Introduction
The increasing awareness of the ability of glycans
to store biological information (coding by sugars) has directed
research efforts toward endogenous lectins (1). In immunology and tumor biology, the
interplay between glycan remodeling and lectin expression
constitutes a potent molecular switch for cell adhesion and growth
regulation, which affects, for example, activated T (effector)
cells or carcinoma cells after the reconstitution of tumor
suppressor p16INK4a (2–8).
Members of the family of galectins (Gals), which share the
β-sandwich fold and reactivity to galactosides, play a prominent
role in this respect (9,10). Of note, they are multifunctional
proteins with an activity spectrum beyond decoding cell surface
glycans (11,12). As our previous studies on galectin
localization in head and neck tumors exemplarily demonstrated, they
can be detected in the cytoplasm and nucleus, with shifts in
localization occurring during progression (13–18).
Gals target distinct counter receptors that are located on the cell
surface and in the extracellular matrix, such as bcl-2, using Gal-3
and -7 as part of their functionality (12,19).
Additionally, our studies have also documented the presence of a
network of these effectors. As a consequence, the study design
should progress from monitoring individual members of this class to
testing a panel of non-cross-reactive antibodies. Database mining
has previously revealed sequence divergence at the promoter level
and variations in the gene copy-number among Gals (20); however, the immunohistochemical
fingerprinting approach will provide insights into the regulation
of individual family members. In the present study, we applied this
technique to comparatively analyze the expression of Gal-1, -3, -4,
-7, -8 and -9, the entire group of human lectins, in naso-sinusal
pathologies. The following diseases were examined: chronic
rhinosinusitis (CRS), nasal polyposis, inverted papillomas and
squamous cell carcinomas. The origin of manifestation can be either
inflammatory, such as in CRS and naso-sinusal polyposis or tumoral
(inverted papillomas and squamous cell carcinomas). For most cases,
the etiology and pathogenesis are not yet completely known.
Therefore, establishing an early diagnosis is difficult,
particularly since symptoms are not specific. CRS affects ~15% of
the population and is defined as inflammation of one or more of the
paranasal sinuses that lasts >12 weeks (21,22).
In general, CRS is divided into three categories: CRS with nasal
polyps, CRS without nasal polyps, and allergic fungal
rhinosinusitis (23). A fourth
group, eosinophilic CRS, that is characterized by the presence of a
high number of activated eosinophils in the mucosa was also
proposed (24,25). The latter group is often associated
with a more severe disease and diminished surgical success
(26). Naso-sinusal polyposis is
characterized by inflammatory outgrowths of paranasal sinus mucosa
caused by chronic mucosal inflammation, typically arising from the
middle meatus and ethmoid region. It is a common disease affecting
up to 4% of the general population (27,28).
In the present study, we focused specifically on non-allergic nasal
polyps and allergic nasal polyps, which present inflammatory
mediators, eosinophils and sensitivity to allergens (29). Typically, patients with CRS or
naso-sinusal polyposis have nasal obstruction, anosmia, rhinorrhea
and facial pain (30). Inverted
papillomas are sinonasal lesions primarily on the lateral nasal
wall that are characterized by recurrence potential and the
propensity for malignancy (31,32).
The term inverted papilloma describes the reversal of epithelial
proliferation, which is endophytic and does not affect the basement
membrane (33). Epstein-Barr virus
(EBV) or human papillomavirus (HPV) are implicated in its
pathogenesis (34). Finally,
squamous cell carcinoma stems from the epithelium of the
respiratory mucosa of the nasal cavity and paranasal sinuses. It is
a rare malignancy, representing <1% of malignant tumors and ~3%
of malignancies affecting the head and neck. Early diagnosis is
difficult because symptoms and signs are not specific but are
similar to those of chronic sinusitis, allergic reactions and nasal
polyps, i.e., symptoms caused by nasal obstruction (35).
Therefore, our disease panel was suited to address
Gal regulation, with potential implications for the diagnosis of 90
cases. We assessed the expression, localization and
semi-quantitative parameters, such as signal intensity, percentage
of stained areas and percentage of positive cells, for each
case.
Materials and methods
Patient characteristics
Specimens were surgically removed from 90 patients
with naso-sinusal pathologies and studied. The specimens included
29 chronic rhinosinusitises, 26 nasal polyps, 29 inverted
papillomas and 6 squamous cell carcinomas (see Table I for clinical data). The specimens
were obtained by a retrospective compilation of the records from
the Department of Pathology at the Hôpital Claude Huriez (Lille,
France), the CHU Saint-Pierre (Brussels, Belgium) and the Centre
Epicura (Baudour, Belgium). The institutional review boards of
these hospitals approved the study (AK/09-09-47/3805AD).
Haematoxylin and eosin-stained (H&E)sections from the 90 cases
were routinely examined by two pathologists to confirm the
diagnosis.
 | Table IClinical data. |
Table I
Clinical data.
|
Characteristics | CRS (n=29) | Nasal polyps
(n=26) | IPs (n=29) | Carcinomas
(n=6) |
|---|
| Age (years) |
| Mean | 37 | 43 | 60 | 72 |
| Range | 18–63 | 10–74 | 27–84 | 53–86 |
| Gender |
| Male | 22 | 18 | 20 | 6 |
| Female | 7 | 8 | 9 | 0 |
| Treatment |
| Surgery | 29 | 26 | 29 | 6 |
| Histology |
|
Non-eosinophilic | 10 | - | - | - |
| Eosinophilic | 19 | - | - | - |
| Non-allergic | - | 9 | - | - |
| Allergic | - | 17 | - | - |
| Squamous cell | - | - | - | 6 |
| TNM stage |
| I | - | - | - | 0 |
| II | - | - | - | 0 |
| III | - | - | - | 0 |
| IV | - | - | - | 6 |
Antibodies
Human Gal-1, -3, -4, -7, -8 and -9 were produced in
bacteria, purified to homogeneity, as confirmed by one-dimensional
and two-dimensional gel electrophoreses, gel filtration, and mass
spectrometry, and used as antigens to develop polyclonal antibodies
in rabbits (36–40). The resulting IgG fractions were
rigorously checked for cross-reactivity among the lectin family,
with systematic testing of human Gal-1, -2, -3, -4, -7, -8 and -9
by western blot analysis and enzyme-linked immunosorbent assay.
Chromatographic affinity depletion was performed on
galectin-presenting Sepharose 4B in the case of positivity,
followed by quality control to ascertain the elimination of
cross-reactivity, as previously described (41–43).
Immunohistochemistry
All tumor samples were fixed in 4% buffered
formaldehyde for 24 h, dehydrated and embedded in paraffin.
Immunohistochemistry was performed on 5-μm thick sections mounted
on silane-coated glass slides (18). Before starting the
immunohistochemistry protocol, deparaffinized tissue sections were
placed in 0.01 M citrate buffer (pH 6.0) and briefly pre-treated in
a 900 W microwave for 2×5 min. The sections were then incubated in
a solution containing 0.06% H2O2 for 5 min to
block endogenous peroxidase activity, rinsed in phosphate-buffered
saline (PBS; 0.04 M Na2HPO4, 0.01 M
KH2PO4 and 0.12 M NaCl, pH 7.4) and
successively exposed to solutions containing avidin (0.1 mg/ml in
PBS) and biotin (0.1 mg/ml in PBS), respectively, for 5 min each to
prevent false-positive staining reactions due to the presence of
endogenous biotin. After thorough washing with PBS, the sections
were incubated for 15 min in a solution containing 0.5% casein in
PBS and sequentially exposed to solutions containing the following
proteins at room temperature: i) the specific primary antibody; ii)
the corresponding biotinylated secondary antibody (polyclonal goat
anti-rabbit IgG); and iii) the avidin-biotin-peroxidase complex
(ABC). The samples were thoroughly washed between incubation steps
to remove unbound proteins. The antigen-dependent presence of the
peroxidase complex in the sections was visualized by incubating
diaminobenzidine and H2O2 with the
chromogenic substrates. After rinsing, the sections were
counterstained with Luxol Fast Blue and mounted in synthetic
medium. To exclude antigen-independent staining in the control
samples, incubation steps with the primary/secondary antibodies
were omitted from the protocol. In all instances, these controls
were negative. The biotinylated secondary antibodies and ABC kit
were obtained from DakoCytomation (Glostrup, Denmark).
Semi-quantitative analysis
For each specimen (15 microscopic fields), we
focused our analysis on the epithelial and stromal components. The
signal intensity of the immunoreactivity (mean intensity, MI) was
scored as either 0 (negative), 1 (weak), 2 (moderate) or 3
(strong), and the population of positive cells (labeling index, LI)
was expressed as the percentage of immunopositive cells. The quick
score (QS) was calculated by multiplying the score for the
reactivity intensity by the percentage of immunopositive cells.
