Introduction
The epithelial-mesenchymal transition (EMT) is a
complex biological process by which the epithelial cells go through
multiple biochemical changes mainly including the loss of
epithelial characteristics and acquisition of a mesenchymal motile
phenotype, which enhance migratory capacity, invasiveness, elevate
resistance to apoptosis and increase production of the
extracellular matrix components (1–5). It
has been confirmed that EMT is closely related to cancer
development and progression. Increasing evidence suggests that the
cancer cell acquisition of EMT phenotype is critically involved in
tumor invasion and metastasis (6,7).
Recently, the EMT characteristics were also found in cancer cells
which acquired chemoresistance after chemotherapy (8). For example, EMT is reportedly
associated with the resistance to gefitinib and erlotinib in NSCLC
(9–11). In breast and ovarian cancer,
chemoresistance can also induce EMT via upregulation of Snail,
which is a key regulator of EMT (12,13).
These data indicated that EMT is strongly involved in cancer cell
drug resistance, but the underlying mechanisms remain unknown.
Breast cancer is the main cause of cancer-related
mortality among women and drug resistance is the major clinical
obstacle to the successful treatment. There are a series of
molecular mechanisms which are involved in breast cancer cell drug
resistance including gene mutation, gene amplification, epigenetic
change and microRNAs (14,15). In our previous study, we found that
BMP6 played a critical role in breast cancer progression and drug
resistance (16). However, during
the acquisition of chemoresistance of breast cancer, the regulation
of BMP6 expression and the function of BMP6 remained unknown. DNA
methylation is an efficacious mechanism to regulate gene expression
during development, differentiation and carcinogenesis of various
tumors. DNA methylation has previously been described to act during
the EMT program in the suppression of epithelial markers and the
conversion of epithelial cells into aggressive, invasive tumor
cells (17,18).
Herein, we performed analyses to identify BMP6 which
was regulated by DNA methylation and which regulated the EMT of
drug-resistant cells. We found that the promoter of BMP6 is
hypermethylated significantly in drug-resistant breast cancer cells
that have undergone EMT. These results suggest that aberrant DNA
methylation of BMP6 is critical in the drug resistance and EMT
phenotype in breast cancer.
Materials and methods
Patients and tissue specimens
A total of 16 fresh breast cancer tissues and
patient-matched adjacent normal breast cancer tissues from West
China Hospital of Sichuan University were used in this study. None
of the patients received chemotherapy or radiotherapy prior to
surgery. The study was approved by the local ethical standards of
the institutional review board.
RNA isolation and quantitative
RT-PCR
The expression of BMP6 was analyzed using SYBR-Green
PCR master mix (Tiangen). Real-time PCR was performed on
Mastercycler ep realplex (Eppendorf). Levels of mRNA expression
were quantified after normalization with endogenous control GAPDH
using the 2-ΔCT method (19). The
primer sequences used for PCR were: GAPDH:
5′-ctttggtatcgtggaaggactc-3′, 5′-gtagaggcagggatgatgttct-3′ (product
size, 132 bp); bmp6: 5′-gtcttacaggagcatcagcacag-3′,
5′-ggagtcacaacccacagattg-3′ (product size, 128 bp); ZEB1:
5′-ctgattccccaggtggcata-3′, 5′-ggg cggtgtagaatcagagt-3′ (product
size, 168 bp); Twist1: 5′-agtggttcttctgcgctact-3′,
5′-gtagggctgctggaaggtaa-3′ (product size, 161 bp); E-cadherin:
5′-cgtagcagtgacgaatgtgg-3′, 5′-ctgggcagtgtaggatgtga-3′ (product
size, 175 bp); Vimentin: 5′-cgccaactacatcgacaagg-3′,
5′-ggctttgtcgttggttagct-3′ (product size, 166 bp). Experiments were
performed independently for each sample and at least 3 technical
replicates were run for each treated sample and controls.
Cell culture
MCF7 cell line was purchased from American Type
Culture Collection (ATCC) and doxorubicin-resistant subline
(MCF7/Adr) was generated by continuously culturing the drug
sensitive parental cell line (MCF7) in medium containing
incrementally increasing concentrations of doxorubicin
(Sigma-Aldrich). The cells were cultured in RPMI-1640 (Gibco)
supplemented with 10% heat inactivated fetal calf serum, 100 U/ml
penicillin, and 100 U/ml streptomycin at 37°C and 5%
CO2. The drug-resistant cell line was cultured in
drug-free medium for >2 weeks before subsequent experiments to
avoid the influence of the drug. MCF-7/ADR were treated with 5 μM
5-aza-2′-deoxycytidine (5-aza-dC; Sigma-Aldrich) or 200 ng/ml
rhBMP-6 (R&D Systems) for 72 h.
BMP6 small hairpin RNA (shRNA)
transfection and isolation of clones stably expressing BMP-6
shRNA
MCF-7 cells grown on 24-well plates were transfected
either with pGPU6/GFP/NEO-BMP6 or negative control plasmid
(Genepharma) using Lipofectamine 2000. After another 12-h
incubation, the cells were re-plated at 1/10 dilution onto the
6-well plates. Selection with G418 (500 μg/ml) started on the next
day and the process lasted for 2 weeks. MCF-7 cell clones with
stably decreased expression of BMP6 as well as the control clones
were obtained for further study. BMP6 knockdown was evaluated by
real-time PCR and western blotting.
