Introduction
Gastric cancer is the leading cause of
cancer-related mortality worldwide and its incidence continues to
rise in both developed and developing countries (1). Population-based studies have shown
that the incidence rate approximates the death rate, which is
>730,000 annually. Hence, most patients who develop gastric
cancer will succumb to this disease (2). Gastric cancer itself is highly
malignant and exhibits an inherited predisposition to infiltrate
and metastasize. At present, the mechanisms underlying gastric
cancer initiation, progression and metastasis are not fully
understood (3).
It is well known that cellular proteins must be
cleaved by protein convertases before maturation. Furin is the best
characterized representative of the mammalian subtilisin-like
family of proprotein convertases. The propeptide-furin complex
leaves the endoplasmic reticulum (ER) and enters the trans-Golgi
network (TGN) for its second activational cleavage (4). This step results in furin, which can
process substrates in multiple compartments in the TGN/endosomal
system (5). Among the known furin
substrates are precursors of hormones, neuropeptides, growth
factors, adhesion molecules, receptors, surface proteins, viral
glycoproteins and bacterial toxins (6–8). Two
cellular migration and invasion proteins, pro-MT1-MMP and vascular
endothelial growth factor (VEGF), must also be cleaved by furin for
their activation (9).
Due to its important role in cleavage-mediated
protein activation, furin is considered a potential prognostic, or
even therapeutic, factor for tumorigenesis. Therefore, many medical
center doctors, including us, have become interested in the
mechanisms by which furin may be involved in so many important
biochemical, clinical and therapeutic functions in tumor research
(10).
As a member of the Src family of non-receptor
tyrosine kinases, c-Src is often upregulated in a variety of human
tumors, including gastric cancer (11–13).
c-Src functions as a critical link between multiple signaling
pathways that regulate proliferation, invasion, survival,
metastasis and angiogenesis (14,15).
Molecular-targeted therapy of c-Src has thus emerged as a promising
treatment of gastric cancer. For example, one potential target is
the Src family kinase (SFK).
However, although c-Src and furin have both been
found to be upregulated in human cancer (16,17),
whether the ubiquitously expressed c-Src participates in the
interactions between furin and its substrates remains unknown.
In the present study, we detected the protein levels
and the interactions between furin and its substrates after either
stimulation with epidermal growth factor receptor (EGFR) ligands or
treatment with c-Src inhibitors in BGC-823 cells. In the present
study, we demonstrated that activation of furin is c-Src dependent
in gastric cancer cells and thus targeting the furin-c-Src
interface could be a promising strategy against gastric cancer
progression and metastasis.
Materials and methods
Cell culture and experimental
reagents
The gastric cancer cell line BGC-823 was cultured in
RPMI-1640 (Invitrogen, USA) supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin. All
cells were cultured in a 5% CO2 humidified atmosphere at
37°C. The EGFR agonist PDGF-BB (20 ng/ml, R&D, USA) or c-Src
inhibitors, PP2 or SU6656 (10 μM each), were added to cells
cultured in serum-free medium.
Primary antibodies against pSrc (Y416), furin,
MT1-MMP, VEGF-C and β-actin were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). The gelatin zymography kit was
from Millipore (USA) while 4-amino-5-(4-chlorophenyl)-7-(t-butyl)
pyrazolo [3,4-d] pyrimidine (PP2) and PDGF-BB were purchased from
Enzo Life Sciences International (USA). SU6656 was purchased from
Sigma (USA).
Gelatin zymography
Levels of the active and latent forms of MMP2 and
MMP9 were analyzed by gelatin zymography as described in the
manufacturer’s instructions. Briefly, BGC-823 cells were washed
with ice-cold PBS and lysed with RIPA buffer for 30 min on ice.
Lysates were then cleared by centrifugation at 12,000 × g for 20
min at 4°C. The supernatant was aliquoted and protein content was
determined using the BCA method (Pierce). Equal amounts of protein
were separated by gel electrophoresis. The gel was subsequently
washed and incubated at 37°C for 24 h, then stained with Coomassie
brilliant blue R250. Bands were examined after the gel was
destained by Coomassie Blue Staining Destaining Solution.
Wound healing assay
BGC-823 cells were grown to confluence in 24-well
plates. The monolayer was then artificially wounded using a sterile
200-μl pipette tip. Cell debris was removed by washing the
monolayer with PBS. The cells were then incubated with c-Src
inhibitors, PP2 or SU6656, at a dose of 10 μM. Wound closure was
monitored by photographing cell migration into the wound at various
time points at the same spot with an inverted microscope equipped
with a digital camera. The extent of healing was defined as the
ratio of the difference between the original and the remaining
wound areas compared with the original wound area.
