Introduction
Multiple myeloma (MM) is a malignant monoclonal
plasma cell disorder that is characterized by end-organ damage such
as anemia, hypocalcemia, renal insufficiency or bone lesions
(1,2). The conventional treatment of MM
includes radiation and chemotherapy. Various chemotherapy drugs,
including doxorubicin, thalidomide, lenalidomide and bortezomib
have improved patient outcomes. However, MM remains incurable, and
the median survival time for MM patients is 3–5 years. Therefore,
it is important to continue the search for novel, safe and
effective reagents to treat MM and improve the treatment efficacy
of this fatal disease. The main thrust of the present study was
towards the identification of small bioactive molecules that have
the greatest potential in combating cancer and to understand the
mechanism of action of these bioactive molecules at the molecular
and cellular levels.
Benzimidazole is a heterocyclic aromatic organic
compound that consists of the fusion of benzene and imidazole. This
particular structure can form hydrogen bonds in vivo with
enzymes and receptors that exhibit varied bioactivities.
Benzimidazole derivatives are currently found in many commercial
drugs that are used in the clinical treatment of numerous diseases
(3–6). The anticancer properties of
benzimidazole-based compounds are also extremely valuable. It has
been reported that benzimidazole derivatives induce anticancer
effects in many human cancer cells (7–10). We
identified
2-(1H-benzimidazol-2-yl)-4,5,6,7-tetrahydro-2H-indazol-3-ol
(BMT-1), a benzimidazole derivative, as a potent anti-myeloma drug
from a drug screening library (Fig.
1A). Thus, the focus of the present study was to explore the
potential of the benzimidazole compound BMT-1 with respect to
apoptosis in MM cells.
Apoptosis is a physiological mechanism for
eliminating malignant cells or cancer cells without eliciting
significant damage to normal cells (11). Thus, agents that suppress the
proliferation of malignant cells by inducing apoptosis may
represent a useful mechanistic approach to both chemoprevention and
chemotherapy of cancer. In the present study, we examined the
cytotoxic effect of BMT-1 on human MM cells. Herein, we
demonstrated that BMT-1 induced the apoptosis of MM1.S and MM1.R
human MM cells via a mitochondrial-mediated mechanism with loss of
mitochondrial membrane potential resulting in the activation of
caspase-8 and -9.
To understand the mechanisms of apoptosis induced by
the small molecule BMT-1, the inhibitory effect of BMT-1 on
H+/K+-ATPase was evaluated since BMT-1 is a
benzimidazole derivative. It has already been observed that most
well-known pharmaceuticals containing the benzimidazole core are
H+/K+-ATPase pump or proton pump inhibitors
(PPIs) (12). The
H+/K+-ATPase is often highly expressed in
cancer cells to maintain the reversed pH gradient, including a
constitutively higher intracellular pH (pHi) and a lower
extracellular pH (pHe) to facilitate the indicated adaptive
behaviors (13,14). As dysregulated pH is also an
adaptive feature of most types of cancers, drugs that inhibit
proton pumps in cancer cells may lower the intracellular pH of
cancer cells and slow proliferation and promote apoptosis in
various cancer cell lines (15).
Many human tumors, including melanoma, osteosarcoma, lymphomas and
various adenocarcinomas, are responsive to PPIs (16). Therefore, the objective of the
present study was to investigate BMT-1-induced apoptosis and
explore whether the inhibition of
H+/K+-ATPase is a potential mechanism by
which BMT-1 inhibits MM cell survival.
Materials and methods
Chemicals
BMT-1 was obtained from Chembridge, Inc. (San Diego,
CA, USA). Dimethyl sulfoxide (DMSO; Sigma-Aldrich Co., St. Louis,
MO, USA) was used to dissolve BMT-1.
Antibodies and reagents
Caspase-3, cleaved capase-3, caspase-8, cleaved
capase-8, poly(ADP-ribose) polymerase (PARP) and glyceraldehyde
3-phosphate dehydrogenase (GAPDH) were obtained from Cell Signaling
Technology (Beverly, MA, USA). The
H+/K+-ATPase β-subunit (ATP4B) antibody was
purchased from Abnova (Abnova, Taipei, Taiwan). The mitochondrial
staining kit was purchased from Sigma (Sigma-Aldrich Co.). The
H+/K+-ATPase kit was from Nanjing Jiancheng
Biochemical Institute (Nanjing, China).
Cells culture and treatment
The MM cell lines, MM1.S, MM1.R, RPMI8226 and U-266,
were obtained from the American Type Culture Collection (ATCC)
(Rockville, MD, USA). The cell lines were maintained in RPMI-1640
medium supplemented with 10% (v/v) fetal bovine serum (FBS) (both
from Gibco, Gaithersburg, MD, USA), L-glutamine and antibiotics
(Gibco Laboratories, Grand Island, NY, USA). The MM cell liness
were treated with varying concentrations of BMT-1 for the indicated
times as described in the figure legends.
Measurement of cell growth inhibition and
data analysis
Cell growth inhibition was performed in accordance
with the protocol from the Drug Evaluation Branch, National Cancer
Institute (17,18). Briefly, cells were plated at an
appropriate density in 96-well plates in RPMI-1640 with 10% (v/v)
FBS and allowed to attach overnight. The cells were exposed to
different drug dilutions for 48 h. Using the absorbance
measurements [time zero (Tz), control growth (C) and test growth in
the presence of drug at the four concentrations (Ti)]; the
percentage of growth was calculated at each of the drug
concentration levels. The percentage of growth inhibition was
calculated as: [(Ti − Tz)/(C − Tz)] × 100 for concentrations for
which Ti≥Tz and [(Ti − Tz)/Tz] × 100 for concentrations for which
Ti≤Tz. Growth inhibition of 50% (GI50) was calculated
from [(Ti − Tz)/(C − Tz)] × 100 = 50, which is the drug
concentration resulting in a 50% reduction in net protein
increase.
Viability of lymphocytes and BMT-1
exposure
To detect the effect of BMT-1 in primary cells,
peripheral blood mononuclear cells (PBMCs) were isolated from whole
human blood following informed consent. The cells were treated with
different concentrations of BMT-1 for 72 h. PBMCs were counted and
analyzed using CellQuest software BD Accuri C6 Flow Cytometer and
analyzed using CFlow software (version 1.0.243.1) (both from BD
Accuri, Ann Arbor, MI, USA). The study protocol was approved by the
Institutional Review Board (IRB) of Chengdu Medical College.
