Introduction
Colorectal cancer (CRC) is one of the most common
malignant diseases worldwide, and the prognosis remains poor
(1,2). The incidence of CRC has increased
during recent decades, and the lifetime risk for CRC in
industrialized countries is ~5% (3). Early diagnosis and more effective
treatment of CRC requires the identification of new biomarkers as
well as insights into the molecular mechanisms of CRC.
Integrins are heterodimeric transmembrane receptors
for ligands in the extracellular matrix. Integrins contain two
distinct chains called the α and β subunits. In mammals, 18 α- and
8 β-subunits have been characterized. Integrins are essential for
both cell adhesion and activation of intracellular signaling
pathways (4). Each integrin
generally consists of a non-covalently linked α- and β-subunit,
with each subunit having a large extracellular domain, a single
membrane-spanning domain and a short, non-catalytic cytoplasmic
tail (5). These subunits combine to
form 24 distinct heterodimers. Splice variants have been identified
for some subunits and the number can reach at least 100 types
(6,7). Integrin signaling plays a key role in
the regulation of cellular growth and tumor progression through the
modulation of gene expression, apoptosis, cell adhesion,
proliferation and migration (8). β1
integrin (ITGB1) is the integrin mainly expressed in normal cells
and in tumor-associated cells, where they control various
developmental processes including angiogenesis, tumor progression,
apoptosis and metastasis (9–14).
Currently, the molecular mechanisms underlying the transcriptional
regulation of ITGB1 expression in CRC are still not fully
understood.
In the present study, we generated a lentivirus
expressing ITGB1 or ITGB1-specific RNAi and an unrelated control
and evaluated their effects on the human colorectal cancer cell
line HT29. The ITGB1 expression, cell proliferation and cell
apoptosis after infection were observed.
Materials and methods
Cells and animals
The human CRC cell line HT29 was purchased from the
Shanghai Cell Collection (Shanghai, China) and maintained in
Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Carlsbad, CA,
USA) containing 10% fetal bovine serum (FBS; Gibco) at 37°C in a
humidified atmosphere with 5% CO2.
Lentivirus packaging
Three precursor miRNA (pre-miRNA) sequences
targeting to ITGB1 (GenBank accession no. NM_133376) were designed
using an Internet application system (Invitrogen). Double-stranded
oligonucleotides encoding pre-miRNA sequence were annealed and
inserted into the lentiviral vector GV248 (Shanghai GeneChem Co.,
Ltd., Shanghai, China). Real-time PCR and western blot analysis
showed that the SR1 yielded the best suppression efficiency (data
not shown); therefore, this construct was chosen to package the
recombinant lentiviral vector for ITGB1 RNAi with the GV248
expression vector. Human ITGB1 was inserted into the GV218
lentiviral vector (Shanghai GeneChem) to upregulate expression of
ITGB1. These two lentiviral vectors possessed a high level of
expression of green fluorescent protein (GFP) and an antipuromycin
site.
Lentiviral infection
The lentiviral vectors were transfected into HT29
cells with a multiplicity of infection (MOI) from 1 to 100 in the
presence of 5 μg/ml polybrene (Sigma-Aldrich, St. Louis, MO, USA).
To produce a stable transfected cell line, the cells were cultured
in a selection medium containing 2.5 μg/ml of puromycin
(Sigma-Aldrich) for 2 weeks. The transfected cells were named
HT29-NC, HT29-RNAi and HT29-ITGB1.
Colony formation assay
For the colony formation assay, following treatment,
adherent HT29, HT29-NC, HT29-RNAi and HT29-ITGB1 cells were
trypsinized, and 1,000 viable cells (depending on the experiment)
were subcultured in 6-well plates (in triplicate). Cells were
allowed to adhere and colonize for 14 days. To visualize the
colonies, the medium was removed, and the cells were fixed in 96%
ethanol for 10 min and stained with crystal violet staining
solution. Where possible, colonies were counted, and are presented
as the mean number of colonies ± SD from at least three independent
experiments.
Hoechst 33258 staining for apoptotic
cells
HT29, HT29-NC, HT29-RNAi or HT29-ITGB1 cells in
exponential growth were placed at a final concentration of
1×105 cells/well in a 6-well plate. The cells were
subsequently fixed, washed three times with PBS, and stained with
Hoechst 33258 (Beyotime Institute of Biotechnology, Nantong, China)
according to the manufacturer’s protocol. Apoptotic features were
assessed by analyzing chromatin condensation and by staining the
fragments under an inverted fluorescence microscope (Olympus).
