Introduction
Worldwide, esophageal cancer is the sixth leading
cause of cancer-related mortality (1). Squamous cell carcinoma (SCC) is the
predominant histologic type of esophageal cancer worldwide
(2), and esophageal squamous cell
carcinoma (ESCC) is often encountered in Asia (3–5),
including Japan. ESCC is one of the least studied and most fatal
cancers (6).
The microenvironment of cancer cells has recently
been shown to strongly influence the biologic properties of cancer
(7). In fact, tumors consist of
tumor cells, fibroblasts, endothelial cells, immune cells and
extracellular matrix. In addition, many types of solid tumors
contain smooth muscle actin (SMA)-positive myofibroblasts [i.e.,
activated fibroblasts, peritumor fibroblasts, and
carcinoma-associated fibroblasts (CAFs)] within the stroma
(8). Molecular features thought to
influence outcomes are generally related to the characteristics of
carcinoma cells (9); however, it
has become increasingly apparent that the components of the tumor
stroma play an important role in promoting tumor progression
(10,11). Myofibroblasts have been reported to
be related to poor outcomes in several types of carcinoma (12–14).
Fibroblasts are associated with cancer cells at all stages of
disease progression, and their production of growth factors,
chemokines, and extracellular matrix facilitates the angiogenic
recruitment of endothelial cells and pericytes (15).
Marsh et al reported that an SMA-positive
myofibroblastic stroma is the strongest predictor of mortality in
patients with oral SCC (9). In the
present study, we focused on the complex interrelations between
ESCC cells and fibroblasts, the main component of cancer stroma.
The levels of α-smooth muscle actin (α-SMA) were determined
immunohistochemically in ESCC lesions, and immunoreactivity was
observed in stromal fibroblasts. We also prepared in vitro
and in vivo experimental systems to evaluate interactions
between ESCC and fibroblasts.
Materials and methods
Tissue collection and processing
From January 1st 2001 to December 31st 2010, a total
of 347 patients underwent resection of ESCC in Jichi Medical
Hospital. We identified and studied patients with tumor invasion
beyond the muscularis mucosa who received neither preoperative
chemotherapy nor radiotherapy. We then retrospectively analyzed the
relations of clinical, pathological and molecular (α-SMA and
vimentin) features to outcomes in 97 patients with ESCC. Data were
collected on age, gender, tumor stage (16,17),
outcomes, the presence or absence of recurrence, lymph node
metastasis, depth of tumor invasion, and the presence or absence of
venous invasion.
Immunohistochemistry (IHC)
Representative paraffin blocks were selected, and
tissue sections (4-μm thick) of the specimens were deparaffinized
in xylene, rehydrated in a graded series of alcohol, and
transferred to phosphate-buffered saline (PBS). The slides were
stained immunohistochemically for α-SMA and vimentin according to
conventional protocols. The antibodies used were α-SMA (clone 1A4)
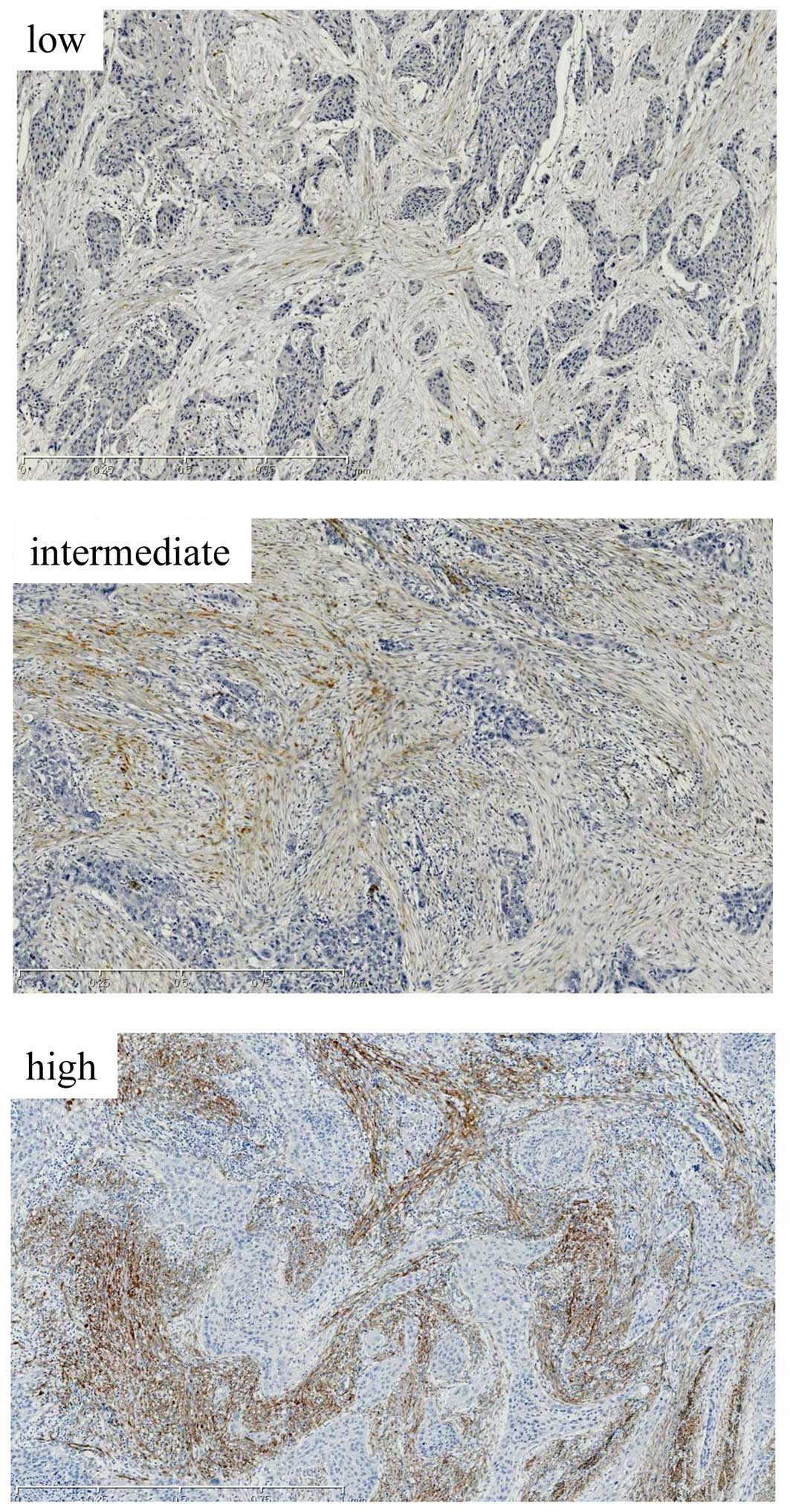
and vimentin (clone V9) (both from Dako, Ely, UK). α-SMA was scored
according to the extent of stromal positivity as low (<5% stroma
stained positive), moderate (patchy/focal expression, 5–50% stroma
stained positive) or high (diffuse expression throughout tumor,
>50% stroma stained positive) (Fig.
