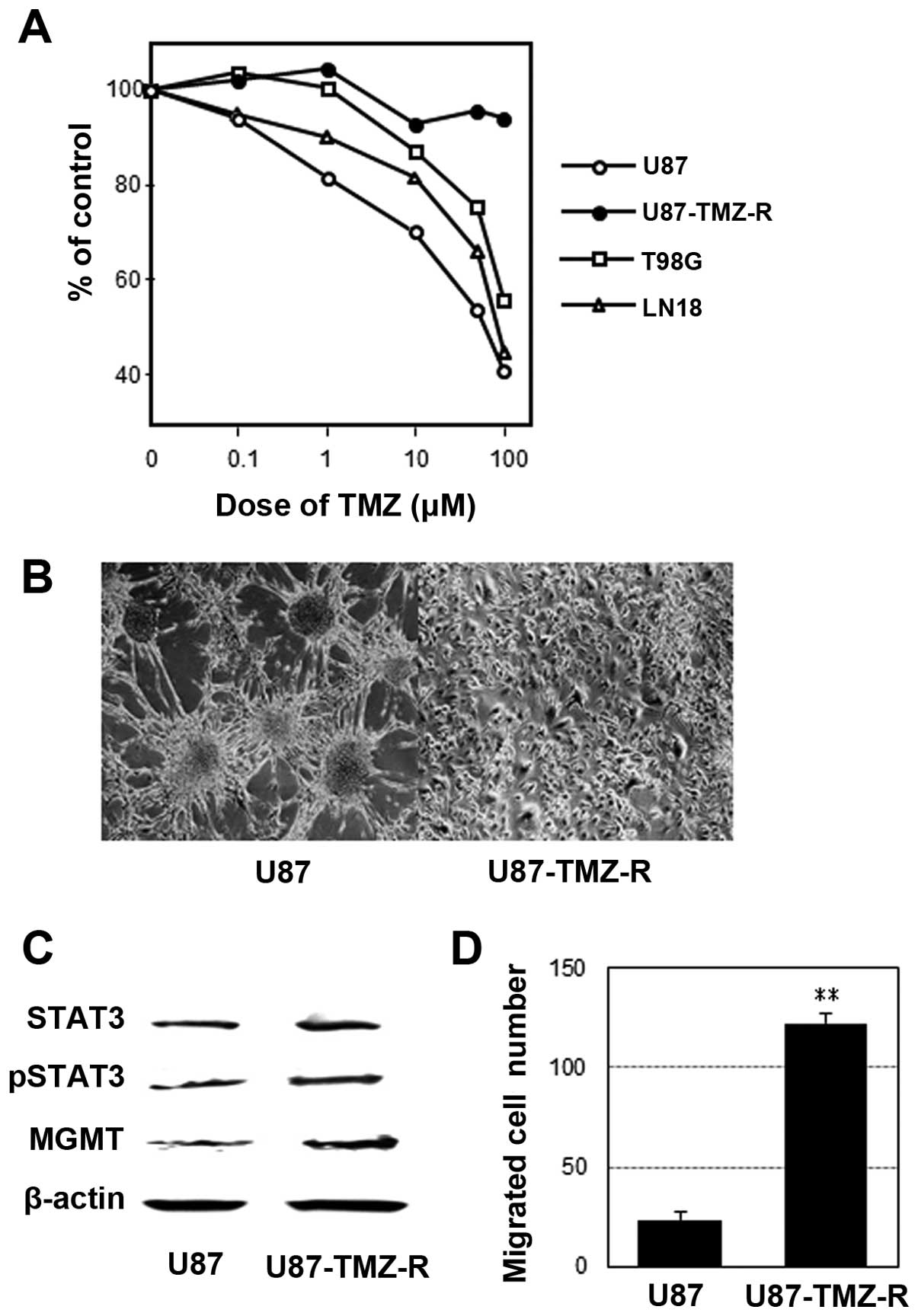
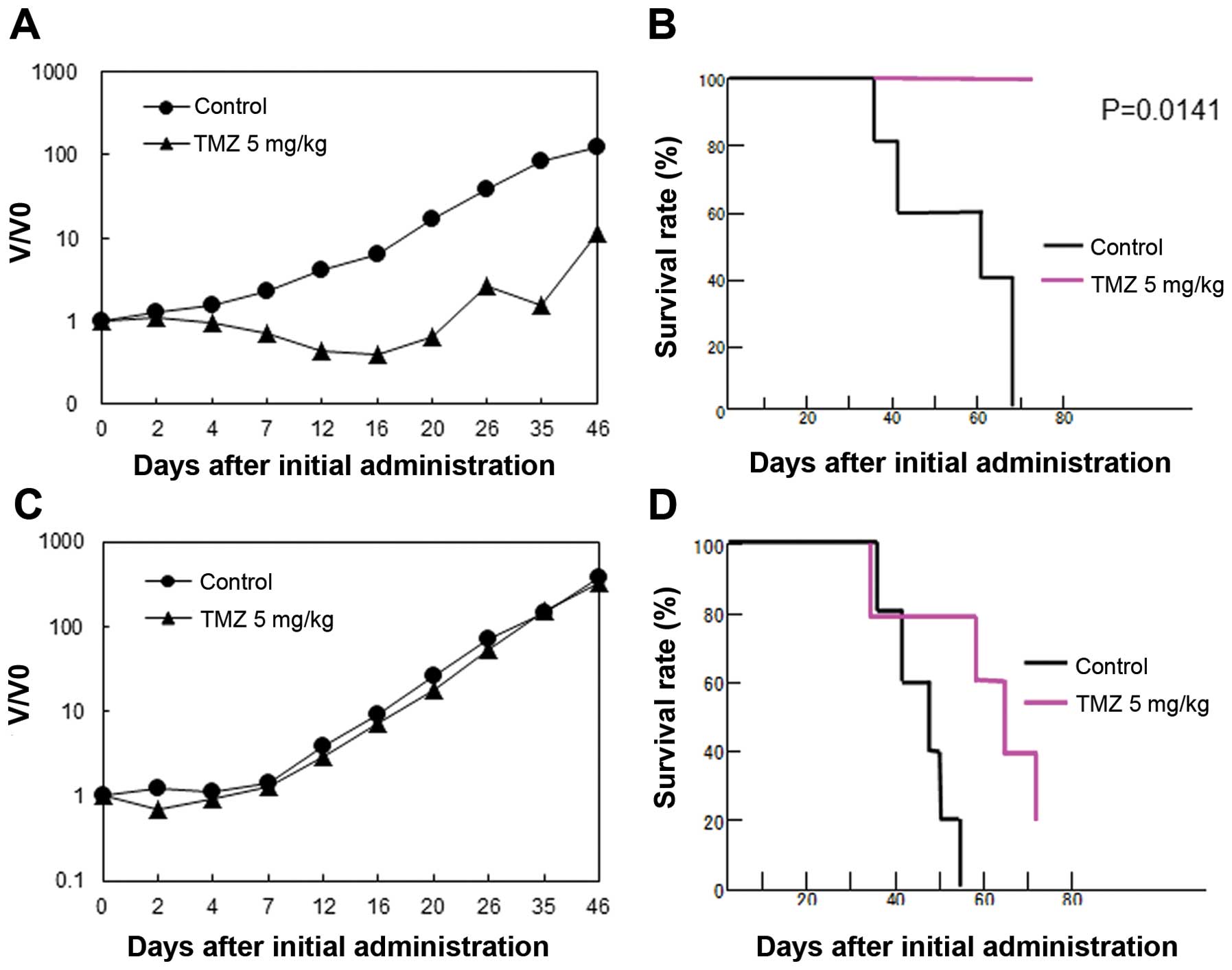
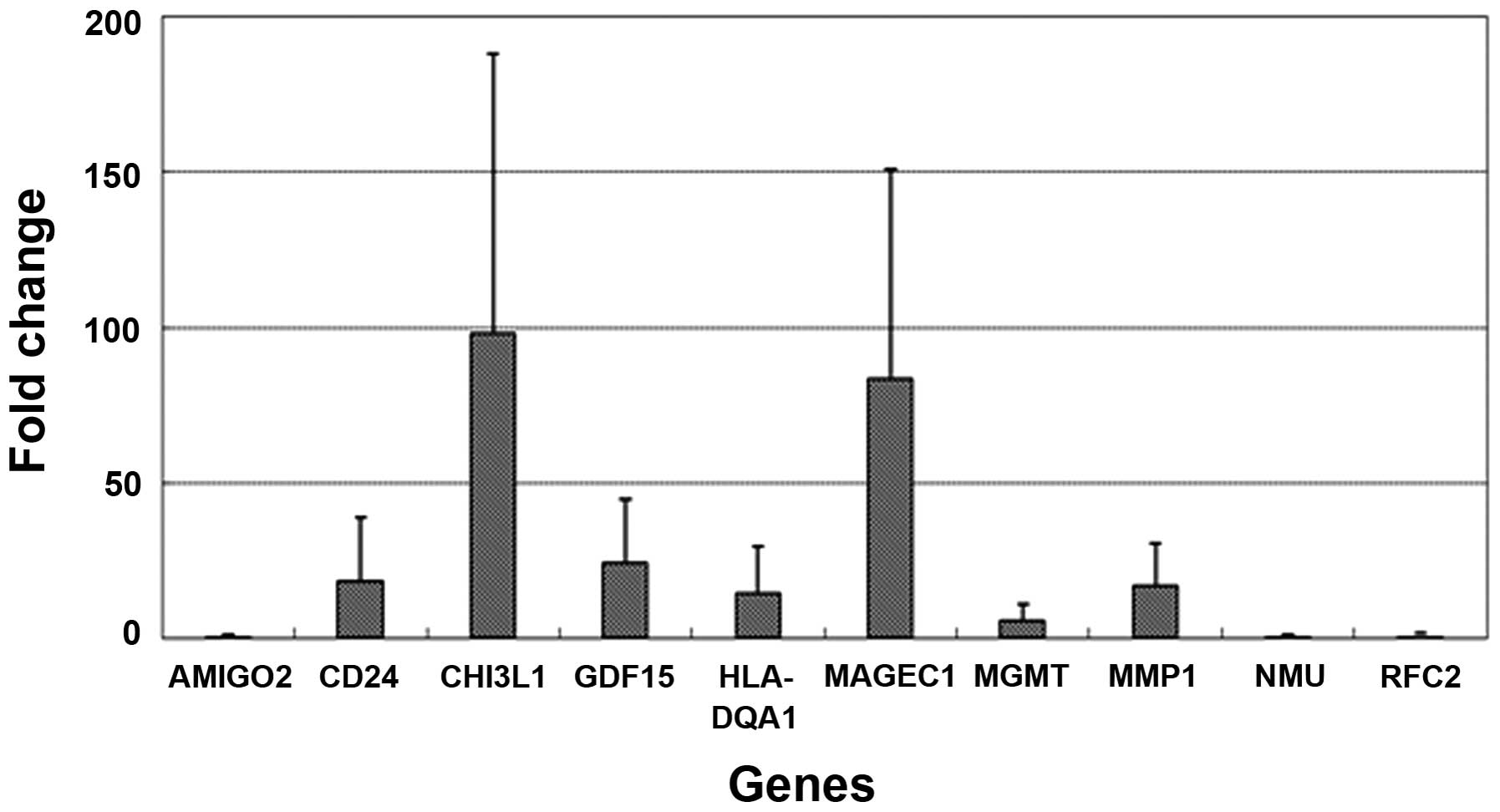
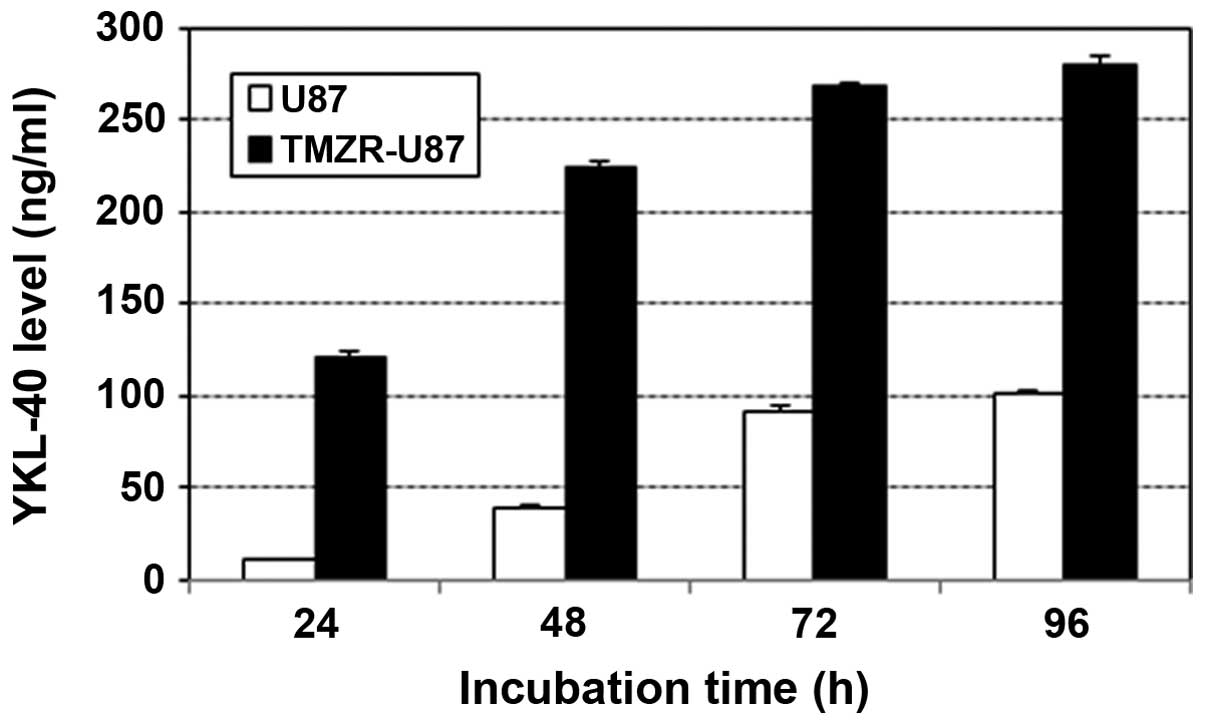
|
1
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirimanoff RO, Gorlia T, Mason W, et al:
Radiotherapy and temozolomide for newly diagnosed glioblastoma:
recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3
phase III randomized trial. J Clin Oncol. 24:2563–2569. 2006.
View Article : Google Scholar
|
|
3
|
Trivedi RN, Almeida KH, Fornsaglio JL,
Schamus S and Sobol RW: The role of base excision repair in the
sensitivity and resistance to temozolomide-mediated cell death.
Cancer Res. 65:6394–6400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hegi ME, Diserens AC, Gorlia T, et al:
MGMT gene silencing and benefit from temozolomide in glioblastoma.