Data analysis
Independent groups of quantitative data were
compared with the non-parametric Kruskall-Wallis test (more than
two groups). In the case of significant results, post-hoc tests
(Dunn procedure) were used to compare pairs of groups (to avoid
multiple comparison effects). A P-value of <0.05 was considered
to indicate a statistically significant result.
Results
The first observation was on the range of galectin
immunohistochemical positivity. In most cases, the application of
the 6 anti-Gal antibodies revealed the presence of lectins. We
decided to describe the galectin fingerprinting of each
naso-sinusal disease separately.
All of the 10 non-eosinophilic chronic
rhinosinusitis (NECRS) cases showed positive staining for the 6
Gals. Of the cases of eosinophilic chronic rhinosinusitis (ECRS)
(19 cases), 100% of the specimens were positive for Gal-1, -3 and
-7, 94% were positive for Gal-8 and 89% were positive for Gal-9.
The immunostaining was predominately nucleocytoplasmic (Table II). In NECRS, the signal intensity
was low except for Gal-7, which was moderately expressed (Fig. 1A, C, E, G, I and K). In fact, the
immunohistochemical profiles of ECRS cases differed from the
non-eosinophilic cases. In detail, a low intensity was detected for
Gal-4 and -8, a low to moderate intensity was recorded for Gal-1,
-3 and -9 and a moderate intensity was observed for Gal-7 (Fig. 2A, C, E, G, I and K). Statistical
analysis shows a decreased positive area for Gal-3 in NECRS
(post-hoc comparison, P=0.02) and Gal-7 in ECRS (post-hoc
comparison, P=0.0003) compared to cases with inverted papillomas
(IPs, 29 cases) (Fig. 7A and C).
Compared to the IPs and squamous cell carcinomas (SCCs, 6 cases),
the percentage of Gal-9-immunopositive cells (Fig. 7E) was downregulated in NECRS
(post-hoc comparisons, P=0.02 for both) and ECRS (post-hoc
comparisons, P=0.0006 and P=0.003, respectively). Concerning the
immunohistochemical detection of Gal-1, -4 and -8, no significant
difference was observed between CRS and the other lesions (Fig. 7B and D). Therefore, ECRS expressed a
low level of Gal-7 and -9, whereas non-eosinophilic lesions
expressed a low level of Gal-3 and -9 (Fig. 7A, C and E).
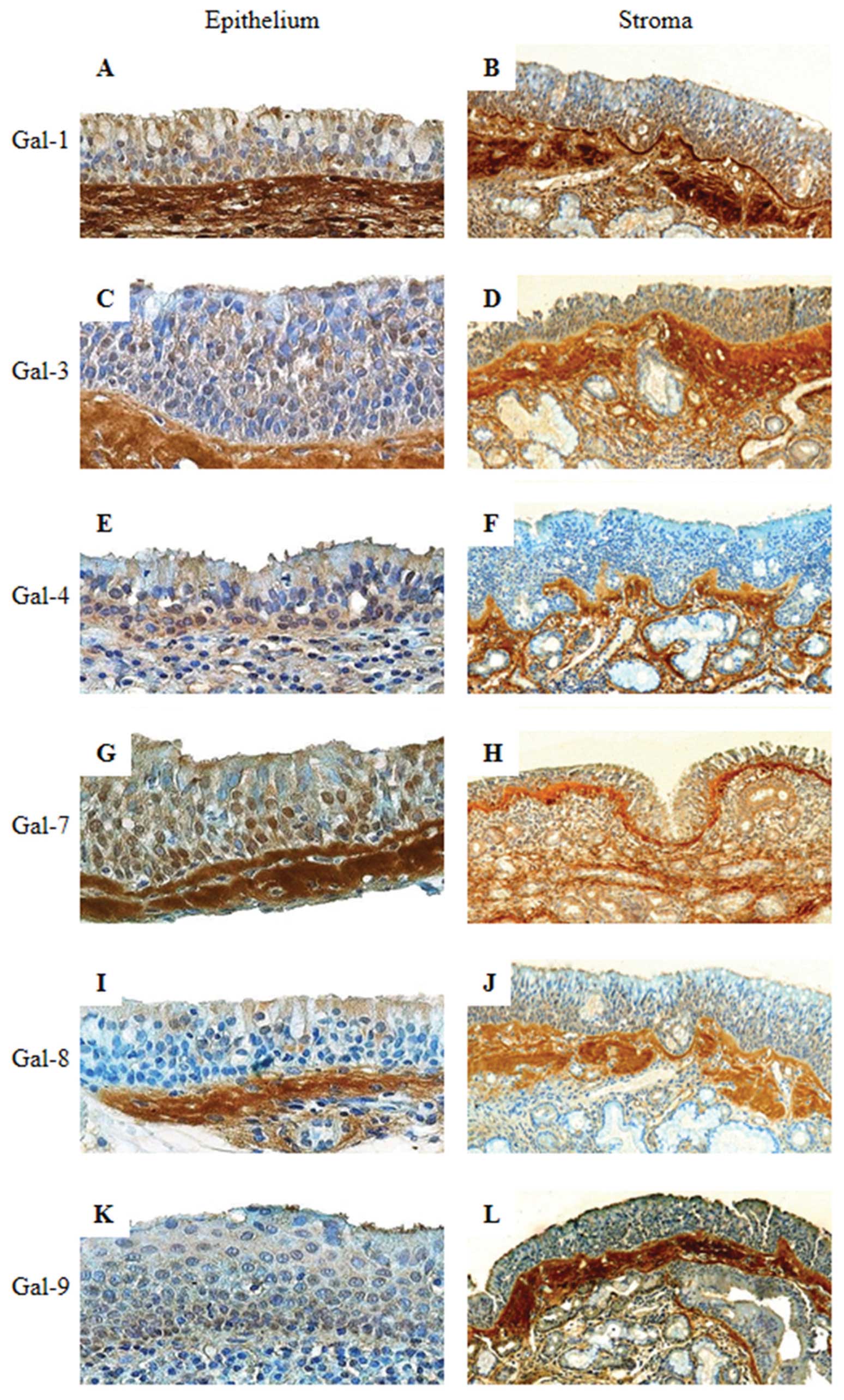 | Figure 1Immunohistochemical expression
profiles for galectin-1, galectin-3, galectin-4, galectin-7,
galectin-8 and galectin-9 in the epithelium (A, C, E, G, I and K;
original magnification, ×400) and stroma (B, D, F, H, J and L;
original magnification, ×100) of non-eosinophilic chronic
rhinosinusitis. |
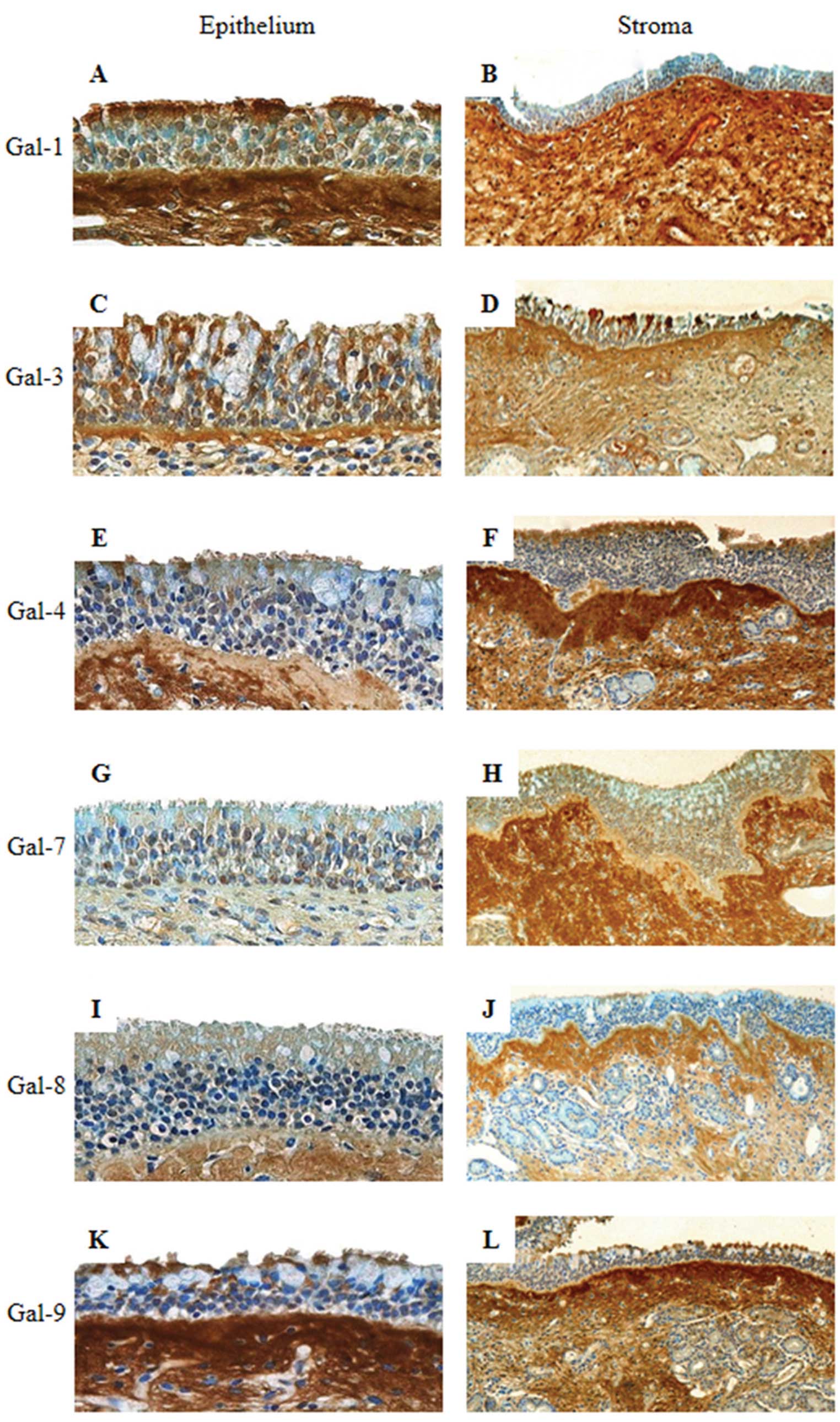 | Figure 2Immunohistochemical expression
profiles for galectin-1, galectin-3, galectin-4, galectin-7,
galectin-8 and galectin-9 in the epithelium (A, C, E, G, I and K;
original magnification, ×400) and stroma (B, D, F, H, J and L;
original magnification, ×100) of eosinophilic chronic
rhinosinusitis. |
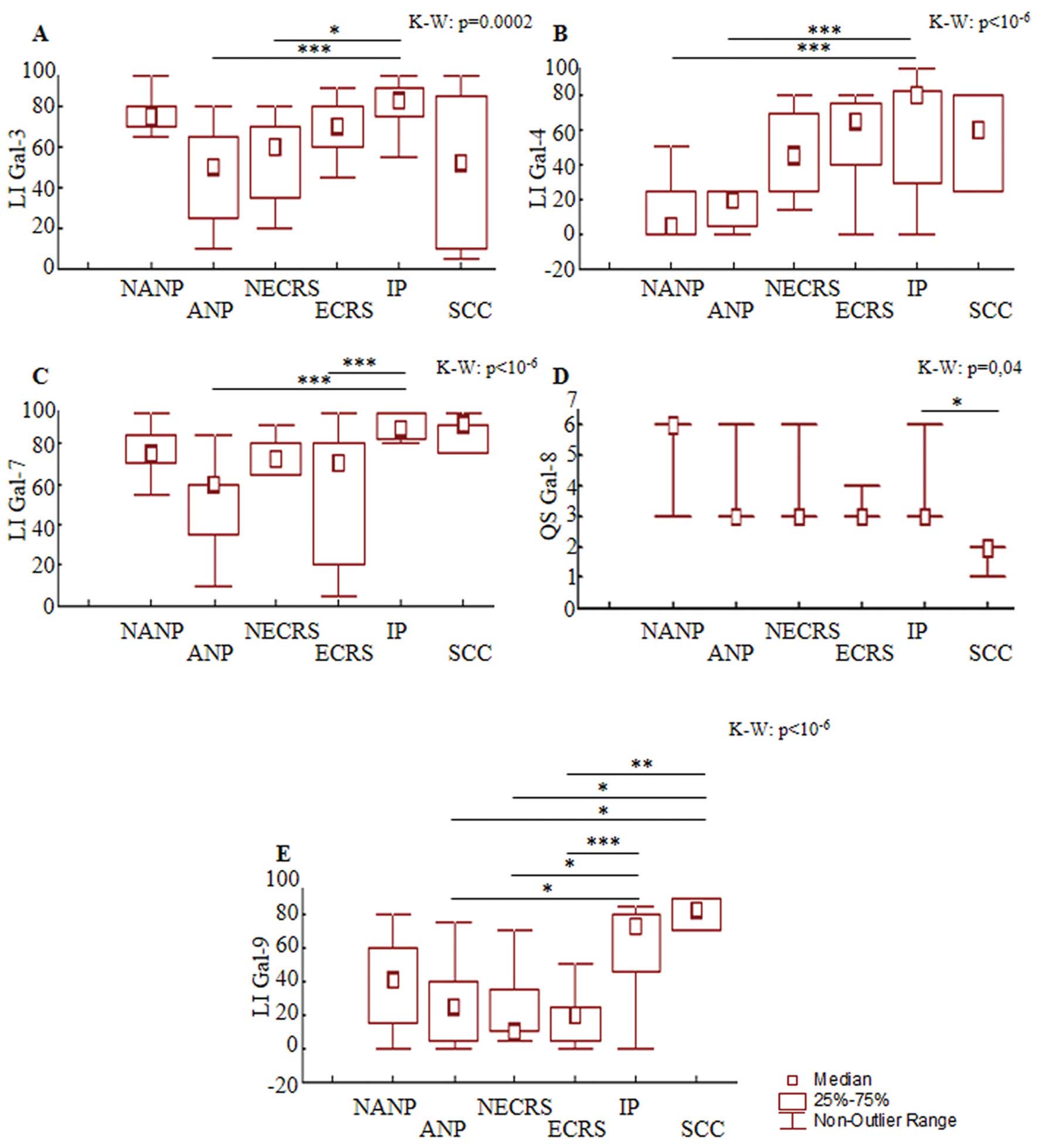 | Figure 7Results of the semi-quantitative
percentage of immunopositive cells for galectin-3, galectin-4,
galectin-7 or galectin-9 [labeling index (LI)] (A, B, C and E) and
the quick score (QS) for galectin-8 (i.e., multiplication of the
signal intensity score by the LI score) (D) for 10 cases of
non-eosinophilic chronic rhinosinusitis (NECRS), 19 cases of
eosinophilic chronic rhinosinusitis (ECRS), 9 cases of non-allergic
nasal polyps (NANPs), 17 cases of allergic nasal polyps (ANPs), 29
cases of inverted papillomas (IPs) and 6 cases of squamous cell
carcinomas (SCCs). Significant post-hoc comparison results are