1) (9). In addition to α-SMA,
vimentin immunostain was used as an ancillary method to help
identify stromal fibroblasts.
Cell lines and media
We used three ESCC cell lines and esophageal stromal
fibroblasts. TE-11 was purchased from RIKEN Cell Bank (Tsukuba,
Japan). KYSE150 and KYSE220 were purchased from Health Science
Research Resources Bank (Osaka, Japan). All cancer cell lines were
maintained in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS; Autogen-Bioclear, Wiltshire, UK), glutamine, 100 U/ml
penicillin and 100 μg/ml streptomycin, in a humidified atmosphere
of 5% CO2 and 95% air. These three cell lines were
selected from among 22 ESCC cell lines on the basis of the results
of cell proliferation induced by fibroblast supernatant
(unpublished observation). Primary human esophageal fibroblasts
designated as HEF75 were isolated from normal human esophageal
tissue specimens resected surgically in the Department of Surgery,
Jichi Medical Hospital. The patients had not received any
neoadjuvant chemotherapy or radiotherapy irradiation. The study was
approved by the Jichi Medical University Ethics Committee, and
written informed consent was obtained from the patients.
To isolate fibroblasts (18,19),
the epithelial tissue was washed twice in PBS and cut into 1- to
2-mm3 pieces. A couple of pieces were placed into the
well of a 6-well plate. The explants were cultured for 48 h in
Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen) supplemented
with 10% FBS, antibiotics, and glutamine at 37°C in a humidified
atmosphere of 5% CO2 and 95% air. After removing the
explants and the non-adherent cells, the remaining cells were
incubated for 1 to 2 weeks. The adherent cells were then
trypsinized and passaged into a new culture flask at a ratio of 1:3
for further expansion. The cells were used for subsequent
experimental studies at the third passage. Some of the human
esophageal fibroblasts were immortalized. Briefly, to induce cell
immortalization, transforming DNA (pCLXSN-hTERT and pVSV-G) was
added to competent cells (Escherichia coli), which were then
spread on LB plates and incubated at 37°C to produce colonies.
Next, in accordance with the Qiagen Plasmid Midi/Maxi kit (Qiagen
KK, Tokyo, Japan) protocol, bacterial cells were collected from the
LB plate colonies. Plasmid DNA was eluted from the bacterial
pellets. The concentration of DNA was adjusted to 1.0 μg/μl. The
two DNA products were digested by adding the restriction enzymes
HindIII and EcoRI, and 1% agarose gel electrophoresis
was performed to confirm that pVSV-G was cut with EcoRI.
In accordance with the protocol of Lipofectamine
2000 reagent (Invitrogen Co., Ltd.), plasmid DNA was transfected
into GP2-293 cell line. After 48 h, the culture supernatant
including the retrovirus was added to fibroblasts to induce
transduction. Selection was performed with G418
(Geneticin®; Invitrogen) 48 h after transduction. In the
present study, cells between the 20 and 25th passages after viral
transduction were used. Immortalized human esophageal fibroblasts
were designated as human embryonic fibroblast (HEF) 75-human
telomerase reverse transcriptase (HEF75-hTERT).