Transwell invasion assay
Matrigel invasion chambers were hydrated for 4 h
before starting the invasion assay. Log-phase cells
(4×104) were then plated in 200 μl RPMI-1640 containing
10% FBS in the upper chambers of the Transwell. The lower chambers
were filled with 500 μl RPMI-1640 containing 10% FBS. After
incubation for 2 h, the cells were treated with 10 μM of either PP2
or SU6656 for 24 h. The cells were then allowed to migrate for 10 h
in the cell culture incubator. Then, the cells were fixed for 15
min at room temperature by replacing the culture medium in the
bottom and top chambers with 4% formaldehyde dissolved in PBS. The
cells that remained on the bottom of the chamber were stained with
0.1% crystal violet and photographed under an optical microscope.
The cell number was counted in 12 different fields of view. Data
were averaged from three parallel experiments, which were
normalized to those of the non-treated control.
Western blot analysis
Cells were lysed in RIPA buffer [50 mM Tris (pH
7.4), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% sodium
deoxycholate, 5 mM EDTA, 100 mM NaF and 1 mM
Na3VO4] containing protease inhibitor
cocktail for 30 min at 4°C. Cell lysates were then cleared by
centrifugation at 4°C at 16,000 × g for 30 min. The protein
concentrations were determined by the BCA (bicinchoninic acid)
method (Pierce, USA) and then equal protein amounts of cell lysates
were fractionated by electrophoresis in SDS-PAGE. Gels of 10% were
used for the analysis of furin and c-Src, while 12% gels were used
to analyze MT1-MMP and VEGF-C. Following electrophoresis, proteins
were electroblotted onto polyvinylidene fluoride (PVDF) membranes
using a wet transblot system (Bio-Rad, Hercules, CA, USA).
Membranes were then blocked with 10% bovine serum albumin (BSA) or
5% non-fat dry milk for 1 h at room temperature. Membranes were
next incubated overnight at 4°C with antibodies against pSrc
(Y416), furin, MT1-MMP, VEGF-C and β-actin, all diluted 1:1,000 in
phosphate-buffered saline with Tween-20 (PBST). After several
washes in PBST, the membranes were incubated for 1 h with
horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse
secondary antibodies, each diluted 1:5,000 in PBST. Membranes were
washed as before and the immunoreactive bands were processed using
the Super Signal West Pico chemiluminescent substrate (Pierce,
USA), followed by exposure to the Fujifilm LAS3000 Imager (Fuji,
Japan). Densitometric analysis was performed with the Image J
densitometer and Excel software.
Co-immunoprecipitation (co-IP)
BGC-823 cells were washed twice with ice-cold PBS,
lysed in 1 ml RIPA buffer for 30 min on ice and clarified by
centrifugation at 4°C at 10,000 × g. The supernatant was then
collected and subjected to IP. Briefly, each cell lysate (500 μg)
was incubated with 2 μg of the appropriate antibody (anti-c-Src or
anti-furin) overnight at 4°C. Protein G (50 μl) was then added and
the mixture was incubated at 4°C for 2 h with gentle agitation. The
pellet was retrieved by centrifugation and washed three times with
RIPA buffer. It was then boiled with 50 μl 2X loading buffer (Tris
pH 6.8, 0.1% SDS, 10% glycerol and 0.025% bromophenol blue, 20 mM
DTT) for 5 min prior to gel loading. Immunoreactive bands were
detected by western blot analysis with antibodies against furin,
c-Src, MT1-MMP, and VEGF-C. In some experiments, the secondary
antibody was substituted by Clean-Blot IP Detection Reagent for
clearer IP/western blot analysis results.
Statistical analysis
Western blots were quantified by measuring the
relative density of protein bands recognized by a particular
antibody using Image J software (NIH, USA). The results were
expressed as mean ± standard deviation. Statistical analysis was
performed with a Student’s t-test for comparison of two groups and
differences with P<0.05 were considered statistically
significant.
