Introduction
Although novel therapies have recently been
introduced into clinical practice for the treatment of advanced
prostate cancer, prostate cancer has remained the second deadliest
cancer in men in the United States in 2014 (1). New therapeutic targets and strategies
are urgently needed to further improve the clinical outcome of
patients with prostate cancer.
One promising potential therapeutic target is
cyclin-dependent kinase 5 (CDK5). CDK5 is a serine/threonine kinase
structurally similar to other CDKs (2). CDK5 does not appear to have a major
role in cell cycle regulation (3,4). It
has been well characterized for its dominant role in the
development of the central nervous system, including roles in
neuronal migration, differentiation and adhesion (5,6). We
and others subsequently showed that CDK5 plays an important role in
cancer development and metastasis (7–12). In
prostate cancer cells, we demonstrated that CDK5 was critical for
cytoskeletal integrity, cell migration and invasion, and in
vivo, for metastasis (7). In
pancreatic cancer, CDK5 is intrinsic to KRAS signaling through the
centrally important RAL signal transduction pathway, thus providing
a potential ‘druggable’ target for mutant KRAS tumors (8). Together, these studies indicate that
inhibition of CDK5, alone or in combination with other agents, may
provide an effective therapeutic strategy for these and other
cancer types.
In the present study we set out to identify agents
that would be particularly effective in combination with CDK5
inhibition in prostate cancer cells. Therefore, we performed a
screen of the Johns Hopkins Drug Library (JHDL). The JHDL is a
collection of 3,360 pharmaceutical compounds that have successfully
completed safety testing in humans for a variety of applications
(13,14). This library has been used
successfully for repurposing of compounds for cancer therapy,
including identification of digoxin as an HIF1α inhibitor (15), and itraconazole as an angiogenesis
inhibitor (16). We previously
employed the JHDL to identify cetrimonium bromide and irinotecan as
compounds with increased antitumor activity against prostate cancer
cells expressing low levels of the metastasis suppressor gene N-myc
downregulated gene 1 (NDRG1) (17).
Here, we performed a similar JHDL screening with prostate cancer
cells which differ in CDK5 activity. Tilorone was identified as a
compound with in vitro synthetical lethality in
CDK5-deficient prostate cancer cells.
Materials and methods
Cell culture
PC3 prostate cancer cell lines were obtained from
ATCC. These cells are derived from a bone metastasis from a 62-year
old prostate cancer patient. Human prostate fibroblasts, kindly
provided by Dr J. Isaacs, were obtained from a prostate biopsy on a
62-year old prostate cancer patient with a Gleason score of 4. Both
cell lines were grown and maintained in RPMI-1640 (Invitrogen)
media supplemented with 10% fetal bovine serum. Cells were cultured
in a humidified incubator at 37°C in a 5% CO2
atmosphere.
Creation of the PC3 CDK5dn cell line
Loss of CDK5 function was accomplished in PC3 cells
by transfection of a dominant-negative construct containing a D144N
mutation, kindly provided by Dr L.H. Tsai (Harvard Medical School)
(18). The protocol used has been
described previously (7). In brief,
the construct was subcloned in a bidirectional Tet vector, pBI-EGFP
(BD Biosciences), which had a zeocin resistance gene added for
selection (kindly provided by Dr K. Schuebel, Johns Hopkins
University School of Medicine). pBI-EGFP empty vector or pBI-EGFP
CDK5dn vector was transfected into PC3 cells which contained a
Tet-Off promoter construct, pTTa (BD Biosciences).
Western blotting
Western blotting was performed as described
previously (19). Ten micrograms of
protein was loaded on the gel. Primary antibodies were dissolved in
blocking buffer [5% milk in TBST (100 mM Tris-HCl pH 7.4, 0.1%
Tween-20, 150 mM NaCl in H2O)]. A 1:1,000 dilution was
used for anti- CDK5 (Sigma-Aldrich); anti-vinculin (Millipore,
Upstate) was diluted 1:4,000. Secondary antibodies were diluted at
a 1:4,000 dilution. Normalization of the band intensity was carried
out with the housekeeper protein vinculin. Developed blots were
scanned using a Microtek scanner.
Wound healing assay
Wound healing assays were performed with confluent
PC3 control (containing the empty pBI-EGFP vector) or PC3 CDK5dn
cells. A rubber-tipped scraper was used to scrape off an area of
cells. Light microscopic images were captured immediately and 24 h
after scraping.
Small-molecule library screening
The JHDL library has been described previously
(13,14,17).
Storage and screening of JHDL compounds were carried out as
described previously (17).