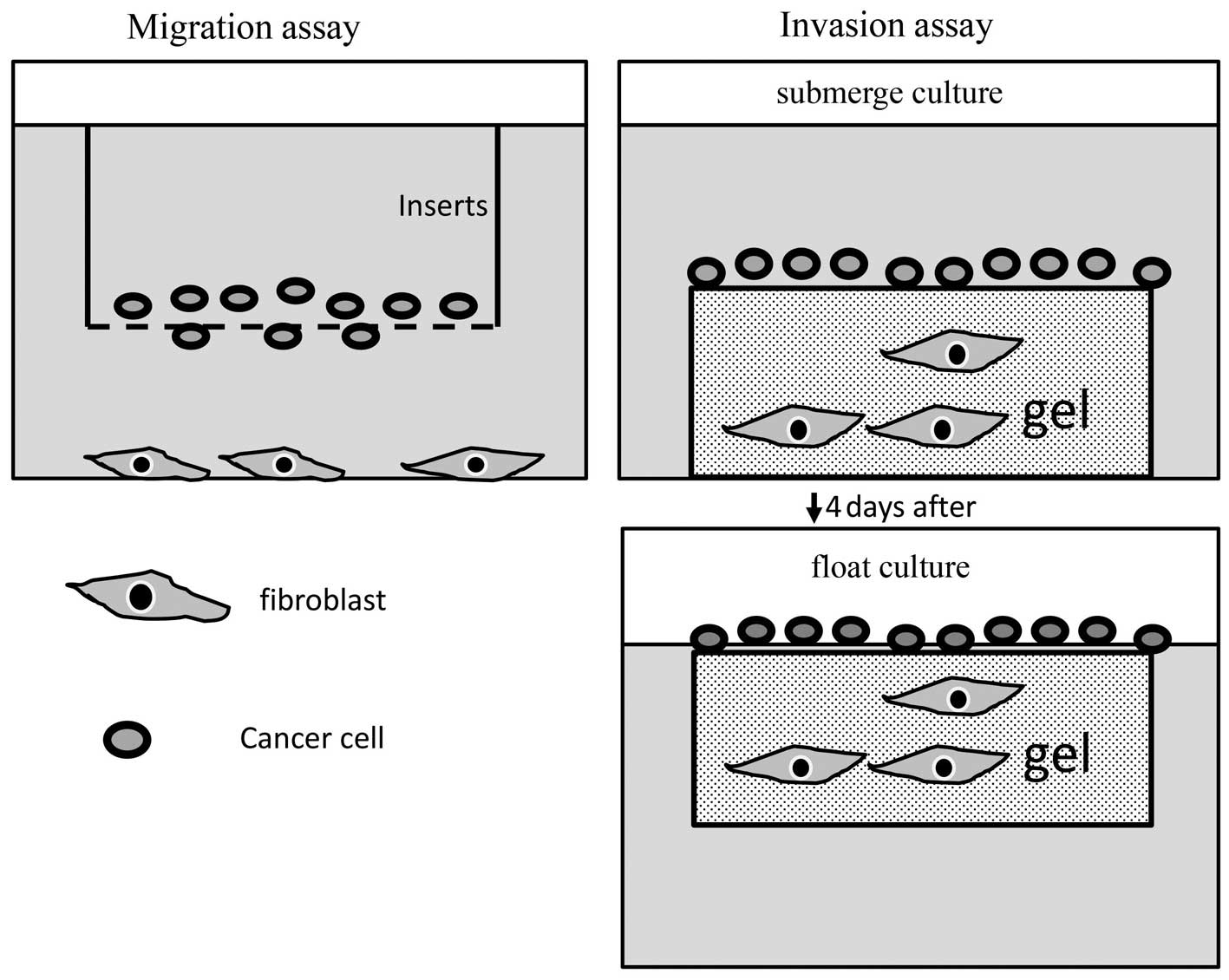
Migration assay and 3D organotypic
culture
Migration and invasion assays were conducted as
described previously (20,21). For migration assay, 24-well cell
culture inserts with a pore size of 8-μm (Falcon) were used. The
inserts were placed in a 24-well plate containing medium with
HEF75. Cancer cells in serum-free medium (containing
5–20×104 cells) were placed in each insert. Migratory
cells pass through membrane and cling to the bottom side. After
cells remaining on the membrane were removed, migratory cells were
stained and captured in 5 different high-power fields. The numbers
of migratory cells were then automatically counted with the use of
a VH analyzer (Keyence, Osaka, Japan). For invasion assay,
HEF75-hTERT fibroblasts were mixed with collagen gel at a
concentration of 1×106 cells/ml and allowed to contract.
Then, 2×105 of cancer cells were plated, using a cloning
ring. The cells were allowed 4 h to attach to the upper surface of
the contracted collagen lattice. The cultures were submerged for 4
days, then subsequently placed into 6-well inserts, raised to an
air-liquid interface, and cultured for an additional 10–14 days.
The schemata of these experiments are shown in Fig. 2.
Co-injection in Nod/Scid mice
Cancer cells and fibroblasts were co-transplanted
subcutaneously in Nod/Scid mice to assess the effects of
fibroblasts on tumorigenicity. ESCC cells alone or together with
human esophageal fibroblasts (HEF75) were suspended in PBS and
injected subcutaneously into the flanks of the mice (two injection
sites per mouse). We subcutaneously injected 200 μl of a cancer
cell suspension with a concentration of 1×106 cells/ml
into the right side of the mice and 200 μl of a suspension
containing a 1:1 mix of cancer cells (2×106 cells/ml)
and HEFs (6×106 cells/ml) into the left side of the
mice. The mice (n=3) were sacrificed 6 weeks after the injections.
We measured the minor axis and major axis and calculated the tumor
volume as an oval sphere shape. The following formula was used to
calculate tumor volume: Volume = 0.5236 ddD (d, minor axis; D,
major axis).
Statistical analysis
The primary end point was death from ESCC. Survival
time was measured from the date of the surgery until the date of
mortality due to ESCC or the date on which the patient was last
confirmed to be alive. Data were censored at the time of death for
patients who died of causes other than ESCC.
Kaplan-Meier survival curves and log-rank tests were
used to assess the statistical significance of differences in
survival. Analyses were performed using the JMP 9 statistical
software package (SAS Institute Inc., Cary, NC, USA). Pearson’s
Chi-square test was used to analyze relations between α-SMA
expression and clinicopathological characteristics. Student’s
t-test was used to compare differences between 2 groups and to
analyze the results of migration assays. Both of these analyses
were performed with the use of JMP 9 software. p<0.05 was
considered to indicate a statistically significant difference.
Results
α-SMA expression is associated with ESCC
mortality in stage I and II
During the period from 2001 to 2010, data were
available for 97 patients with ESCC. Table I shows the characteristics of these
patients. There were 15 women (15.4%) and 82 men (84.5%). The mean
age at surgery (± standard deviation) was 67.2±7.7 years (range,
44–82 years). The median follow-up was 1,244 days, and the minimum
follow-up was 94 days. Of the 97 patients, 30 were confirmed to
have succumbed to ESCC. More than half of the patients (62/97, 64%)
presented with relatively early-stage disease (stage I-II according
to the TNM classification) (17).
Fig. 1 shows typical examples of
tumors with low, intermediate and high SMA expression. The
percentages of patients with low, intermediate and high α-SMA
expression were 47.4, 24.7 and 27.8%, respectively.