N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quinn JA, Desjardins A, Weingart J, et al:
Phase I trial of temozolomide plus
O6-benzylguanine for patients with recurrent or
progressive malignant glioma. J Clin Oncol. 23:7178–7187.
2005.PubMed/NCBI
|
|
6
|
Quinn JA, Jiang SX, Reardon DA, et al:
Phase II trial of temozolomide plus
O6-benzylguanine in adults with recurrent,
temozolomide-resistant malignant glioma. J Clin Oncol.
27:1262–1267. 2009.PubMed/NCBI
|
|
7
|
Murat A, Migliavacca E, Gorlia T, et al:
Stem cell-related ‘self-renewal’ signature and high epidermal
growth factor receptor expression associated with resistance to
concomitant chemoradiotherapy in glioblastoma. J Clin Oncol.
26:3015–3024. 2008.
|
|
8
|
Hunter C, Smith R, Cahill DP, et al: A
hypermutation phenotype and somatic MSH6 mutations in recurrent
human malignant gliomas after alkylator chemotherapy. Cancer Res.
66:3987–3991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun S, Lee D, Ho AS, et al: Inhibition of
prolyl 4-hydroxylase, beta polypeptide (P4HB) attenuates
temozolomide resistance in malignant glioma via the endoplasmic
reticulum stress response (ERSR) pathways. Neuro Oncol. 15:562–577.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mukherjee B, McEllin B, Camacho CV, et al:
EGFRvIII and DNA double-strand break repair: a molecular mechanism
for radioresistance in glioblastoma. Cancer Res. 69:4252–4259.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitange GJ, Carlson BL, Schroeder MA, et
al: Expression of CD74 in high grade gliomas: a potential role in
temozolomide resistance. J Neurooncol. 100:177–186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vlachostergios PJ, Hatzidaki E, Befani CD,
Liakos P and Papandreou CN: Bortezomib overcomes MGMT-related
resistance of glioblastoma cell lines to temozolomide in a
schedule-dependent manner. Invest New Drugs. 31:1169–1181. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanzawa T, Germano IM, Kondo Y, Ito H, Kyo
S and Kondo S: Inhibition of telomerase activity in malignant
glioma cells correlates with their sensitivity to temozolomide. Br
J Cancer. 89:922–929. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng M, Bocangel D, Ramesh R, et al:
Interleukin-24 overcomes temozolomide resistance and enhances cell
death by down-regulation of O6-methylguanine-DNA
methyltransferase in human melanoma cells. Mol Cancer Ther.