Analysis of changes in mitochondrial
membrane potential
Cells (5×105) treated with varying
concentrations of BMT-1 were stained with JC-1 from the
mitochondrial staining kit (Sigma) at 37°C for 20 min following the
manufacturer’s procedures. Changes in cellular mitochondrial
membrane potential were quantified by measuring the fluorescence
intensity emission ratios at 590 nm (red)/525 nm (green) using the
BD Accuri C6 Flow Cytometer and CFlow software (version 1.0.227.4;
BD Accuri). The widely used mitochondrial membrane potential
disruptor valinomycin was used as a positive control.
Cell cycle analysis and detection of
apoptosis
MM cells (5×105) were cultured for 24 h
in media alone or with varying concentrations of BMT-1. The cells
were harvested, washed with ice-cold phosphate-buffered saline
(PBS), fixed with 70% (v/v) ethanol for 2 h, and pretreated with
100 μg/ml RNAse (Sigma) for 1 h. Cells were stained with propidium
iodide (PI) (20 μg/ml; Sigma), and the cell cycle profile was
determined using the BD Accuri C6 Flow Cytometer and CFlow software
(version 1.0.227.4). Dual staining with Fluos-labeled Annexin V and
PI was carried out to detect the induction of apoptotic cell death.
Following treatment of 2×105 cells with a test agent for
24 h, cells were washed with PBS and re-suspended in 100 μl binding
buffer containing Annexin V-Fluos and PI (Annexin V-Fluos staining
kit; Roche). After 15 min of incubation at room temperature, cells
were analyzed on a BD Accuri C6 Flow Cytometer for the presence of
Annexin V-Fluos-positive/PI-negative apoptotic cell
populations.
Western blot analysis
MM cells were treated with varying concentrations of
BMT-1 for the indicated times, as described in the figure legends.
Cells were lysed for 30 min in ice-cold lysis buffer (BioTeke
Corporation, Beijing, China). The cell lysates were cleared by
centrifugation for 15 min at 12,000 × g and used for
immunoprecipitation. Proteins (50 μg/lane) were resolved by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
then transferred to a PVDF membrane. The membranes were blocked
with a 5% (w/v) milk solution for 1 h and incubated in the
following primary antibodies: caspase-3, cleaved capase-3,
caspase-8, cleaved capase-8, PARP and GAPDH.
Evaluation of cytosolic pH
The effects of BMT-1 on cytosolic pH were evaluated
by flow cytometry using the pH-sensitive fluorescent probe BCECF-AM
(Beyotime Institute of Biotechnology, China). Approximately
1×106 cells were incubated at 37°C for 30 min in 1 ml