Western blot analysis
Tissue samples or cell pellets were lysed in RIPA
buffer containing complete protease inhibitor cocktail (Millipore).
Proteins were quantified with BCA Protein Assay (Bio-Rad) and
separated by SDS-PAGE. Blots were probed using anti-BMP6 (ab155963,
Abcam), anti-E-cadherin (ab53033, Abcam) and anti-Vimentin
(sc-6260, Santa Cruz). Proteins were detected using horseradish
peroxidase-conjugated secondary antibodies.
Quantitative DNA methylation
analyses
Genomic DNA of 16 cancers and adjacent normal
colorectal tissues or cells were extracted by Tissue DNA kit
(Qiagen) according to the manufacturer’s instructions. A total of
200 ng genomic DNA from each sample was bisulfite-treated with the
Methylamp DNA Modification kit (Epigentek). The quality of the
bisulfite conversion was controlled by using PCR products. The
Sequenom MassARRAY platform was used to perform the quantitative
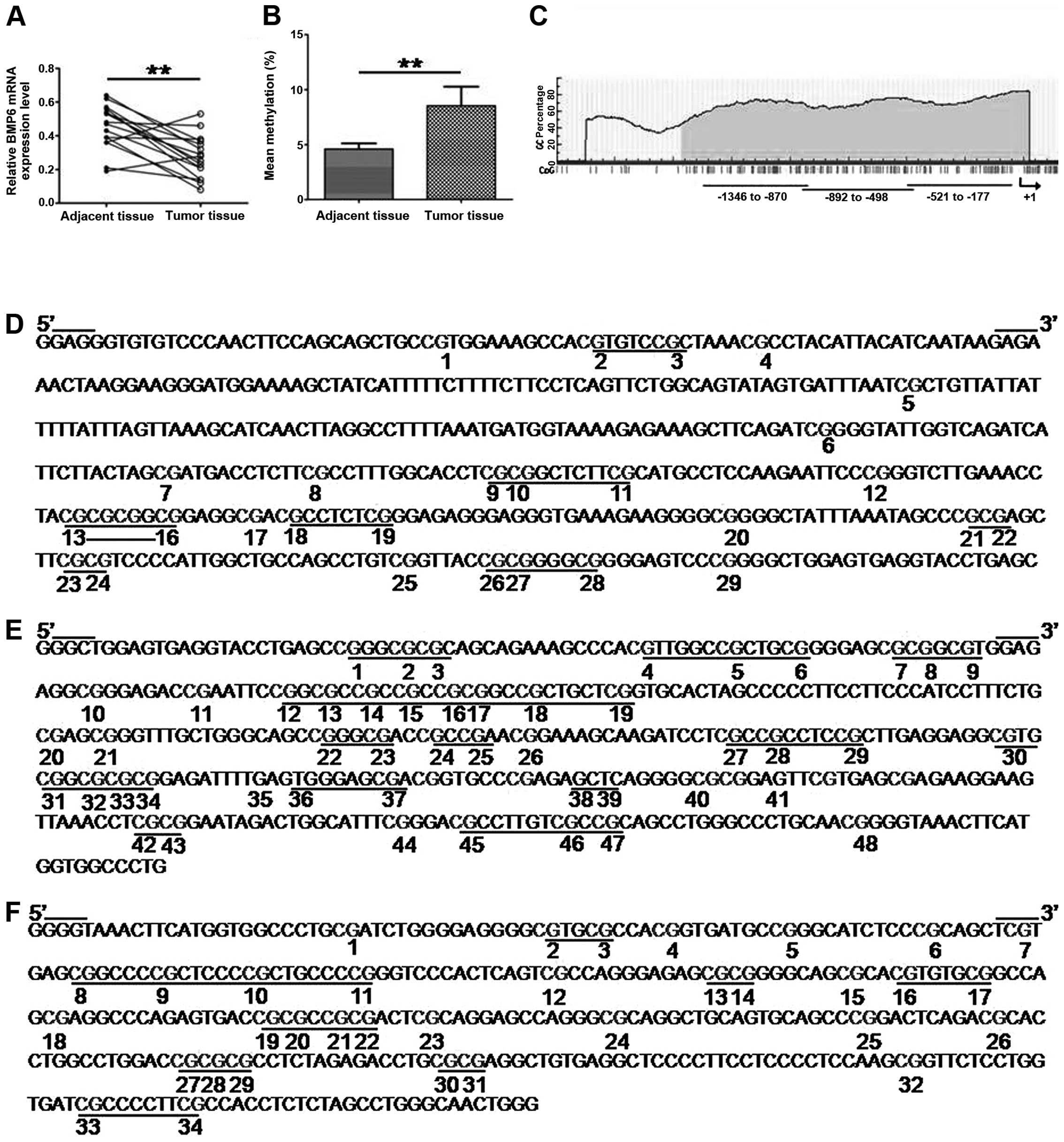
methylation analysis of BMP6. We analyzed BMP6 promoter region and
selected three fragments in CpG islands, the following primers were
designed. The first (to amplify fragment 1 base pairs between −1346
to −870) was 5′-aggaagagagGGAGGGTGTGTTTTAATTTTTAGTA-3′ and
3′-cagtaatacgactcactatagggagaaggctACTCAAATACCTCACTCCAACCC-5′. The
second (to amplify fragment 2 base pairs between −892 to −498) was
5′-aggaagagagGGGTTGGAGTGAGGTATTTGAGT-3′ and
3′-cagtaatacgactcactatagggagaaggctCAAAACCACCATAAAATTTACCCC -5′. The
third (to amplify fragment 3 base pairs between −521 to −177) was
5′-aggaagagagGGGGTAAATTTTATGGTGGTTTT-3′ and
3′-cagtaatacgactcactatagggagaaggctCCCAATTACCCAAACTAAAAAAATAA-5′.