Results
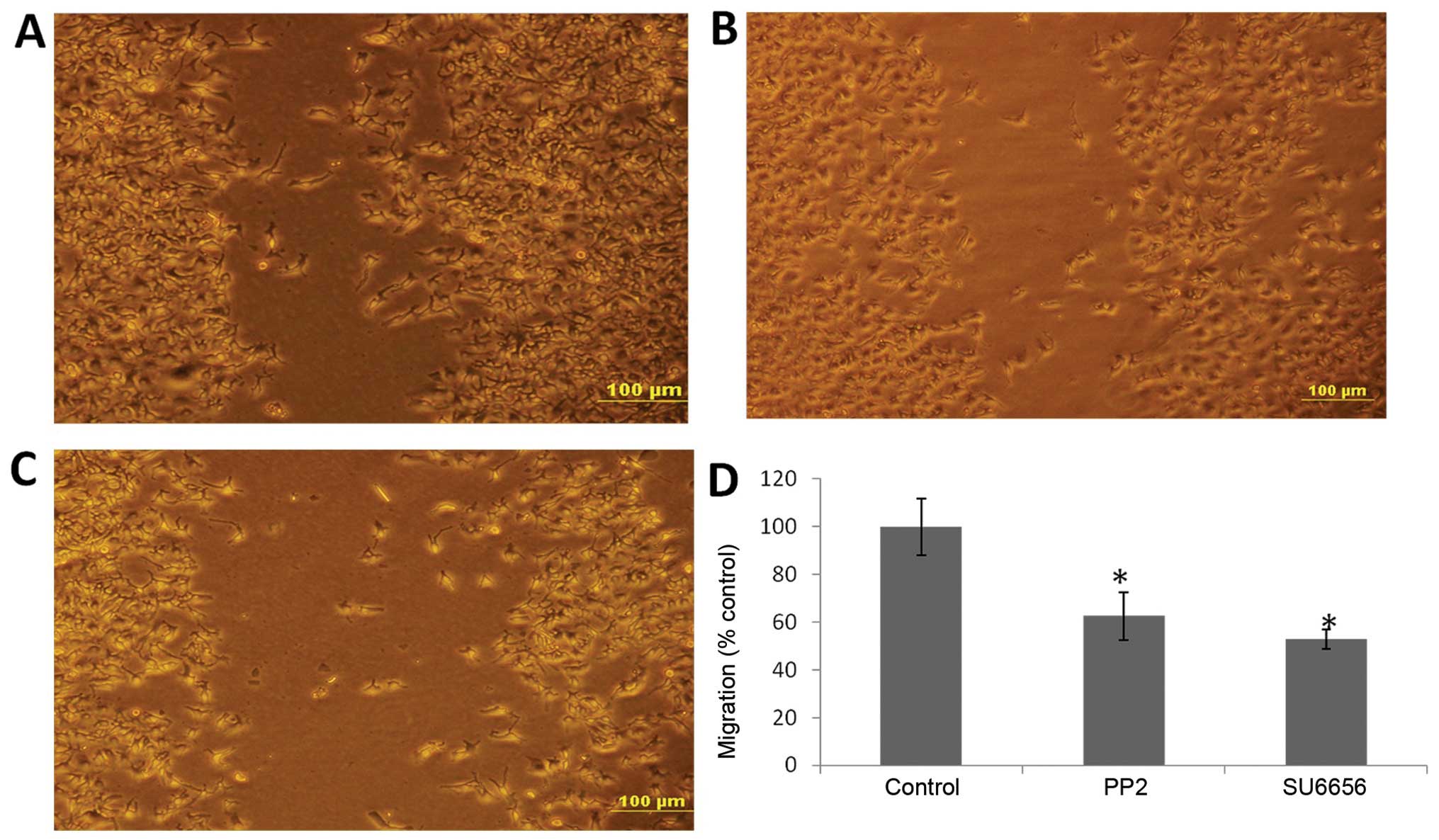
c-Src inhibitors decrease the invasive
and migratory capacity of BGC-823 cells
To detect whether the invasion and migration of
BGC-823 cells were regulated by c-Src activity, we performed wound
healing and Transwell assays. We observed that the invasive and
migratory abilities of cells treated with either PP2 or SU6656
decreased significantly compared with those of the control
(Figs. 1 and 2).
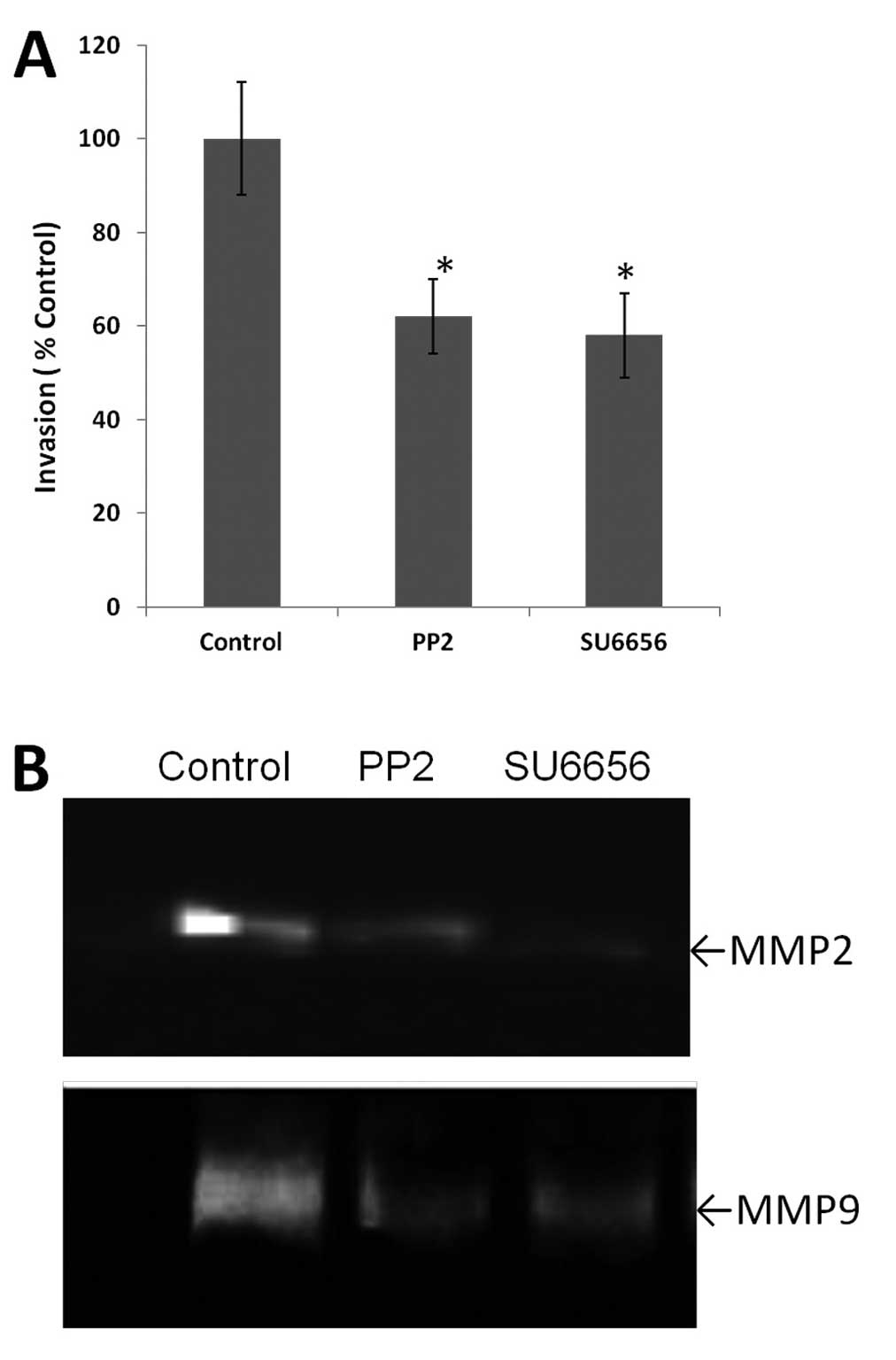
Role of c-Src inhibitors in the activity
of MMP2 and MMP9
The gelatin zymography assay was used to detect the