Briefly, PC3 control and CDK5dn cells were seeded in 96-well plates
(1×103 cells/well) and allowed to adhere overnight. Then
5 μl of drugs, stored as stock solutions of 200 μM in
DMSO/H2O, was added to complete RPMI media, so that
cells were treated at a final concentration of 10 μM. After 48 h of
treatment, 20 μl of MTS reagent from the CellTiter 96™ Aqueous
Non-Radioactive Cell Proliferation Assay [a reagent containing
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) and phenazine methosulfate (PMS); Promega] was added to each
well for a duration of 2–4 h at 37°C. Plates were analyzed using a
SoftMax Pro plate reader (Molecular Devices). Proliferation of
treated cells was compared with proliferation of DMSO-treated PC3
control or CDK5dn cells (proliferation index). Proliferation
indices of PC3 CDK5dn cells were compared to the proliferation
indices of PC3 control cells. A PubMed study was performed to
assess the clinical use of potential hits.
MTS assays
MTS assays were performed to measure the
antiproliferative effect of tilorone treatment. Tilorone
dihydrochloride (Sigma-Aldrich) was stored as a 10 mM stock
solution in DMSO at −20°C. One thousand PC3 cells were plated in
96-well plates containing 100 μl complete RPMI media. At circa 50%
confluence, tilorone dihydrochloride was administered. For
experiments the compound was diluted in complete RPMI media to
obtain the desired final concentration. After treatment for 72 h
(tilorone monotherapy), MTS reagent was added, and absorption at
490 nm was determined using a SoftMax Pro plate reader.
Proliferation indices were calculated; untreated PC3 control or
CDK5dn cells (in 103 μl complete RPMI media) were used as a
control. Student’s t-tests were performed to assess p-values.
Clonogenic assays
Clonogenic assays were performed to assess long-term
survival after tilorone treatment. Prostate cancer cells were
plated in 60 mm dishes and allowed to adhere. At 50–60% confluency,
cells were treated with tilorone for 72 h. Subsequently,
1×103 cells from each dish were plated in triplicate in