Western blot analysis
Cell lysates were prepared with RIPA lysis buffer
(Beyotime Institute of Biotechnology) with phenylmethylsulfonyl
fluoride (PMSF; Beyotime Institute of Biotechnology). The cell
extracted protein was subjected to SDS-PAGE, transferred to
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA), and blocked with 5% non-fat milk and 0.05% Tween. The
membranes were immunoblotted with the following rabbit polyclonal
antibodies: anti-ITGB1 (9699), anti-Bcl2 (2870), anti-Bax (5023),
anti-p21 (2947), anti-cyclin D1 (2978), anti-caspase-9 (9502),
anti-caspase-3 (9662) or anti-GAPDH (2118) (all from Cell Signaling
Technology, Beverly, MA, USA).
The blots were rinsed three times in TBST and
incubated with a 1:10,000 diluted goat anti-rabbit secondary
antibody (LI-COR Biosciences, Lincoln, NE, USA) for 1 h at room
temperature before they were washed extensively with TBST. Finally,
the membranes were scanned with a two-color infrared imaging system
(Odyssey, Lincoln, NE, USA). Membranes were also probed for GAPDH
as an additional loading control.
Flow cytometric analysis of cell
apoptosis
The HT29, HT29-NC, HT29-RNAi and HT29-ITGB1 cells
were washed with PBS, centrifuged at 12,000 × g for 5 min and then
resuspended in 500 μl binding buffer. Double staining with Annexin
V-PE and 7-ADD was performed using the Annexin V-FITC kit (Beyotime
Institute of Biotechnology) according to the manufacturer’s
recommendations, and the cells were then analyzed by FACS (BD
FACSAria III; BD Biosciences, San Jose, CA, USA). The data were
analyzed using FlowJo 7.6 software (Tree Star Inc., Ashland, OR,
USA).
Xenograft tumor model
Male nude mice (4–6 weeks of age, n=24) (Beijing HFK
Bioscience Co., Ltd., Beijing China) were used in the experiments.
After alcohol preparation of the skin, 1×106 HT29,
HT29-NC, HT29-RNAi or HT29-ITGB1 cells suspended in 100 μl PBS,
were subcutaneously inoculated into the dorsal area of the nude
mice. At the end of the experiment, tumors were harvested and
weighed. All experiments were performed according to the
recommendations of the Institutional Animal Care and Use Committee,
and the study protocol was approved by the Ethics Committee for