7:3842–3851. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang G, Wei ZP, Pei DS, Xin Y, Liu YQ and
Zheng JN: A novel approach to overcome temozolomide resistance in
glioma and melanoma: Inactivation of MGMT by gene therapy. Biochem
Biophys Res Commun. 406:311–314. 2011. View Article : Google Scholar
|
|
16
|
Tso CL, Shintaku P, Chen J, et al: Primary
glioblastomas express mesenchymal stem-like properties. Mol Cancer
Res. 4:607–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phillips HS, Kharbanda S, Chen R, et al:
Molecular subclasses of high-grade glioma predict prognosis,
delineate a pattern of disease progression, and resemble stages in
neurogenesis. Cancer Cell. 9:157–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carro MS, Lim WK, Alvarez MJ, et al: The
transcriptional network for mesenchymal transformation of brain
tumors. Nature. 463:318–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verhaak RG, Hoadley KA, Purdom E, et al:
Integrated genomic analysis identifies clinically relevant subtypes
of glioblastoma characterized by abnormalities in PDGFRA, IDH1,
EGFR, and NF1. Cancer Cell. 17:98–110. 2010. View Article : Google Scholar
|
|
20
|
Ashizawa T, Miyata H, Iizuka A, et al:
Effect of the STAT3 inhibitor STX-0119 on the proliferation of
cancer stem-like cells derived from recurrent glioblastoma. Int J
Oncol. 43:219–227. 2013.PubMed/NCBI
|
|
21
|
Ashizawa T, Miyata H, Ishii H, et al:
Antitumor activity of a novel small molecule STAT3 inhibitor
against a human lymphoma cell line with high STAT3 activation. Int
J Oncol. 38:1245–1252. 2011.PubMed/NCBI
|
|
22
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double A, Everitt J, Farningham DAH,
Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson
S, Wedge SR and Eccles SA: An ad hoc committee of the National
Cancer Research Institute: Guidelines for the welfare and use of
animals in cancer research. Br J Cancer. 102:1555–1577. 2010.
View Article : Google Scholar
|
|
23
|
Kohsaka S, Wang L, Yachi K, et al: STAT3
inhibition overcomes temozolomide resistance in glioblastoma by
downregulating MGMT expression. Mol Cancer Ther. 11:1289–1299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh SK, Bhardwaj R, Wilczynska KM, Dumur