Clinicopathologically, α-SMA expression correlated with venous
invasion, nodal involvement and recurrence (p<0.01, p=0.02 and
p=0.01, respectively) (Table II).
The mean survival time of the 97 patients with ESCC was 1,720±83
days.
 | Table ICharacteristics of 97 patients with
ESCC. |
Table I
Characteristics of 97 patients with
ESCC.
| No. | % |
|---|
| Age (years) |
| 40–59 | 18 | 18.5 |
| 60–79 | 76 | 78.4 |
| 80–99 | 3 | 3.1 |
| Average | 67.2±7.7 | |
| Gender |
| Female | 15 | 15.5 |
| Male | 82 | 84.5 |
| Recurrence |
| Present | 34 | 35.1 |
| Absent | 63 | 64.9 |
| Outcome |
| AWOD | 62 | 63.9 |
| AWD | 4 | 4.1 |
| DOAD | 1 | 1.1 |
| DOD | 30 | 30.9 |
| Disease stagea |
| I | 29 | 29.9 |
| II | 32 | 33.0 |
| III | 32 | 33.0 |
| IV | 4 | 4.1 |
| Disease
stageb |
| I (A, B) | 36 (29, 7) | 37.1 |
| II (A, B) | 26 (9, 17) | 26.8 |
| III (A, B, C) | 31 (18, 8, 5) | 32 |
| IV | 4 | 4.1 |
| Depth of
invasion |
| T1b | 44 | 45.4 |
| T2 | 15 | 15.5 |
| T3 | 37 | 38.1 |
| T4 | 1 | 1.0 |
| Nodal
involvement |
| Present | 51 | 52.6 |
| Absent | 46 | 47.4 |
| α-SMA |
| Low | 46 | 47.4 |
| Intermediate | 24 | 24.7 |
| High | 27 | 27.8 |
 | Table IIRelationship between SMA expression
and clinicopathological features. |
Table II
Relationship between SMA expression
and clinicopathological features.
| α-SMA
expression | | |
|---|
| |
| | |
|---|
| Clinicopathological
features | n | Low | Intermediate | High | r | P-value |
|---|
| Venous
invasion | | | | | 0.33 | <0.01 |
| v0 | 24 | 21 | 2 | 1 | | |
| v+ | 73 | 25 | 22 | 26 | | |
| Nodal
involvement | | | | | 0.18 | 0.02 |
| pN0 | 46 | 28 | 10 | 8 | | |
| pN+ | 51 | 18 | 14 | 19 | | |
| Recurrence | | | | | 0.2 | 0.01 |
| − | 64 | 37 | 13 | 14 | | |
| + | 33 | 9 | 11 | 13 | | |
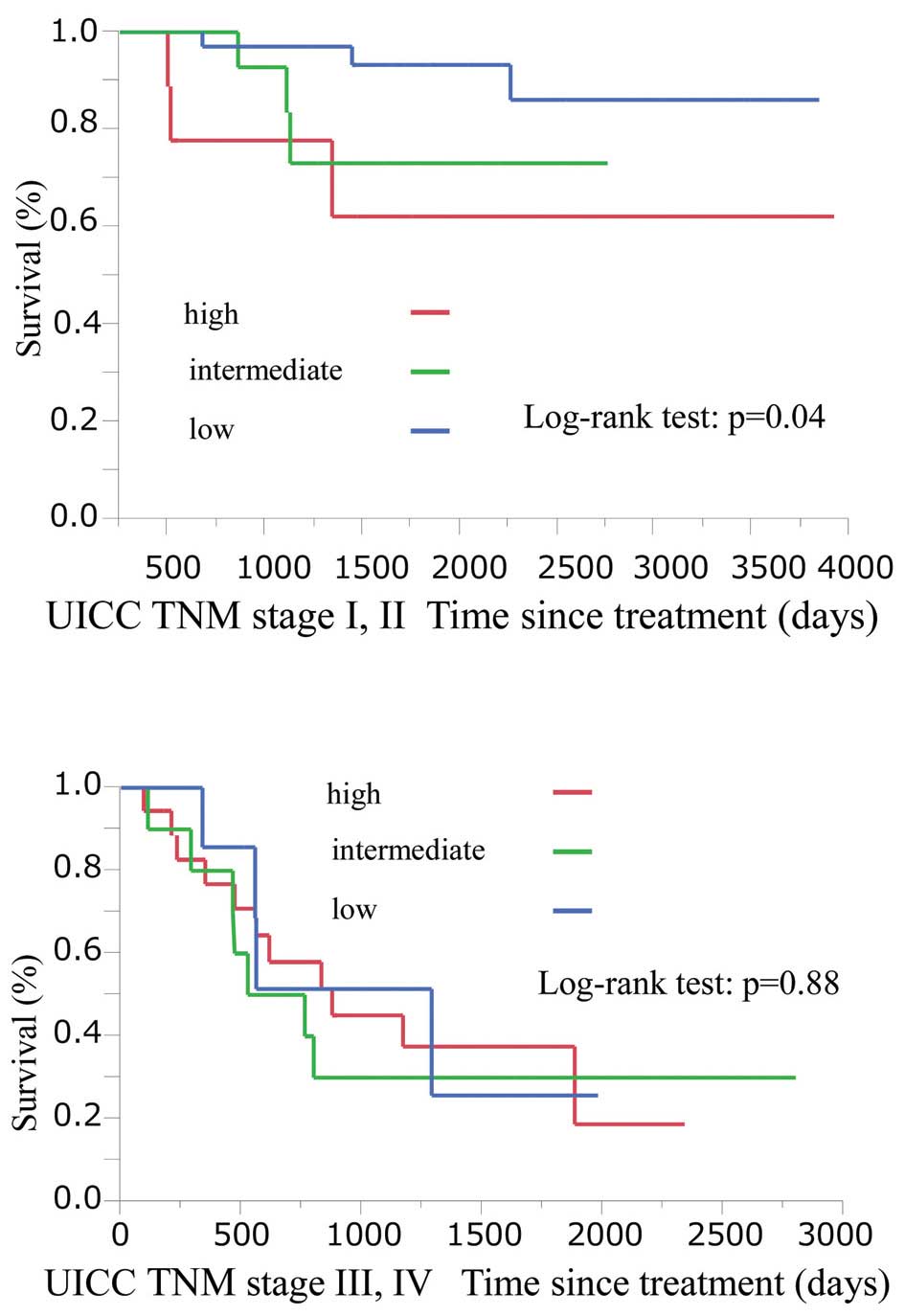
Among patients with stage I and II disease, the
Kaplan-Meier survival curves differed significantly according to
α-SMA expression (p=0.04). In patients with advanced stage III and
IV disease, however, there was no difference in survival according
to SMA expression (p=0.88) (Fig.