RPMI containing 1 μmol/l BCECF-AM. The cells were then washed in
Hank’s balanced salt solution (HBSS), placed on ice, and analyzed
with a FACSCalibur equipped with a 488-nm argon laser collecting
the emission of BCECF-AM in the FL1 and FL2 channels (19,20).
The relative cytosolic pH of individual cells was displayed in a
two-dimensional dot-plot showing their fluorescence intensity at
533/30 nm (FL1, base) and 585/40 nm (FL2, acid).
Measurements of ATPase activity in MM
cells
Cells (5×107) were collected by
centrifugation at 600 × g for 5 min at 4°C, and washed with
ice-cold 0.9% (w/v) saline solution. Cells were then homogenized on
ice with a dounce tissue grinder, and the supernatant was
transferred into tubes with final BMT-1 concentrations of 0, 25, 50
and 100 μM. To analyze the activity of
H+/K+-ATPase, the OD value at 660 nm was
detected according to the procedure of the
H+/K+-ATPase kit (Nanjing Jiancheng
Biochemical Institute, Nanjing, China) using the formula: Activity
of H+/K+-ATPase (U/mg-prot) =
(ODtest − ODcontrol)/ODstandard ×
standard concentration (0.5 μmol/ml) × (60 min/10 min) × dilution
ratio (4.8)/protein concentration (mg/ml).
Results
Effect of BMT-1 on MM and normal
cells
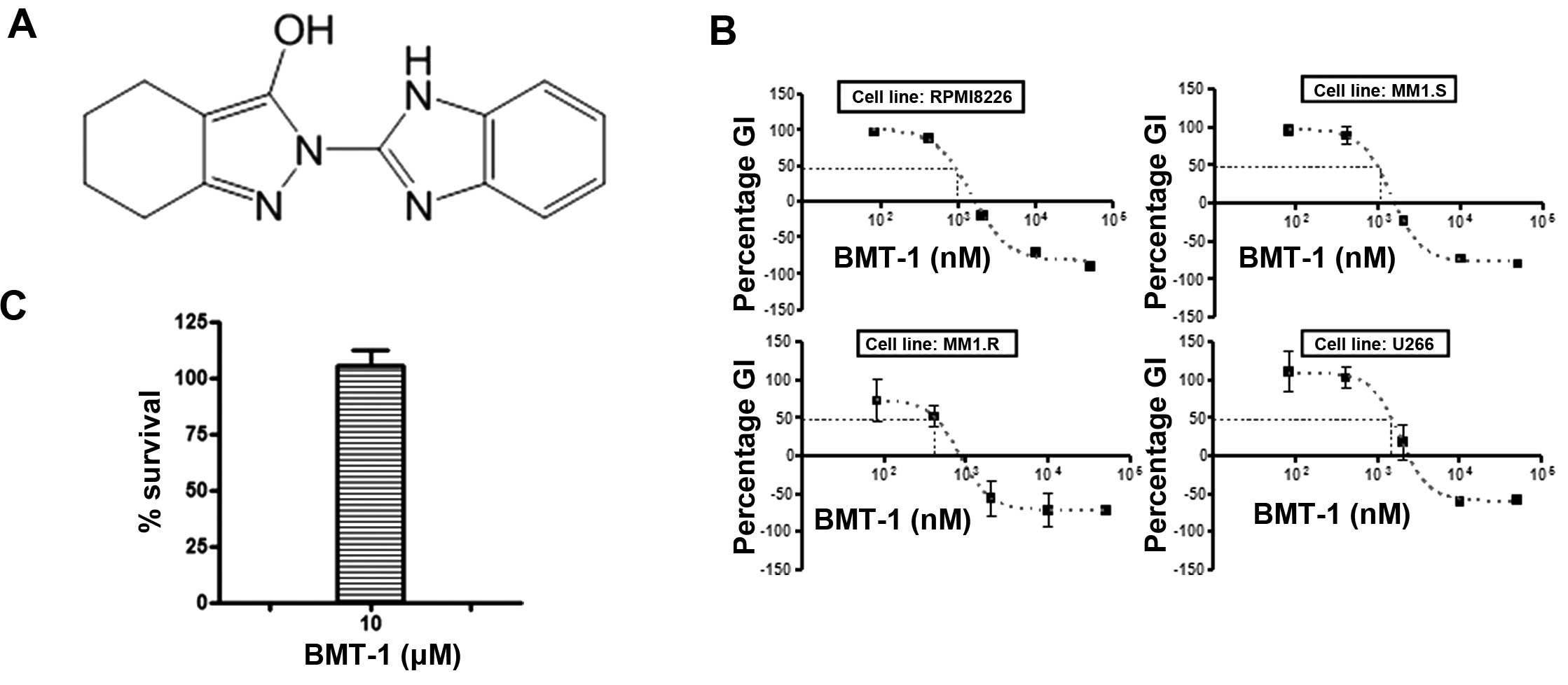
We first examined the growth inhibitory effect of
BMT-1 on MM cell lines. By using the CCK-8 assay, we found that
BMT-1 markedly inhibited the growth of U266, RPMI-8226, MM.1S and
MM.1R cells with IC50 values of 889 to 1476 nM (Fig. 1B). To examine the inhibition of
BMT-1 on cancer cell growth that was not caused by toxicity, we
performed in vitro analyses using cultured PBMCs in the
absence and presence of BMT-1. As shown in Fig. 1C, non-significant lymphotoxicity was
observed after exposure to BMT-1 at a concentration of 10 μM.
Overall, these results suggest that BMT-1 inhibits the growth of MM
cells that is not related to toxicity.
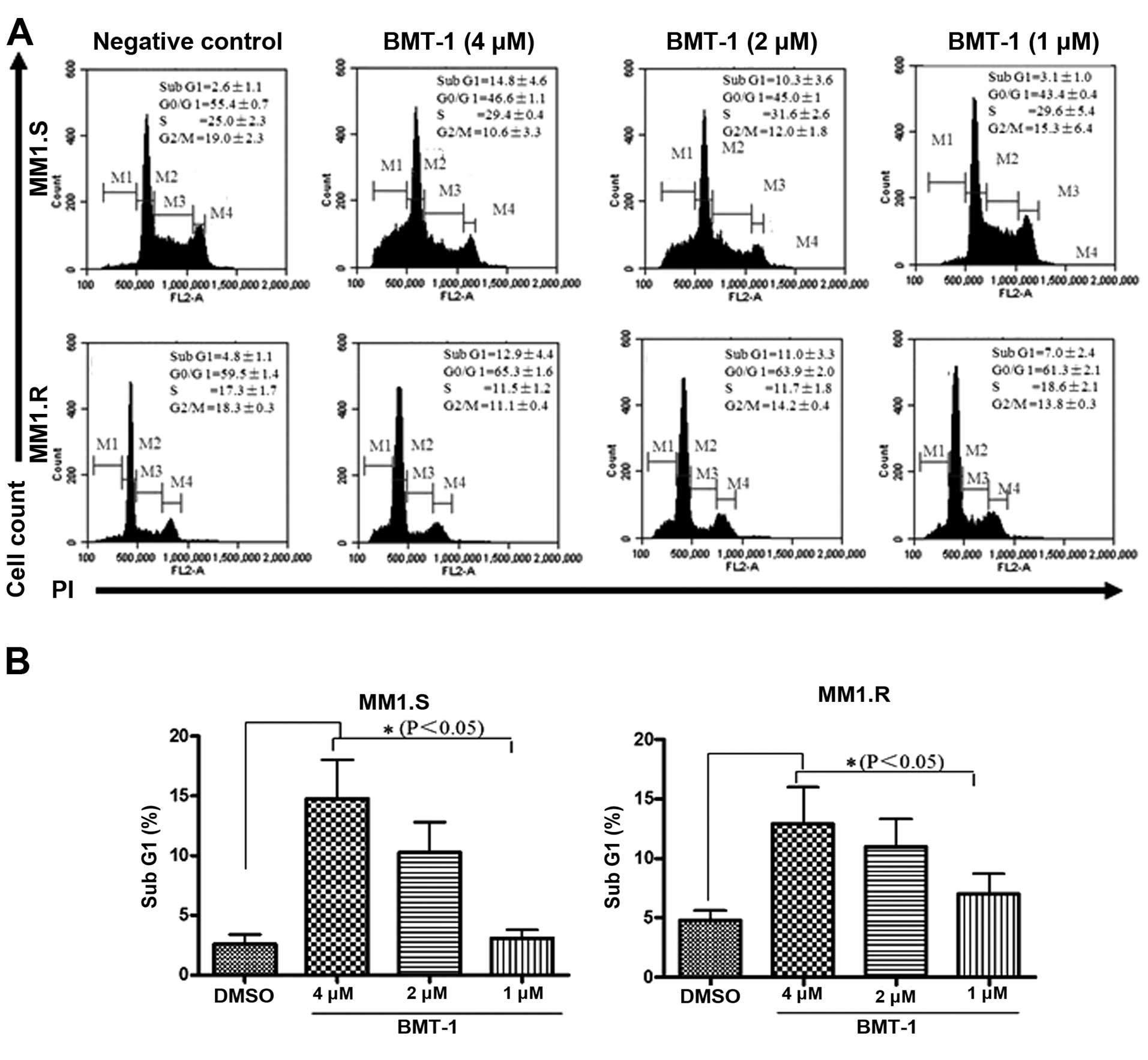
BMT-1 causes accumulation of MM cells in
the sub-G1 phase
To further confirm that BMT-1 inhibits proliferation
of MM cells through induction of cell cycle arrest, we analyzed the
cell cycle distribution after PI staining. The percentage of MM1.S
and MM1.R cells in the sub-G1 (apoptotic cells) phase was
significantly elevated as the concentration of BMT-1 increased. For
example, 10.3±3.6 and 14.8±4.6% of MM1.S cells existed in the
sub-G1 region following treatment with 2 and 4 μM BMT-1 for 24 h,
respectively (Fig. 2A and B).
