in breast cancer
(24). Therefore, we conclude that
miR-502-5p serves as a tumor-suppressor gene via regulation of the
interaction of TRAF2 and TRAF4, leading to inhibition of the
NF-κB-mediated signaling pathway in breast cancer. This suggests
that miR-502-5p may provide a promising therapeutic strategy for
breast cancer treatment.
Acknowledgements
The present study was supported by the Research Fund
for the Doctoral Program of Higher Education (20112104110017).
References
|
1
|
Chung JY, Park YC, Ye H and Wu H: All
TRAFs are not created equal: common and distinct molecular
mechanisms of TRAF-mediated signal transduction. J Cell Sci.
115:679–688. 2002.PubMed/NCBI
|
|
2
|
Xia ZP and Chen ZJ: TRAF2: a double-edged
sword? Sci STKE. 2005:pe72005.PubMed/NCBI
|
|
3
|
Arch RH and Thompson CB: 4-1BB and Ox40
are members of a tumor necrosis factor (TNF)-nerve growth factor
receptor subfamily that bind TNF receptor-associated factors and
activate nuclear factor κB. Mol Cell Biol. 18:558–565.
1998.PubMed/NCBI
|
|
4
|
Duckett CS, Gedrich RW, Gilfillan MC and
Thompson CB: Induction of nuclear factor kappaB by the CD30
receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol.
17:1535–1542. 1997.PubMed/NCBI
|
|
5
|
Hsu H, Shu HB, Pan MG and Goeddel DV:
TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF
receptor 1 signal transduction pathways. Cell. 82:299–308. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reinhard C, Shamoon B, Shyamala V and
Williams LT: Tumor necrosis factor α-induced activation of c-jun
N-terminal kinase is mediated by TRAF2. EMBO J. 16:1080–1092.
1997.
|
|
7
|
Rothe M, Sarma V, Dixit VM and Goeddel DV:
TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40.
Science. 269:1424–1427. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeuchi M, Rothe M and Goeddel DV:
Distinct domains for nuclear factor-κB activation and association
with tumor necrosis factor signaling proteins. J Biol Chem.
271:19935–19942. 1996.
|
|
9
|
Liu ZG, Hsu H, Goeddel DV and Karin M:
Dissection of TNF receptor 1 effector functions: JNK activation is
not linked to apoptosis while NF-κB activation prevents cell death.
Cell. 87:565–576. 1996.PubMed/NCBI
|
|
10
|
Natoli G, Costanzo A, Ianni A, Templeton
DJ, Woodgett JR, Balsano C and Levrero M: Activation of SAPK/JNK by
TNF receptor 1 through a noncytotoxic TRAF2-dependent pathway.
Science. 275:200–203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao Z, Henzel WJ and Gao X: IRAK: a kinase
associated with the interleukin-1 receptor. Science. 271:1128–1131.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song HY, Regnier CH, Kirschning CJ,
Goeddel DV and Rothe M: Tumor necrosis factor (TNF)-mediated kinase
cascades: bifurcation of nuclear factor-kappaB and c-jun N-terminal
kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2.
Proc Natl Acad Sci USA. 94:9792–9796. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jing Q, Huang S, Guth S, et al:
Involvement of microRNA in AU-rich element-mediated mRNA
instabiliby. Cell. 120:623–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He L and Hannon GJ: MicroRNAs: small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lecellier CH, Dunoyer P, Arar K, et al: A
cellular microRNA mediates antiviral defense in human cells.
Science. 308:557–560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sullivan CS, Grundhoff AT, Tevethia S,
Pipas JM and Ganem D: SV40-encoded microRNAs regulate viral gene
expression and reduce susceptibility to cytotoxic T cells. Nature.
435:682–686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pallante P, Visone R, Ferracin M, et al:
MicroRNA deregulation in human thyroid papillary carcinomas. Endocr
Relat Cancer. 13:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. N Engl Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Mi X, Liu N, Fang C, Teng Y and
Zhao R: Expression of TRAF2 and its relationship with invasion in
breast cancer. J China Med Univ. 37:52008.
|
|
24
|
Zhang X, Wen Z, Sun L, Wang J, Song M,
Wang E and Mi X: TRAF2 regulates the cytoplasmic/nuclear
distribution of TRAF4 and its biological function in breast cancer
cells. Biochem Biophys Res Commun. 436:344–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haghighat S, Akbari ME, Ghaffari S and
Yavari P: Standardized breast cancer mortality rate compared to the
general female population of Iran. Asian Pac J Cancer Prev.
13:5525–5528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blondal T, Jensby Nielsen S, Baker A,
Andreasen D, Mouritzen P, Wrang Teilum M and Dahlsveen IK:
Assessing sample and miRNA profile quality in serum and plasma or
other biofluids. Methods. 59:S1–S6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lorenzen JM and Thum T: Circulating and
urinary microRNAs in kidney disease. Clin J Am Soc Nephrol.
7:1528–1533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhai H, Song B, Xu X, Zhu W and Ju J:
Inhibition of autophagy and tumor growth in colon cancer by
miR-502. Oncogene. 32:1570–1579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding C, Li R, Peng J, Li S and Guo Z: A
polymorphism at the miR-502 binding site in the 3′ untranslated
region of the SET8 gene is associated with the outcome of
small-cell lung cancer. Exp Ther Med. 3:689–692. 2012.
|