activity of MMP2 and MMP9 after c-Src inhibitor treatment in
BGC-823 cells. As shown in Fig. 2B,
the activity of MMP2 and MMP9 was decreased following treatment of
BGC-823 cells with either PP2 or SU6656 for 30 min prior to
stimulation with PDGF-BB.
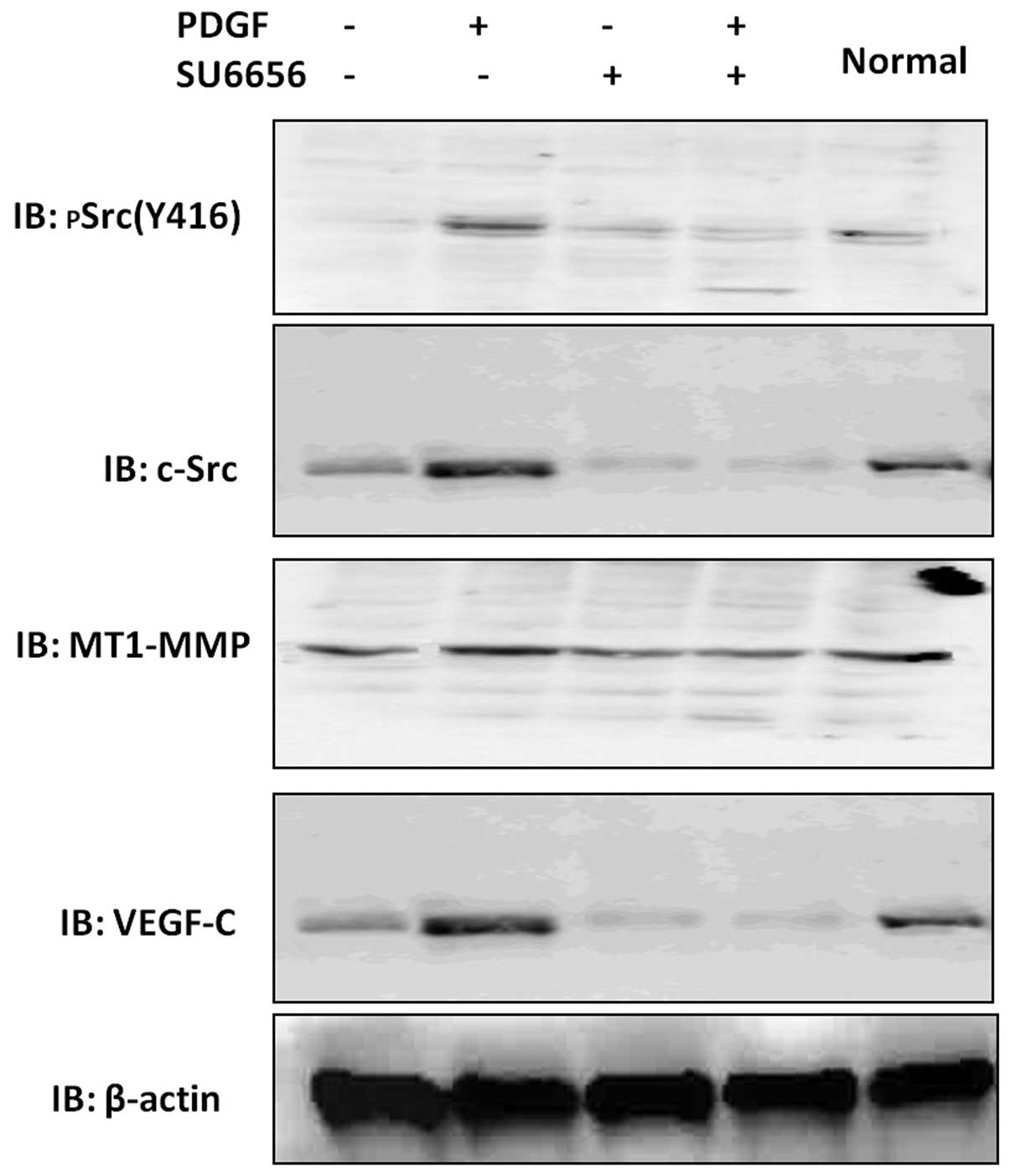
Effects of c-Src inhibitors on the
expression of furin and its substrates in BGC-823 cells
The expression levels of pSrc (Y416), MT1-MMP and
VEGF-C were detected by immunoblot analysis in BGC-823 cells
treated with 10 μM of either PP2 or SU6656 for 24 h. Treatment with
PP2 or SU6656 resulted in a quantitative decrease in the pSrc
(Y416) band intensities (Fig. 3).