Collectively, we observed a significant increase in the sub-G1 cell
population indicating the occurrence of apoptosis that was
associated with a reduction in the G0–G1 fraction.
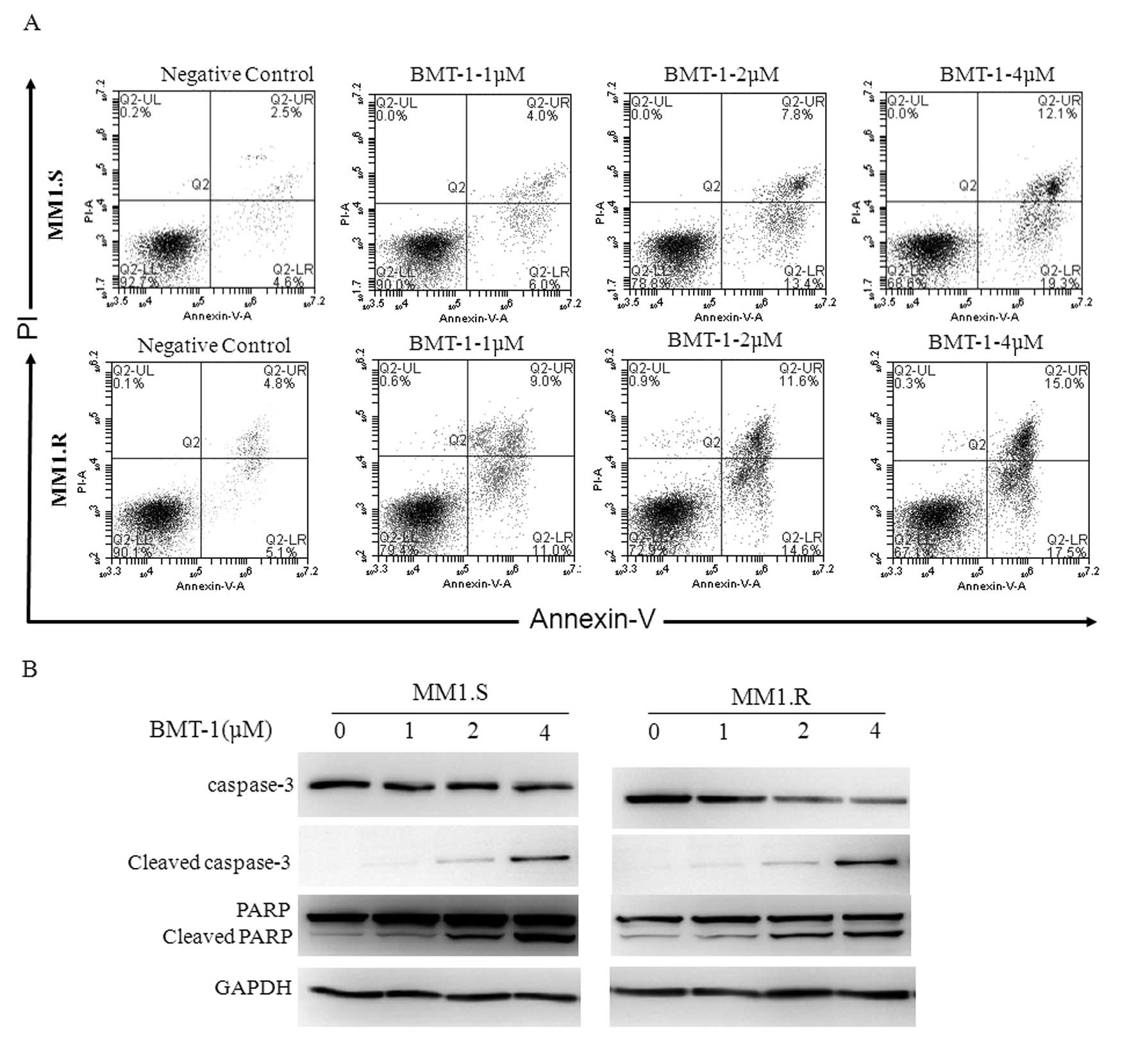
BMT-1 causes apoptosis in MM cells
To confirm whether the growth inhibition of BMT-1
was caused by apoptosis, the percentage of Annexin V-positive cells
was measured using flow cytometry. A dose-dependent increase in the
population of apoptotic cells was observed (Fig. 3A). BMT-1 at a concentration of 4 μM
induced 17.5 and 19.3% apoptosis in the MM1.S and MM1.R cells,
respectively (cells positive for Annexin V, negative for PI) after
24 h when compared with 4.6% (MM1.S) and 5.1% (MM1.R) in the
negative control (DMSO). To evaluate the contribution of active
caspases in BMT-1-induced apoptosis, we evaluated cells carrying
activated caspase-3 and PARP and found that pro-caspase-3 and
pro-PARP were cleaved to yield smaller fragments in MM cells
following BMT-1 treatment (Fig.
3B). These data indicated that BMT-1 indeed induced apoptosis
in cancer cells, which was consistent with the results of the
growth-inhibition assay.
