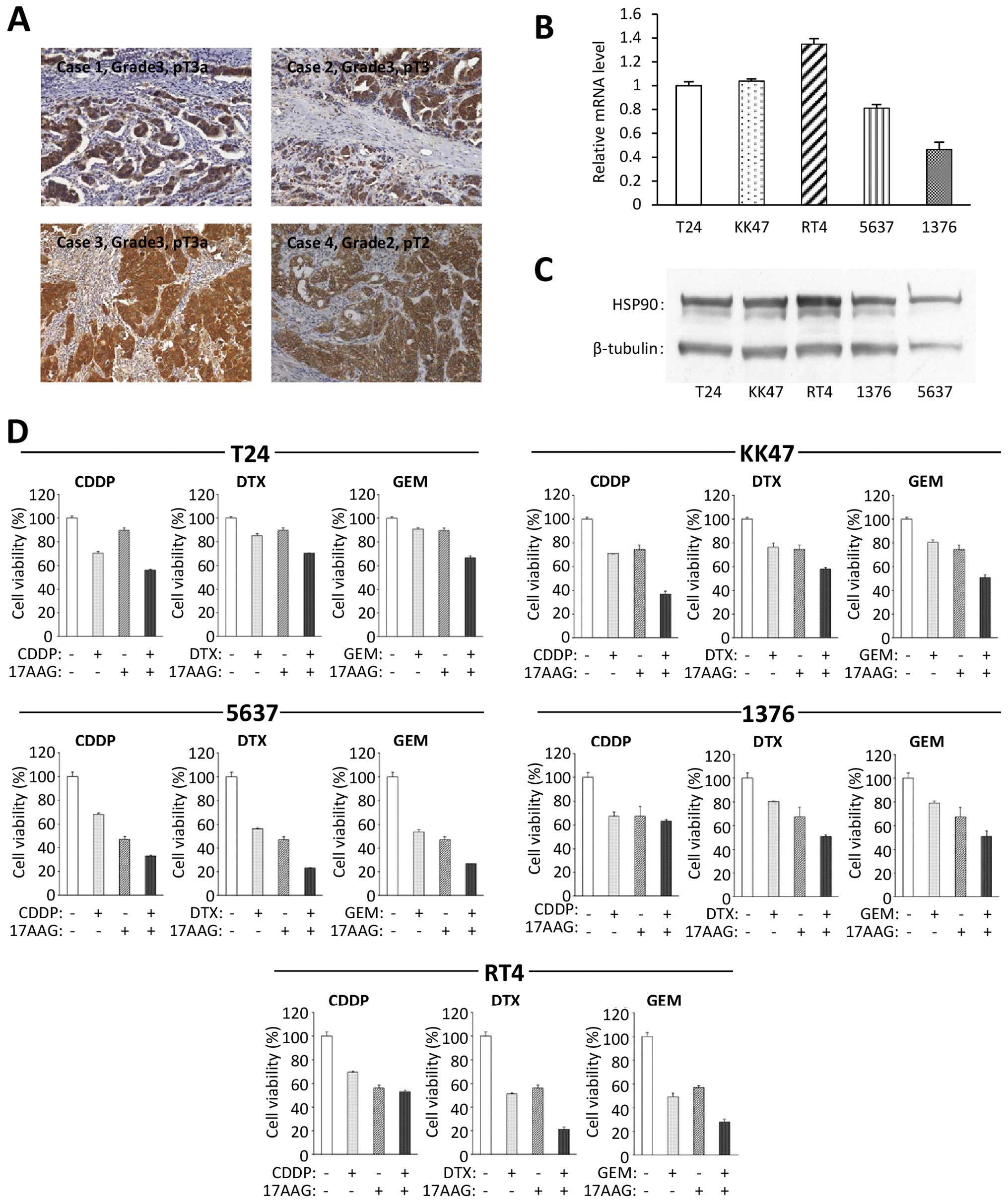
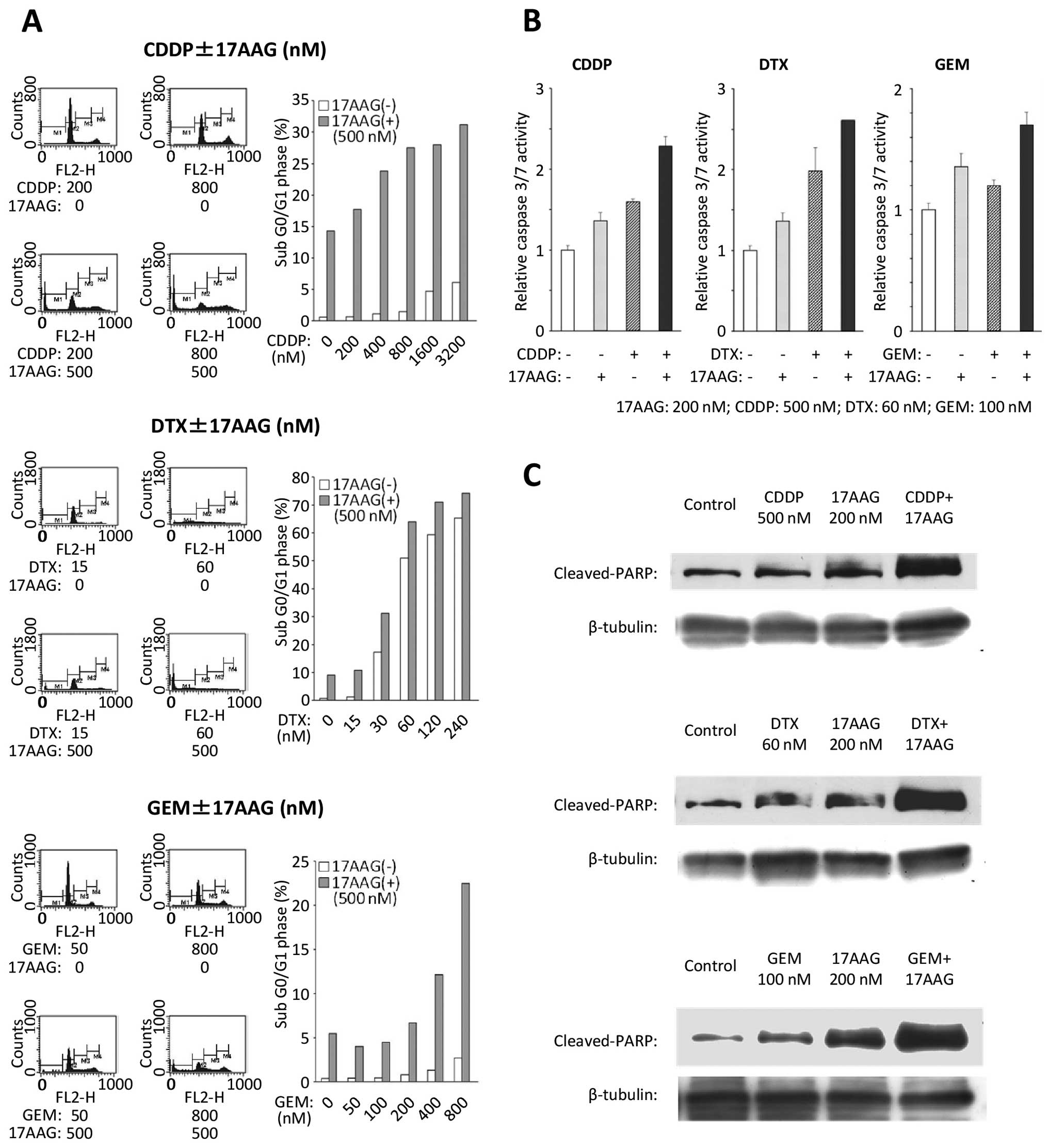
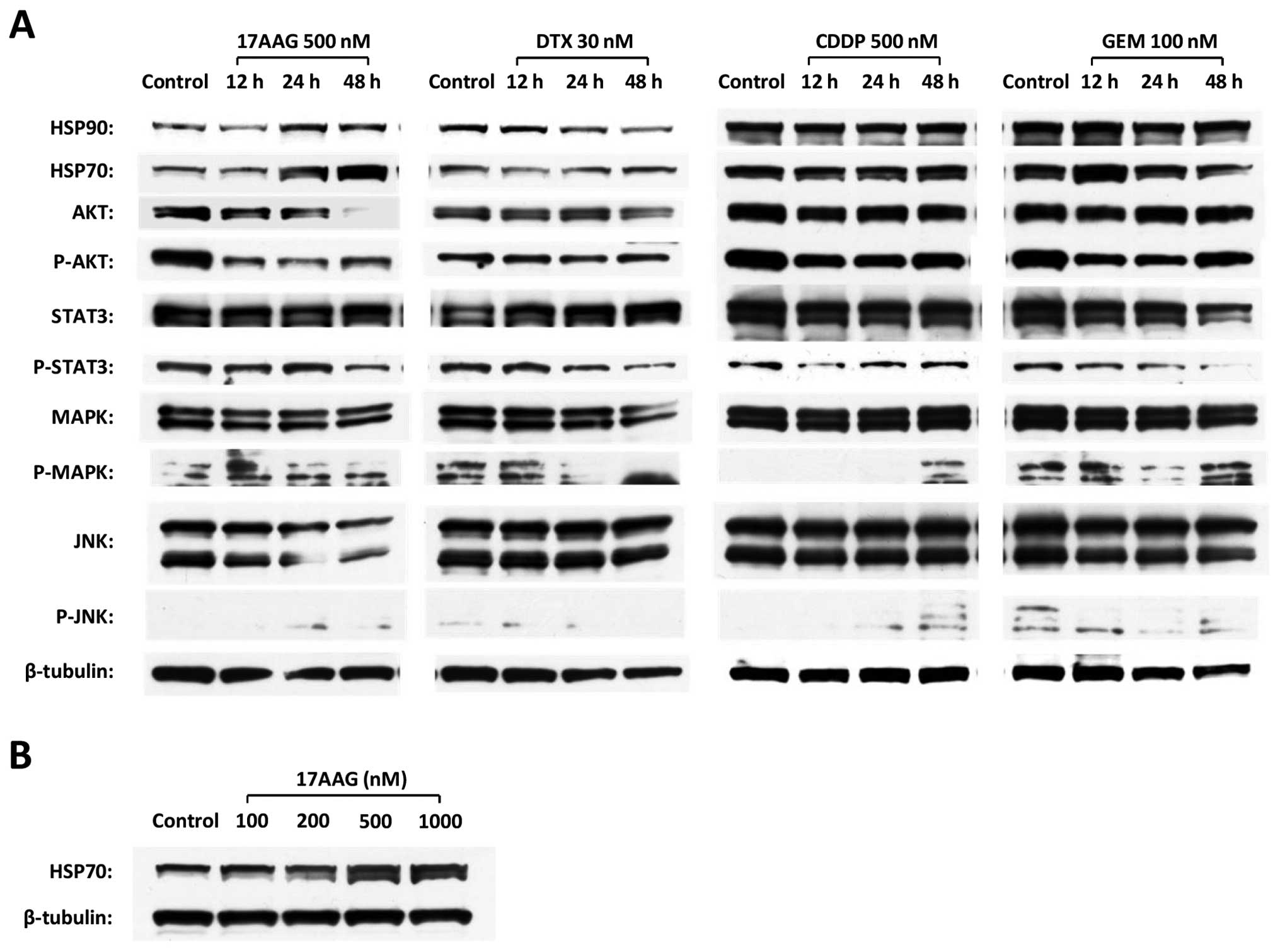
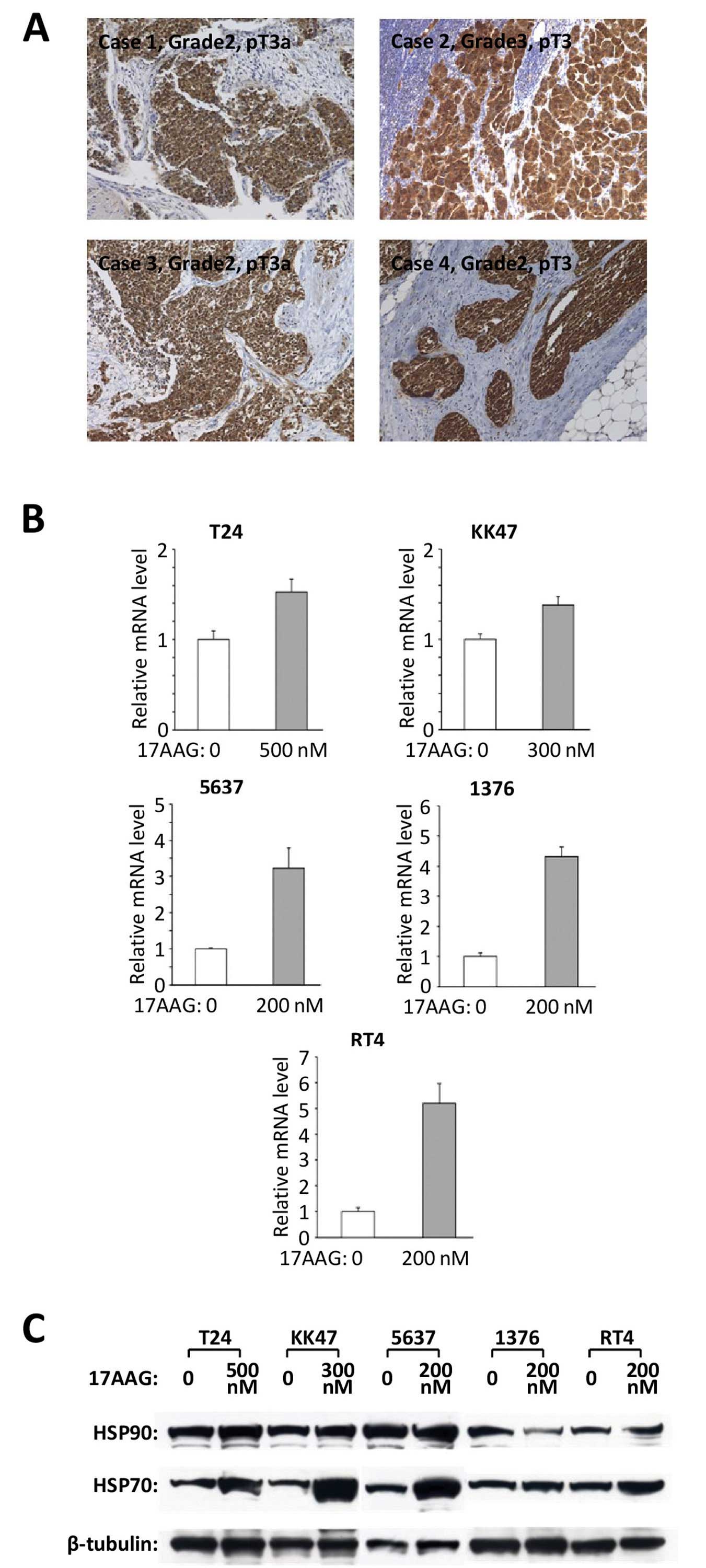
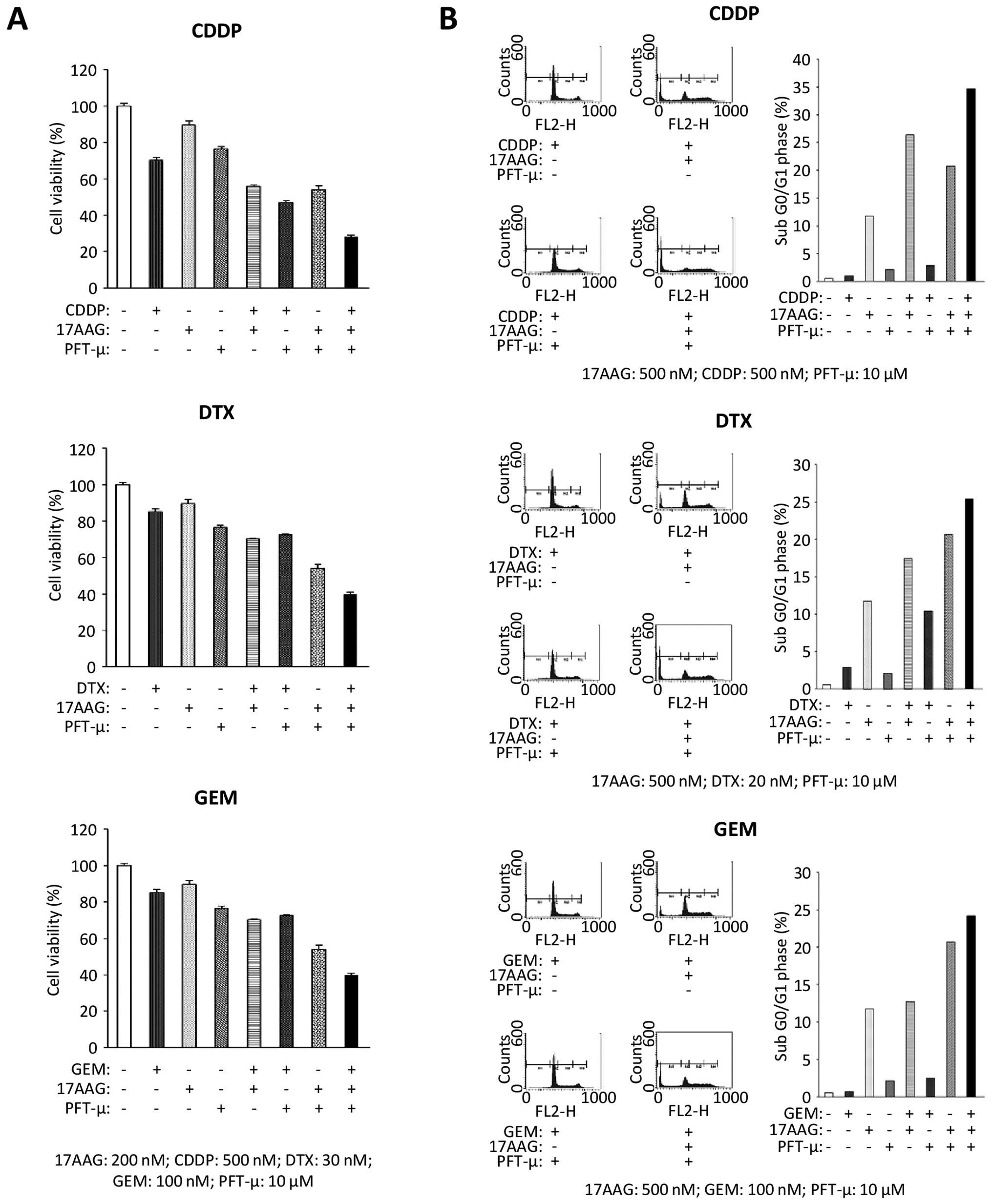