The spectra methylation ratios were generated by Epityper software
version 1.0 (Sequenom).
Transwell migration assay
Cell migration was assessed using 24-well inserts
(Corning Inc., Corning, NY, USA) with 8-μm pores according to the
manufacturer’s protocol. Briefly, cells (1.0×105 cells)
were seeded into the upper chamber of the system. After 72 h of
incubation, the cells in the upper chamber were removed, and the
cells which invaded through the Matrigel matrix membrane were
stained with Giemsa assay. After slightly washing, the migrating
cells were examined by microscopy at a magnification of ×40 with a
Nikon microscope (ts100-F, Japan).
Statistical analyses
Data are expressed as the mean ± SD. The paired
t-test was applied for statistical analysis by using the SPSS
software (version 13.0). Results were considered statistically
significant if 2-tailed P-values were <0.05.
Results
DNA methylation suppresses BMP6
expression in primary breast tumors
To determine the epigenetic silencing of the BMP6
gene in primary breast cancer, firstly, we analyzed BMP6 expression
in 16 paired breast cancer samples and matched adjacent normal
tissues. Real-time PCR showed that 12/16 (75%) breast cancer
tissues decreased expression of BMP6 compared to paired adjacent
normal tissues; there was a significant difference between tumor
tissues and the paired adjacent normal tissues (Fig. 1A). Secondly, we detected BMP6
methylation status in primary breast cancer tissues using Sequenom
MassARRAY platform. The mean methylation level of BMP6 of breast
cancer tissues was significantly higher than paired adjacent normal
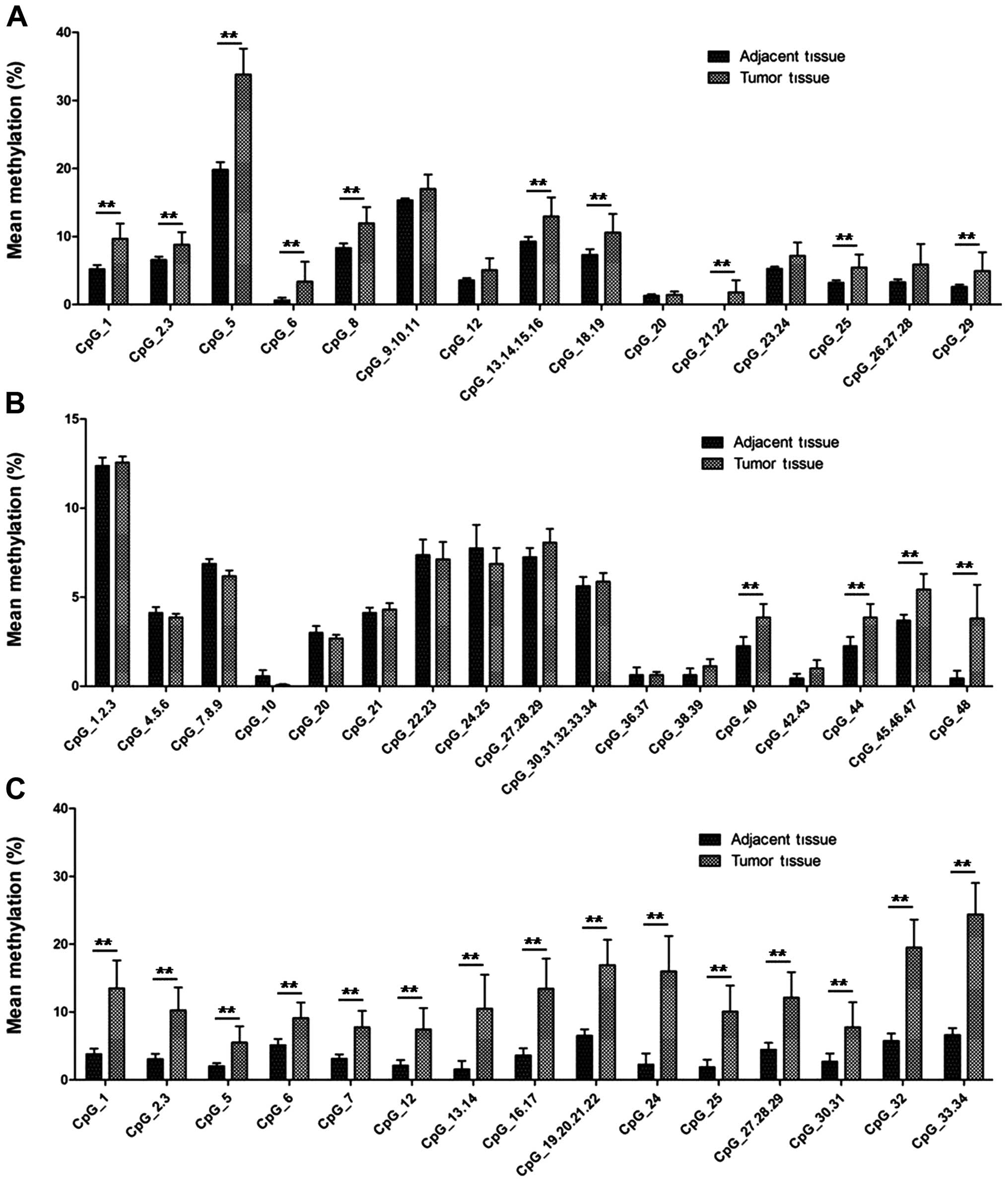
tissues (Fig. 1B). Fig. 2 shows the methylation level at
different CpG sites of tumor tissues and adjacent normal tissues
and 29/51 (56.86%) CpG sites showed a higher methylation level in
tumor tissues compared to the paired adjacent normal tissues.
DNA hypermethylation of BMP6 is related
to breast cancer cell drug resistance
In our previous study (16), we found that BMP6 expression was
downregulated in the multidrug-resistant breast cancer cell line
MCF-7/ADR compared to its parental MCF-7 cells and knockdown of the
expression of BMP6 in MCF-7 cells enhanced the chemoresistance to
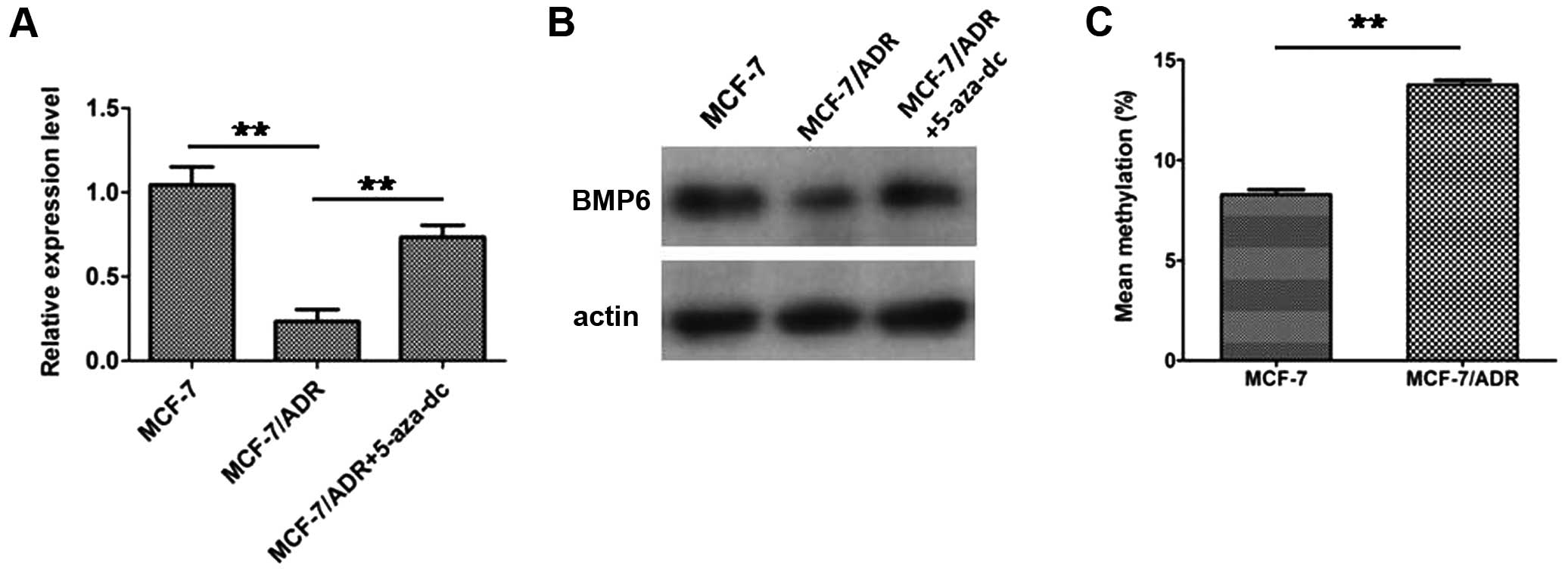
doxorubicin. Here, we found that downregulation of BMP6 in
MCF-7/ADR was reversed by treatment of DNA methyltransferase
inhibitor 5-aza-dC (Fig. 3A and B).
