Introduction
Epithelial ovarian cancer is one of the most common
causes of cancer-related death in women. The survival rate of
ovarian cancer patients has not improved although diagnosis and
treatment have advanced significantly in recent years. Therefore,
advances in ovarian cancer treatment must be supported by a deep
understanding of its biological features. Vasculogenic mimicry (VM)
was derived from aggressive uveal melanoma microcirculation by
Maniotis et al (1). VM is
the ability of aggressive cancer cells to form vasculogenic-like
networks in vivo. Endothelial cells are not apparent in VM
vessels upon CD31 immunostaining, while cells lining the VM vessels
are, as expected of cells of hepatic origin and periodic
acid-Schiff (PAS)-positive. The presence of red blood cells
indicates that blood circulates in VM vessels (2). VM has been observed in several
malignant tumor types, such as breast, prostate and liver cancer,
glioma, melanoma and bidirectional differentiated malignant tumors
(3). Our previous studies
demonstrated that the prognosis of patients with VM is
significantly worse than that of patients without VM (4), and these findings suggest that
epithelial-mesenchymal transition (EMT) is important in tumor
progression and VM (5). However,
the molecular mechanism of VM remains unclear.
The Wnt families are crucial for tumorigenesis and
chick embryo development; these families include canonical
(β-catenin-dependent) Wnt signaling pathways and non-canonical
(β-catenin-independent) Wnt signaling pathways (6). Our studies showed that activation of
the Wnt-β-catenin signaling pathway in colon cancer cells is
associated with VM (7). However,
the function of the non-canonical Wnt pathway in VM is
unidentified. Wnt5a is an important non-canonical Wnt pathway that
was first identified in Drosophila developmental studies
(8). Dissanayake et al
(9), and Weeraratna et al
(10) reported that Wnt5a signals
can activate phospholipase C via frizzled. This activation causes
phospholipid turnover in the membrane, thereby releasing calcium
from intracellular stores and increasing protein kinase C (PKC)
activity. The authors also induced Wnt5a overexpression and
downregulation, and conducted microarray analysis to demonstrate
that Wnt5a/PKC stimulates melanoma cell motility by inducing genes
involved in melanoma EMT. However, the function of Wnt5a/PKC in
epithelial ovarian cancer is unknown. The present study aimed to
confirm the relationship between Wnt5a and VM and its mechanism in
epithelial ovarian cancer.
Materials and methods
Tissue samples
Seventy-nine cases of ovarian cancer were selected
from Tianjin Cancer Hospital and Tianjin Medical University General
Hospital between 1991 and 1999. All specimens were obtained by
tumor surgical operation, were formalin-fixed and processed for
paraffin embedding, and were sectioned and stained with hematoxylin
and eosin, and diagnosed by two pathologists.
A total of 79 patients with epithelial ovarian
cancer were enrolled in this study, and their clinicopathological
characteristics are summarized in Table
I. The ages ranged from 21 to 80 years (median age, 54.4
years); 28 patients (35.4%) were <50 years and 51 patients
(64.6%) were ≥50 years of age. The size of tumors ranged from 1 to
20 cm (median, 5.6 cm); 41 cases (51.9%) were <5 cm, 38 cases
(48.1%) were ≥5 cm. The FIGO stages at initial diagnosis were as
follows: stage I in 24 cases (30.4%), stage II in 12 cases (15.2%),
and stage III in 43 cases (54.4%). The histological types were
serous tumors in 54 cases (68.4%), mucous tumors in 11 cases
(13.9%), and endometrioid tumors in 14 cases (17.7%). Distant
metastasis was detected in 43 cases (54.4%), and ascites was
present in 56 cases (70.9%). The study protocol was approved by the
Ethics Committee of Tianjin Medical University.
 | Table IWnt5a expression in 79 epithelial
ovarian cancer cases and the correlation with clinicopathological
characteristics. |
Table I
Wnt5a expression in 79 epithelial
ovarian cancer cases and the correlation with clinicopathological
characteristics.