The protein levels of MT1-MMP and VEGF-C were also significantly
decreased, whereas furin protein expression showed no obvious
variation (data not shown).
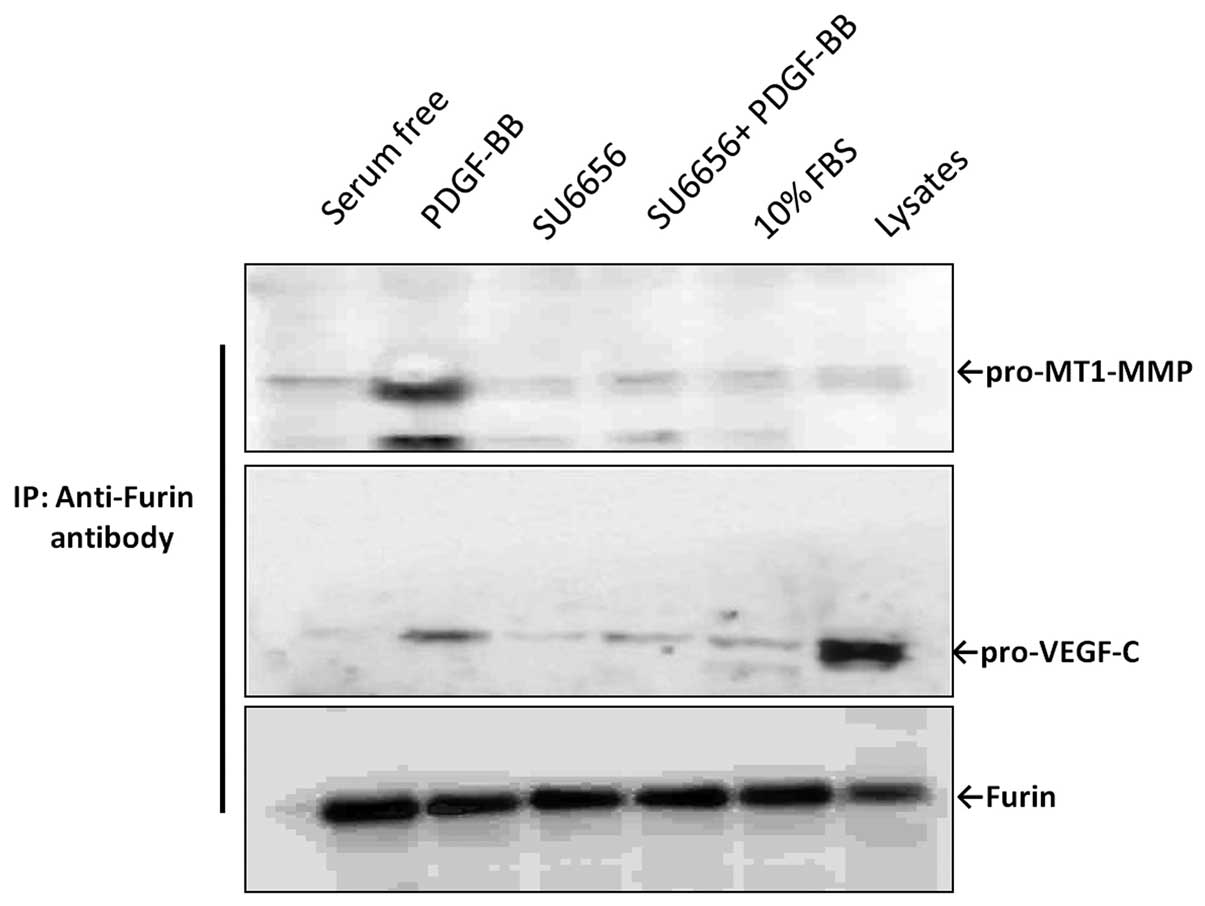
c-Src activity is required for efficient
association between furin and its substrates
To explore a possible role for c-Src in the
modulation of furin interaction with its substrates, we analyzed
the binding between furin and two substrates, pro-MT1-MMP or
pro-VEGF-C, in the presence or absence of the Src inhibitors,
through co-IP experiments (Fig. 4).