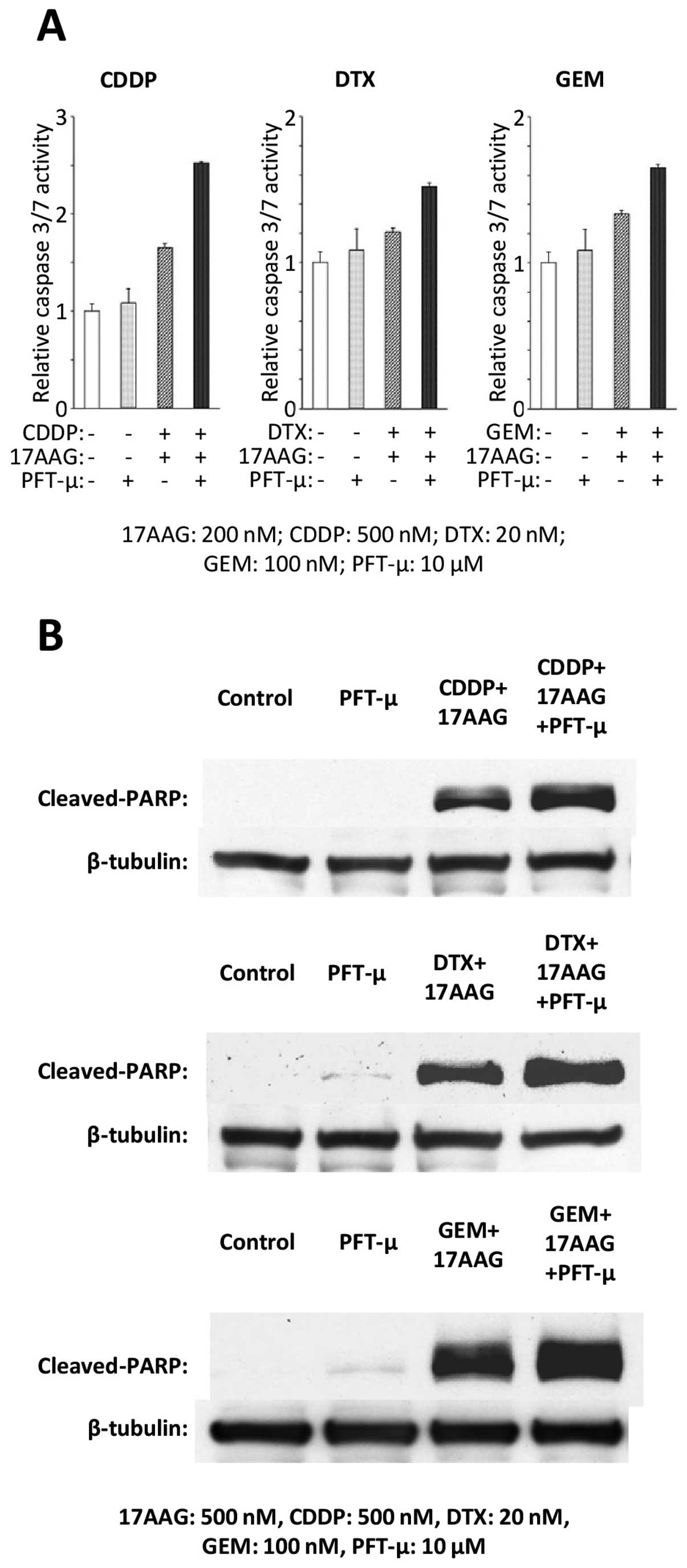
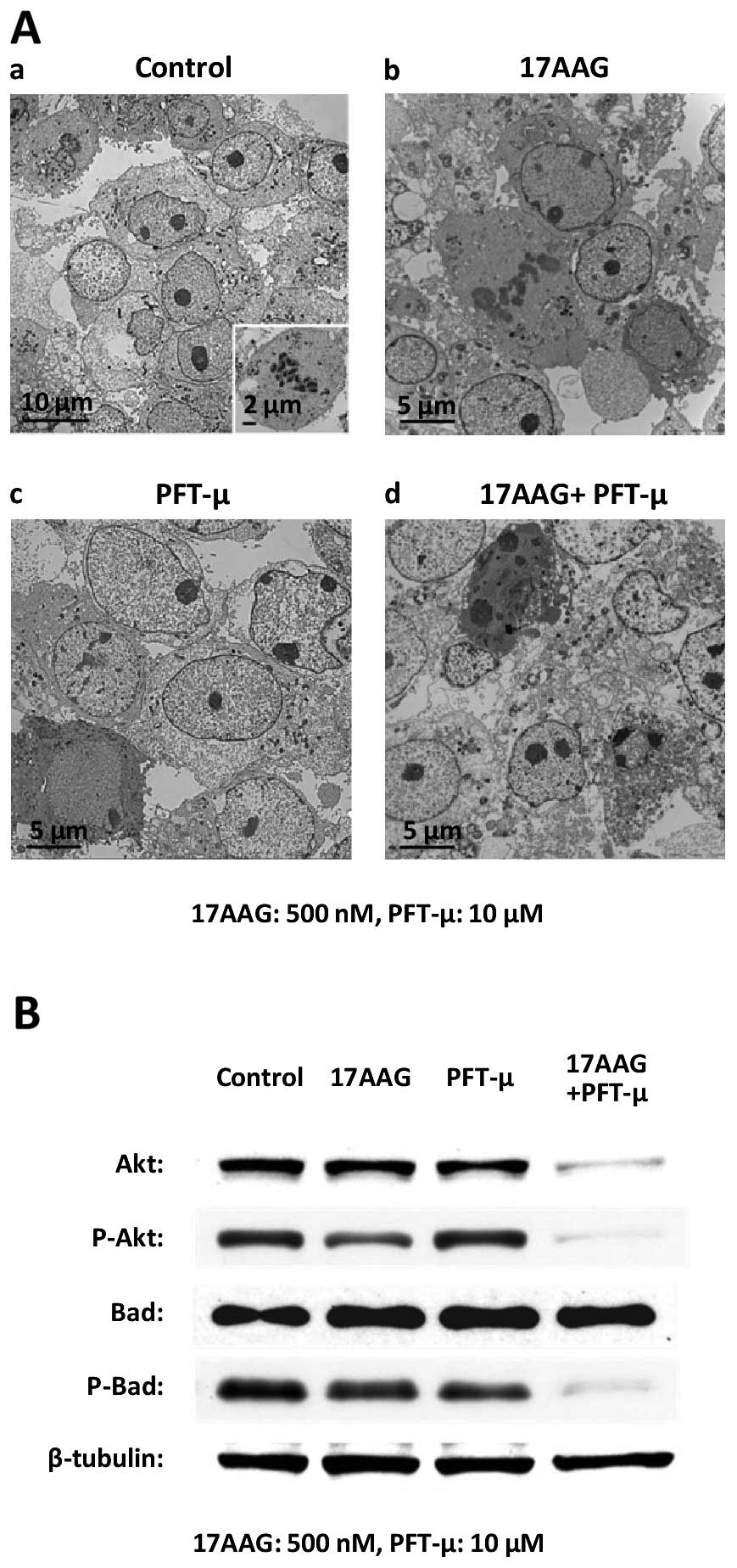
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mitra AP, Datar RH and Cote RJ: Molecular
staging of bladder cancer. BJU Int. 96:7–12. 2005. View Article : Google Scholar
|
|
4
|
Bambury RM and Rosenberg JE: Advanced
urothelial carcinoma: overcoming treatment resistance through novel
treatment approaches. Front Pharmacol. 4:32013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo T, Yu J, Nguyen J, et al: Electron
transfer-based combination therapy of cisplatin with
tetramethyl-p-phenylenediamine for ovarian, cervical, and
lung cancers. Proc Natl Acad Sci USA. 109:10175–10180. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Drayton RM and Catto JW: Molecular
mechanisms of cisplatin resistance in bladder cancer. Expert Rev
Anticancer Ther. 12:271–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin M, Pienkowski T, Mackey J, et al:
Adjuvant docetaxel for node-positive breast cancer. N Engl J Med.