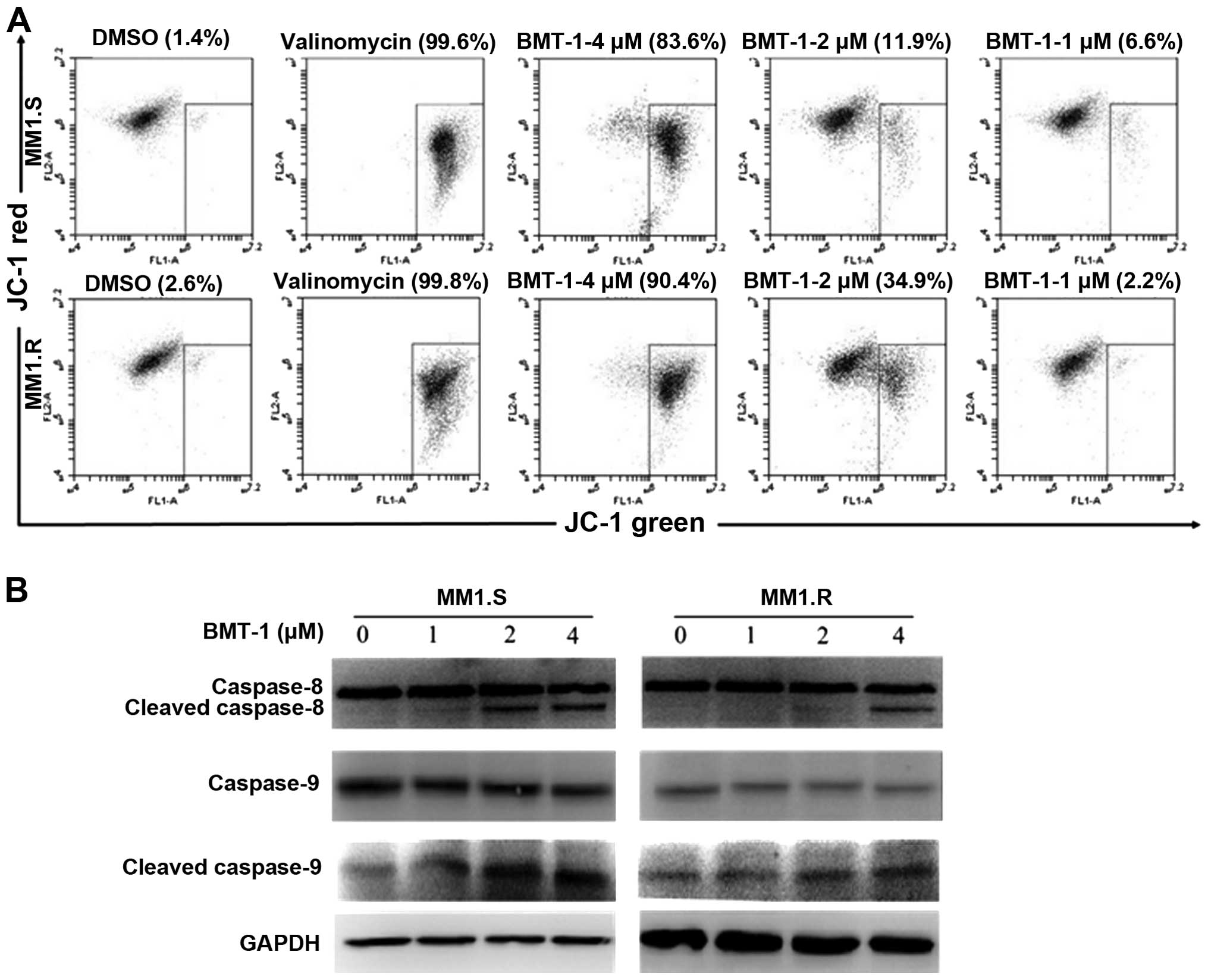
Effect of BMT-1 on mitochondrial membrane
potential
The effect of BMT-1 on mitochondrial membrane
potential was monitored. Any event that dissipates mitochondrial
membrane potential prevents the accumulation of JC-1 in the
mitochondria and thus, the dye is dispersed throughout the entire
cell leading to a shift from red (J-aggregates) to green
fluorescence (JC-1 monomers). As shown in Fig. 4A, after an 8-h drug exposure, BMT-1
depolarized the mitochondrial membranes in a
concentration-dependent manner, a time-point when no cellular
morphological change could be observed. Most control cells
fluoresced red, while BMT-1-treated cells turned green. During
mitochondrial-dependent apoptosis, the instability of mitochondria
initiates the formation of the apoptosome and the sequential
activation of caspase-8 and -9 (21,22).
The present study found that BMT-1 induced the loss of
mitochondrial membrane potential resulting in activation of
caspase-8 and -9 (Fig. 4B). These
results suggest that the mitochondrial dependent pathway is
involved in BMT-1-trigged MM cell apoptosis.
As the energy power plants of the cell, mitochondria
generate ATP by utilizing the proton electrochemical gradient
potential (23). Thus,
H+/K+-ATPase is extremely important to
mitochondria since it uses energy to transport protons from the
matrix of the mitochondrion to the inner and outer mitochondrial
membranes. Therefore, H+/K+-ATPase inhibition
disrupts the proton electrochemical gradient to induce a loss of
mitochondrial function. In the present study, BMT-1 strongly
depolarized mitochondrial membranes. Furthermore, all of the
currently marketed H+/K+-ATPase inhibitors
(e.g., omeprazole, lansoprazole and pantoprazole) are benzimidazole
derivatives (24–26). Therefore, we proposed that BMT-1
would also inhibit H+/K+-ATPase activity.