Briefly, BGC-823 cells were cultured in serum-free medium overnight
and were then stimulated with 20 ng/ml PDGF-BB for 30 min, with or
without pre-treatment with 10 μM of either PP2 or SU6656. Whole
cell lysates were then collected and immunoprecipitated with the
anti-furin antibody and the expression levels of MT1-MMP and VEGF-C
were investigated using specific antibodies. Our results showed
that MT1-MMP was found only in the PDGF-BB stimulation group, while
almost no band was detected in the SU6656 pre-treated group.
Similar results were observed for VEGF-C. Thus, these data suggest
that c-Src activity is necessary for efficient interaction between
furin and its substrates.
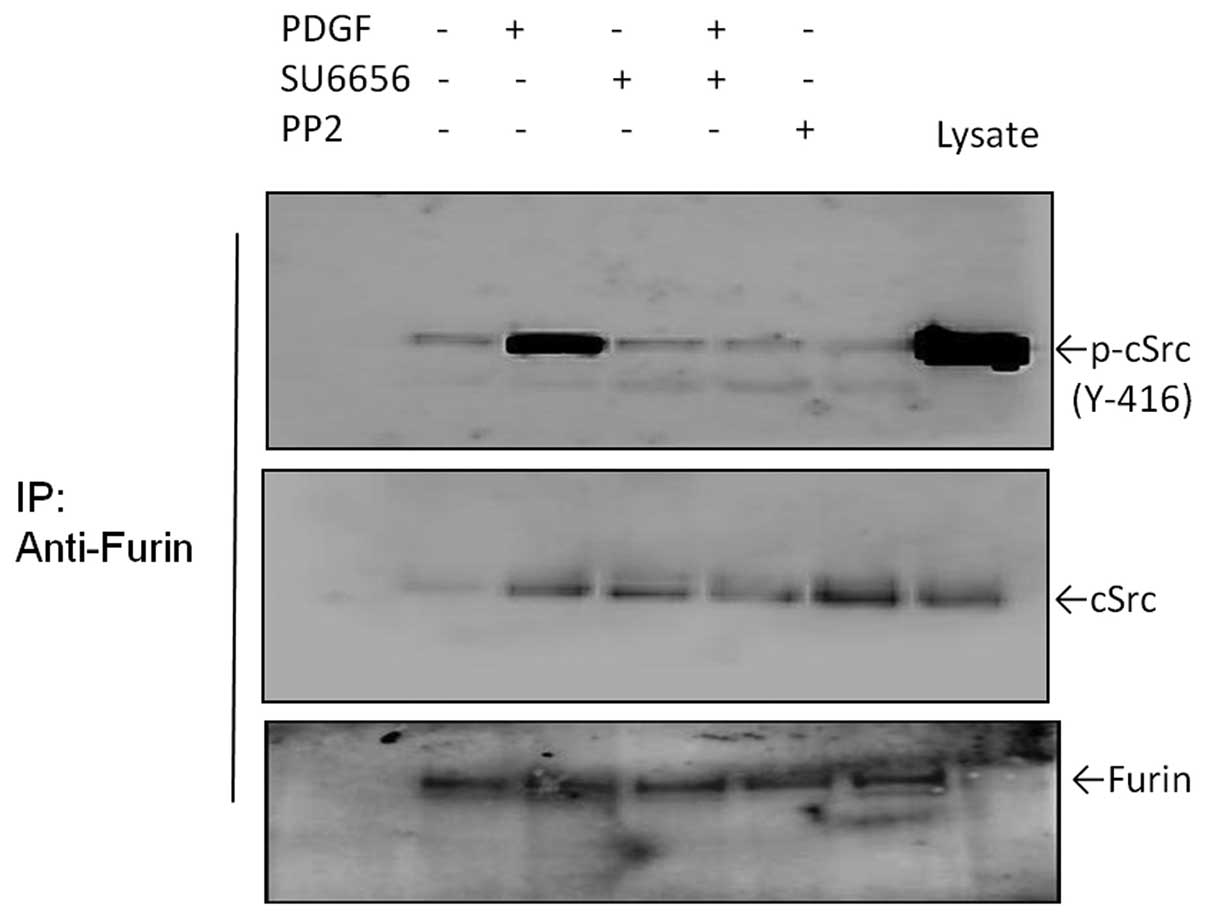
c-Src directly binds to furin in
vivo
It is unclear whether binding between c-Src and
furin exists and, if so, how it affects furin interaction with its
substrates in BGC-823 cells. Thus, we performed co-IP experiments
to test whether c-Src and furin are directly associated in BGC-823
cells. As shown in Fig. 5, we found
that significant endogenous amounts of c-Src and furin were
specifically immunoprecipitated with their respective antibodies.