60-mm dishes and incubated in complete RPMI media for 12 days.
Colonies were fixed and stained with a solution containing 90%
methanol and 10% crystal violet solution (2.3% crystal violet, 0.1%
ammonium oxalate and 20% ethyl alcohol; Sigma). Colonies were
scanned with a computer scanner (Microtek) and counted manually.
Student’s t-tests were performed to evaluate whether differences
between cell lines were statistically significant.
3D growth assay
3D growth assays were performed utilizing the same
protocol as described previously (17). In short, spheroids were generated by
culturing PC3 cells for 16 h as a hanging drop over a humidified
plate in a CO2 incubator in complete RPMI media
containing 0.5% methylcellulose. Spheroids were embedded in
collagen matrix (BD Biosciences), treated with tilorone, and imaged
using a Nikon Eclipse Ti microscope (Nikon) on the day of treatment
and six days after treatment start. Spheroid and total (spheroid
plus sprouts) areas were measured with ImageJ. Fold increases were
calculated by dividing the spheroid/total area at day 6 by the
spheroid/total area on day 0 for each individual spheroid. For each
cell line and time point, fold increases of four spheroids were
averaged. Statistical analyses were performed using Student’s
t-tests.
Results
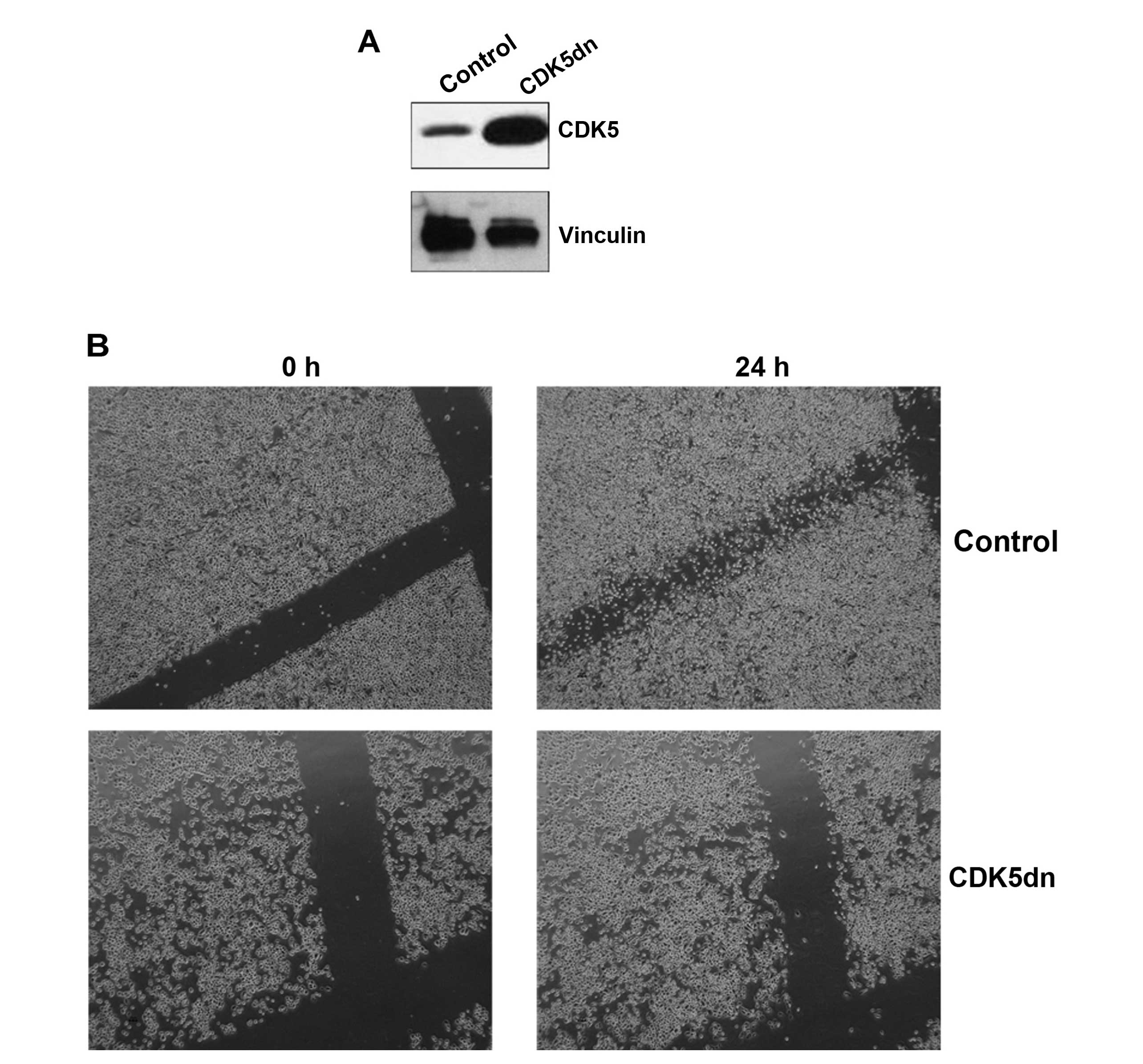
Suppression of CDK5 activity
PC3 prostate cancer cells were chosen for the JHDL
compound screen due to their highly metastatic potential and
androgen independence, thereby resembling aggressive metastatic
castrate-resistant prostate cancer. CDK5 activity was inhibited by
transfection and selection of a dominant-negative mutation (CDK5
144N). These PC3 CDK5dn cells had a higher protein level of total
CDK5 as compared to the PC3 control cells (PC3 cells transfected
with an empty vector) (Fig. 1A). A
wound healing assay (8) confirmed
that CDK5 was functionally inactive in these cells; unlike the PC3
control cells, PC3 CDK5dn cells did not have the ability to invade
the scraped surface area (Fig.
1B).
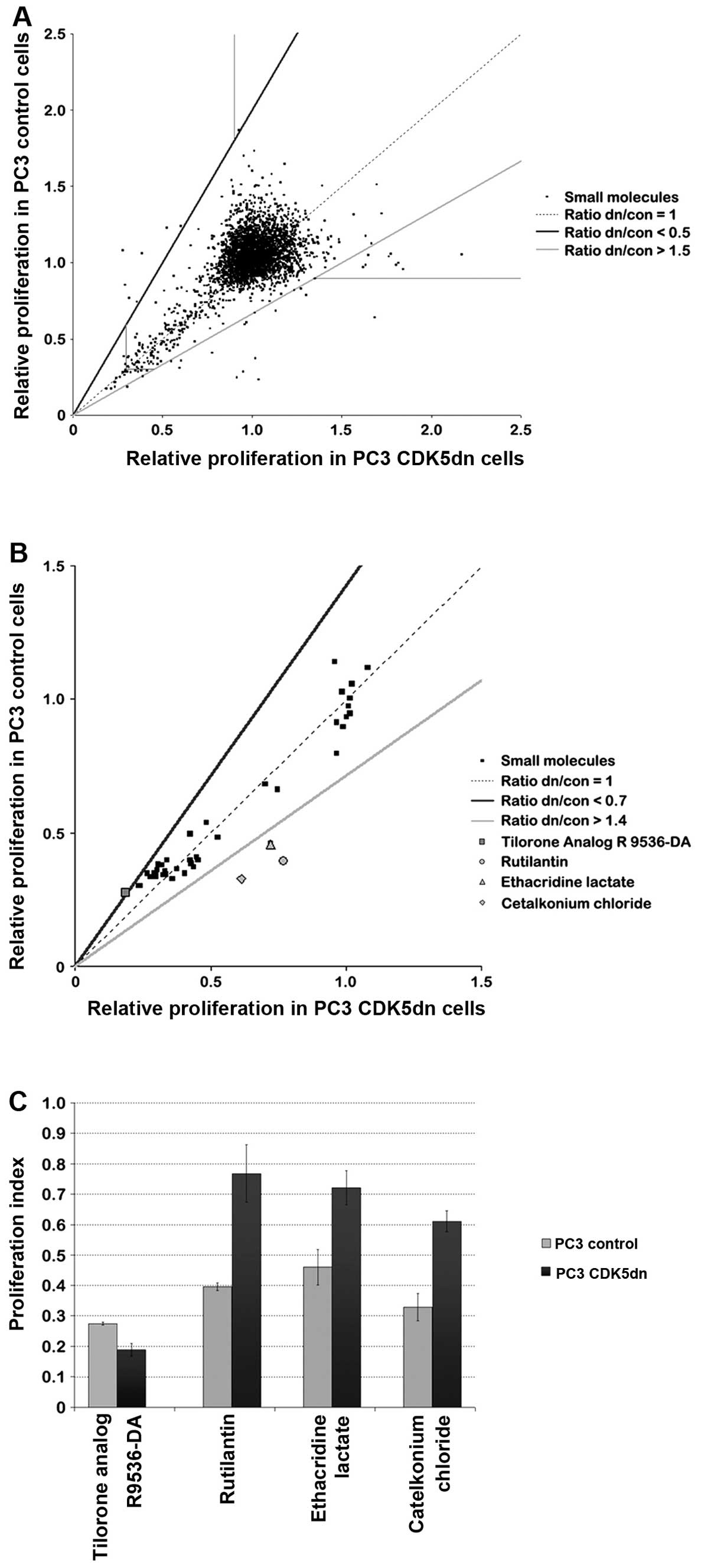