H+/K+-ATPase
inhibition is associated with acidification of cytosolic pH in
BMT-1-treated cells
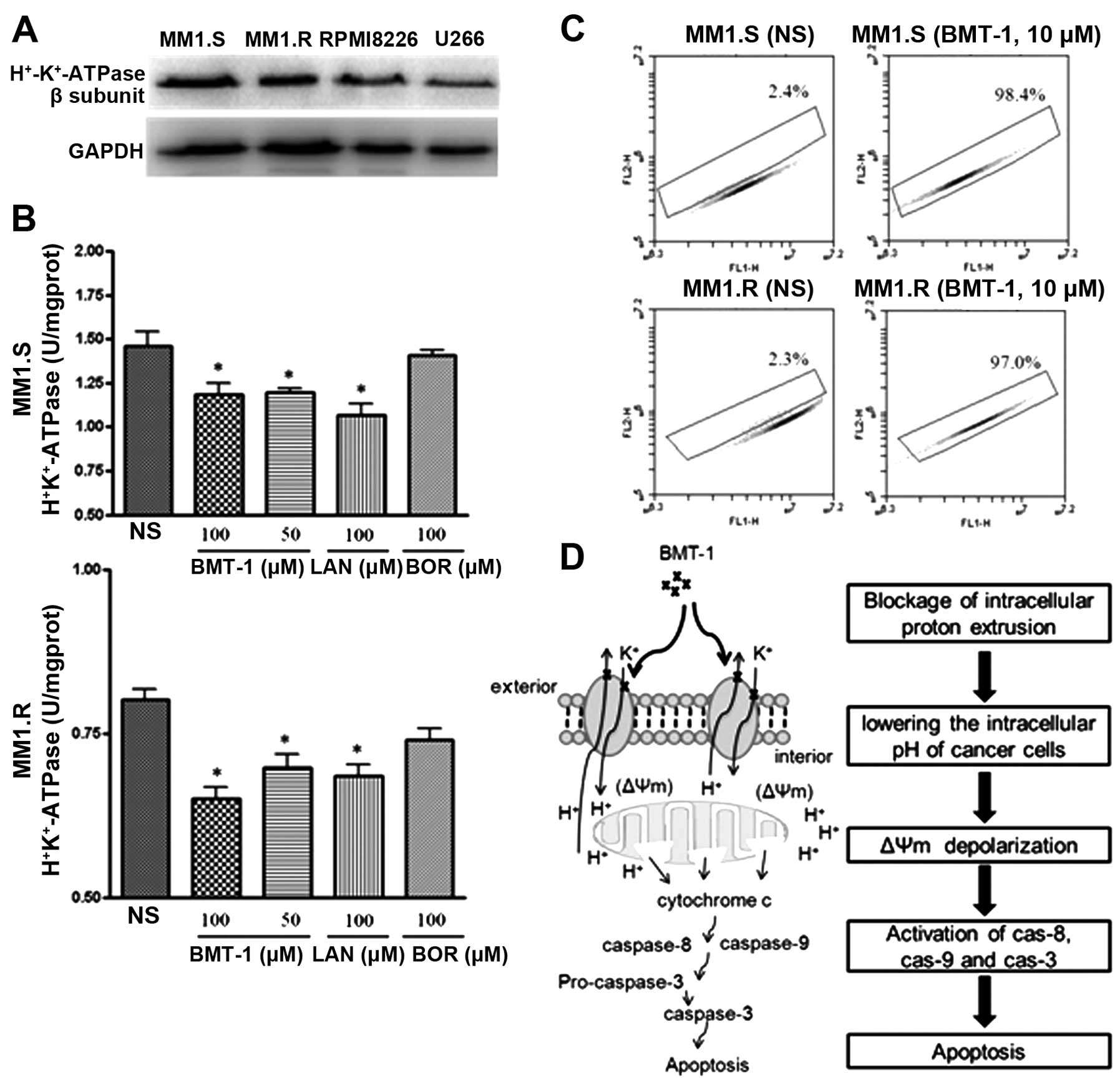
The expression of H+/K+-ATPase
in MM cells was determined by western blot analysis (Fig. 5A). To further confirm whether
inhibition of H+/K+-ATPase activity is
induced by BMT-1, the homogenized MM cells were treated with
different concentrations of BMT-1 for 30 min and the activity of
H+/K+-ATPase was detected. Compared with the
control group (0 μM), BMT-1 at every concentration and lansoprazole
(LAN), a positive inhibitor of H+/K+-ATPase,
both inhibited H+/K+-ATPase from the MM1.S
and MM1.R cells (Fig. 5B), while
the activity of H+/K+-ATPases was not
inhibited by the negative control inhibitor bortezomib (BOR).
H+/K+-ATPases are ion pumps that use the
energy of ATP hydrolysis to transport protons (H+) in
exchange for potassium ions against their concentration gradients.
Thus, the inhibition of H+/K+-ATPases by
BMT-1 could block H+ extrusion, resulting in proton
accumulation and intracellular acidification, which would terminate
or limit the growth of cancer cells (19,27).
To explore this possibility, the effect of BMT-1 on intracellular
pH changes was examined; we used BCECF-AM as a pH-dependent
fluorescent dye. Flow cytometry was used to measure intracellular
fluorescence of BCECF-labeled MM cells, which were exposed to
BMT-1. Treated cells exhibited a strong decrease in the FL1/FL2
ratio (indicating an acidic cytosolic pH), whereas little change in
the emission ratio of control cells was observed. Hence, the
results indicated that the intracellular pH was reduced in the
cells exposed to BMT-1 (Fig.
5C).
Discussion
In drug discovery, low toxicity and high efficacy
are the main criteria in selecting a ‘hit’ compound (28). BMT-1 is one such compound, and its
potential suitability for use in the treatment of cancer was
investigated in the present study. Cultures of PBMCs were used to
determine the effect of BMT-1 on normal primary cells. The data
showed that BMT-1 displayed higher efficacy against MM cancer cells
than normal PBMCs with 10-fold selectivity. BMT-1 exhibited no
cytotoxicity in the primary cells at a concentration of 10 μM even
following a 96-h treatment. Therefore, BMT-1 selectively induced
cell death in cancer cells with low cytotoxicity to normal cells,
which suggests it could be used as a potential chemotherapeutic
agent against cancer.
Benzimidazole derivatives provide useful precursors
or subunits for the development of molecules of pharmaceutical or
biological interest. It has previously been reported that
benzimidazole derivatives induce anti-mitotic and antitumor effects
in many human cancer cell lines (29–32).
However, the antitumor effects of benzimidazole derivatives on MM
cells are mostly unknown. Our experimental results showed that
BMT-1 exhibits obvious cytotoxicity against MM1.S and MM1.R cells,
based on the significant IC50 value of 1 μM (Fig. 1). The present study clearly and
convincingly showed that BMT-1 induced MM cell apoptosis in a
dose-dependent and time-dependent manner.
Mitochondrial depolarization is a well-characterized
event in apoptosis. In this process, the electrochemical gradient
across the mitochondrial membrane breaks down. To elucidate the
mechanism of apoptosis, we investigated the effects of BMT-1 on
mitochondrial membrane potential. Our results showed that BMT-1
strongly depolarized mitochondrial membranes in a
concentration-dependent manner in these cell lines (Fig. 2), which is in agreement with our
Annexin V and cell cycle results.