This indicated that downregulation of BMP6 in the drug-resistant
cell line MCF-7/ADR might be involved in epigenetic mechanisms.
Subsequently, we examined BMP6 methylation status in MCF-7 and
MCF-7/ADR cells. Higher level methylation status was detected in
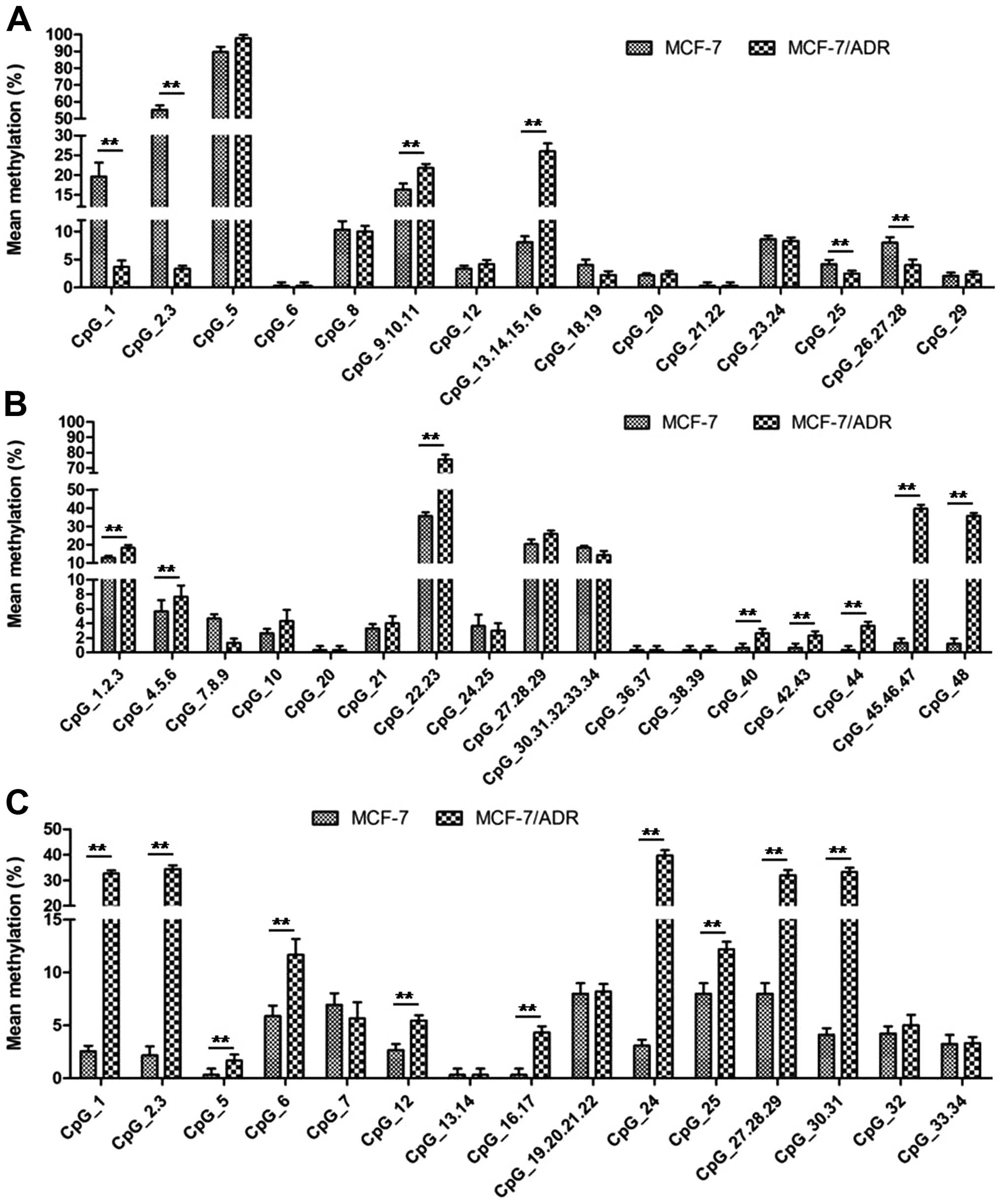
MCF-7/ADR cells compared to the MCF-7 cells (Fig. 3C). In addition, we found that 20/51
(39.21%) CpG sites showed a higher methylation level in MCF-7/ADR
compared to the MCF-7 cells (Fig.
4). It is of note that the methylation level of CpG site 1, 2,
3, 25, 26, 27 and 28 in fragment 1 of MCF-7 cells was higher than
MCF-7/ADR cells (Fig. 4A).