Animal Research of Wuhan University, China.
TdT-mediated dUTP-biotin nick
end-labeling assay
The TUNEL technique was performed to detect and
quantitate apoptotic cell death using the In Situ Cell Death
Detection kit (Roche Diagnostics Corporation, Indianapolis, IN,
USA) following the provided instructions. Briefly, chamber slides
were fixed with 4% paraformaldehyde and permeabilized in 0.1%
Triton X-100. The slides were then incubated with TUNEL reaction
mixture for 1 h at 37°C. After washing with PBS, the slides were
incubated with peroxidase-conjugated antibody for 30 min at 37°C
and were developed with the DAB system. Under microscopy, 6 fields
were randomly selected from every sample and 100 cells were
randomly selected from every field. The apoptotic rate = (number of
total apoptotic cells/100) × 100%.
Statistical analysis
Data are expressed as the mean ± SD. The difference
among groups was determined by ANOVA, and the difference between
groups was analyzed by the Student’s t-test using SPSS 17.0 for
Microsoft Windows (SPSS Inc., Chicago, IL, USA). A value of
P<0.05 was considered to indicate a statistically significant
result.
Results
Increased transduction of the lentiviral
vector in vitro and in vivo
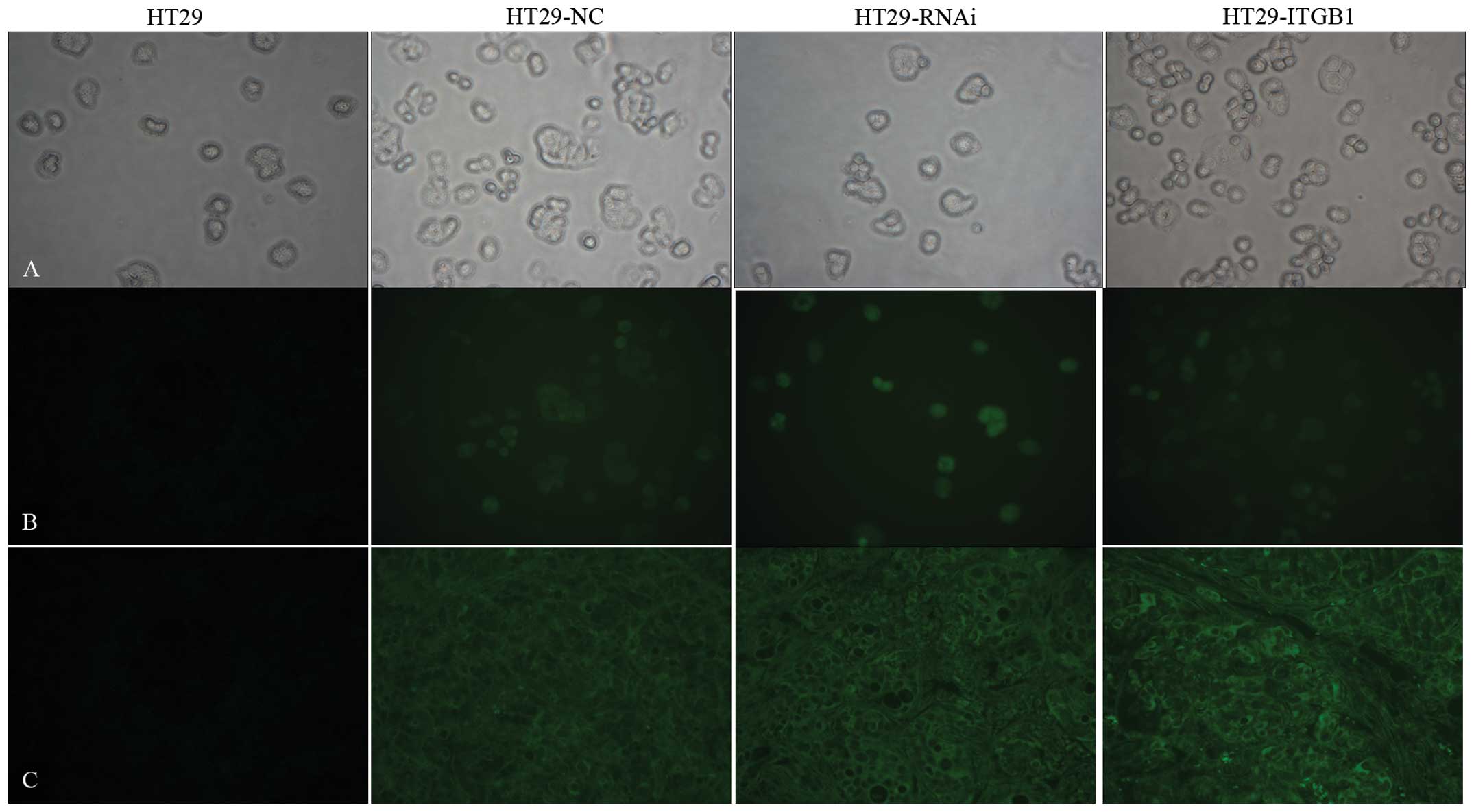
EGFP was used as a marker to detect whether the
recombinant lentiviral vectors were successfully transduced in
vitro and in vivo. The transfection efficiency (Fig. 1) in vitro approached 95% and
in vivo the efficiency was at least 90%.
Lentiviral-mediated ITGB1 expression in
HT29 cells
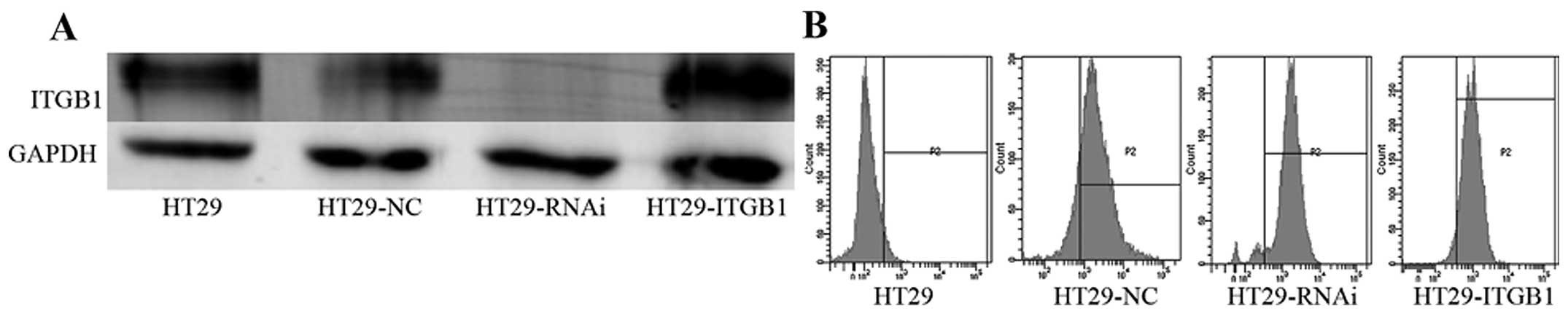
Western blot analyses revealed that the level of the
ITGB1 transcripts in the HT29-ITGB1 cells was significantly
increased (P<0.05). The level of ITGB1 transcripts in the
HT29-RNAi cells was decreased compared to the level in the HT29 and
HT29-NC cells (P<0.05). As expected, infection with the control
lentivirus did not affect the expression of ITGB1 (Fig. 2).
In addition, as shown in Fig. 2, transfection efficiency was
detected by flow cytometric analysis. The transfection rate was
90.2, 93.8 and 94.8% in the HT29-NC, HT29-RNAi and HT29-ITGB1
cells, respectively.