3).
Isolated human esophageal fibroblast
promotes ESCC cell line progression in vitro and in vivo
We used in vitro and in vivo
experimental systems to evaluate interactions between ESCC cells
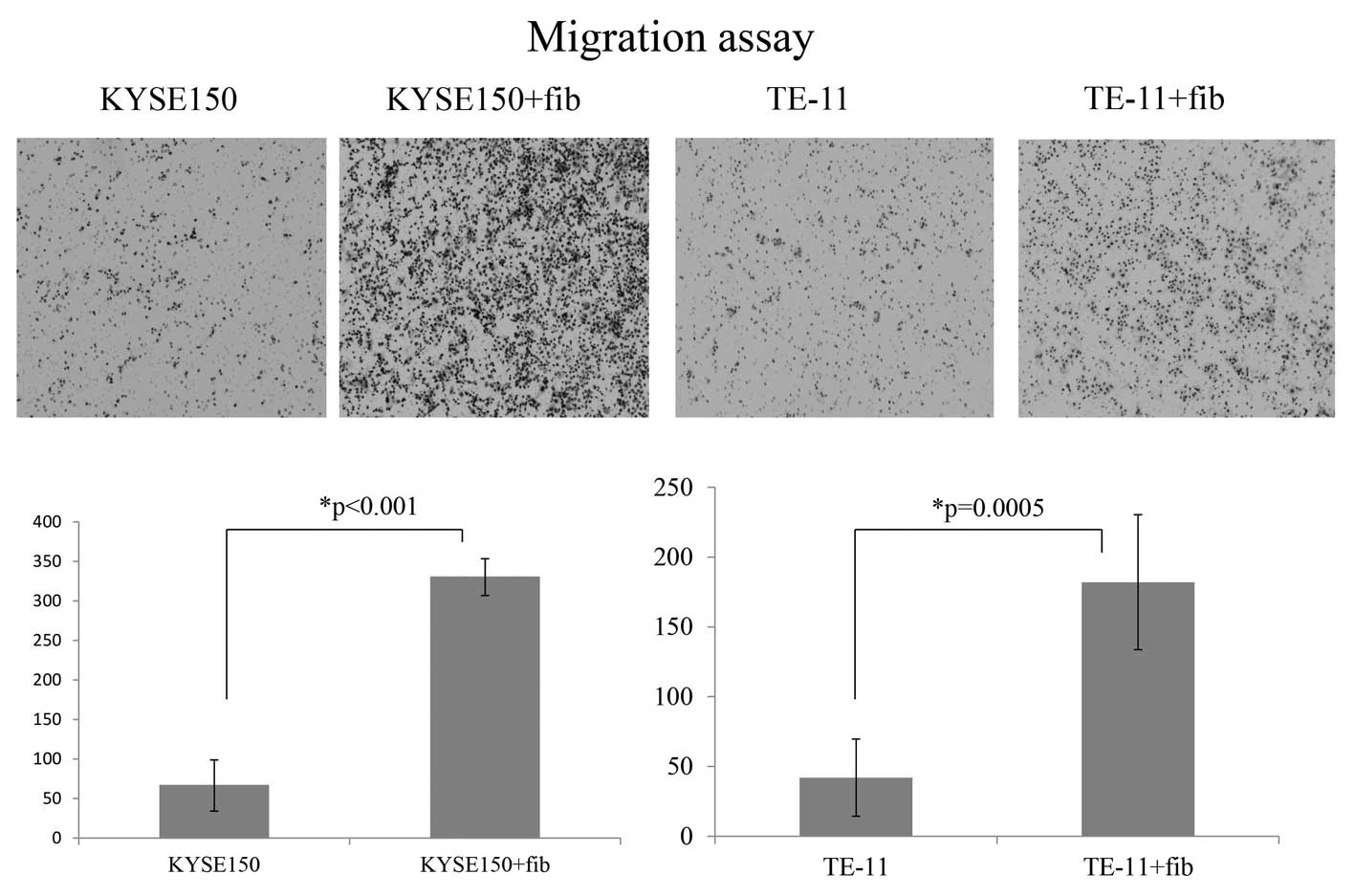
and stromal fibroblasts. In the migration assay, we compared
stained images acquired from the bottom membrane of the cell
culture inserts according to the presence and absence of
fibroblasts. The presence of fibroblasts (HEF75) significantly
promoted migration of KYSE150 and TE-11 cells (Fig. 4). For KYSE220, there was virtually
no migration of cancer cells through the membrane in the absence of
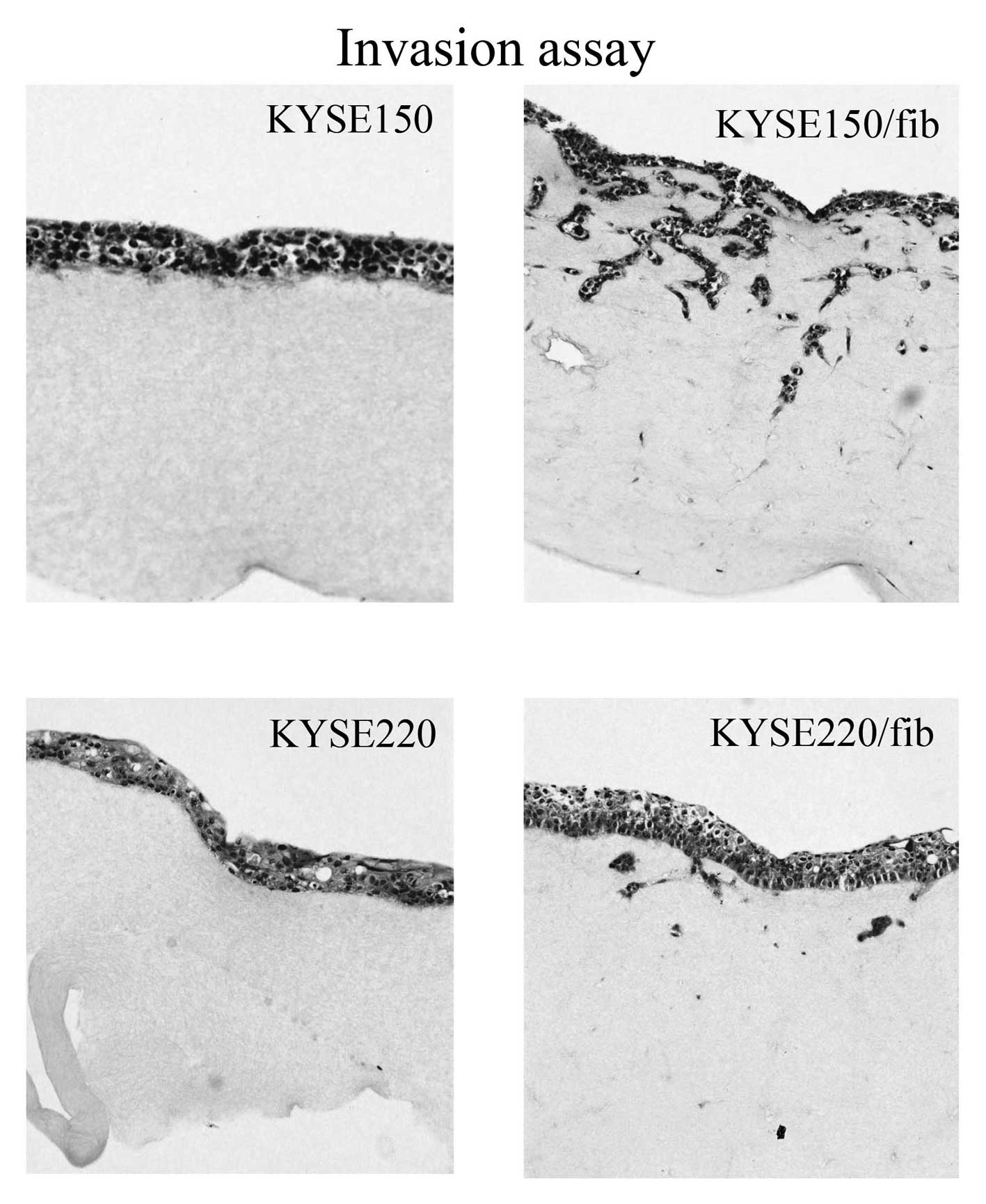
fibroblasts (data not shown). The KYSE150 and KYSE220 cell lines
showed invasion into the extracellular matrix gel mixed with human
immortalized esophageal fibroblasts (HEF75-hTERT), while cancer
cells alone showed no such effect (Fig.
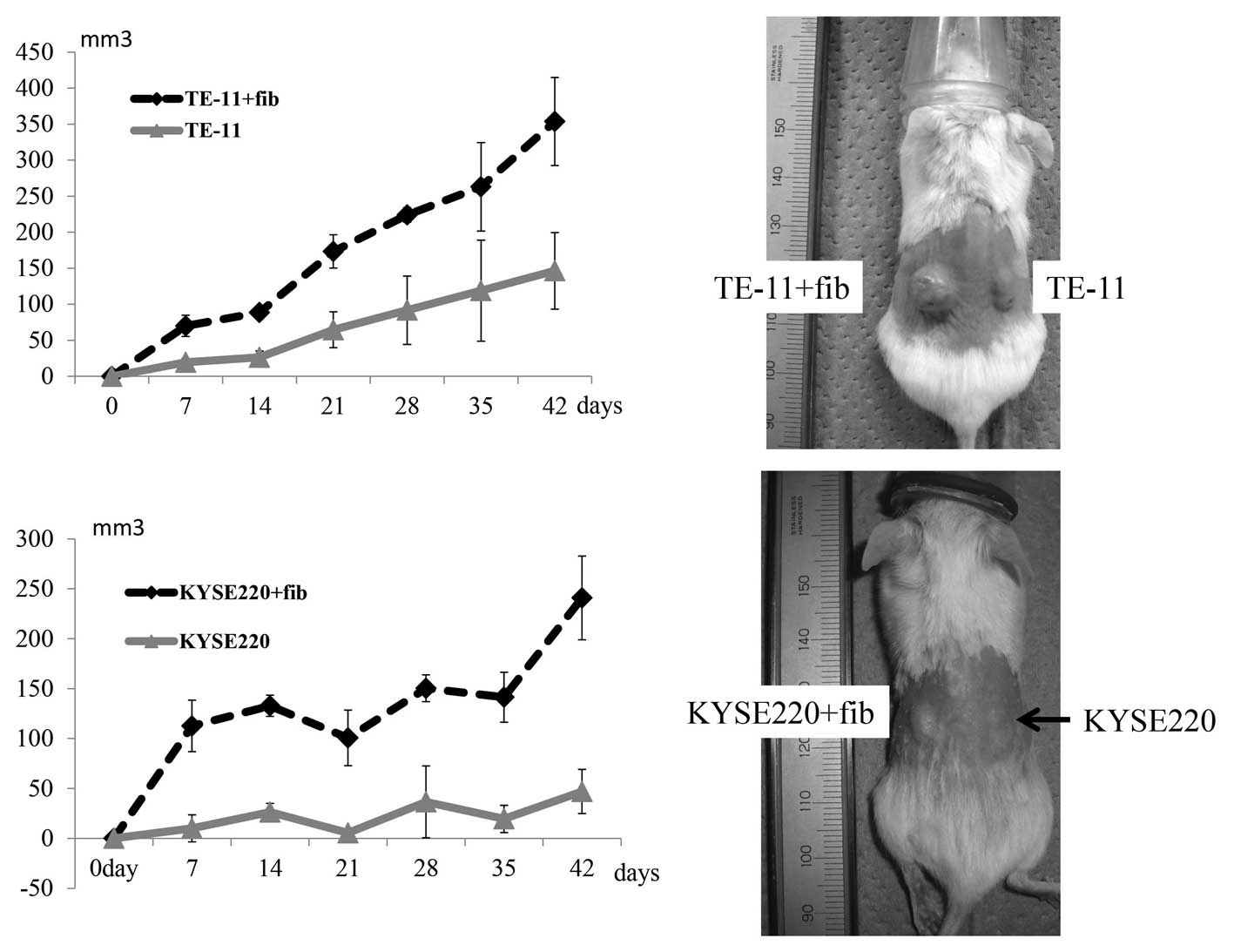
5). Finally, ESCC cells (TE-11 and KYSE220) alone or together
with immortalized human esophageal fibroblasts (HEF75-hTERT) were
suspended in PBS and injected subcutaneously into the flanks of the
Nod/Scid mice to assess the effects of fibroblasts on
tumorigenicity. Tumors developed in a fibroblast-dependent manner
after the implantation of the two ESCC cell lines (Fig. 6).
Discussion
Cancer results from the accumulated effects of many
genetic alterations, and the specific combination of changes is
reflected in the unique characteristics of each tumor. However, the
microenvironment of cancer cells has recently been shown to
strongly influence the biologic properties of cancer (7). Activated fibroblasts in tumor stroma,
referred to as CAFs, have been associated with poor outcomes in
several types of carcinoma (9).
α-SMA is the most widely used marker of CAFs (22,23).