CI and Kordula T: A complex of nuclear factor I-X3 and STAT3
regulates astrocyte and glioma migration through the secreted
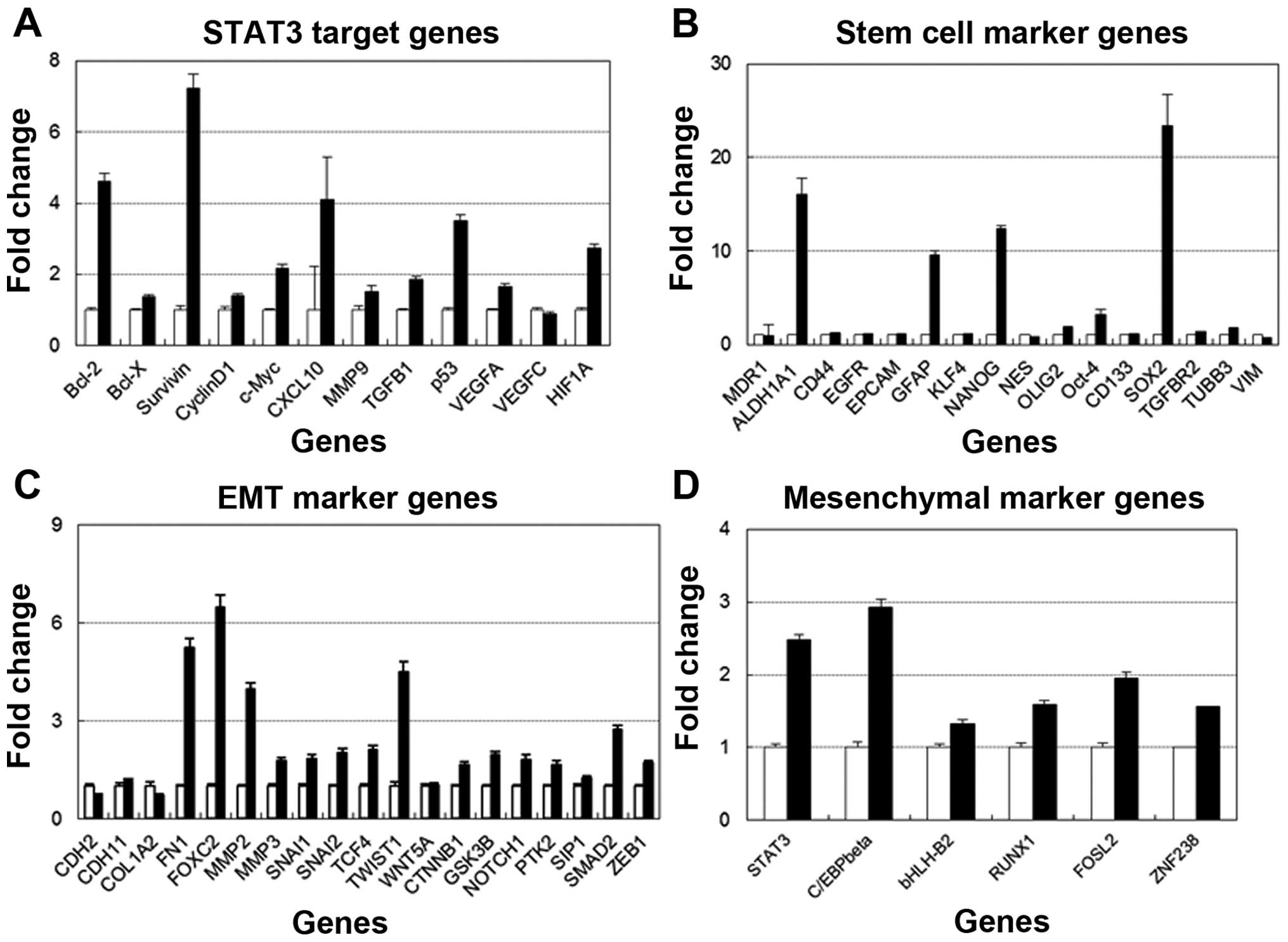
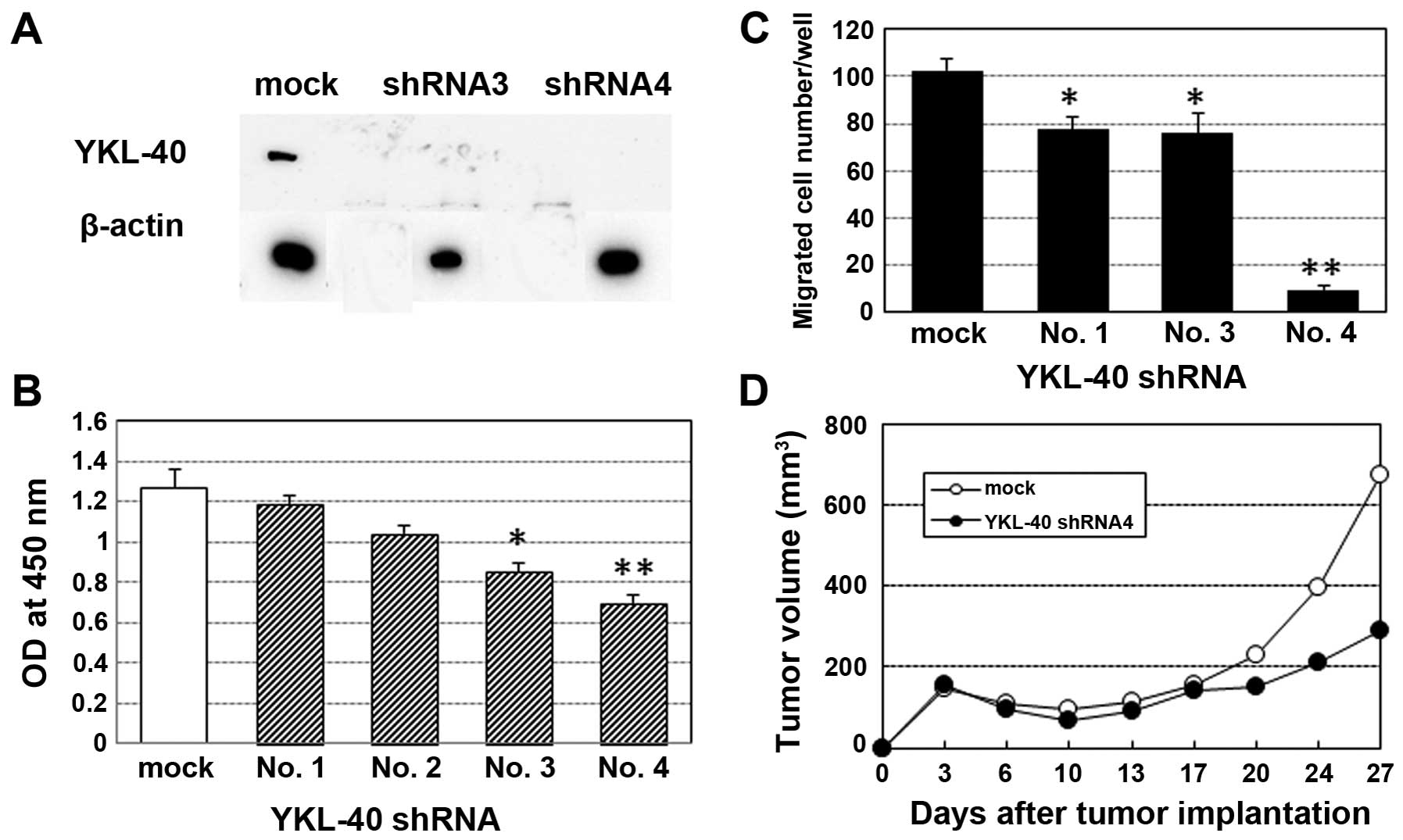
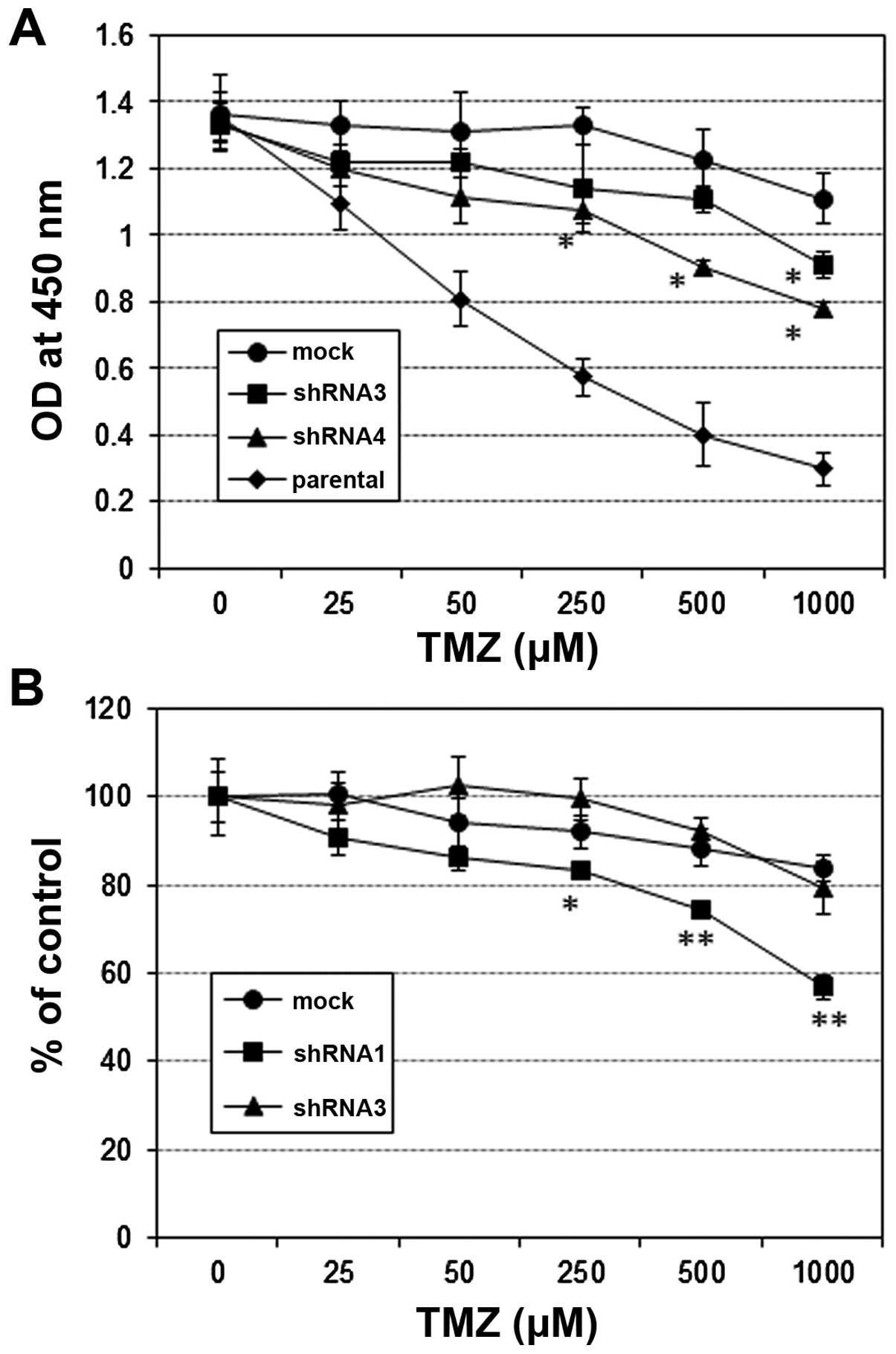
glycoprotein YKL-40. J Biol Chem. 286:39893–39903. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kzhyshkowska J, Gratchev A and Goerdt S:
Human chitinases and chitinase-like ptoteins as indicators for
inflammation and cancer. Biomark Insights. 3:128–146.
2007.PubMed/NCBI
|
|
26
|
Shao R, Hamel K, Petersen L, et al:
YKL-40, a secreted glycoprotein, promotes tumor angiogenesis.
Oncogene. 28:4456–4468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Francescone RA, Scully S, Faibish M, et
al: Role of YKL-40 in the angiogenesis, radioresistance, and
progression of glioblastoma. J Biol Chem. 286:15332–15343. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanwar MK, Gilbert MR and Holland EC: Gene
expression microarray analysis reveals YKL-40 to be a potential
serum marker for malignant character in human glioma. Cancer Res.
62:4364–4368. 2002.
|
|
29
|
Høgdall EV, Johansen JS, Kjaer SK, et al:
High plasma YKL-40 level in patients with ovarian cancer stage III
is related to shorter survival. Oncol Rep. 10:1535–1538.
2003.PubMed/NCBI
|
|
30
|
Hormigo A, Gu B, Karimi S, et al: YKL-40
and matrix metalloproteinase-9 as potential serum biomarkers for
patients with high-grade gliomas. Clin Cancer Res. 12:5698–5704.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thöm I, Andritzky B, Schuch G, et al:
Elevated pretreatment serum concentration of YKL-40 - An
independent prognostic biomarker for poor survival in patients with
metastatic nonsmall cell lung cancer. Cancer. 116:4114–4121.
2010.
|
|
32
|
Zhang W, Kawanishi M, Miyake K, et al:
Association between YKL-40 and adult primary astrocytoma. Cancer.
116:2688–2697. 2010.PubMed/NCBI
|
|
33
|
Iwamoto FM, Hottinger AF, Karimi S, et al:
Serum YKL-40 is a marker of prognosis and disease status in
high-grade gliomas. Neuro Oncol. 13:1244–1251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bernardi D, Padoan A, Ballin A, et al:
Serum YKL-40 following resection for cerebral glioblastoma. J
Neurooncol. 107:299–305. 2012. View Article : Google Scholar : PubMed/NCBI
|