Effect of ITGB1 expression on the
proliferation of HT29 cells
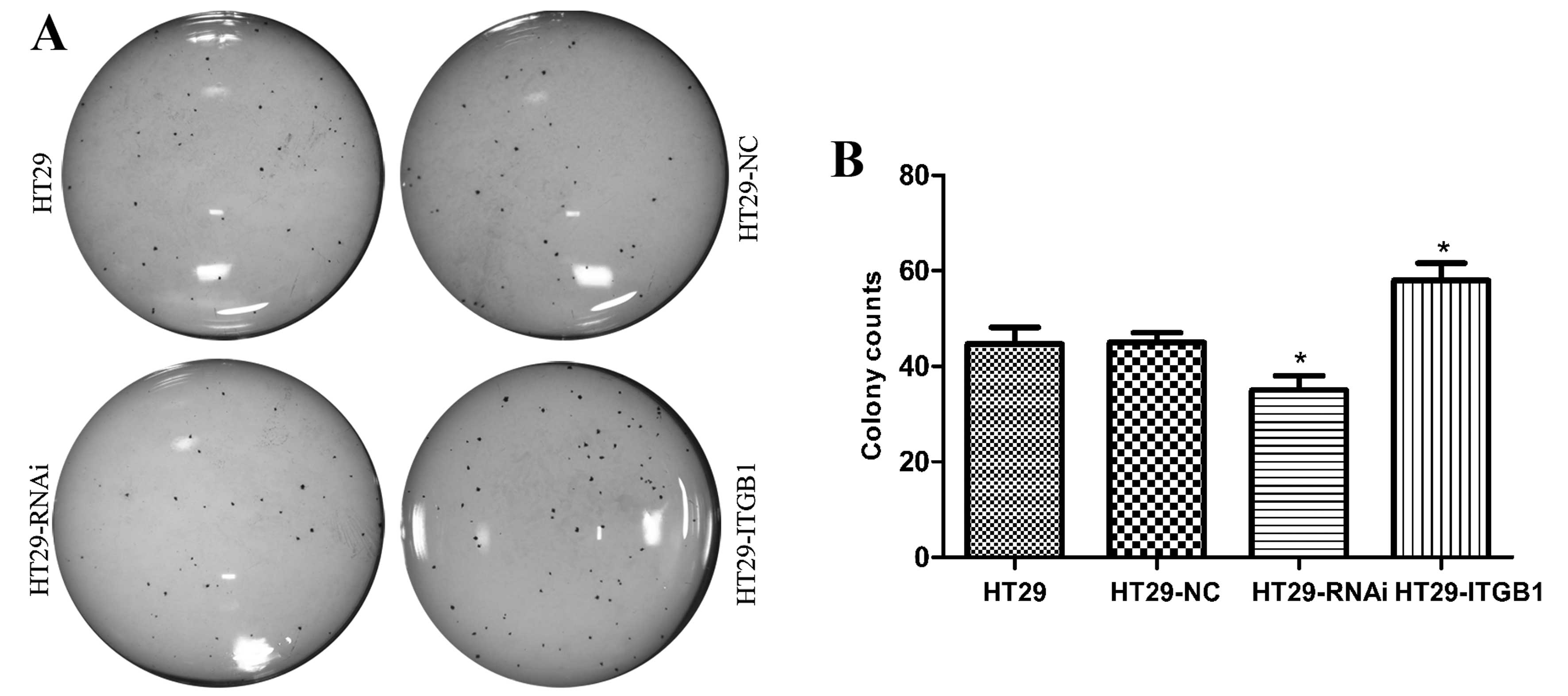
To determine the effect of ITGB1 expression on the
growth of HT29 cells, the proliferation of HT29, HT29-NC, HT29-RNAi
and HT29-ITGB1 cells was determined by clone formation assay
(Fig. 3). Following incubation for
2 weeks, 45±3.5, 45±2.0, 35±3.0 and 58±3.6 colonies were generated
from the HT29, HT29-NC, HT29-RNAi and HT29-ITGB1 cells,
respectively. The HT29-ITGB1 cells showed increased proliferation
when compared to the HT29 and HT29-NC cells. In contrast, the
proliferation of HT29-RNAi cells was much slower as compared with
the other two control groups (P<0.05).