In the present study, we initially examined expression levels of
α-SMA in tumor stroma of resected ESCC specimens.
Clinicopathologically, lymph node metastasis, venous invasion and
recurrence were significantly related to α-SMA expression levels.
Furthermore, increased α-SMA expression was associated with poorer
outcomes in patients with earlier stage ESCC. In contrast, there
was no relationship between α-SMA expression and outcomes in
patients with more advanced disease. These findings suggested that
increased stromal α-SMA expression may be a predictor of mortality
in patients with earlier stage ESCC.
Next, we conducted migration and invasion assays to
assess the effects of stromal fibroblasts. The presence of
fibroblasts (HEF75) strongly promoted migration of KYSE150, KYSE220
and TE-11 cells (Fig. 4).
Furthermore, the KYSE150 and KYSE220 cell lines invaded into the
extracellular matrix gel mixed with human immortalized esophageal
fibroblasts (HEF75-hTERT) (Fig. 5).
These assays showed that isolated esophageal fibroblasts could
promote cancer-cell migration and invasion. Tumor stroma has been
suggested to play a critical role in the progression of human ESCC
(24). Growth factors such as
hepatocyte growth factor (HGF) and fibroblast growth factors (FGF)
secreted by cancer stroma can promote ESCC development and
progression (7,25–28).
In the present study, growth factors and cytokines (29) secreted by isolated fibroblasts may
have played an important role in cancer-cell migration and
invasion. Gaggioli et al reported that fibroblasts may
generate a track within the matrix that SCC cells then use to
invade in organotypic culture model experiments (30). Not only paracrine systems induced by
growth factors secreted by fibroblasts, but also the ability of
fibroblasts to create tracks in the matrix may have promoted