H+/K+-ATPases or proton pumps
play a key role in the function of the mitochondria, as they use
the energy made available from electron transfer reactions to
transport protons across the inner mitochondrial membrane and
create an electrochemical gradient used for the production of ATP
(33). Thus,
H+/K+-ATPase inhibition could disrupt the
proton electrochemical gradient and lead to the destruction of
mitochondria. In the present study, BMT-1 was observed to strongly
depolarize the mitochondrial membrane potential. We wanted to
investigate whether BMT-1 possesses the characteristic of many
other benzimidazole derivatives in being able to inhibit
H+/K+-ATPase activity. Our results showed
that BMT-1 and the positive H+/K+-ATPase
inhibitor, LAN, significantly inhibited
H+/K+-ATPases from the MM1.S and MM1.R cells
in a dose-dependent manner (Fig.
5B), while the activity of H+/K+-ATPases
was not inhibited by a negative control inhibitor BOR. Furthermore,
it was believed that impaired inhibition of
H+/K+-ATPases by BMT-1 would lead to
intracellular acidification, limiting cancer cell growth. To
explore this possibility, the effect of BMT-1 on intracellular pH
changes was examined; we used BCECF as a pH-dependent fluorescent
dye. The results indicated that the intracellular pH was reduced in
the cells exposed to BMT-1 (Fig.
5C).
Cancer cells have an inverse pH gradient when
compared with normal differentiated adult cells, including a
constitutively higher intracellular pH (pHi) and a lower
extracellular pH (pHe), which facilitate the indicated adaptive
behaviors (13,14). To avoid intracellular acidification
under such conditions, tumor cells express high levels of proton
pumps, including H+/K+-ATPases and V-ATPase
(16,19,34,35).
Thus, PPIs, which target the acidic tumor mass, block proton
trafficking and represent a class of drugs suitable for this
purpose (19). In the present
study, we used a primary antibody against the
H+/K+-ATPase B-subunit (ATP4B), and specific
bands at ~70 kDa were detected in both MM1.S and MM1.R cells
(Fig. 5A). This result indicated
that BMT-1 may have intracellular compartments as their sites of
action, thus causing H+/K+-ATPase inhibition.
This H+/K+-ATPase inhibition subsequently
triggered rapid cell apoptosis as a result of intracellular
acidification, mitochondrial depolarization and caspase activation.
Collectively, our data also showed that BMT-1-induced apoptosis was
associated with the activation of caspase-3, -8 and -9, as
determined by the appearance of the processed forms by western
blotting (Fig. 4B). These findings
indicate the involvement of caspases in BMT-1-induced apoptosis in
MM cells.
In conclusion, our data suggest that BMT-1 is an
effective anticancer agent. BMT-1 causes mitochondrial-dependent
apoptosis, as indicated by sequential events including
H+/K+-ATPase inhibition, intracellular
acidification, mitochondrial depolarization and activation of
caspase-9, -8 and -3 (Fig. 5D). We
believe that our data contribute to the development of
benzimidazole derivatives as lead compounds for the design and
development of new agents for the treatment of cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81201668, 81273530,
81302786), from the Sichuan Youth Science and Technology Fund (no.
2010JQ0035), from the Scientific Research Fund of Sichuan
Provincial Education Department (nos. 11ZA201 and 11ZB169), from
the Science and Technology Bureau of Sichuan Province (no.
2012JY0031), from the Foundation of Chengdu Military General
Hospital (nos. 2011YG-B02 and 2013YG-A004), from the Research Fund
of Chengdu Medical College (CYZ12-001), from the Scientific
Research Fund of Sichuan Provincial Health Department (130308),
from the National Undergraduates Innovating Experimentation Project
(201313705008) and from the Sichuan Province Undergraduates
Innovating Experimentation Project (201313705008).
References
|
1
|
Kyle RA and Rajkumar SV: Criteria for
diagnosis, staging, risk stratification and response assessment of
multiple myeloma. Leukemia. 23:3–9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mahindra A, Laubach J, Raje N, Munshi N,
Richardson PG and Anderson K: Latest advances and current
challenges in the treatment of multiple myeloma. Nat Rev Clin
Oncol. 9:135–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jáuregui I, García-Lirio E, Soriano AM,
Gamboa PM and Antépara I: An overview of the novel H1-antihistamine
bilastine in allergic rhinitis and urticaria. Expert Rev Clin
Immunol. 8:33–41. 2012.PubMed/NCBI
|
|
4
|
Wexler RR, Greenlee WJ, Irvin JD, et al:
Nonpeptide angiotensin II receptor antagonists: the next generation
in antihypertensive therapy. J Med Chem. 39:625–656. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Navarrete-Vázquez G, Cedillo R,
Hernández-Campos A, et al: Synthesis and antiparasitic activity of
2-(trifluoromethyl)-benzimidazole derivatives. Bioorg Med Chem
Lett. 11:187–190. 2001.PubMed/NCBI
|
|
6
|
Sachs G, Shin JM, Vagin O, Lambrecht N,
Yakubov I and Munson K: The gastric H,K ATPase as a drug target:
past, present, and future. J Clin Gastroenterol. 41(Suppl 2):
S226–S242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SK, Ahn CM, Choi SJ, Park YS, Cho HC
and Koh CM: The growth inhibitiory effect of new
pyrrolo[1,2-α]benzimidazole derivatives on human gastric cancer
cells. Arch Pharm Res. 20:410–413. 1997.PubMed/NCBI
|
|
8
|
Grimaudo S, Tolomeo M, Chimirri A, Zappala
M, Gancitano RA and D’Alessandro N: Selective induction of
apoptosis in multidrug resistant HL60R cells by the
thiazolobenzoimidazole derivative 1-(2,6-difluorophenyl)-1
H,3H-thiazolo [3,4-a] benzimidazole (TBZ). Eur J
Cancer. 34:1756–1763. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Douh MH, Sahib HB, Osman H, Abd Hamid S
and Salhimi SM: Anti-proliferation effects of benzimidazole
derivatives on HCT-116 colon cancer and MCF-7 breast cancer cell
lines. Asian Pac J Cancer Prev. 13:4075–4079. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Youssef AM, Malki A, Badr MH, Elbayaa RY
and Sultan AS: Synthesis and anticancer activity of novel
benzimidazole and benzothiazole derivatives against HepG2 liver
cancer cells. Med Chem. 8:151–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin ML, Chen SS, Lu YC, et al: Rhein
induces apoptosis through induction of endoplasmic reticulum stress
and Ca2+-dependent mitochondrial death pathway in human
nasopharyngeal carcinoma cells. Anticancer Res. 27:3313–3322.