ITGB1 inhibits expression of
apoptosis-related proteins in HT29 cells
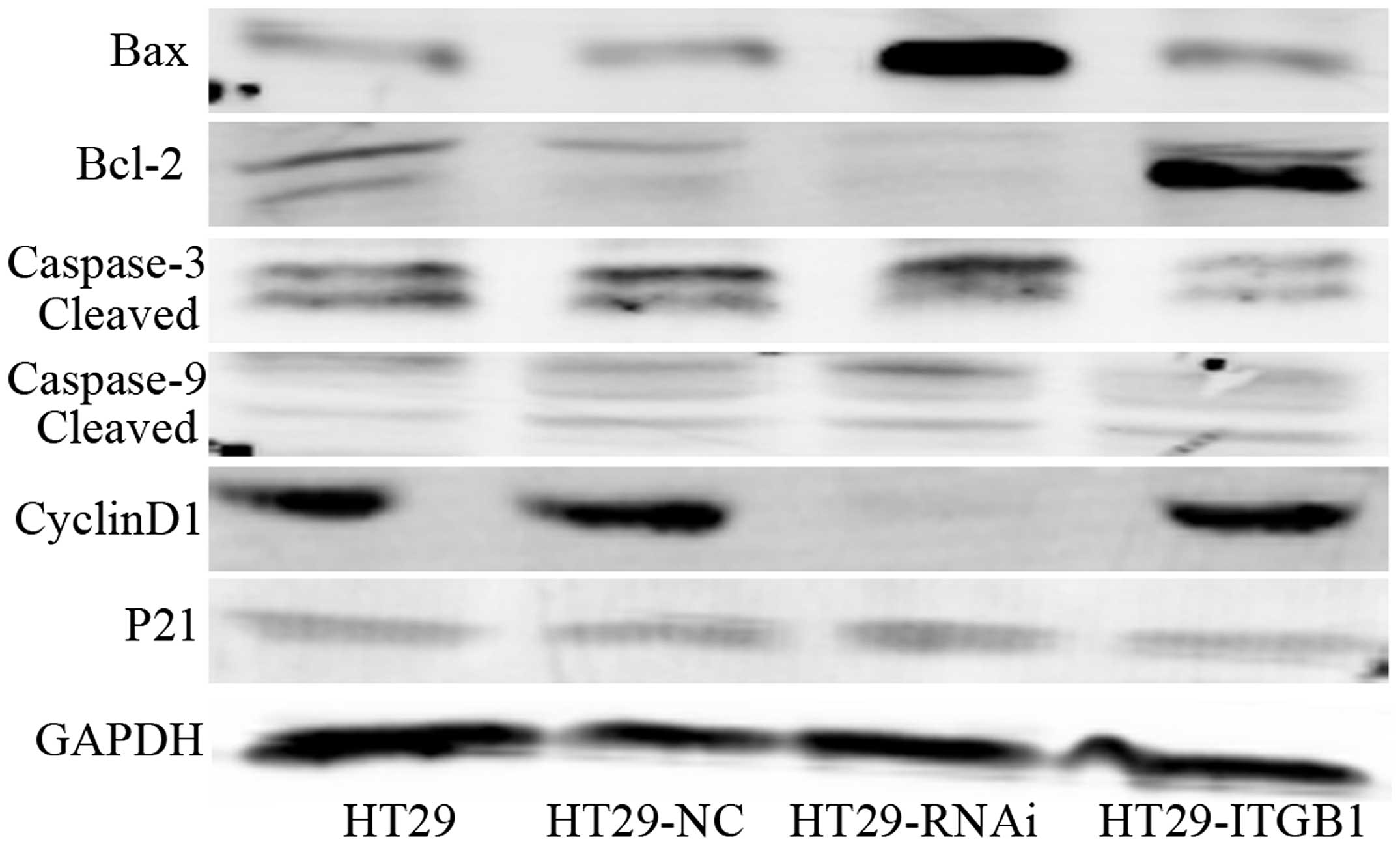
We assessed the effects of Bcl-2, Bax, caspase-3,
caspase-9, cyclin D1 and p21 protein by western blotting in the
HT29, HT29-NC, HT29-RNAi and HT29-ITGB1 cells. The levels of Bcl-2
and cyclin D1 proteins were upregulated while Bax, caspase-3,
caspase-9 and p21 levels were downregulated in the HT29-ITGB1 cells
when compared to the controls (Fig.
4). However, the results were found to be opposite in the
HT29-RNAi cells. Taken together, these findings indicate that ITGB1
activates the mitochondrial signaling pathway in human HT29
cells.
Effect of ITGB1 on HT29 cell
apoptosis
By using flow cytometric analysis, we assessed the
effect of ITGB1 on HT29 cell apoptosis. HT29, HT29-NC, HT29-RNAi
and HT29-ITGB1 cells were incubated with Annexin V-PE in a buffer
containing 7-AAD and were analyzed by flow cytometric analysis.
The results in Fig.
5 show that the HT29-RNAi cells exhibited a higher apoptosis
rate up to 30.6±5.1% (P<0.05) when compared with the HT29
(12.1±0.9%) and HT29-NC cells (10.8±1.3%). However, the HT29-ITGB1
cells had an apoptosis rate of only 12.3±2.5%, and as expected, no
difference was noted between the HT29 and HT29-NC cells
(P>0.05).