indicated by the lines (indicating the pairs of groups being
compared) (*P<0.05, **P<0.01,
***P<0.001). The result of the Kruskall-Wallis test
is indicated in the top-left corner of each frame. |
 | Table IIIntracellular localization of
galectin-1, -3, -4, -7, -8 and -9.a |
Table II
Intracellular localization of
galectin-1, -3, -4, -7, -8 and -9.a
| Galectin-3
localization | Galectin-3
localization | Galectin-4
localization | Galectin-7
localization | Galectin-8
localization | Galectin-9
localization |
|---|
|
|
|
|
|
|
|
|---|
| Type of tissue | C
n/total (%) | N
n/total (%) | C + N
n/total (%) | C
n/total (%) | N
n/total (%) | C + N
n/total (%) | C
n/total (%) | N
n/total (%) | C + N
n/total (%) | C
n/total (%) | N
n/total (%) | C + N
n/total (%) | C
n/total (%) | N
n/total (%) | C + N
n/total (%) | C
n/total (%) | N
n/total (%) | C + N
n/total (%) |
|---|
| Non-eosinophilic
chronic rhinosinusitis (n=10) | 0/10 (0) | 0/10 (0) | 10/10 (100) | 0/10 (0) | 0/10 (0) | 10/10 (100) | 2/10 (20) | 0/10 (0) | 8/10 (80) | 0/10 (0) | 0/10 (0) | 10/10 (100) | 0/10 (0) | 0/10 (0) | 10/10 (100) | 2/10 (20) | 0/10 (0) | 8/10 (80) |
| Eosinophilic
chronic rhinosinusitis (n=19) | 0/19 (0) | 0/19 (0) | 19/19 (100) | 0/19 (0) | 0/19 (0) | 19/19 (100) | 2/17 (12) | 0/17 (0) | 15/17 (88) | 0/19 (0) | 0/19 (0) | 19/19 (100) | 0/17 (0) | 0/17 (0) | 17/17 (100) | 11/17 (65) | 0/17 (0) | 6/17 (35) |
| Non-allergic nasal
polyps (n=9) | 2/8 (25) | 0/8 (0) | 6/8 (75) | 0/9 (0) | 0/9 (0) | 9/9 (100) | 0/5 (0) | 0/5 (0) | 5/5 (100) | 0/9 (0) | 0/9 (0) | 9/9 (100) | 0/9 (0) | 0/9 (0) | 9/9 (100) | 0/8 (0) | 0/8 (0) | 8/8 (100) |
| Allergic nasal
polyps (n=17) | 2/17 (12) | 0/17 (0) | 15/17 (88) | 0/17 (0) | 0/17 (0) | 17/17 (100) | 5/15 (33) | 0/15 (0) | 10/15 (67) | 0/17 (0) | 0/17 (0) | 17/17 (100) | 2/16 (12) | 0/16 (0) | 14/16 (88) | 3/16 (19) | 0/16 (0) | 13/16 (81) |
| Inverted papillomas
(n=29) | 2/28 (7) | 0/28 (0) | 26/28 (93) | 1/24 (4) | 0/24 (0) | 23/24 (96) | 18/27 (67) | 0/27 (0) | 9/27 (33) | 0/27 (0) | 0/27 (0) | 27/27 (100) | 4/27 (15) | 0/27 (0) | 23/27 (85) | 6/27 (22) | 2/27 (8) | 19/27 (70) |
| Squamous cell
carcinomas (n=6) | 6/6 (100) | 0/6 (0) | 0/6 (0) | 5/6 (83) | 0/6 (0) | 1/6 (16) | 2/6 (33) | 0/6 (0) | 4/6 (67) | 2/6 (33) | 0/6 (0) | 4/6 (67) | 2/5 (40) | 0/5 (0) | 3/5 (60) | 6/6 (100) | 0/6 (0) | 0/6 (0) |
The polyp group was subdivided into non-allergic
nasal polyps (NANPs; 9 cases) and allergic nasal polyps (ANPs; 17
cases). All epithelial cells were moderately positive for Gal-3 and
-7 with nucleocytoplasmic distribution (Figs. 3C and G; 4C and G) (Table II). For the other 4 Gals, the
percentage of positive cases (i.e., with LI >0%) did not
significantly vary according to the type of lesion (NANP vs. ANP),
except for Gal-4 (55 vs. 88% of positive cases). These two types of
lesions are shown in Fig. 3 and
4, which reveal low to moderate
nucleocytoplasmic or cytoplasmic positivity, respectively. As
illustrated in Fig. 7B, a lower
percentage of Gal-4-immunopositive cells was detected in ANPs and
NANPs (post-hoc comparisons, P=0.0004 and P=0.0007, respectively)
compared to IPs. Downregulation of Gal-3 and -7 (post-hoc
comparisons, P=0.0003 and P=0.000002, respectively) was detected in
ANPs compared to IPs (Fig. 7A and
C). We also observed a lower percentage of Gal-9-positive cells
in ANPs than in IPs or SCCs (post-hoc comparison, P=0.01 and
P=0.02, respectively) (Fig. 7E). A
statistically significant difference was not observed between the
expression of Gal-8 in nasal polyps and the expression of Gal-8 in
the other pathologies (Fig. 7D).