352:2302–2313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tannock IF, de Wit R, Berry WR, et al:
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fossella F, Pereira JR, von Pawel J, et
al: Randomized, multinational, phase III study of docetaxel plus
platinum combinations versus vinorelbine plus cisplatin for
advanced non-small-cell lung cancer: the TAX 326 study group. J
Clin Oncol. 21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McKiernan JM, Masson P, Murphy AM, et al:
Phase I trial of intravesical docetaxel in the management of
superficial bladder cancer refractory to standard intravesical
therapy. J Clin Oncol. 24:3075–3080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Wit R, Kruit WH, Stoter G, de Boer M,
Kerger J and Verweij J: Docetaxel (Taxotere): an active agent in
metastatic urothelial cancer; results of a phase II study in
non-chemotherapy-pretreated patients. Br J Cancer. 78:1342–1345.
1998.PubMed/NCBI
|
|
12
|
McCaffrey JA, Hilton S, Mazumdar M, Sadan
S, Kelly WK, Scher HI and Bajorin DF: Phase II trial of docetaxel
in patients with advanced or metastatic transitional-cell
carcinoma. J Clin Oncol. 15:1853–1857. 1997.PubMed/NCBI
|
|
13
|
Toschi L, Finocchiaro G, Bartolini S,
Gioia V and Cappuzzo F: Role of gemcitabine in cancer therapy.
Future Oncol. 1:7–17. 2005. View Article : Google Scholar
|
|
14
|
Bellmunt J, Albiol S, de Olano AR, Pujadas
J and Maroto P; Spanish Oncology Genitourinary Group (SOGUG).
Gemcitabine in the treatment of advanced transitional cell
carcinoma of the urothelium. Ann Oncol. 17(Suppl 5): v113–v117.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cognetti F, Ruggeri EM, Felici A, et al:
Adjuvant chemotherapy with cisplatin and gemcitabine versus
chemotherapy at relapse in patients with muscle-invasive bladder
cancer submitted to radical cystectomy: an Italian, multicenter,
randomized phase III trial. Ann Oncol. 23:695–700. 2012. View Article : Google Scholar
|
|
16
|
Kim JJ: Recent advances in treatment of
advanced urothelial carcinoma. Curr Urol Rep. 13:147–152. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neri B, Vannini L, Giordano C, et al:
Gemcitabine plus docetaxel as first-line biweekly therapy in
locally advanced and/or metastatic urothelial carcinoma: a phase II
study. Anticancer Drugs. 18:1207–1211. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boukovinas I, Androulakis N, Vamvakas L,
et al: Sequential gemcitabine and cisplatin followed by docetaxel
as first-line treatment of advanced urothelial carcinoma: a
multicenter phase II study of the Hellenic Oncology Research Group.
Ann Oncol. 17:1687–1692. 2006. View Article : Google Scholar
|
|
19
|
Maloney A and Workman P: HSP90 as a new
therapeutic target for cancer therapy: the story unfolds. Expert
Opin Biol Ther. 2:3–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamal A, Thao L, Sensintaffar J, Zhang L,
Boehm MF, Fritz LC and Burrows FJ: A high-affinity conformation of
Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature.
425:407–410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schulte TW and Neckers LM: The
benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds
to HSP90 and shares important biologic activities with
geldanamycin. Cancer Chemother Pharmacol. 42:273–279. 1998.
View Article : Google Scholar
|
|
22
|
Karkoulis PK, Stravopodis DJ, Margaritis
LH and Voutsinas GE: 17-Allylamino-17-demethoxygeldanamycin induces
downregulation of critical Hsp90 protein clients and results in
cell cycle arrest and apoptosis of human urinary bladder cancer
cells. BMC Cancer. 10:4812010. View Article : Google Scholar
|
|
23
|
Barluenga S, Fontaine JG, Wang C, et al:
Inhibition of HSP90 with pochoximes: SAR and structure-based
insights. Chembiochem. 10:2753–2759. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barluenga S, Wang C, Fontaine JG, et al:
Divergent synthesis of a pochonin library targeting HSP90 and in
vivo efficacy of an identified inhibitor. Angew Chem Int Ed Engl.
47:4432–4435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Solit DB, Osman I, Polsky D, et al: Phase
II trial of 17-allylamino-17-demethoxygeldanamycin in patients with
metastatic melanoma. Clin Cancer Res. 14:8302–8307. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weng SH, Tseng SC, Huang YC, Chen HJ and
Lin YW: Inhibition of thymidine phosphorylase expression by using
an HSP90 inhibitor potentiates the cytotoxic effect of cisplatin in
non-small-cell lung cancer cells. Biochem Pharmacol. 84:126–136.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vasilevskaya IA, Rakitina TV and O’Dwyer
PJ: Quantitative effects on c-Jun N-terminal protein kinase
signaling determine synergistic interaction of cisplatin and
17-allylamino-17-demethoxygeldanamycin in colon cancer cell lines.