Notably, we found readily detectable levels of activated c-Src in
the immunoprecipitations. These results suggest that endogenous
c-Src may physically associate with furin in vitro and this
binding may be required for the activity of c-Src.
Discussion
In the present study, we demonstrated that the
ability of BGC-823 cells to invade and migrate is decreased upon
treatment with c-Src inhibitors. Moreover, our results indicate
that c-Src activity may directly regulate BGC-823 cell invasion and
migration through modulation of the maturation of MT1-MMP and
VEGF-C.
Furin plays a crucial role in tumorigenesis
(16,17) and it has been suggested that it
could be a valuable marker for tumor progression and for predicting
the outcome of this disease (18).
Furin is a Ca2+-dependent cellular endoprotease that
activates a large number of precursor proteins in secretory pathway
compartments (19). Inhibition of
furin activity decreases substrate activation, which has been shown
to lead to both a reduced proliferation rate and invasive potential
of cancer cells. Thus, furin could be a potentially useful target
for anticancer therapeutics (20).
MT1-MMP and VEGF-C have been demonstrated to play
vital roles in the regulation of cancer cell invasion and migration
(21–23). Upregulation of MT1-MMP can
effectively elevate invasiveness in human cancer cells, including
gastric cancer (24–26). However, to be active, the zymogens
of MT1-MMP or VEGF must be cleaved from the propeptides by the
protein convertase furin (7,9,27).
Stawowy et al demonstrated that furin-like
proprotein convertase PC5 is strongly upregulated by PDGF-BB
through the PI3-kinase/p70s6-kinase pathway (28). We hypothesized that a similar
mechanism may apply to the convertase furin. Thus, we first
investigated whether furin or furin activity was regulated by
PDGF-BB through c-Src kinase and, second, how furin activity is
controlled to mediate the processing of two of its substrates,
MT1-MMP and VEGF-C.
To this end, we explored the effects of c-Src
inhibitors, PP2 and SU6656, on the regulation of cell migration,
invasion and the protein expression of MT1-MMP and VEGF-C in
BGC-823 cells. The results showed that MT1-MMP and VEGF-C protein
expression levels were decreased significantly in accordance with
reduced c-Src activity, while the protein level of furin remained
clearly unchanged (Figs. 3 and
4). These results indicated that
the regulation of MT1-MMP or VEGF-C was not dependent on the
alteration of furin protein expression levels. Therefore, another
mechanism should exist. Based on the above findings and
accumulating evidence in the literature, we proposed that c-Src may
have a potential role in the regulation of furin-mediated
maturation of its substrates.
Indeed, our results showed that while activation of
c-Src with PDGF-BB enhanced formation of a complex between furin
and pro-MT1-MMP, SU6656 treatment resulted in the reversion of this
interaction. Therefore, these data suggest that c-Src activity is
required for efficient association between furin and its substrate
pro-MT1-MMP. Similar results were observed when the interaction
between furin and VEGF-C was examined. Notably, we found that c-Src
directly interacts with furin in vivo in BGC-823 cells. This
interaction may have a potential role in the regulation of
furin-mediated maturation of its substrates.
In conclusion, our present study indicates that
binding between furin and pro-MT1-MMP/pro-VEGF is enhanced upon
c-Src activation. In contrast, the binding is decreased
significantly following c-Src inhibitor treatment. Hence, c-Src
activity may be used as a potential anticancer research approach.
Therefore, the binding between furin with pro-MT1-MMP or pro-VEGF-C
or other tumor-associated enzyme precursors can be regulated by
c-Src activity, thereby reducing or changing the expression of
these enzymes in order to inhibit the development of gastric cancer
invasion and metastasis.
Acknowledgements
This study was supported by a grant (no. 30972887)
from the National Natural Science Foundation of China.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Namikawa T and Hanazaki K:
Clinicopathological features and treatment outcomes of metastatic
tumors in the stomach. Surg Today. Jul 30–2013.(Epub ahead of
print).
|
|
3
|
He CZ and Zhang KH: Serum protein and
genetic tumor markers of gastric carcinoma. Asian Pac J Cancer
Prev. 14:3437–3442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vey M, Schafer W, Berghofer S, Klenk HD
and Garten W: Maturation of the trans-Golgi network protease furin:
compartmentalization of propeptide removal, substrate cleavage, and
COOH-terminal truncation. J Cell Biol. 127:1829–1842. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson ED, VanSlyke JK, Thulin CD, Jean
F and Thomas G: Activation of the furin endoprotease is a
multiple-step process: requirements for acidification and internal
propeptide cleavage. EMBO J. 16:1508–1518. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Lartigue J, Polson H, Feldman M, et al:
PIKfyve regulation of endosome-linked pathways. Traffic.