The immunohistochemical profile of nasal polyps differed with
respect to the allergic status of the patients. In fact, the
observations show that ANPs were low in Gal-3, -4, -7 and -9,
whereas NANPs were only low in Gal-4.
 | Figure 3Immunohistochemical expression
profiles for galectin-1, galectin-3, galectin-4, galectin-7,
galectin-8 and galectin-9 in the epithelium (A, C, E, G, I and K;
original magnification, ×400) and stroma (B, D, F, H, J and L;
original magnification, ×100) of a non-allergic nasal polyp. |
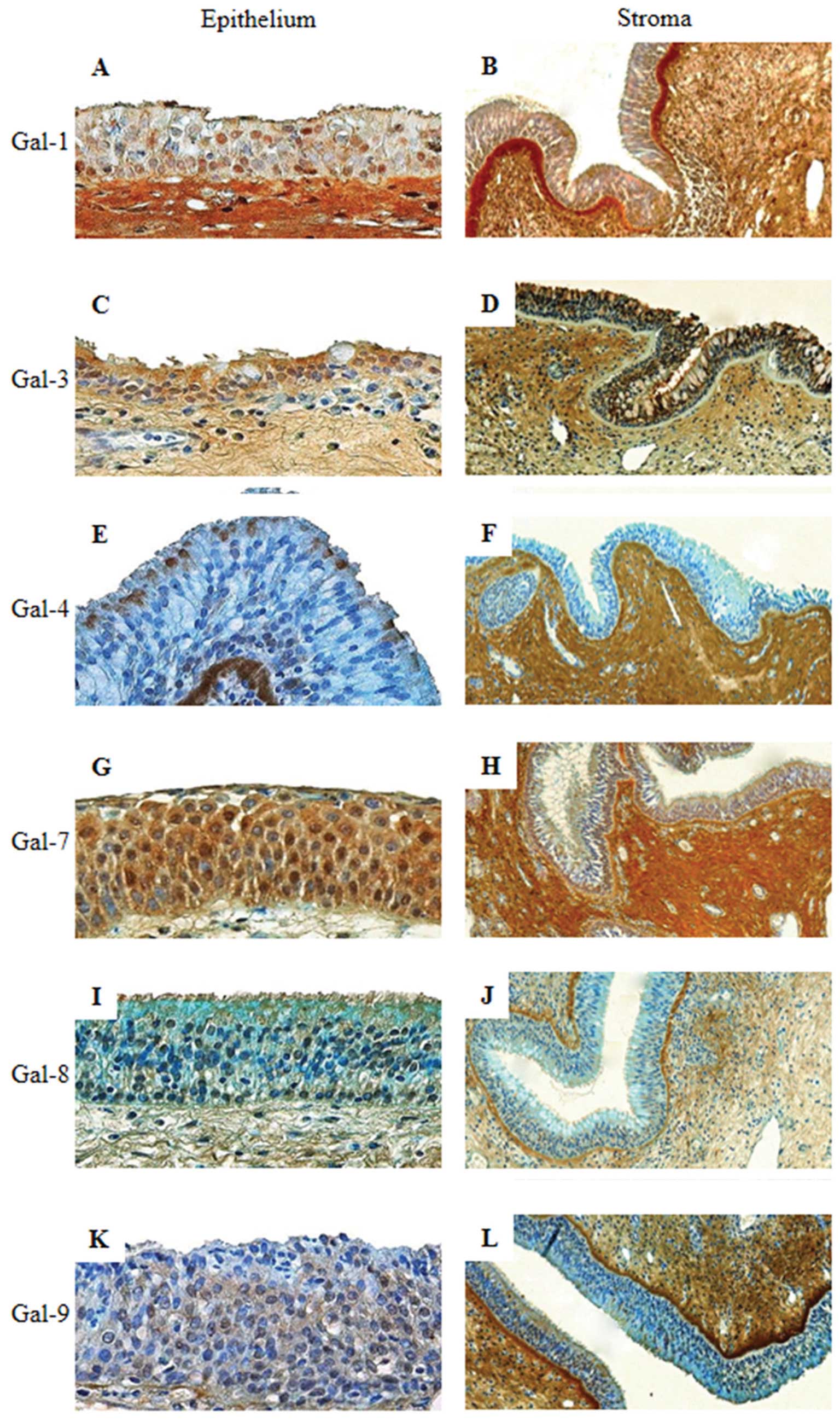 | Figure 4Immunohistochemical expression
profiles for galectin-1, galectin-3, galectin-4, galectin-7,
galectin-8 and galectin-9 in the epithelium (A, C, E, G, I, K;
original magnification, ×400) and stroma (B, D, F, H, J, L;
original magnification, ×100) of an allergic nasal polyp. |
In inverted papillomas (IPs), nucleocytoplasmic
immunostaining of Gal-1, -3 and -7 was detected in 100% of
epithelial cells. Slightly lower percentages of 96 and 93% were
detected for Gal-4 and both Gal-8 and -9, respectively. Galectins
were present in both the nuclei and cytoplasm. Only Gal-9 was
invariably present in epithelial cells (Table II). As shown in Fig. 5 for epithelial cells, a low to
moderate signal was detected for Gal-1, -4 and -8, and a moderate
to intense signal was detected for Gal-3, -7 and -9. An analysis of
the quantitative data showed an increased percentage of positivity
for the following Gals in the epithelium of IPs: Gal-3 compared to
ANPs and NECRS (post-hoc comparison, P=0.0003 and P=0.002,
respectively) (Fig. 7A), Gal-4
compared to ANPs and NANPs (post-hoc comparison, P=0.0004 and
P=0.0007, respectively) (Fig. 7B),
Gal-7 compared to ANPs and ECRS (post-hoc comparison, P=0.000002
and P=0.0003, respectively) (Fig.
7C), Gal-8 compared to SCCs (post-hoc comparison, P=0.004)
(Fig. 7D) and Gal-9 compared to
ANPs, ECRS and NECRS (post-hoc comparison, P=0.01, P=0.0006 and
P=0.02, respectively) (Fig. 7E).
IPs are thus characterized by the overexpression of Gal-3, -4, -7,
-8, -9 but not Gal-1.