Antitumor efficacy of ITGB1 in nude
mice
On the basis of the in vitro data above, we
investigated the effects of lentivirus treatment on tumor growth
in vivo. The development of solid HT29 tumors was monitored
for 30 days. As shown in Fig. 6,
solid tumors were first visible ~5 days post inoculation and grew
rapidly afterwards in the HT29-ITGB1 group (1454±95.65
mm3). In contrast, the HT29-RNAi group had a slower
solid tumor growth rate and a smaller mean tumor volume
(889.3±61.49 mm3) compared to the control groups
(1162±144.8 and 1174±58.41 mm3; P<0.05) (Fig. 6A and B). These results indicate that
HT29-ITGB1 cells have high tumorigenicity following inoculation in
immunocompromised nude mice.
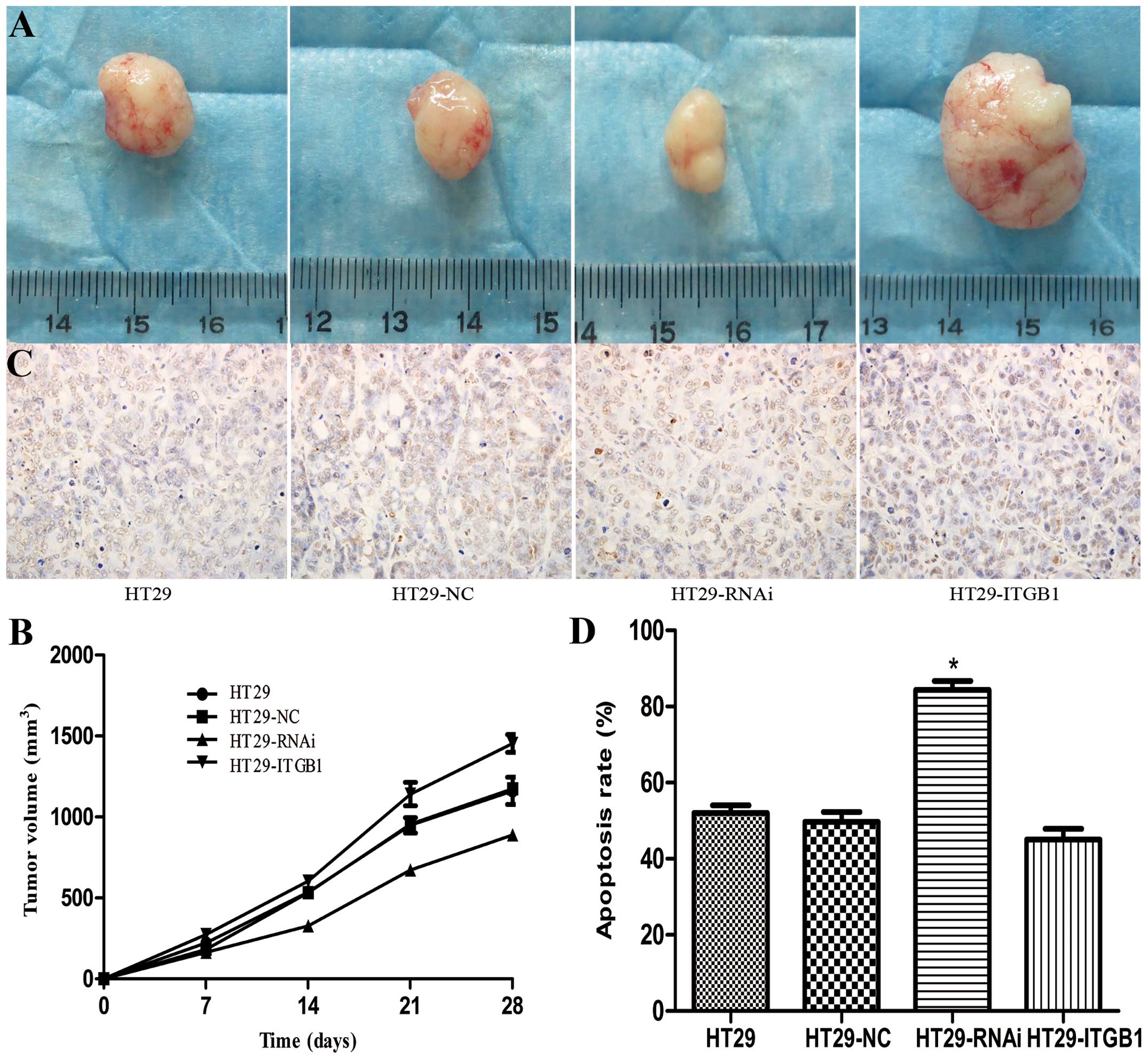 | Figure 6Effects of ITGB1 on mouse xenograft
tumors in vivo. (A) Cells were injected subcutaneously into
nude mice. One group of mice received HT29, HT29-NC, HT29-RNAi or
HT29-ITGB1 cells while a control group of mice received PBS.
Representative images of a tumor in each group post inoculation.