Downregulation of BMP6 is involved in EMT
in MCF-7/ADR cells
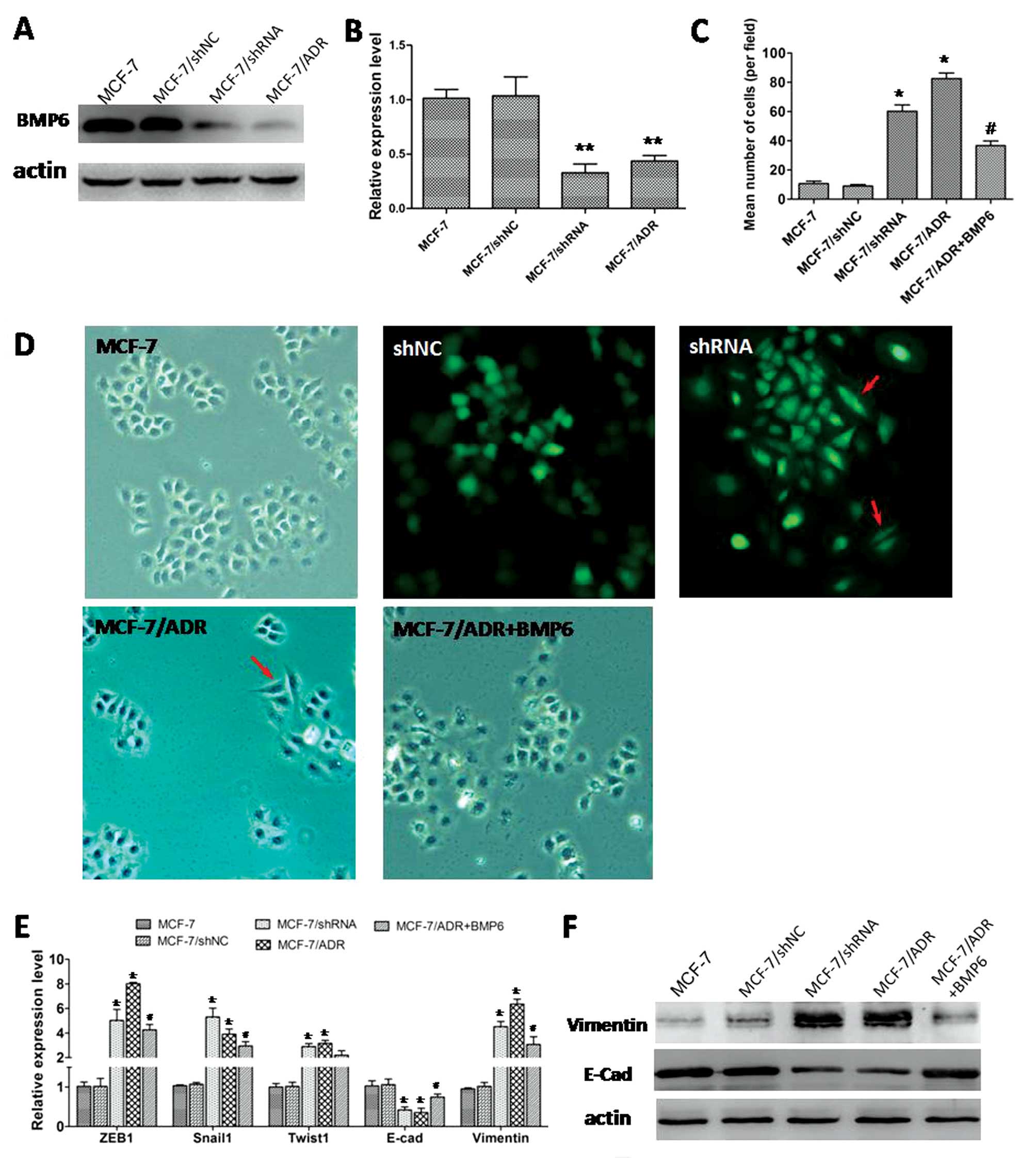
In the drug-resistant breast cancer cells MCF-7/ADR,
the BMP6 expression was significantly lower than in the MCF-7 cell
lines (Fig. 5A and B). In addition,
our previous results indicated that downregulation of BMP6
contributed to the breast cancer cell drug resistance. In the
present study, low expression BMP6 cells MCF-7/ADR or MCF-7 cells
transfected BMP6-specific shRNA showed EMT phenotype. We found that
higher BMP6 expression cells (MCF-7 or MCF-7/shNC cells) displayed
a cobblestone-like appearance and tight cell-cell junctions
(Fig. 5D). In contrast, the lower
BMP6 expression cells MCF-7/ADR cells or MCF-7 cells transfected
with BMP6-specific shRNA vector) appeared to have spindle-cell
morphology; MCF-7/ADR cells treated with BMP6 protein reversed the
morphologic changes (Fig. 5D).
Moreover, Transwell assay showed MCF-7/shRNA or
MCF-7/ADR cells displayed markedly increased migration ability in
comparison with their parental MCF-7 cells and BMP6 recombinant
protein treatment significantly inhibited the migration ability of
MCF-7/ADR cells (Fig. 5C).
Real-time PCR showed that EMT transcription factors such as ZEB1,
Snail1 and Twist1 expression were significantly upregulated in
MCF-7/ADR or MCF-7 cells transfected with BMP6 specific-shRNA
vector (Fig. 5E). The epithelial
cell marker E-cadherin expression was significantly downregulated
but the mesenchymal marker vimentin was significantly upregulated
(Fig. 5E and F). MCF-7/ADR cells
treated with BMP6 protein effectively reversed the expression of
marker of EMT (Fig. 5E and F).