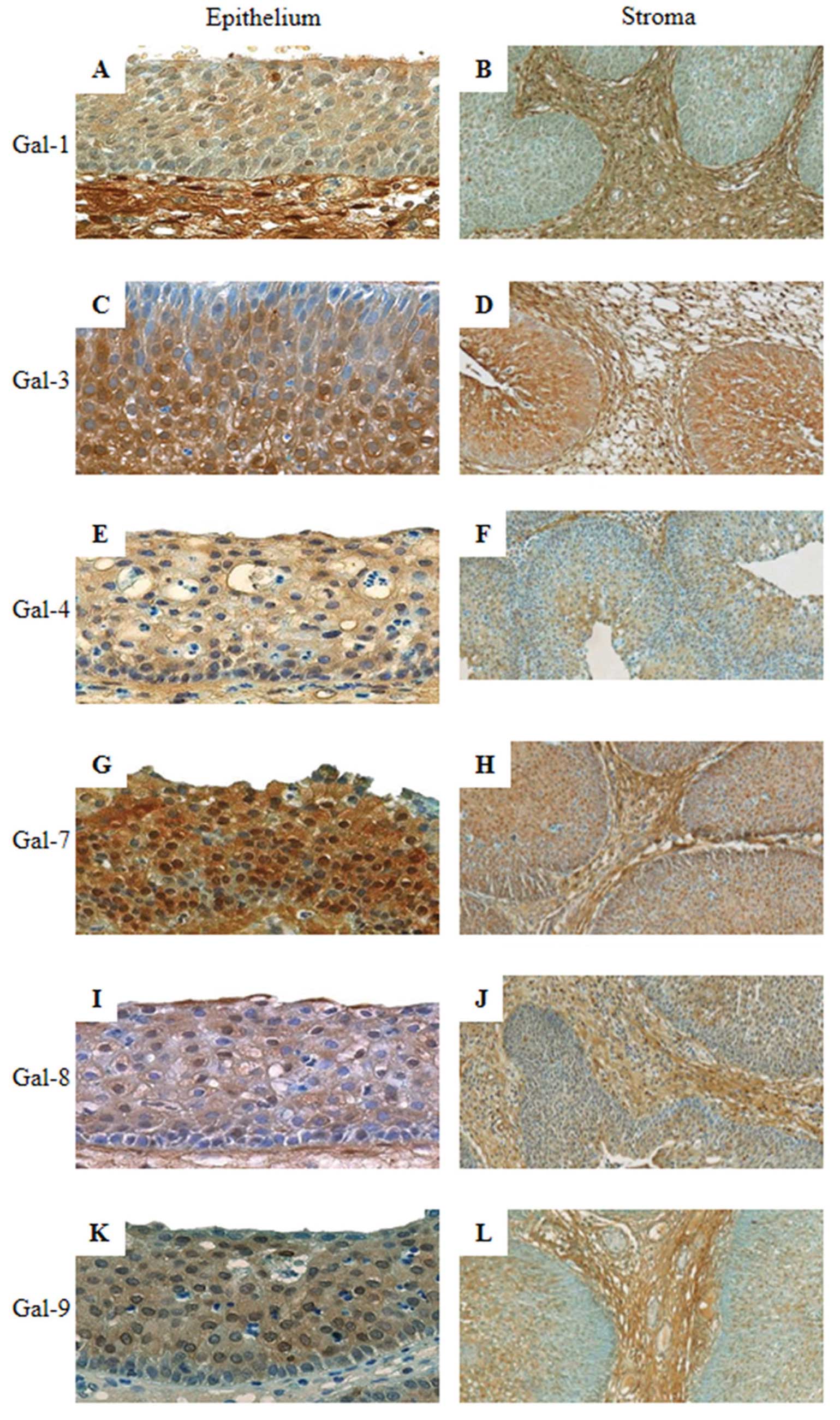 | Figure 5Immunohistochemical expression
profiles for galectin-1, galectin-3, galectin-4, galectin-7,
galectin-8 and galectin-9 in the epithelium (A, C, E, G, I and K;
original magnification, ×400) and stroma (B, D, F, H, J, and L;
original magnification, ×100) of an inverted papilloma. |
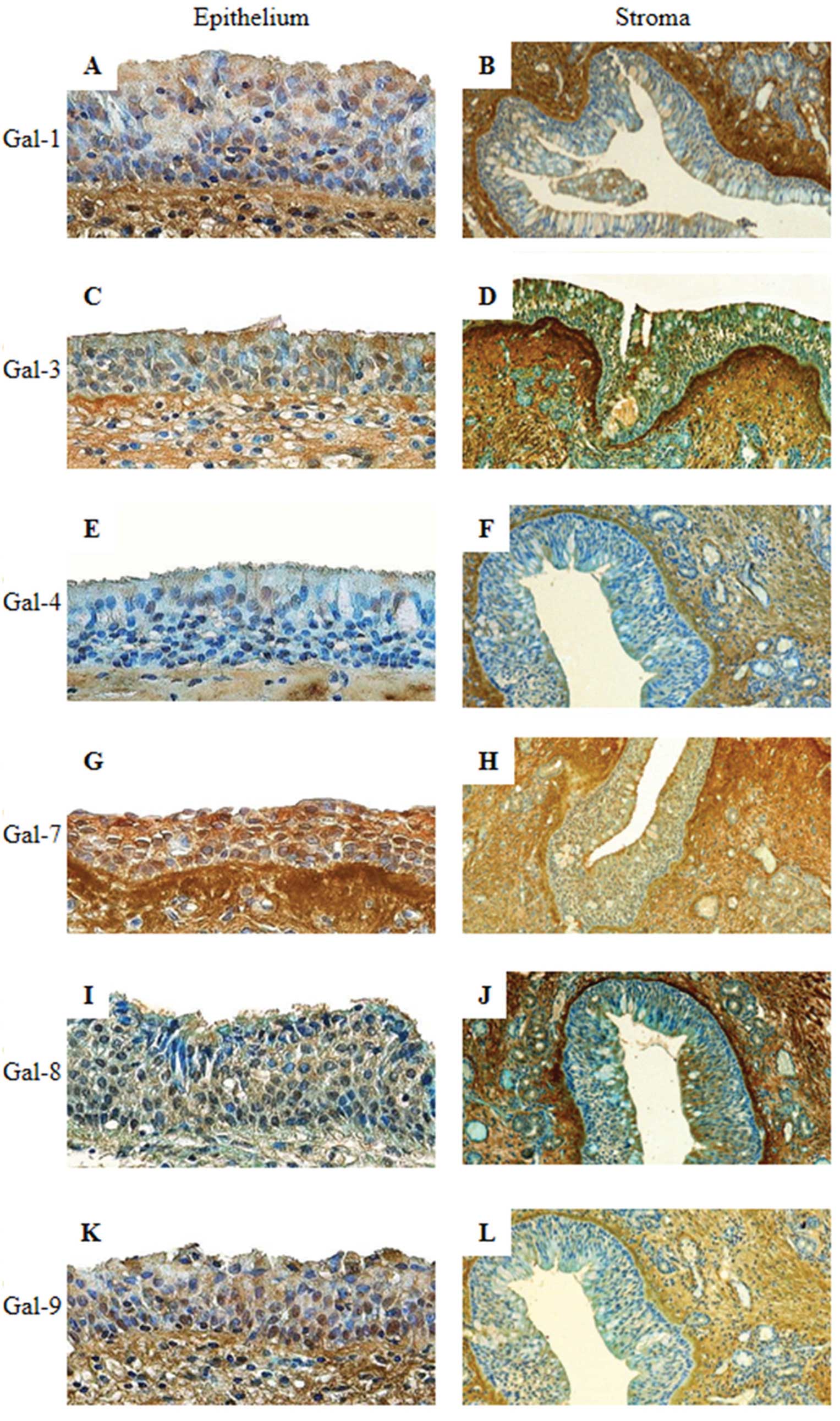
The epithelial cells in naso-sinusal SCCs were
positive for all of the galectins, except Gal-8 (83%). The
localization depends on the type of lectin. The nucleocytoplasmic
or cytoplasmic expression pattern contrasted with the exclusively
cytoplasmic positivity observed for Gal-1 and -9 (Fig. 6; Table
II). The signal intensity detected was moderate for Gal-1 and
low to moderate for the other Gals, as shown in Fig. 6A higher percentage of Gal-9-positive
cells was detected in SCCs compared to ANPs, NECRS and ECRS
(post-hoc comparisons, P=0.02, P=0.02 and P=0.003, respectively)
(Fig. 7E). In contrast, a lower
Gal-8 quick score was determined for SCCs compared to IPs (post-hoc
comparison, P=0.04; Fig. 7D). No
significant difference was found for the presence of Gal-1, -3, -4
and -7 (Fig. 7A–C). Thus, the
immunohistochemical profile of nasal carcinomas appears to be
characterized by a high-level of Gal-9 and a low-level of
Gal-8.