10:883–893. 2009.PubMed/NCBI
|
|
7
|
Kappert K, Meyborg H, Fritzsche J, et al:
Proprotein convertase subtilisin/kexin type 3 promotes adipose
tissue-driven macrophage chemotaxis and is increased in obesity.
PLoS One. 8:e705422013. View Article : Google Scholar
|
|
8
|
Lopez de Cicco R, Bassi DE, Zucker S, et
al: Human carcinoma cell growth and invasiveness is impaired by the
propeptide of the ubiquitous proprotein convertase furin. Cancer
Res. 65:4162–4171. 2005.PubMed/NCBI
|
|
9
|
Komiyama T, Coppola JM, Larsen MJ, et al:
Inhibition of furin/proprotein convertase-catalyzed surface and
intracellular processing by small molecules. J Biol Chem.
284:15729–15738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hilbig A: Src kinase and pancreatic
cancer. Recent Results Cancer Res. 177:179–185. 2008. View Article : Google Scholar
|
|
11
|
Okamoto W, Okamoto I, Yoshida T, et al:
Identification of c-Src as a potential therapeutic target for
gastric cancer and of MET activation as a cause of resistance to
c-Src inhibition. Mol Cancer Ther. 9:1188–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nam HJ, Im SA, Oh DY, et al: Antitumor
activity of saracatinib (AZD0530), a c-Src/Abl kinase inhibitor,
alone or in combination with chemotherapeutic agents in gastric
cancer. Mol Cancer Ther. 12:16–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Summy JM and Gallick GE: Treatment for
advanced tumors: SRC reclaims center stage. Clin Cancer Res.
12:1398–1401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee M, Choy WC and Abid MR: Direct sensing
of endothelial oxidants by vascular endothelial growth factor
receptor-2 and c-Src. PLoS One. 6:e284542011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tekedereli I, Alpay SN, Tavares CD, et al:
Targeted silencing of elongation factor 2 kinase suppresses growth
and sensitizes tumors to doxorubicin in an orthotopic model of
breast cancer. PLoS One. 7:e411712012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demidyuk IV, Shubin AV, Gasanov EV, et al:
Alterations in gene expression of proprotein convertases in human
lung cancer have a limited number of scenarios. PLoS One.
8:e557522013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Page RE, Klein-Szanto AJ, Litwin S, et al:
Increased expression of the pro-protein convertase furin predicts
decreased survival in ovarian cancer. Cell Oncol. 29:289–299.
2007.PubMed/NCBI
|
|
18
|
Thomas G: Furin at the cutting edge: from
protein traffic to embryogenesis and disease. Nat Rev Mol Cell
Biol. 3:753–766. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bassi DE, Mahloogi H, Lopez De Cicco R, et
al: Increased furin activity enhances the malignant phenotype of
human head and neck cancer cells. Am J Pathol. 162:439–447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strongin AY: Proteolytic and
non-proteolytic roles of membrane type-1 matrix metalloproteinase
in malignancy. Biochim Biophys Acta. 1803.133–141. 2010.PubMed/NCBI
|
|
21
|
Suboj P, Babykutty S, Valiyaparambil G,
Nair RS, Srinivas P and Gopala S: Aloe emodin inhibits colon cancer
cell migration/angiogenesis by downregulating MMP-2/9, RhoB and
VEGF via reduced DNA binding activity of NF-κB. Eur J Pharm Sci.
45:581–591. 2012.PubMed/NCBI
|
|
22
|
Stacker SA, Caesar C and Baldwin ME:
VEGF-D promotes the metastatic spread of tumor cells via the
lymphatics. Nat Med. 7:186–191. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tolde O, Rosel D, Mierke CT, et al:
Neoplastic progression of the human breast cancer cell line G3S1 is
associated with elevation of cytoskeletal dynamics and upregulation
of MT1-MMP. Int J Oncol. 36:833–839. 2010.PubMed/NCBI
|
|
24
|
Iravani O, Tay BW, Chua PJ, Yip GW and Bay
BH: Claudins and gastric carcinogenesis. Exp Biol Med (Maywood).
238:344–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zucker S, Pei D, Cao J and Lopez-Otin C:
Membrane type-matrix metalloproteinases (MT-MMP). Curr Top Dev
Biol. 54:1–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakanishi Y, Ohara M, Domen H, Shichinohe
T, Hirano S and Ishizaka M: Differences in risk factors between
patterns of recurrence in patients after curative resection for
advanced gastric carcinoma. World J Surg Oncol. 11:982013.
View Article : Google Scholar
|
|
27
|
Parker MW, Hellman LM, Xu P, et al: Furin
processing of semaphorin 3F determines its anti-angiogenic activity
by regulating direct binding and competition for neuropilin.
Biochemistry. 49:4068–4075. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stawowy P, Blaschke F and Kilimnik A:
Proprotein convertase PC5 regulation by PDGF-BB involves
PI3-kinase/p70s6-kinase activation in vascular smooth
muscle cells. Hypertension. 39:399–406. 2002. View Article : Google Scholar : PubMed/NCBI
|