Discussion
In the present study, we found that DNA methylation
can regulate the expression of BMP6 in breast cancer. These
findings showed a decreased expression of BMP6 and an increase of
methylation level of its promoter in breast cancer tissue compared
to the paired adjacent normal tissues. In the breast cancer cell
line, doxorubicin-resistant MCF-7/ADR cells showed a higher
methylation level of BMP6 promoter compared with their parental
MCF-7 cells accompanied by the lower expression of BMP6. Moreover,
the cells with lower expression of BMP6, MCF-7/ADR cells or MCF-7
cells transfected with the BMP6-specific shRNA vector, showed a
typical EMT phenotype. Collectively, our results indicated that
hypermethylation of promoter downregulated the expression of BMP6
and then induced the cells to undergo EMT during the acquisition of
drug resistance of breast cancer cells.
BMP6 is a member of the signaling molecules of the
TGF-β superfamily that are important regulators of cell
proliferation and apoptosis in various types of cells including
breast cancer, myeloma and lymphoma (16,20,21).
BMP6 transfer signals through ligation of type I and type II
serine-threonine kinase receptor, which then propagate the signal
downstream by phosphorylating receptor-activated Smad protein
(22). Previous studies by us and
others showed that BMP6 might act as a suppressor of cancer to
inhibit the cancer cell proliferation and metastasis (23). Furthermore, we also confirmed that
BMP6 played a critical role in breast cancer cell aberrant
proliferation and chemoresistance. With the development of breast
cancer cell drug resistance, the expression of BMP6 was markedly
decreased. However, the regulation of BMP6 downregulation remains
unknown in breast cancer since the promoter sequence of BMP6 was
identified as a target for aberrant DNA methylation (24).
Herein, we analyzed the methylation status of BMP6
promoter region in primary breast cancer tissues and breast cancer
cell line MCF-7 and the drug-resistant cell line MCF-7/ADR. We
found the level of methylation of BMP6 in cancer tissues was
significantly higher than the adjacent normal tissues. In cell
lines, the MCF-7 cells showed a lower level of methylation of BMP6
than the MCF-7/ADR cells. These indicated that hypermethylation of
BMP6 promoter might be involved in breast tumorigenesis and drug
resistance. In fact, previous studies also confirmed that aberrant
methylation of BMP6 is involved in various cancers including
malignant lymphoma, malignant pleural mesothelioma, T-cell leukemia
and multiple myeloma (20,21,25–27).
In the present study, we noted that aberrant methylation of BMP6
promoter was involved in breast cancer cell drug resistance. The
findings suggest that DNA methylation may be important molecular
events in the acquisition of drug resistance in breast cancer and
the use of hypomethylation agents may be a novel therapeutic
strategy for reversal of drug resistance.
Epithelial-mesenchymal transition (EMT) is an
essential process for embryonic development and is implicated in
tumor progression, invasion and metastasis (28). Previous studies suggested that EMT
phenotype was closely related to chemoresistance in cancer cells.
An EMT phenotype has been detected in gemcitabine-resistant
pancreatic cancer cells (29,30),
gefitinib-resistant non-small cell lung cancer (11), oxaliplatin-resistant colorectal
cancer cells (31),
paclitaxel-resistant ovarian cancer cells (32) and 5-fluorouracil or
tamoxifen-resistant breast cancer cells (12,33).
In our study, we found BMP6 expression inhibition caused an EMT
phenotype of MCF-7 cells and resistance to doxorubicin. MCF-7/ADR
cells or MCF-7 cells transfected with BMP6-specific shRNA vector
displayed elongated, irregular fibroblastoid morphology. In
contrast, MCF-7 cells or MCF-7 cells transfected with control shRNA
vector had a rounded shape, typical of epithelial cells. These
changes in phenotype suggest that MCF-7/ADR cells underwent EMT.
Results from real-time PCR and western blotting also confirmed the
phenotype of EMT in MCF-7/ADR cells. When the MCF-7/ADR cells were
treated with the BMP6 recombinant protein, the morphologic change
and EMT-related gene expression could be reversed. These indicated
that BMP6 was an important regulator of EMT during the breast
cancer cell acquisition of drug resistance.
In conclusion, our data highlight the importance of
DNA methylation modifications in determining the expression of
BMP6. In addition, downregulation of BMP6 caused by
hypermethylation induced an EMT phenotype subsequently during the
breast cancer cell acquisition drug resistance. To the best of our
knowledge, this is the first study on BMP6 suppression by DNA
methylation that modulates the breast cancer chemosensitivity via
EMT. The findings suggest that DNA methylation and EMT may be
important molecular events in the acquisition of drug resistance in
breast cancer and the use of hypomethylation agents may be a novel
therapeutic strategy for the reversal of drug resistance.
References
|
1
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: new insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hollier BG, Evans K and Mani SA: The
epithelial-to-mesenchymal transition and cancer stem cells: a
coalition against cancer therapies. J Mammary Gland Biol Neoplasia.