Mol Pharmacol. 65:235–243. 2004. View Article : Google Scholar
|
|
28
|
Solit DB, Basso AD, Olshen AB, Scher HI
and Rosen N: Inhibition of heat shock protein 90 function
down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer
Res. 63:2139–2144. 2003.PubMed/NCBI
|
|
29
|
Arlander SJ, Eapen AK, Vroman BT, McDonald
RJ, Toft DO and Karnitz LM: Hsp90 inhibition depletes Chk1 and
sensitizes tumor cells to replication stress. J Biol Chem.
278:52572–52577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghoshal S, Rao I, Earp JC, Jusko WJ and
Wetzler M: Down-regulation of heat shock protein 70 improves
arsenic trioxide and 17-DMAG effects on constitutive signal
transducer and activator of transcription 3 activity. Cancer
Chemother Pharmacol. 66:681–689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang RE: Targeting heat shock proteins
70/90 and proteasome for cancer therapy. Curr Med Chem.
18:4250–4264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leu JI, Pimkina J, Frank A, Murphy ME and
George DL: A small molecule inhibitor of inducible heat shock
protein 70. Mol Cell. 36:15–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Newman B, Liu Y, Lee HF, Sun D and Wang Y:
HSP90 inhibitor 17-AAG selectively eradicates lymphoma stem cells.
Cancer Res. 72:4551–4561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang H and Burrows F: Targeting multiple
signal transduction pathways through inhibition of Hsp90. J Mol
Med. 82:488–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida S, Koga F, Tatokoro M, et al:
Low-dose Hsp90 inhibitors tumor-selectively sensitize bladder
cancer cells to chemoradiotherapy. Cell Cycle. 10:4291–4299. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tatokoro M, Koga F, Yoshida S, Kawakami S,
Fujii Y, Neckers L and Kihara K: Potential role of Hsp90 inhibitors
in overcoming cisplatin resistance of bladder cancer-initiating
cells. Int J Cancer. 131:987–996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Page C, Lin HJ, Jin Y, Castle VP, Nunez G,
Huang M and Lin J: Overexpression of Akt/AKT can modulate
chemotherapy-induced apoptosis. Anticancer Res. 20:407–416.
2000.PubMed/NCBI
|
|
38
|
Li X, Luo R, Jiang R, Meng X, Wu X, Zhang
S and Hua W: The role of the Hsp90/Akt pathway in myocardial
calpain-induced caspase-3 activation and apoptosis during sepsis.
BMC Cardiovasc Disord. 13:82013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Evans CG, Chang L and Gestwicki JE: Heat
shock protein 70 (Hsp70) as an emerging drug target. J Med Chem.
53:4585–4602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pocaly M, Lagarde V, Etienne G, et al:
Overexpression of the heat-shock protein 70 is associated to
imatinib resistance in chronic myeloid leukemia. Leukemia.
21:93–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gabai VL, Budagova KR and Sherman MY:
Increased expression of the major heat shock protein Hsp72 in human
prostate carcinoma cells is dispensable for their viability but
confers resistance to a variety of anticancer agents. Oncogene.
24:3328–3338. 2005. View Article : Google Scholar
|
|
42
|
Guo F, Rocha K, Bali P, et al: Abrogation
of heat shock protein 70 induction as a strategy to increase
antileukemia activity of heat shock protein 90 inhibitor
17-allylamino-demethoxy geldanamycin. Cancer Res. 65:10536–10544.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Håvik B and Bramham CR: Additive
viability-loss following hsp70/hsc70 double interference and
Hsp90 inhibition in two breast cancer cell lines. Oncol Rep.
17:1501–1510. 2007.
|
|
44
|
Gao T and Newton AC: The turn motif is a
phosphorylation switch that regulates the binding of Hsp70 to
protein kinase C. J Biol Chem. 277:31585–31592. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu H, Zhang T, Chen R, McConkey DJ, Ward
JF and Curley SA: Multiple kinase pathways involved in the
different de novo sensitivity of pancreatic cancer cell lines to
17-AAG. J Surg Res. 176:147–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pommier Y, Sordet O, Antony S, Hayward RL
and Kohn KW: Apoptosis defects and chemotherapy resistance:
molecular interaction maps and networks. Oncogene. 23:2934–2949.
2004. View Article : Google Scholar : PubMed/NCBI
|