2007.PubMed/NCBI
|
|
12
|
Shin JM and Kim N: Pharmacokinetics and
pharmacodynamics of the proton pump inhibitors. J
Neurogastroenterol Motil. 19:25–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Webb BA, Chimenti M, Jacobson MP and
Barber DL: Dysregulated pH: a perfect storm for cancer progression.
Nat Rev Cancer. 11:671–677. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen M, Zou X, Luo H, et al: Effects and
mechanisms of proton pump inhibitors as a novel chemosensitizer on
human gastric adenocarcinoma (SGC7901) cells. Cell Biol Int.
33:1008–1019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCarty MF and Whitaker J: Manipulating
tumor acidification as a cancer treatment strategy. Altern Med Rev.
15:264–272. 2010.PubMed/NCBI
|
|
16
|
Fais S: Proton pump inhibitor-induced
tumour cell death by inhibition of a detoxification mechanism. J
Intern Med. 267:515–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Monks A, Scudiero D, Skehan P, et al:
Feasibility of a high-flux anticancer drug screen using a diverse
panel of cultured human tumor cell lines. J Natl Cancer Inst.
83:757–766. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karali N: Synthesis and primary
cytotoxicity evaluation of new 5-nitroindole-2,3-dione derivatives.
Eur J Med Chem. 37:909–918. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Milito A, Iessi E, Logozzi M, et al:
Proton pump inhibitors induce apoptosis of human B-cell tumors
through a caspase-independent mechanism involving reactive oxygen
species. Cancer Res. 67:5408–5417. 2007.PubMed/NCBI
|
|
20
|
Nilsson C, Kågedal K, Johansson U and
Ollinger K: Analysis of cytosolic and lysosomal pH in apoptotic
cells by flow cytometry. Methods Cell Sci. 25:185–194. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cowling V and Downward J: Caspase-6 is the
direct activator of caspase-8 in the cytochrome c-induced
apoptosis pathway: absolute requirement for removal of caspase-6
prodomain. Cell Death Differ. 9:1046–1056. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen M, Guerrero AD, Huang L, et al:
Caspase-9-induced mitochondrial disruption through cleavage of
anti-apoptotic BCL-2 family members. J Biol Chem. 282:33888–33895.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perry SW, Norman JP, Barbieri J, Brown EB
and Gelbard HA: Mitochondrial membrane potential probes and the
proton gradient: a practical usage guide. Biotechniques. 50:98–115.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smolka AJ, Goldenring JR, Gupta S and
Hammond CE: Inhibition of gastric H,K-ATPase activity and gastric
epithelial cell IL-8 secretion by the pyrrolizine derivative ML
3000. BMC Gastroenterol. 4:42004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morii M, Takata H, Fujisaki H and
Takeguchi N: The potency of substituted benzimidazoles such as
E3810, omeprazole, Ro 18-5364 to inhibit gastric H+,
K+-ATPase is correlated with the rate of acid-activation
of the inhibitor. Biochem Pharmacol. 39:661–667. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beil W and Sewing KF: Inhibition of
partially purified K+/H+-ATPase from
guinea-pig isolated and enriched parietal cells by substituted
benzimidazoles. Br J Pharmacol. 82:651–657. 1984.PubMed/NCBI
|
|
27
|
Yeo M, Kim DK, Park HJ, Cho SW, Cheong JY
and Lee KJ: Blockage of intracellular proton extrusion with proton
extrusions with proton pump inhibitor induces apoptosis in gastric
cancer. Cancer Sci. 99:1852008.
|
|
28
|
Pinto MC, Dias DF, Del Puerto HL, et al:
Discovery of cytotoxic and pro-apoptotic compounds against leukemia
cells: Tert-butyl-4-[(3-nitrophenoxy)
methyl]-2,2-dimethyloxazolidine-3-carboxylate. Life Sci.
89:786–794. 2011.PubMed/NCBI
|
|
29
|
Chang WL, Chang CS, Chiang PC, et al:
2-Phenyl-5-(pyrrolidin-1-yl)-1-(3,4,5-trimethoxybenzyl)-1H-benzimidazole,
a benzimidazole derivative, inhibits growth of human prostate
cancer cells by affecting tubulin and c-Jun N-terminal kinase. Br J
Pharmacol. 160:1677–1689. 2010.PubMed/NCBI
|
|
30
|
Liu JF, Huang YL, Yang WH, Chang CS and
Tang CH: 1-Benzyl-2-phenylbenzimidazole (BPB), a benzimidazole
derivative, induces cell apoptosis in human chondrosarcoma through
intrinsic and extrinsic pathways. Int J Mol Sci. 13:16472–16488.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vasaitis T, Belosay A, Schayowitz A, et
al: Androgen receptor inactivation contributes to antitumor
efficacy of 17α-hydroxylase/17,20-lyase inhibitor
3β-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in
prostate cancer. Mol Cancer Ther. 7:2348–2357. 2008.PubMed/NCBI
|
|
32
|
Liu JF, Chang CS, Fong YC, Kuo SC and Tang
CH: FPipTB, a benzimidazole derivative, induces chondrosarcoma cell
apoptosis via endoplasmic reticulum stress and apoptosis
signal-regulating kinase 1. Mol Carcinog. May 18–2011.(Epub ahead
of print).
|
|
33
|
Schultz BE and Chan SI: Structures and
proton-pumping strategies of mitochondrial respiratory enzymes.
Annu Rev Biophys Biomol Struct. 30:23–65. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murakami T, Shibuya I, Ise T, et al:
Elevated expression of vacuolar proton pump genes and cellular pH
in cisplatin resistance. Int J Cancer. 93:869–874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Streif D, Iglseder E, Hauser-Kronberger C,
Fink KG, Jakab M and Ritter M: Expression of the non-gastric
H+/K+ ATPase ATP12A in normal and
pathological human prostate tissue. Cell Physiol Biochem.
28:1287–1294. 2011.PubMed/NCBI
|