Library screen for compounds targeting
PC3 cells based on CDK5 activity
A high-throughput screening assay was performed to
select compounds that target PC3 cells based on CDK5 activity. PC3
control and CDK5dn cells were treated with all compounds of the
JHDL at 10 μM for 48 h. To identify hits that selectively target
PC3 cells based on CDK5 expression, we selected all compounds in
which the proliferation index ratio (CDK5dn/control) was below 0.5
or above 1.5 (Fig. 2A).
Furthermore, hits had to inhibit cell proliferation of PC3 cells by
at least 10%, as we were specifically interested in compounds that
inhibited cell growth (horizontal and vertical line in graph). We
also selected all compounds that inhibited cell proliferation in
PC3 cells by 70% (bottom left corner of the graph), as we were
interested in identifying potential highly effective antitumor
agents. In total, 41 hits were selected for further evaluation.
A secondary screen was performed in which selected
hits from the primary screen were added at 10 μM for 48 h to PC3
control and CDK5dn cells in triplicate, to weed out false positive
results (Fig. 2B). Cutoff values
were slightly less strict than in the primary screen; compounds
were considered a hit when the ratio of proliferation indices
(CDK5dn/control) was below 0.7 or above 1.4. This resulted in the
identification of three compounds that selectively target
CDK5-expressing PC3 cells: rutilantin, ethacridine lactate and
cetalkonium chloride (Fig. 2C).
These compounds have not been used as antitumor agents and their
potential clinical use as intravenous antitumor agents seems
limited (20–23). Another compound, tilorone analog
R9536-DA, was highly effective in inhibiting both isogenic PC3 cell
lines (>70% inhibition), but it inhibited proliferation of PC3
CDK5dn cells somewhat more effectively (ratio CDK5dn/control:
0.687). Tilorone and its analogs have antiviral activity, acting at
least in part as interferon inducers (24–26)
and have been shown preclinically and clinically to have antitumor
activity as well (27,28).