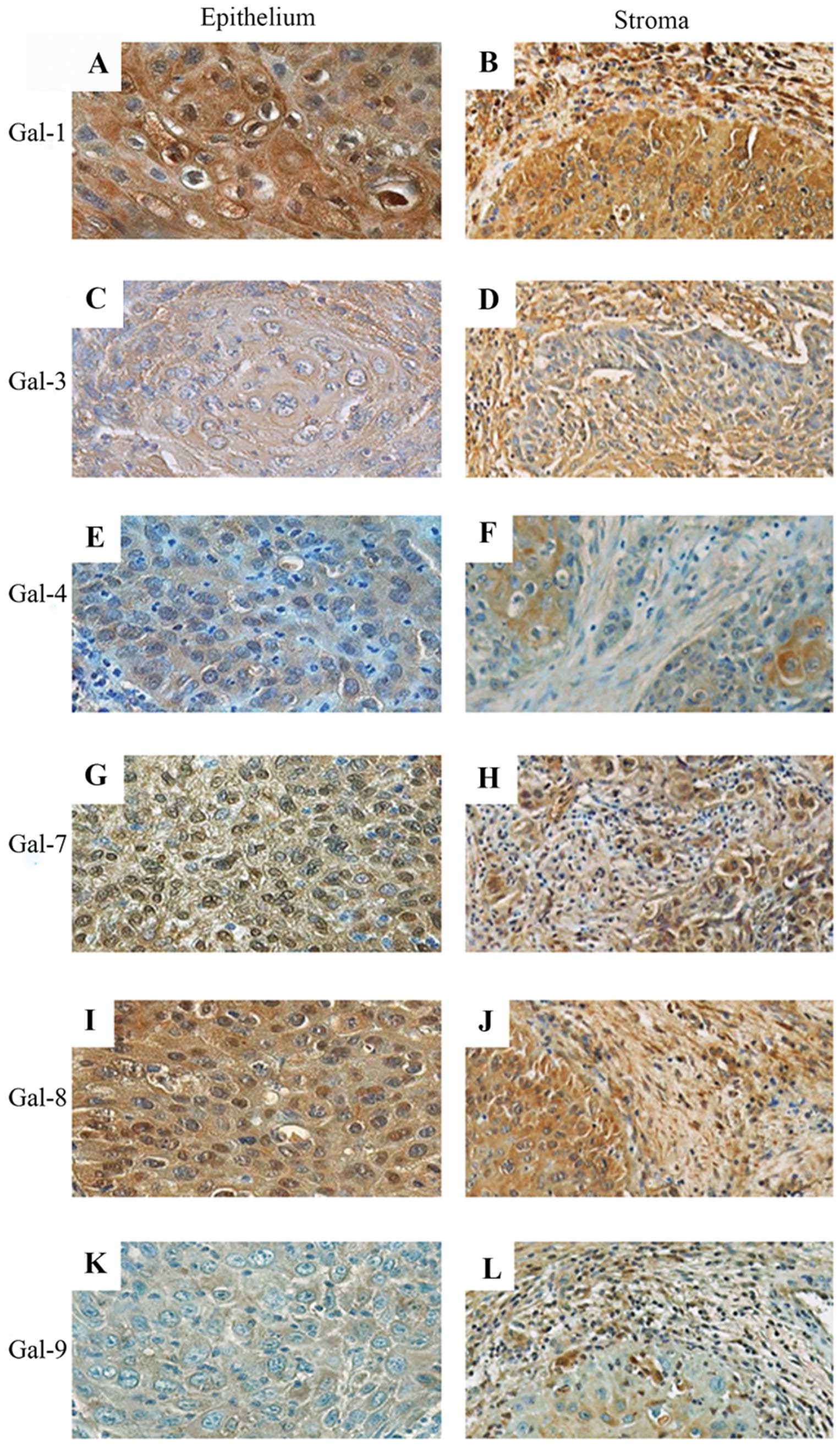 | Figure 6Immunohistochemical expression
profiles for galectin-1, galectin-3, galectin-4, galectin-7,
galectin-8 and galectin-9 in the epithelium (A, C, E, G, I and K;
original magnification, ×400) and stroma (B, D, F, H, J and L;
magnification, ×200) of squamous cell carcinoma. |
In the 90 cases studied, almost all of the stromal
cells exhibited staining for the 6 galectins. Similar
immunohistochemical profiles were revealed for NECRS and ANPs,
i.e., low to moderate positivity (Figs.
1B, D, F, H, J and L; 4B, D, F, H,
J and L). Gal-1 and -4 were moderately positive in ECRS, NANPs
and IPs (Figs. 2B and F; 3B and F; 5B
and F). The staining intensity of Gal-3, -7, -8 and -9 was
either low, low to moderate or moderate depending on the type of
lesion, i.e., ECRS, NANPs and IPs (Figs. 2D, H, J and L; 3D, H, J and L; 5D, H, J and L). In contrast, galectin
expression was relatively low in the SCCs studied (Fig. 6B, D, F, H, J and L).
Discussion
Galectin expression is often studied using
immunohistochemistry, which focuses on individual family members.
The accumulating evidence of this work suggests that effectors can
establish a network that warrants combined analysis. The present
study explored the characteristic signatures of galectin in several
naso-sinusal pathologies. Previous studies of our laboratory that
were dedicated to head and neck carcinomas revealed an association
between the presence of Gal-1, -3 and -7 and the progression to
malignancy, the inverse shifts between nuclear and cytoplasmic
localization, and the upregulation of Gal-8 in hypopharyngeal and
laryngeal squamous cell carcinomas. These observations contrast
with the downregulation that is often encountered in other tumor
types, thereby strongly indicating an intriguing difference among
galectins (14,17,44).
In the present study on a large series of salivary gland tumors,
galectin fingerprinting provided a new, significant tool for the
differential diagnosis of mucoepidermoid and acinic cell
carcinomas. In fact, we observed a unique profile that included the
cytoplasmic localization of Gal-1, -3, -7 and -8 in the
intermediate cells of mucoepidermoid carcinomas and the absence of
Gal-7 expression in acinic cell carcinomas (18). To pursue these observations, the
following methodological approach was used in this study: i)
monitoring of the expression of 6 galectins in parallel using
immunohistochemistry; ii) determination of their localization
profiles; and iii) assessment of the semi-quantitative expression
parameters. In detail, we compared the parameters for Gal-1 and
Gal-7 (proto-type), Gal-3 (chimera-type), and Gal-8 and -9
(tandem-repeat-type) to provide several key insights, such as the
non-uniform regulation of individual homologous proteins. The study
also further verifies the nucleocytoplasmic existence of galectins,
which is a common location for these proteins (44,45).
We determined that allergies do not seem to affect
galectin presence. Our results showed no significant difference
between NANPs and ANPs for all galectins studied, which is
consistent with previous results obtained for Gal-1 and -3
(27). Indeed, they showed that the
expression of galectin-1 was significantly higher in nasal polyps
than in middle turbinates. They also detected the increased
expression of galectin-3 in nasal polyps compared to middle and
inferior turbinates. However, they showed no relationship between
the allergic status of the patient and the expression of these
galectins (27). Galectin-3,
formerly identified as IgE-binding protein, is also suspected to
play a role in allergy pathways (46). It is remarkable that the allergic
status of the patient had no apparent effect on the expression of
this galectin. Another study by Sena and colleagues (47) used an immunohistochemical and
molecular approach to investigate the expression of Gal-1 and
glucocorticoid-regulated protein Annexin 1 (ANXA1). They observed
the upregulation of ANXA1 and the downregulation of Gal-1 in
polypoid tissue compared to normal mucosa. The authors suggested
that this observation could be associated with a specific mechanism
in NPs. Regarding the presence of Gals in CRS, no significant
difference between NECRS and ECRS was observed. Although Gal-9 is
considered a chemoattractant for eosinophils (48), its expression is not modulated by
eosinophils. Notably, nuclear Gal-9 can physically interact with
NF-IL6 (C/EBP-β), a transcription factor of the basic leucine
zipper family, in monocytic cells (49). If we consider all inflammatory
conditions, no significant difference was observed between nasal
polyposis and chronic rhinosinusitis. Sinonasal polyposis is the
final stage of chronic rhinosinusitis, and the expression of
galectin does not seem to be affected by the severity of the
inflammatory process.
In the present study, we describe the significant
upregulation and downregulation of Gals in naso-sinusal tumoral
progression. An increased number of epithelial cells positive for
Gal-4, -7 and -9 was detected in inverted papillomas and carcinomas
compared to non-malignant disease. The anti-apoptotic activity of
intracellular Gal-3 may contribute to tumor cell survival.
Concerning squamous cell carcinoma, a decrease in nuclear but not
cytoplasmic Gal-3 was observed. The overexpression of galectin-9,
which is known to suppress the adhesion of tumor cells to the
extracellular matrix and vascular endothelium, could be
advantageous for the treatment of squamous cell carcinomas because
it limits the formation of metastases (50). Exosomal packaging, which has been
shown to induce apoptosis of EBV-specific CD4+ cells in
nasopharyngeal carcinomas (51–53),
can describe a route for intracellular Gal-9 to become an
extracellular effector. Finally, a decrease in galectin-8 in
carcinomas, but not IPs, was shown. Hadari and colleagues (54) showed that galectin-8, which is
involved in anoikis, was able to interact with integrins and,
therefore, inhibit adhesion to the extracellular matrix and promote
apoptosis. Based on these data, a greater amount of galectin-8 in
IPs suggests that galectin-8 is involved in the prevention of
carcinogenesis by inhibiting cell-extracellular matrix
interactions.