14:29–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwatsuki M, Mimori K, Yokobori T, et al:
Epithelial-mesenchymal transition in cancer development and its
clinical significance. Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frederick BA, Helfrich BA, Coldren CD,
Zheng D, Chan D, Bunn PA Jr and Raben D: Epithelial to mesenchymal
transition predicts gefitinib resistance in cell lines of head and
neck squamous cellcarcinoma and non-small cell lung carcinoma. Mol
Cancer Ther. 6:1683–1691. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Uramoto H, Iwata T, Onitsuka T, Shimokawa
H, Hanagiri T and Oyama T: Epithelial-mesenchymal transition in
EGFR-TKI acquired resistant lung adenocarcinoma. Anticancer Res.
30:2513–2517. 2010.PubMed/NCBI
|
|
11
|
Rho JK, Choi YJ, Lee JK, et al: Epithelial
to mesenchymal transition derived from repeated exposure to
gefitinib determines the sensitivity to EGFR inhibitors in A549, a
non-small cell lung cancer cell line. Lung Cancer. 63:219–226.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Feng M, Zheng G, et al:
Chemoresistance to 5-fluorouracil induces epithelial-mesenchymal
transition via up-regulation of Snail in MCF7 human breast cancer
cells. Biochem Biophys Res Commun. 417:679–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haslehurst AM, Koti M, Dharsee M, et al:
EMT transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fojo T: Multiple paths to a drug
resistance phenotype: mutations, translocations, deletions and
amplification of coding genes or promoter regions, epigenetic
changes and microRNAs. Drug Resist Updat. 10:59–67. 2007.
View Article : Google Scholar
|
|
15
|
Chen GQ, Zhao ZW, Zhou HY, Liu YJ and Yang
HJ: Systematic analysis of microRNA involved in resistance of the
MCF-7 human breast cancer cell to doxorubicin. Med Oncol.
27:406–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lian WJ, Liu G, Liu YJ, Zhao ZW, Yi T and
Zhou HY: Downregulation of BMP6 enhances cell proliferation and
chemoresistance via activation of the ERK signaling pathway in
breast cancer. Oncol Rep. 30:193–200. 2013.PubMed/NCBI
|
|
17
|
Wang Y and Shang Y: Epigenetic control of
epithelial-to-mesenchymal transition and cancer metastasis. Exp
Cell Res. 319:160–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kiesslich T, Pichler M and Neureiter D:
Epigenetic control of epithelial-mesenchymal-transition in human
cancer (Review). Mol Clin Oncol. 1:3–11. 2013.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
20
|
Daibata M, Nemoto Y, Bandobashi K, et al:
Promoter hypermethylation of the bone morphogenetic protein-6 gene
in malignant lymphoma. Clin Cancer Res. 13:3528–3535. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hashida Y, Nemoto Y, Imajoh M, et al:
Promoter methylation of the bone morphogenetic protein 6 gene in
multiple myeloma. Oncol Rep. 27:825–830. 2012.PubMed/NCBI
|
|
22
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kersten C, Dosen G, Myklebust JH,
Sivertsen EA, Hystad ME, Smeland EB and Rian E: BMP-6 inhibits
human bone marrow B lymphopoiesis - upregulation of Id1 and Id3.
Exp Hematol. 34:72–81. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamada H, Kitazawa R, Gohji K, Kamidono S,
Maeda S and Kitazawa S: Molecular cloning and analysis of the
5′-flanking region of the human bone morphogenetic protein-6
(BMP-6). Biochim Biophys Acta. 1395:247–251. 1998.
|
|
25
|
Barekati Z, Radpour R, Lu Q, et al:
Methylation signature of lymph node metastases in breast cancer
patients. BMC Cancer. 12:2442012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Taniguchi A, Nemoto Y, Yokoyama A, Kotani
N, Imai S, Shuin T and Daibata M: Promoter methylation of the bone
morphogenetic protein-6 gene in association with adult T-cell
leukemia. Int J Cancer. 123:1824–1831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kimura K, Toyooka S, Tsukuda K, et al: The
aberrant promoter methylation of BMP3b and BMP6 in malignant
pleural mesotheliomas. Oncol Rep. 20:1265–1268. 2008.PubMed/NCBI
|
|
28
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013.PubMed/NCBI
|
|
29
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Li Y, Kong D, et al: Acquisition
of epithelial-mesenchymal transition phenotype of
gemcitabine-resistant pancreatic cancer cells is linked with
activation of the notch signaling pathway. Cancer Res.
69:2400–2407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang AD, Fan F, Camp ER, et al: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kajiyama H, Shibata K, Terauchi M,
Yamashita M, Ino K, Nawa A and Kikkawa F: Chemoresistance to
paclitaxel induces epithelial-mesenchymal transition and enhances
metastatic potential for epithelial ovarian carcinoma cells. Int J
Oncol. 31:277–283. 2007.
|
|
33
|
Hiscox S, Jiang WG, Obermeier K, et al:
Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and
involves modulation of beta-catenin phosphorylation. Int J Cancer.
118:290–301. 2006. View Article : Google Scholar : PubMed/NCBI
|