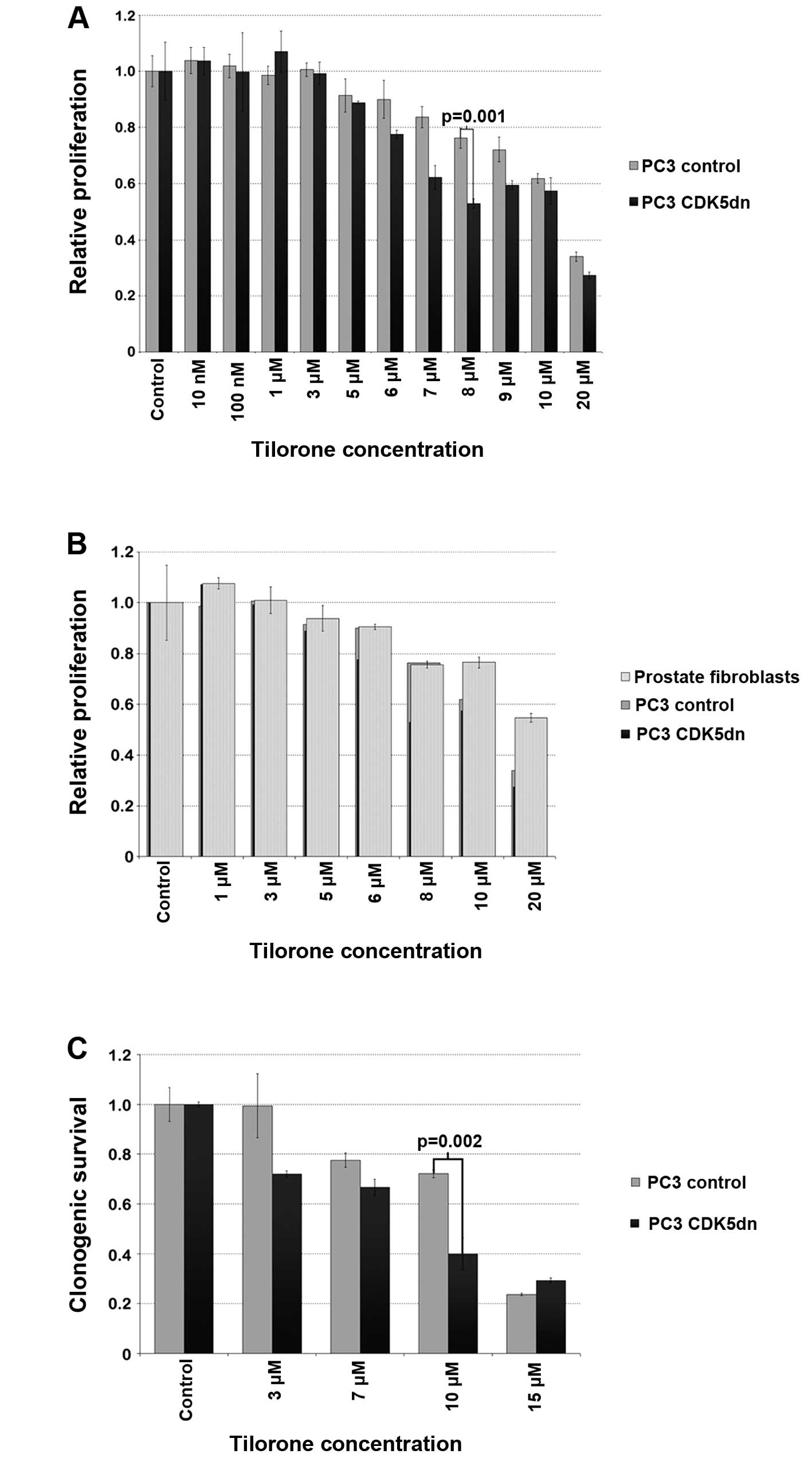
Tilorone selectively targets PC3 cells
with low CDK5 activity
We continued our experiments with freshly dissolved
tilorone dihydrochloride. After 72 h of tilorone treatment at
various concentrations, its IC50 was established at 8–12
μM in PC3 CDK5dn cells and 15 μM in PC3 control cells in MTS assays
(Fig. 3A). At 8 μM, proliferation
activity was decreased by 24 and 47% in the PC3 control and CDK5dn
cells, respectively (p=0.001). To assess toxicity of tilorone in
normal prostate cells, MTS assays were performed with tilorone
treatment of human prostate fibroblasts (Fig. 3B). Sensitivity of these cells to
tilorone was similar to that of the PC3 control cells.
The inhibitory effect of tilorone in PC3 cells was
further assessed by performing clonogenic assays (Fig. 3C). PC3 CDK5dn cells were also
significantly more sensitive than PC3 control cells to tilorone in
this assay. Treatment with 10 μM tilorone resulted in clonogenic
survival of 40% in PC3 CDK5dn cells and 72% in PC3 control cells
(p=0.002).