At this stage, our analysis documents
semi-quantitative changes in the cellular expression of Gals. These
results suggest a functional correlation by the recognition of
distinct counter receptor(s). Methodologically, the application of
labeled Gals is an approach to mapping the location of accessible
binding sites, which has previously been demonstrated (55,56).
Recently, prognostic information was delineated from intracellular
Gal-3 reactivity in colon cancer (57). Using the presented map of protein
localization, it would be informative to study the reactivity
profiles using endogenous lectins as probes. Investigation of
galectin-specific signaling pathways in inverted papillomas and
carcinomas in future studies are warranted.
Acknowledgements
The present study was generously supported by the EC
(GlycoHIT; contract no. 260600).
Abbreviations:
|
ANP
|
allergic nasal polyp
|
|
NANP
|
non-allergic nasal polyp
|
|
CRS
|
chronic rhinosinusitis
|
|
ECRS
|
eosinophilic chronic
rhinosinusitis
|
|
NECRS
|
non-eosinophilic chronic
rhinosinusitis
|
|
IP
|
inverted papilloma
|
|
SCC
|
squamous cell carcinoma
|
References
|
1
|
Gabius HJ: The Sugar Code Fundamentals of
Glycosciences. Wiley-VCH; Weinheim, Germany: 2009
|
|
2
|
Sperandio M: Selectins and
glycosyltransferases in leukocyte rolling in vivo. FEBS J.
273:4377–4389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
André S, Sanchez-Ruderisch H, Nakagawa H,
Buchholz M, Kopitz J, Forberich P, Kemmner W, Böck C, Deguchi K,
Detjen KM, Wiedenmann B, von Knebel Doeberitz M, Gress TM,
Nishimura S, Rosewicz S and Gabius HJ: Tumor suppressor
p16INK4a- modulator of glycomic profile and galectin-1
expression to increase susceptibility to carbohydrate-dependent
induction of anoikis in pancreatic carcinoma cells. FEBS J.
274:3233–3256. 2007.
|
|
4
|
Sanchez-Ruderisch H, Fischer C, Detjen KM,
Welzel M, Wimmel A, Manning JC, André S and Gabius HJ: Tumor
suppressor p16INK4a: downregulation of galectin-3, an
endogenous competitor of the pro-anoikis effector galectin-1, in a
pancreatic carcinoma model. FEBS J. 277:3552–3563. 2010.
|
|
5
|
Wu G, Lu ZH, Gabius HJ, Ledeen RW and
Bleich D: Ganglioside GM1 deficiency in effector T cells from NOD
mice induces resistance to regulatory T cell suppression. Diabetes.
60:2341–2349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amano M, Eriksson H, Manning JC, Detjen
KM, André S, Nishimura SI, Lehtiö J and Gabius HJ: Tumour
suppressor p16INK4a: anoikis-favouring decrease in
N/O-glycan/cell surface sialylation by down-regulation of enzymes
in sialic acid biosynthesis in tandem in a pancreatic carcinoma
model. FEBS J. 279:4062–4080. 2012.PubMed/NCBI
|
|
7
|
Clark MC and Baum LG: T cells modulate
glycans on CD43 and CD45 during development and activation, signal
regulation, and survival. Ann NY Acad Sci. 1253:58–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu FT, Yang RY and Hsu DK: Galectins in
acute and chronic inflammation. Ann NY Acad Sci. 1253:80–91. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cooper DN: Galectinomics: finding themes
in complexity. Biochim Biophys Acta. 1572:209–231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaltner H and Gabius HJ: A toolbox of
lectins for translating the sugar code: the galectin network in
phylogenesis and tumors. Histol Histopathol. 27:397–416.
2012.PubMed/NCBI
|
|
11
|
Haudek KC, Spronk KJ, Voss PG, Patterson
RJ, Wang JL and Arnoys EJ: Dynamics of galectin-3 in the nucleus
and cytoplasm. Biochim Biophys Acta. 1800.181–189. 2010.PubMed/NCBI
|
|
12
|
Smetana K Jr, Andre S, Kaltner H, Kopitz J
and Gabius HJ: Context-dependent multifunctionality of galectin-1:
a challenge for defining the lectin as therapeutic target. Expert
Opin Ther Targets. 17:379–392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saussez S, Cucu DR, Decaestecker C,
Chevalier D, Kaltner H, André S, Wacreniez A, Toubeau G, Camby I,
Gabius HJ and Kiss R: Galectin-7 (p53-induced gene-1): a new
prognostic predictor of recurrence and survival in stage IV
hypopharyngeal cancer. Ann Surg Oncol. 13:999–1009. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saussez S, Decaestecker C, Lorfevre F,
Chevalier D, Mortuaire G, Kaltner H, André S, Toubeau G, Gabius HJ
and Leroy X: Increased expression and altered intracellular
distribution of adhesion/growth-regulatory lectins galectins-1 and
-7 during tumour progression in hypopharyngeal and laryngeal
squamous cell carcinomas. Histopathology. 52:483–493. 2008.
View Article : Google Scholar
|
|
15
|
Saussez S, Decaestecker C, Mahillon V,
Cludts S, Capouillez A, Chevalier D, Vet HK, Andre S, Toubeau G,
Leroy X and Gabius HJ: Galectin-3 upregulation during tumor
progression in head and neck cancer. Laryngoscope. 118:1583–1590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saussez S, de Leval L, Decaestecker C,
Sirtaine N, Cludts S, Duray A, Chevalier D, André S, Gabius HJ,
Remmelink M and Leroy X: Galectin fingerprinting in Warthin’s
tumors: lectin-bases approach to trace its origin? Histol
Histopathol. 25:541–550. 2010.
|
|
17
|
Cludts S, Decaestecker C, Mahillon V,
Chevalier D, Kaltner H, André S, Remmelink M, Leroy X, Gabius HJ
and Saussez S: Galectin-8 up-regulation during hypopharyngeal and
laryngeal tumor progression and comparison with galectin-1, -3 and
-7. Anticancer Res. 29:4933–4940. 2009.PubMed/NCBI
|
|
18
|
Remmelink M, de Leval L, Decaestecker C,
Duray A, Crompot E, Sirtaine N, André S, Kaltner H, Leroy X, Gabius
HJ and Saussez S: Quantitative immunohistochemical fingerprinting
of adhesion/growth-regulatory galectins in salivary gland tumours:
divergent profiles with diagnostic potential. Histopathology.
58:543–556. 2011. View Article : Google Scholar
|
|
19
|
Liu FT and Rabinovich G: Galectins as
modulators of tumour progression. Nat Rev Cancer. 5:29–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaltner H, Raschta AS, Manning JC and
Gabius HJ: Copy-number variation of functional galectin genes:
studying animal galectin-7 (p53-induced gene 1 in man) and
tandem-repeat-type galectins-4 and -9. Glycobiology. 23:1152–1163.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teclu A and Lacroix JS: Chronic
rhinosinusitis and nasal polyposis - a review. Otorinolaringol.
53:89–97. 2003.
|
|
22
|
Wood AJ and Douglas RG: Pathogenesis and
treatment of chronic rhinosinusitis. Postgrad Med J. 86:359–364.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Georgy MS and Peters AT: Chapter 8:
Rhinosinusitis. Allergy Asthma Proc. 33:24–27. 2012. View Article : Google Scholar
|
|
24
|
Sok JC and Ferguson BJ: Differential
diagnosis of eosinophilic chronic rhinosinusitis. Clin Allergy
Immunol. 19:69–85. 2007.PubMed/NCBI
|
|
25
|
Takeno S, Hirakawa K and Ishino T:
Pathological mechanisms and clinical features of eosinophilic
chronic rhinosinusitis in the Japanese population. Allergol Int.
59:247–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferguson BJ: Categorization of
eosinophilic chronic rhinosinusitis. Curr Opin Otolaryngol Head
Neck Surg. 12:237–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Delbrouck C, Gabius HJ, Kaltner H,
Decaestecker C, Kiss R and Hassid S: Expression patterns of
galectin-1 and galectin-3 in nasal polyps and middle and inferior
turbinates in relation to growth regulation and immunosuppression.
Arch Otolaryngol Head Neck Surg. 129:665–669. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Georgy MS and Peters AT: Chapter 7: Nasal
polyps. Allergy Asthma Proc. 33:22–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng W, Zheng C, Tian J and Shi G: T
helper cell population and eosinophilia in nasal polyps. J Investig
Allergol Clin Immunol. 17:297–301. 2007.PubMed/NCBI
|
|
30
|
Tomassen P, Van Zele T, Zhang N,
Perez-Novo C, Van Bruaene N, Gevaert P and Bachert C:
Pathophysiology of chronic rhinosinusitis. Proc Am Thorac Soc.