cancer-cell invasion in our organotypic assay. In vivo,
fibroblasts may also have helped cancer cells grow in a
fibroblast-dependent manner after the implantation of two ESCC cell
lines (Fig. 6). We also
subcutaneously transplanted HEF75 and HEF75-hTERT into the Nod/Scid
mice and sacrificed the animals 2, 4, and 6 weeks after injection.
Immunohistochemical studies of vimentin (clone V9; Dako), which
does not react with cells of mice (31), showed that the survival periods of
transplanted normal fibroblasts and immortalized fibroblasts were 2
and 4 weeks, respectively (data not shown). These findings
suggested that interactions between ESCC cells and fibroblasts play
a critical role in the relatively early period after
co-transplantation. This hypothesis may be consistent with our
finding that higher stromal α-SMA expression was related to poorer
survival in patients with earlier stage ESCC.
In conclusion, our data provide evidence that
stromal fibroblasts and tumor cells interact to promote tumor
progression in ESCC. In patients with earlier stage ESCC, α-SMA may
be a predictor of mortality. Our findings also suggest that
inhibition of paracrine systems related to fibroblasts and cancer
cells may slow or reverse tumor progression.
References
|
1
|
Pisani P, Parkin DM, Bray F and Ferlay J:
Estimates of the worldwide mortality from 25 cancers in 1990. Int J
Cancer. 83:18–29. 1999. View Article : Google Scholar
|
|
2
|
Cook MB: Non-acid reflux: the missing link
between gastric atrophy and esophageal squamous cell carcinoma? Am
J Gastroenterol. 106:1930–1932. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Higuchi K, Koizumi W, Tanabe S, et al:
Current management of esophageal squamous-cell carcinoma in Japan
and other countries. Gastrointest Cancer Res. 3:153–161.