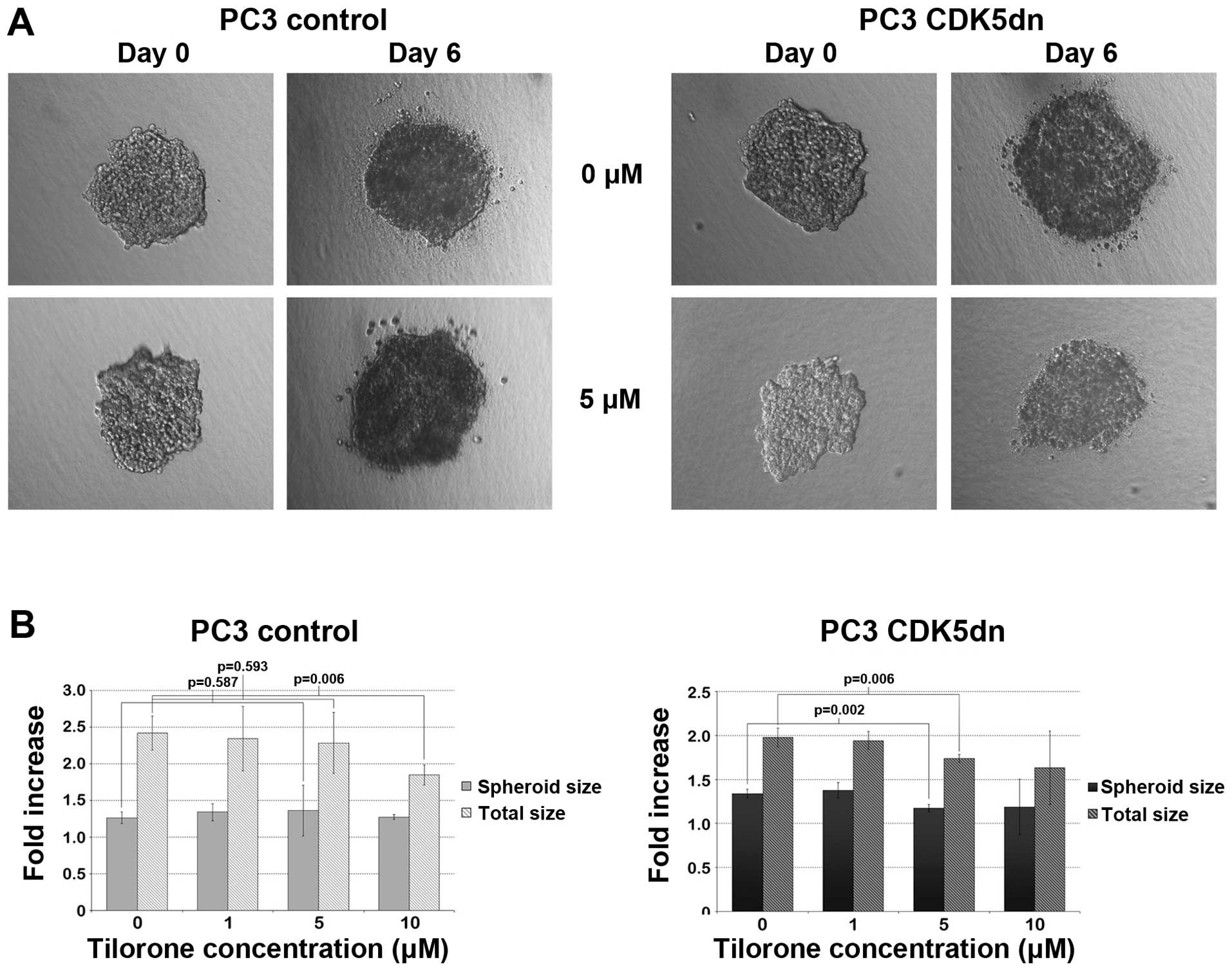
A spheroid growth assay was performed to assess 3D
tumor growth and invasion of PC3 cells upon tilorone treatment
(Fig. 4). Both PC3 control and PC3
CDK5dn cells had comparable increases in spheroid size over six
days. However, total size (the size of spheroids plus sprouts) had
a higher fold increase in PC3 control cells, confirming that
untreated PC3 CDK5dn cells had a decreased invasive potential as
compared to PC3 control cells. When tilorone was administered at 5
μM, PC3 control spheroids had a similar growth and invasive pattern
as the untreated PC3 control cells (p=0.59) (Fig. 4B, left graph). However, when
tilorone was administered at the same concentration to PC3 CDK5dn
cells, a significant decrease in both spheroid size and total size
was observed (p<0.01), suggesting that tilorone successfully
inhibits spheroid growth and invasion of PC3 CDK5dn cells when
administered at 5 μM (Fig. 4B,
right graph). At 10 μM, both isogenic cell lines had a decreased
invasive potential.
Discussion
The JHDL, a library of well characterized
pharmaceutical compounds, was developed to facilitate drug
repurposing studies (29). The
extensive in vivo toxicity and pharmacokinetic profiles of
compounds in the library allow rapid subsequent development of
these compounds. Several compounds from the JHDL have been advanced
to clinical trials for cancer and other therapeutic applications
(13,14,16,17,30–32).