8:115–120. 2011. View Article : Google Scholar
|
|
31
|
Nadir H, Prades JM, Dumoliard JM and
Martin CH: Papillomes inverses des cavités naso-sinusiennes: A
propos de 19 patients. Journal Français d’oto-rhino-laryngologie.
52:81–86. 2003.(In French).
|
|
32
|
Anari S and Carrie S: Sinonasal inverted
papilloma: narrative review. J Laryngol Otol. 124:705–715. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brasnu D, Ayache D, Hans S, Hartl D and
Papon JF: Traité d’ORL. Flammarion, Paris: pp. 212–220. 2008
|
|
34
|
Altavilla G, Staffieri A, Busatto G,
Canesso A, Giacomelli L and Marioni G: Expression of p53,
p16INK4A, pRB, p21WAF1/CIP1,
p27KIP1, cyclin D1, Ki-67 and HPV DNA in sinonasal
endophytic Schneiderian (inverted) papilloma. Acta Otolaryngol.
129:1242–1249. 2009.
|
|
35
|
Thompson LDR: Head and Neck Pathology.
Churchill Livingstone/Elsevier; Philadelphia: pp. 124–132. pp.
155–160. 2006
|
|
36
|
Kopitz J, André S, von Reitzenstein C,
Versluis K, Kaltner H, Pieters RJ, Wasano K, Kuwabara I, Liu FT,
Cantz M, Heck AJ and Gabius HJ: Homodimeric galectin-7 (p53-induced
gene 1) is a negative growth regulator for human neuroblastoma
cells. Oncogene. 22:6277–6288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Purkrábková T, Smetana K Jr, Dvoránková B,
Holíková Z, Böck C, Lensch M, André S, Pytlík R, Liu FT, Klíma J,
Smetana K, Motlik J and Gabius HJ: New aspects of galectin
functionality in nuclei of cultured bone marrow stromal and
epidermal cells: biotinylated galectins as tool to detect specific
binding sites. Biol Cell. 95:535–545. 2003.PubMed/NCBI
|
|
38
|
Langbein S, Brade J, Badawi JK, Hatzinger
M, Kaltner H, Lensch M, Specht K, André S, Brinck U, Alken P and
Gabius HJ: Gene-expression signature of adhesion/growth-regulatory
tissue lectins (galectins) in transitional cell cancer and its
prognostic relevance. Histopathology. 51:681–690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaltner H, Kübler D, López-Merino L, Lohr
M, Manning JC, Lensch M, Seidler J, Lehmann WD, André S, Solís D
and Gabius HJ: Toward comprehensive analysis of the galectin
network in chicken: unique diversity of galectin-3 and comparison
of its localization profile in organs of adult animals to the other
four members of this lectin family. Anat Rec. 294:427–444. 2011.
View Article : Google Scholar
|
|
40
|
Fík Z, Valach J, Chovanec M, Mazánek J,
Kodet R, Kodet O, Tachezy R, Foltynova E, André S, Kaltner H,
Gabius HJ and Smetana K Jr: Loss of adhesion/growth-regulatory
galectin-9 from squamous cell epithelium in head and neck
carcinomas. J Oral Pathol Med. 42:166–1673. 2013.PubMed/NCBI
|
|
41
|
Saal I, Nagy N, Lensch M, Lohr M, Manning
JC, Decaestecker C, André S, Kiss R, Salmon I and Gabius HJ: Human
galectin-2: expression profiling by RT-PCR/immunohistochemistry and
its introduction as histochemical tool for ligand localization.
Histol Histopathol. 20:1191–1208. 2005.PubMed/NCBI
|
|
42
|
Lohr M, Kaltner H, Lensch M, André S,
Sinowatz F and Gabius HJ: Cell-type-specific expression of murine
multifunctional galectin-3 and its association with follicular
atresia/luteolysis in contrast to pro-apoptotic galectins-1 and -7.
Histochem Cell Biol. 130:567–581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sarter K, Janko C, Andre S, Munoz LE,
Schorn C, Winkler S, Rech J, Kaltner H, Lorenz HM, Schiller M,
Andreoli L, Manfredi AA, Isenberg DA, Schett G, Herrmann M and
Gabius HJ: Autoantibodies against galectins are associated with
antiphospholipid syndrome in patients with systemic lupus
erythematosus. Glycobiology. 23:12–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Danguy A, Rorive S, Decaestecker C,
Bronckart Y, Kaltner H, Hadari YR, Goren R, Zich Y, Petein M,
Salmon I, Gabius HJ and Kiss R: Immunohistochemical profile of
galectin-8 expression in benign and malignant tumors of epithelial,
mesenchymatous and adipous origins, and of the nervous system.
Histol Histopathol. 16:861–868. 2001.PubMed/NCBI
|
|
45
|
Wang JL, Gray RM, Haudek KC and Patterson
RJ: Nucleocytoplasmic lectins. Biochim Biophys Acta. 1673:75–93.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rubinstein N, Ilarregui JM, Toscano MA and
Rabinovich GA: The role of galectins in the initiation,
amplification and resolution of the inflammatory response. Tissue
Antigens. 64:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sena AAS, Provazzi PJS, Fernandes AM, Cury
PM, Rahal P and Oliani SM: Spatial expression of two
anti-inflammatory mediators, annexin 1 and galectin-1, in nasal
polyposis. Clin Exp Allergy. 36:1260–1267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Iino Y, Miyazama T, Kakizaki K, Saigusa H,
Katano H, Shiga J and Kanegasaki S: Expression of ecalectin, a
novel eosinophil chemoattractant, in nasal polyps. Acta
Otolaryngol. 126:43–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Matsuura A, Tsukada J, Mizobe T, Higashi
T, Mouri F, Tanikawa R, Yamauchi A, Hirashima M and Tanaka Y:
Intracellular galectin-9 activates inflammatory cytokines in
monocytes. Genes Cells. 14:511–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nobumoto A, Nagahara K, Oomizu S, Katoh S,
Nishi N, Takeshita K, Niki T, Tominaga A, Yamauchi A and Hirashima
M: Galectin-9 suppresses tumor metastasis by blocking adhesion to
endothelium and extracellular matrices. Glycobiology. 18:735–744.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pioche-Durieu C, Keryer C, Souquere S,
Bosq J, Faigle W, Loew D, Hirashima M, Nishi N, Middeldorp J and
Busson P: In nasopharyngeal carcinoma cells, Epstein-Barr virus
LMP1 interacts with galectin 9 in membrane raft elements resistant
to simvastatin. J Virol. 79:13326–13337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Keryer-Bibens C, Pioche-Durieu C,
Villemant C, Souquere S, Nishi N, Hirashima M, Middeldorp J and
Busson P: Exosomes released by EBV-infected nasopharyngeal
carcinoma cells convey the viral latent membrane protein 1 and the
immunomodulatory protein galectin 9. BMC Cancer. 6:2832006.
View Article : Google Scholar
|
|
53
|
Klibi J, Niki T, Riedel A, Pioche-Durieu
C, Souquere S, Rubinstein E, Le Moulec S, Guigay J, Hirashima M,
Guemira F, Adhikary D, Mautner J and Busson P: Blood diffusion and
Th1-suppressive effects of galectin-9-containing exosomes released
by Epstein-Barr virus-infected nasopharyngeal carcinoma cells.
Blood. 113:1957–1966. 2009. View Article : Google Scholar
|
|
54
|
Hadari YR, Arbel-Goren R, Levy Y,
Amsterdam A, Alon R, Zakut R and Zick Y: Galectin-8 binding to
integrins inhibits cell adhesion and induces apoptosis. J Cell Sci.
113:2385–2397. 2000.PubMed/NCBI
|
|
55
|
Saussez S, Lorfevre F, Nonclercq D,
Laurent G, André S, Journé F, Kiss R, Toubeau G and Gabius HJ:
Towards functional glycomics by localization of binding sites for
tissue lectins: lectin histochemical reactivity for galectins
during diethylstilbestrol-induced kidney tumorigenesis in male
Syrian hamster. Histochem Cell Biol. 126:57–69. 2006. View Article : Google Scholar
|
|
56
|
Vansthertem D, Cludts S, Nonclercq D,
Gossiaux A, Saussez S, Legrand A, Gabius HJ and Toubeau G:
Immunohistochemical localization of galectins-1 and -3 and
monitoring of tissue galectin-binding sites during tubular
regeneration after renal ischemia reperfusion in the rat. Histol
Histopathol. 25:1417–1429. 2010.
|
|
57
|
Dawson H, André S, Karamitopoulou E,
Zlobec I and Gabius HJ: The growing galectin network in colon
cancer and clinical relevance of cytoplasmic galectin-3 reactivity.
Anticancer Res. 33:3053–3059. 2013.PubMed/NCBI
|





