| | Wnt5a
expression | | |
|---|
| |
| | |
|---|
| Variables | Cases n=79 | Negative n (%) | Positive n (%) | χ2 | P-value |
|---|
| Age, years | | | | | 0.802 |
| <50 | 28 | 20 (71.4) | 8 (28.6) | 0.189 | |
| ≥50 | 51 | 34 (73.8) | 17 (26.2) | | |
| Tumor size
(cm) | | | | | 0.226 |
| <5 | 41 | 31 (75.6) | 10 (24.4) | 2.074 | |
| ≥5 | 38 | 23 (60.5) | 15 (39.5) | | |
| Histological
type | | | | | 0.581 |
| Serous | 54 | 35 (64.8) | 19 (35.1) | 1.086 | |
| Mucous | 11 | 8 (72.7) | 3 (27.3) | | |
| Endometrioid | 14 | 11 (78.6) | 3 (21.4) | | |
| FIGO stage | | | | | 0.115 |
| I | 24 | 17 (70.8) | 7 (29.2) | 4.320 | |
| II | 12 | 11 (91.7) | 1 (8.3) | | |
| III | 43 | 26 (60.5) | 17 (39.5) | | |
| Metastasis | | | | | 0.008a |
| Absent | 36 | 31 (86.1) | 5 (13.9) | 7.419 | |
| Present | 43 | 23 (53.5) | 20 (46.5) | | |
| Ascites | | | | | 0.292 |
| Absent | 23 | 18 (78.3) | 5 (21.7) | 1.472 | |
| Present | 56 | 36 (64.3) | 20 (35.7) | | |
Inmunohistochemistry
The sections were pretreated with a microwave,
blocked and incubated using a series of antibodies: Wnt5a antibody
(dilution 1:100; R&D Biosystems, Minneapolis, MN, USA) and the
protein kinase Cα (PKCα) antibody (dilution 1:100; Zhongshan
Chemical Co., Beijing, China). The staining systems used in the
present study were PV6000 and Elivision Plus (Zhongshan Chemical
Co.).
Cell lines
The cells used in this study were human ovarian
adenocarcinoma cells OVCAR3 and SKOV3 (American Type Culture
Collection, Rockville, MD, USA). The cells were cultured in a
mixture of RPMI-1640 medium, antibiotics and 10% fetal bovine serum
(FBS) (both from HyClone, Thermo Scientific), and were incubated at
37°C in a 5% CO2 incubator.
Plasmid constructs and generation of
stable cell clones
The plasmids carrying Wnt5a and Wnt5a shRNA
(shWnt5a) as well as a control scrambled plasmid were purchased
from GeneChem (Shanghai, China). Wnt5a shRNA and the control
scrambled plasmid were labeled with GFP. The vectors were
transfected into cells by percutaneous ethanol injection (cat. no.
23966; Polysciences, Inc.). More than 60% efficiency was utilized
in the transient transfection experiments. We used G418 (600 mg/l)
in the stable transfection experiments.
Western blot analysis
The antibodies used were Wnt5a (dilution 1:100;
R&D Biosystems); Snail, PI3K (dilution 1:200; Santa Cruz,
Dallas, TX, USA); PKCα, E-cadherin, vimentin, β-catenin (dilution
1:100), GAPDH and β-actin (dilution 1:2,000) (all from Zhongshan
Chemical Co.). GAPDH or β-actin were used as a protein-loading
control.
Wound assay to assess cell motility
SKOV3 and OVCAR3 cells (1×105) were
plated in 12-well plates for 24 h and were then transfected with
the plasmids. PMA (200 nM) or PKC inhibitor (1 μM) (Calbiochem) was
added to the conditioned medium. After 48 h when the cells reached
90% confluency, sterile pipette tips were used to scratch the wound
uniformly. Then, the medium was replaced with fresh RPMI-1640
without FBS. The cells were photographed with a microscope (Nikon,
Japan) and counted in several pre-marked areas at 0, 4, 8, 12 and
24 h.
Transwell assay
Transwell chambers (6.5 mm) (Corning Costar,
Cambridge, MA, USA) with polycarbonate membranes (with 8.0-μm
pores) were treated with 10 μl Matrigel. SKOV3, SKOV3-Wnt5a, OVCAR3
and OVCAR3-pGFP-shWnt5a cells (5×104/well) were
incubated in the upper chamber at 37°C in a 5% CO2
incubator, and the medium, serum-free, was added to the lower
chamber to allow the cells to migrate. Cells from the top of the
Transwell chambers were removed by a cotton swab, and the cells
that had migrated to the lower surface were fixed with 4%
formaldehyde and stained with crystal violet. Cells in the lower
chamber were counted in three random microscopic fields using an
inverted microscope (Nikon, Japan).
3D culture
A total of 50 μl of Matrigel basement membrane
matrix and cells (as mentioned above) were coated on a 96-well
plate for 4 h at 37°C. Then, 50 μl of SKOV3 or OVCAR3 cells
(1×105) was added. The cells were incubated for 24 h at
37°C in a 5% CO2 incubator. Tube-like structures of
cells that formed after 6 h were counted under an inverted
microscope (Nikon, Japan).
Immunofluorescence
The antibodies used were E-cadherin and vimentin (as
mentioned above). Cells were observed under a confocal microscope
(Nikon, Japan).
Statistical analysis
All data were evaluated by SPSS version 17.0. Data
are representative of at least triplicate independent
determinations. The relationship between Wnt5a expression and
clinicopathological characteristics and the expression of VM and
PKCα was analyzed using Chi-square and Pearson’s correlation test.
For univariate survival analysis, survival curves were obtained
using the Kaplan-Meier method. Differences in survival curves were
assessed according to the log-rank test. GraphPad Prism 6 (GraphPad
software) was used for western blotting and cellular function
analysis. Differences were considered significant at values of
P<0.05.