In the present study we screened the JHDL for
compounds that differentially inhibit cancer cell growth in the
presence of CDK5 inhibition; tilorone and a tilorone analog were
identified as agents that selectively target CDK5-deficient PC3
prostate cancer cells. Tilorone (Amixin IC) is employed clinically
in some countries as an orally active antiviral agent (25). Tilorone has been tested in humans
for the treatment of cerebral gliomas, laryngeal papillomatosis and
breast cancer (28,33,34).
Although antitumor efficacy was reported, interest in tilorone for
cancer therapy has subsided. Recently, Zhou et al reported
new tilorone analogs with improved anticancer activity (35). These analogs may be promising to
examine, particularly in combination with CDK5 inhibition.
In addition to the possibility that tilorone may be
promising in combination with CDK5 inhibition, the identification
of tilorone as an agent that selectively targets cells with
inactive CDK5 suggests potential classes of drugs to potentiate the
efficacy of CDK5 inhibition. Tilorone has been characterized as an
interferon inducer (24). This
suggests that interferon itself, or an alternative interferon
inducer such as a TLR agonist, may be useful in combination with a
CDK5 inhibitor. Nevertheless, other mechanisms may be involved. For
example, tilorone is a DNA intercalating agent as well (24) and one may envision that it may
modulate chromatin structure and gene expression. Other functions
of tilorone, including signaling pathway and transcription factor
interactions (36,37), may also be involved. Further studies
are needed to unravel the exact mechanism of action by which
tilorone selectively targets CDK5-negative prostate cancer
cells.
Acknowledgements
The authors wish to thank Professors P.J. Van Diest
and E. Van der Wall for their support and discussion and Professors
M.A. Carducci and J.T. Isaacs for their provision of laboratory
materials. This study was supported by the Flight Attendant Medical
Research Institute, NCI R01 CA085567, R01 CA134767, DOD grant
W81XWH-06-1-0139 and NCI SPORE grant P50 CA58236. M.D.W. was
supported by the Dr Saal van Zwanenbergstichting and Huygens
Scholarship Programme.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
2
|
Tarricone C, Dhavan R, Peng J, Areces LB,
Tsai LH and Musacchio A: Structure and regulation of the
CDK5-p25(nck5a) complex. Mol Cell. 8:657–669. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosales JL and Lee KY: Extraneuronal roles
of cyclin-dependent kinase 5. Bioessays. 28:1023–1034. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lalioti V, Pulido D and Sandoval IV: Cdk5,
the multifunctional surveyor. Cell Cycle. 9:284–311. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dhavan R and Tsai LH: A decade of CDK5.
Nat Rev Mol Cell Biol. 2:749–759. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cicero S and Herrup K: Cyclin-dependent
kinase 5 is essential for neuronal cell cycle arrest and
differentiation. J Neurosci. 25:9658–9668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strock CJ, Park JI, Nakakura EK, Bova GS,
Isaacs JT, Ball DW and Nelkin BD: Cyclin-dependent kinase 5
activity controls cell motility and metastatic potential of
prostate cancer cells. Cancer Res. 66:7509–7515. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feldmann G, Mishra A, Hong SM, et al:
Inhibiting the cyclin-dependent kinase CDK5 blocks pancreatic
cancer formation and progression through the suppression of Ras-Ral
signaling. Cancer Res. 70:7509–7515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu FN, Chen MC, Chiang MC, et al:
Regulation of androgen receptor and prostate cancer growth by
cyclin-dependent kinase 5. J Biol Chem. 286:33141–33149. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demelash A, Rudrabhatla P, Pant HC, et al:
Achaete-scute homologue-1 (ASH1) stimulates migration of lung
cancer cells through Cdk5/p35 pathway. Mol Biol Cell. 23:2856–2866.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu FN, Chen MC, Lin KC, et al:
Cyclin-dependent kinase 5 modulates STAT3 and androgen receptor
activation through phosphorylation of Ser727on STAT3 in
prostate cancer cells. Am J Physiol Endocrinol Metab.
305:E975–E986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pozo K, Castro-Rivera E, Tan C, et al: The
role of Cdk5 in neuroendocrine thyroid cancer. Cancer Cell.