(C) Apoptotic cells, shown by TUNEL staining, were significantly
increased in the HT29-RNAi group (original magnification, ×400).
(B) Tumor volumes of the xenograft tumors derived from HT29,
HT29-NC, HT29-RNAi or HT29-ITGB1 cells. (D) Quantitative analysis
of apoptotic cells in the HT29, HT29-NC, HT29-RNAi and HT29-ITGB1
groups. Data are presented as mean ± SD. *P<0.05,
compared to the HT29 and HT29-NC groups. |
Apoptotic cells in the tumor sections were analyzed
by TUNEL staining. TUNEL staining showed markedly more positive
cells in the HT29-RNAi group (84.3±4.0%), which were more
significant than in the HT29-ITGB1 group (48.3±2.9%) and the other
two control groups, HT29 (52.0±3.6%) and HT29-NC (49.7±4.5%)
(Fig. 6C and D).
Discussion
ITGB1 is the most widely expressed integrin in cells
and it has been suggested to play a role in predicting the clinical
course and prognosis of several types of cancers (15,16). A
number of studies suggest that ITGB1 is important but not essential
for metastasis in different types of cancer (17,18).
In another study ITGB1 inhibition was found to induce apoptosis in
breast cancer cells (19). The
current series of experiments demonstrated the essential role of
ITGB1 in maintaining cell survival and promoting tumorigenesis in
cancer cells. In the present study, we generated a lentivirus
expressing ITGB1 or ITGB1-specific RNAi and an unrelated control
and evaluated their effects on the human colorectal cancer cell
line HT29. We found that upregulation of ITGB1 expression greatly
induced proliferation of HT29 cells in vitro, as shown by
higher proliferation efficiency in HT29-ITGB1 cells compared to
controls. In addition, the average volume of tumors in the
HT29-ITGB1 group was significantly increased compared to that of
the other three groups. In the present study, we found a
relationship between expression of ITGB1 and proliferation.
However, the mechanism(s) underlying the action of ITGB1 and the
downstream effects on the ITGB1 target genes remain unclear.
Cell proliferation and apoptosis are two essential
conditions for cell transformation and malignant tumorigenesis. The
B-cell leukemia/lymphoma 2 (Bcl-2) family plays a pivotal role in
the control of apoptosis and can be classified into two
functionally distinct groups: anti-apoptotic proteins and
pro-apoptotic proteins (20).
Bcl-2, an anti-apoptotic member of the Bcl family, was initially
identified as a proto-oncogene detected in human B-cell follicular
lymphoma (21). Bcl-2-associated X
protein (Bax), a pro-apoptotic protein is expressed abundantly and
selectively during apoptosis and promotes cell death (22). An increased ratio of Bcl-2 to Bax
has commonly been used to determine the induction of apoptosis in a
number of tissue types (23).
In the present study, we explored whether ITGB1
modulates the apoptosis of HT29 cells in vitro and found
that HT29-RNAi cells underwent rapid apoptosis when compared with
the other groups. Therefore, we conclude that downregulation of
ITGB1 expression markedly enhances the apoptosis capacity of HT29
cells in vitro. Western blot analysis showed that the level
of Bcl-2 was upregulated while Bax, caspase-3 and caspase-9 levels
were downregulated in the HT29-ITGB1 cells when compared to the
controls. The significant effect of ITGB1 on HT29 cells suggests
that ITGB1 may play an important role in the apoptosis of CRC
cells.
Cell cycle progression is likely to play an
important role in gastrointestinal carcinogenesis. Cell cycle
progression is governed by a family of cyclin-dependent kinases
(CDKs), which are activated by binding to cyclin proteins and
inhibited by CDK inhibitors (24).
Cyclin D1 is a key regulator of the G1 phase of the cell cycle and
is located on chromosome 11q13. A significant proportion of
dysplasias contain molecular abnormalities that may result in
cyclin D1 overexpression (25). Our
data showed that ITGB1 upregulated cyclin D1 protein while the p21
level was downregulated in the HT29-ITGB1 cells when compared with
the other groups. This indicates that ITGB1 may participate in the
cell cycle regulation of CRC cells, but the mechanism(s) underlying
the action of ITGB1 remain unclear.
Taken together, based on our data, we demonstraed
that ITGB1 may regulate the growth and apoptosis of HT29 cells both
in vitro and in vivo. Our findings extend our
knowledge concerning the pathogenesis of CRC and may assist in
developing therapies for human CRC.
Acknowledgements
The present study was supported by research grants
from the National Natural Science Foundation of China (no.
81172069) and the Science and Technology Department of Hubei
Province (no. 2010CDA043).
References
|
1
|
Zheng S and Shanrong C: Colorectal cancer
epidemiology and prevention study in China. Chin Ger J Clin Oncol.