2009.PubMed/NCBI
|
|
4
|
Chang CY, Cook MB, Lee YC, et al: Current
status of Barrett’s esophagus research in Asia. J Gastroenterol
Hepatol. 26:240–246. 2011.
|
|
5
|
Hongo M, Nagasaki Y and Shoji T:
Epidemiology of esophageal cancer: Orient to Occident. Effects of
chronology, geography and ethnicity. J Gastroenterol Hepatol.
24:729–735. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mueller MM and Fusenig NE: Friends or foes
- bipolar effects of the tumour stroma in cancer. Nat Rev Cancer.
4:839–849. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Radisky DC, Kenny PA and Bissell MJ:
Fibrosis and cancer: do myofibroblasts come also from epithelial
cells via EMT? J Cell Biochem. 101:830–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marsh D, Suchak K, Moutasim KA, et al:
Stromal features are predictive of disease mortality in oral cancer
patients. J Pathol. 223:470–481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Wever O and Mareel M: Role of tissue
stroma in cancer cell invasion. J Pathol. 200:429–447.
2003.PubMed/NCBI
|
|
11
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Surowiak P, Murawa D, Materna V, et al:
Occurence of stromal myofibroblasts in the invasive ductal breast
cancer tissue is an unfavourable prognostic factor. Anticancer Res.
27:2917–2924. 2007.PubMed/NCBI
|
|
13
|
Kellermann MG, Sobral LM, da Silva SD, et
al: Myofibroblasts in the stroma of oral squamous cell carcinoma
are associated with poor prognosis. Histopathology. 51:849–853.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsujino T, Seshimo I, Yamamoto H, et al:
Stromal myofibroblasts predict disease recurrence for colorectal
cancer. Clin Cancer Res. 13:2082–2090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar
|
|
16
|
Japanese Esophageal Society. Japanese
Classification of Esophageal Cancer, tenth edition: part I.
Esophagus. 6:1–25. 2009. View Article : Google Scholar
|
|
17
|
Sobin LH GM, Gospodarowicz MK and
Wittekind CH: International Union Against Cancer (UICC) TNM
Classification of Malignant Tumors. 7th edition. Wiley-Blackwell;
2009
|
|
18
|
Andl CD, Mizushima T, Nakagawa H, et al:
Epidermal growth factor receptor mediates increased cell
proliferation, migration, and aggregation in esophageal
keratinocytes in vitro and in vivo. J Biol Chem. 278:1824–1830.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Underwood TJ, Derouet MF, White MJ, et al:
A comparison of primary oesophageal squamous epithelial cells with
HET-1A in organotypic culture. Biol Cell. 102:635–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aoki S, Takezawa T, Uchihashi K, Sugihara
H and Toda S: Non-skin mesenchymal cell types support epidermal
regeneration in a mesenchymal stem cell or myofibroblast
phenotype-independent manner. Pathol Int. 59:368–375. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okawa T, Michaylira CZ, Kalabis J, et al:
The functional interplay between EGFR overexpression, hTERT
activation, and p53 mutation in esophageal epithelial cells with
activation of stromal fibroblasts induces tumor development,
invasion, and differentiation. Genes Dev. 21:2788–2803. 2007.
View Article : Google Scholar
|
|
22
|
Erez N, Truitt M, Olson P, Arron ST and
Hanahan D: Cancer-associated fibroblasts are activated in incipient
neoplasia to orchestrate tumor-promoting inflammation in an
NF-κB-dependent manner. Cancer Cell. 17:135–147. 2010.PubMed/NCBI
|
|
23
|
Sugimoto H, Mundel TM, Kieran MW and
Kalluri R: Identification of fibroblast heterogeneity in the tumor
microenvironment. Cancer Biol Ther. 5:1640–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noma K, Smalley KS, Lioni M, et al: The
essential role of fibroblasts in esophageal squamous cell
carcinoma-induced angiogenesis. Gastroenterology. 134:1981–1993.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshino M, Ishiwata T, Watanabe M, et al:
Expression and roles of keratinocyte growth factor and its receptor
in esophageal cancer cells. Int J Oncol. 31:721–728.
2007.PubMed/NCBI
|
|
26
|
Ren Y, Cao B, Law S, et al: Hepatocyte
growth factor promotes cancer cell migration and angiogenic factors
expression: a prognostic marker of human esophageal squamous cell
carcinomas. Clin Cancer Res. 11:6190–6197. 2005. View Article : Google Scholar
|
|
27
|
Grugan KD, Miller CG, Yao Y, et al:
Fibroblast-secreted hepatocyte growth factor plays a functional
role in esophageal squamous cell carcinoma invasion. Proc Natl Acad
Sci USA. 107:11026–11031. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C, Fu L, Fu J, et al: Fibroblast
growth factor receptor 2-positive fibroblasts provide a suitable
microenvironment for tumor development and progression in
esophageal carcinoma. Clin Cancer Res. 15:4017–4027. 2009.
View Article : Google Scholar
|
|
29
|
Lederle W, Depner S, Schnur S, et al: IL-6
promotes malignant growth of skin SCCs by regulating a network of
autocrine and paracrine cytokines. Int J Cancer. 128:2803–2814.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gaggioli C, Hooper S, Hidalgo-Carcedo C,
et al: Fibroblast-led collective invasion of carcinoma cells with
differing roles for RhoGTPases in leading and following cells. Nat
Cell Biol. 9:1392–1400. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bohn W, Wiegers W, Beuttenmüller M and
Traub P: Species-specific recognition patterns of monoclonal
antibodies directed against vimentin. Exp Cell Res. 201:1–7. 1992.
View Article : Google Scholar : PubMed/NCBI
|