24:499–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chong CR, Qian DZ, Pan F, Wei Y, Pili R,
Sullivan DJ and Liu JO: Identification of type 1 inosine
monophosphate dehydrogenase as an antiangiogenic drug target. J Med
Chem. 49:2677–2680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ
and Liu JO: Inhibition of angiogenesis by the antifungal drug
itraconazole. ACS Chem Biol. 2:263–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rudin CM, Liu W, Desai A, et al:
Pharmacogenomic and pharmacokinetic determinants of erlotinib
toxicity. J Clin Oncol. 26:1119–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang H, Qian DZ, Tan YS, et al: Digoxin
and other cardiac glycosides inhibit HIF-1alpha synthesis and block
tumor growth. Proc Natl Acad Sci USA. 105:19579–19586. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wissing MD, Mendonca J, Kim E, et al:
Identification of cetrimonium bromide and irinotecan as compounds
with synthetic lethality against NDRG1 deficient prostate cancer
cells. Cancer Biol Ther. 14:401–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nikolic M, Dudek H, Kwon YT, Ramos YF and
Tsai LH: The cdk5/p35 kinase is essential for neurite outgrowth
during neuronal differentiation. Genes Dev. 10:816–825. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kachhap SK, Rosmus N, Collis SJ, et al:
Downregulation of homologous recombination DNA repair genes by HDAC
inhibition in prostate cancer is mediated through the E2F1
transcription factor. PLoS One. 5:e112082010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O’Meara S, Al-Kurdi D, Ologun Y and
Ovington LG: Antibiotics and antiseptics for venous leg ulcers.
Cochrane Database Syst Rev. CD0035572010.PubMed/NCBI
|
|
21
|
Hou SP, Fang AH, Chen QF, Huang YM, Chen
OJ and Cheng LN: Termination of second-trimester pregnancy by
mifepristone combined with misoprostol versus intra-amniotic
injection of ethacridine lactate (Rivanol®): a
systematic review of Chinese trials. Contraception. 84:214–223.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Daull P, Lallemand F and Garrigue JS:
Benefits of cetalkonium chloride cationic oil-in-water
nanoemulsions for topical ophthalmic drug delivery. J Pharm
Pharmacol. May 26–2013.(Epub ahead of print). View Article : Google Scholar
|
|
23
|
Hume V, Westwood JC and Appleyard G: The
anti-viral action of Rutilantin A. J Gen Microbiol. 38:143–151.
1965. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krueger RE and Mayer GD: Tilorone
hydrochloride: an orally active antiviral agent. Science.
169:1213–1214. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mayer GD and Krueger RF: Tilorone
hydrochloride: mode of action. Science. 169:1214–1215. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tazulakhova EB, Parshina OV, Guseva TS and
Ershov FI: Russian experience in screening, analysis, and clinical
application of novel interferon inducers. J Interferon Cytokine
Res. 21:65–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Adamson RH: Antitumor activity of tilorone
hydrochloride against some rodent tumors: preliminary report. J
Natl Cancer Inst. 46:431–434. 1971.PubMed/NCBI
|
|
28
|
Cummings FJ, Gelman R, Skeel RT, Kuperminc
M, Israel L, Colsky J and Tormey D: Phase II trials of Baker’s
antifol, bleomycin, CCNU, streptozotocin, tilorone, and
5-fluorodeoxyuridine plus arabinosyl cytosine in metastatic breast
cancer. Cancer. 48:681–685. 1981.
|
|
29
|
Chong CR and Sullivan DJ: New uses for old
drugs. Nature. 448:645–646. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shim JS, Matsui Y, Bhat S, et al: Effect
of nitroxoline on angiogenesis and growth of human bladder cancer.
J Natl Cancer Inst. 102:1855–1873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shim JS, Rao R, Beebe K, Neckers L, Han I,
Nahta R and Liu JO: Selective inhibition of HER2-positive breast
cancer cells by the HIV protease inhibitor nelfinavir. J Natl
Cancer Inst. 104:1576–1590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang HC, Xing S, Shan L, et al:
Small-molecule screening using a human primary cell model of HIV
latency identifies compounds that reverse latency without cellular
activation. J Clin Invest. 119:3473–3486. 2009.PubMed/NCBI
|
|
33
|
Lisianyi MI and Skitiak SA: Use of amiksin
in complex therapy of cerebral gliomas. Lik Sprava. 121–123.
2002.(In Ukranian).
|
|
34
|
Karimova FS, Ivanchenko GF and Grigorian
SS: The treatment of laryngeal papillomatosis with interferon
inducers. Vestn Otorinolaringol. 54–57. 2000.(In Russian).
|
|
35
|
Zhou D, Tuo W, Hu H, et al: Synthesis and
activity evaluation of tilorone analogs as potential anticancer
agents. Eur J Med Chem. 64:432–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ratan RR, Siddiq A, Aminova L, et al:
Small molecule activation of adaptive gene expression: tilorone or
its analogs are novel potent activators of hypoxia inducible
factor-1 that provide prophylaxis against stroke and spinal cord
injury. Ann NY Acad Sci. 1147:383–394. 2008. View Article : Google Scholar
|
|
37
|
Schrimpf MR, Sippy KB, Briggs CA, et al:
SAR of α7 nicotinic receptor agonists derived from tilorone:
exploration of a novel nicotinic pharmacophore. Bioorg Med Chem
Lett. 22:1633–1638. 2012.
|