2:72–75. 2003. View Article : Google Scholar
|
|
2
|
Yang L, Parkin DM, Ferlay J, Li L and Chen
Y: Estimates of cancer incidence in China for 2000 and projections
for 2005. Cancer Epidemiol Biomarkers Prev. 14:243–250.
2005.PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
4
|
Hynes RO: Integrins: bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hood JD and Cheresh DA: Role of integrins
in cell invasion and migration. Nat Rev Cancer. 2:91–100. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Melker AA and Sonnenberg A: Integrins:
alternative splicing as a mechanism to regulate ligand binding and
integrin signaling events. Bioessays. 21:499–509. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gilcrease MZ: Integrin signaling in
epithelial cells. Cancer Lett. 247:1–25. 2007. View Article : Google Scholar
|
|
8
|
Felding-Habermann B: Integrin adhesion
receptors in tumor metastasis. Clin Exp Metastasis. 20:203–213.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li N, Zhang Y, Naylor MJ, Schatzmann F,
Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH and
Hynes NE: β integrins regulates mammary proliferation and maintain
the integrity of mammary alveoli. EMBO J. 24:1942–1953. 2005.
|
|
10
|
Arao S, Masumoto A and Otsuki M: β1
integrins play an essential role in adhesion and invasion of
pancreatic carcinoma cells. Pancreas. 20:129–137. 2000.
|
|
11
|
Aumailley M, Pesch M, Tunggal L, Gaill F
and Fässler R: Altered synthesis of laminin 1 and absence of
basement membrane component deposition in β1 integrin deficient
embryoid bodies. J Cell Sci. 113:259–268. 2001.PubMed/NCBI
|
|
12
|
Stroeke PJ, van Rijthoven EA, Boer E,
Geerts D and Roos E: Cytoplasmic domain mutants of β1 integrin,
expressed in β1-knockout lymphoma cells, have distinct effects on
adhesion, invasion and metastasis. Oncogene. 19:1232–1238.
2000.
|
|
13
|
Brakebusch C and Fassler R: β1 integrin
function in vivo: adhesion, migration and more. Cancer
Metastasis Rev. 24:403–411. 2005.
|
|
14
|
Wang W, Goswami S, Lapidus K, Wells AL,
Wyckoff JB, Sahai E, Singer RH, Segall JE and Condeelis JS:
Identification and testing of a gene expression signature of
invasive carcinoma cells within primary mammary tumors. Cancer Res.
64:8585–8594. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao ES, Zhang H, Chen YY, Lee B, Chew K,
Moore D and Park C: Increased β1 integrin is associated with
decreased survival in invasive breast cancer. Cancer Res.
67:659–664. 2007.
|
|
16
|
Wang D, Müller S, Amin AR, Huang D, Su L,
Hu Z, Rahman MA, Nannapaneni S, Koenig L, Chen Z, Tighiouart M,
Shin DM and Chen ZG: The pivotal role of integrin β1 in metastasis
of head and neck squamous cell carcinoma. Clin Cancer Res.
18:4589–4599. 2012.
|
|
17
|
Adachi M, Taki T, Higashiyama M, Kohno N,
Inufusa H and Miyake M: Significance of integrin α5 gene
expression as a prognostic factor in node-negative non-small cell
lung cancer. Clin Cancer Res. 6:96–101. 2000.
|
|
18
|
Ahmed N, Riley C, Rice G and Quinn M: Role
of integrin receptors for fibronectin, collagen and laminin in the
regulation of ovarian carcinoma functions in response to a matrix
microenvironment. Clin Exp Metastasis. 22:391–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park CC, Zhang H, Pallavicini M, Gray JW,
Baehner F, Park CJ and Bissell MJ: β1 integrin inhibitory antibody
induces apoptosis of breast cancer cells, inhibits growth and
distinguishes malignant from normal phenotype in three dimensional
cultures and in vivo. Cancer Res. 66:1526–1535. 2006.
|
|
20
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bakhshi A, Jensen JP, Goldman P, Wright
JJ, McBride OW, Epstein AL and Korsmeyer SJ: Cloning the
chromosomal breakpoint of t(14;18) human lymphomas: clustering
around JH on chromosome 14 and near a transcriptional unit on 18.
Cell. 41:899–906. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Puthalakath H and Strasser A: Keeping
killers on a tight leash: transcriptional and post-translational
control of the pro-apoptotic activity of BH3-only proteins
(Review). Cell Death Differ. 9:505–512. 2002. View Article : Google Scholar
|
|
23
|
Thome M and Tschopp J: Regulation of
lymphocyte proliferation and death by FLIP. Nat Rev Immunol.
1:50–58. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sherr CJ: G1 phase progression: Cycling on
cue. Cell. 79:551–555. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang S, Chen CS and Ingber DE: Control of
cyclin D1, p27Kip1, and cell cycle progression in human
capillary endothelial cells by cell shape and cytoskeletal tension.
Mol Biol Cell. 9:3179–3193. 1998.
|