Results
Wnt5a expression is significantly
associated with VM and PKCα expression in epithelial ovarian cancer
and clinicopathological characteristics
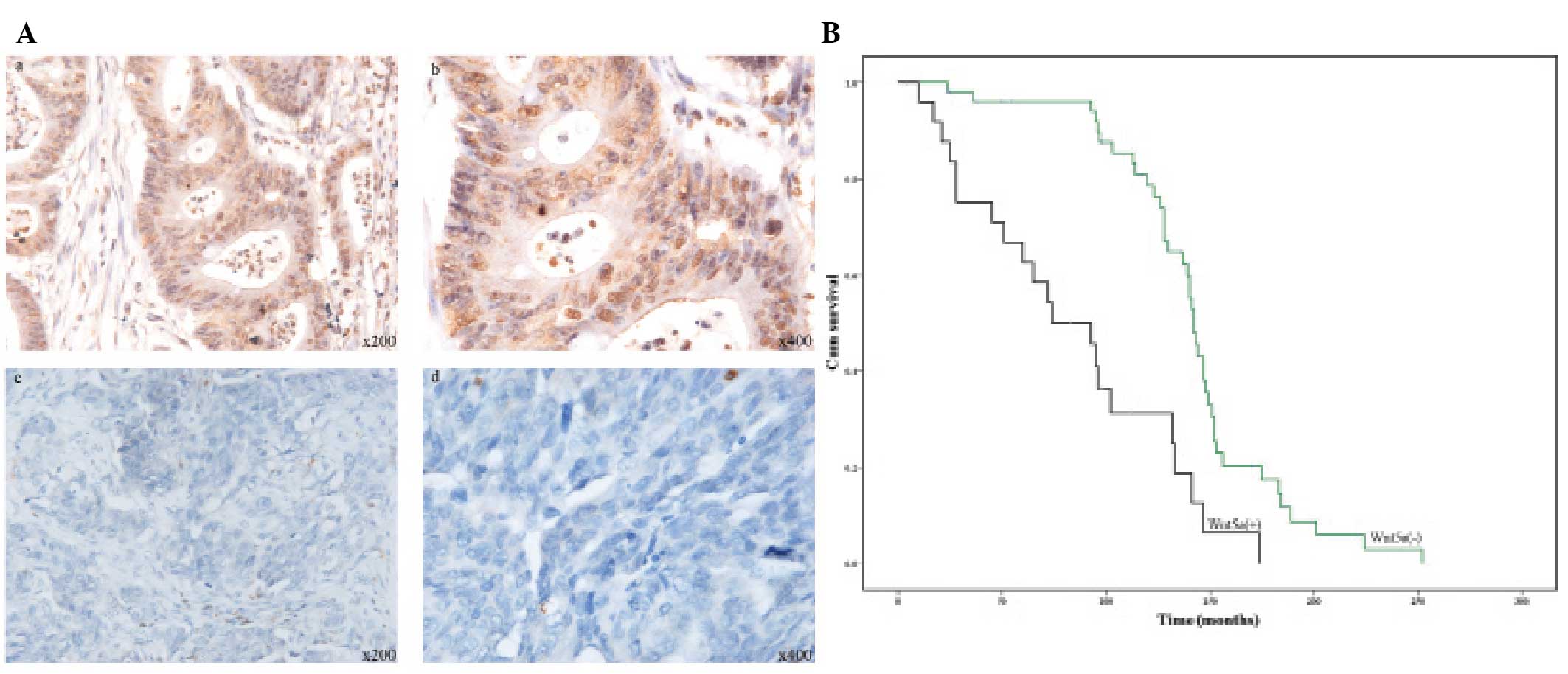
Immunohistochemistry was used to evaluate Wnt5a
protein expression in the ovarian cancer cases. Wnt5a was
distributed in the cytoplasm and nucleus (Fig. 1A). Of the 79 ovarian cancer cases in
the present study, 25 (31.6%) exhibited positive Wnt5a expression
and 54 (68.4%) exhibited negative Wnt5a expression. Groups were
classified as positive or negative according to Wnt5a expression,
and SPSS was used to analyze the relationship between Wnt5a and
clinicopathological characteristics. The results showed that Wnt5a
expression was not correlated with age, tumor size, histological
type, FIGO stage and the presence of ascites, but was highly
correlated with cancer metastasis (Table I, P=0.008), indicating that Wnt5a
promotes tumor metastasis. The mean survival time of the 79 ovarian
cancer patients was 137 months (range, 4–252 months), and
Wnt5a-negative patients were found to have a longer survival than
Wnt5a-positive patients (Fig. 1B,
P=0.00).
We assessed the relationship between Wnt5a and VM in
ovarian cancer. Our previous study showed that the prognosis of
patients with VM was significantly worse than the prognosis of
patients without VM (4). The
expression of Wnt5a in ovarian cancer cases with or without VM was
analyzed, and Wnt5a staining was shown to be significantly
correlated with VM (Table II,
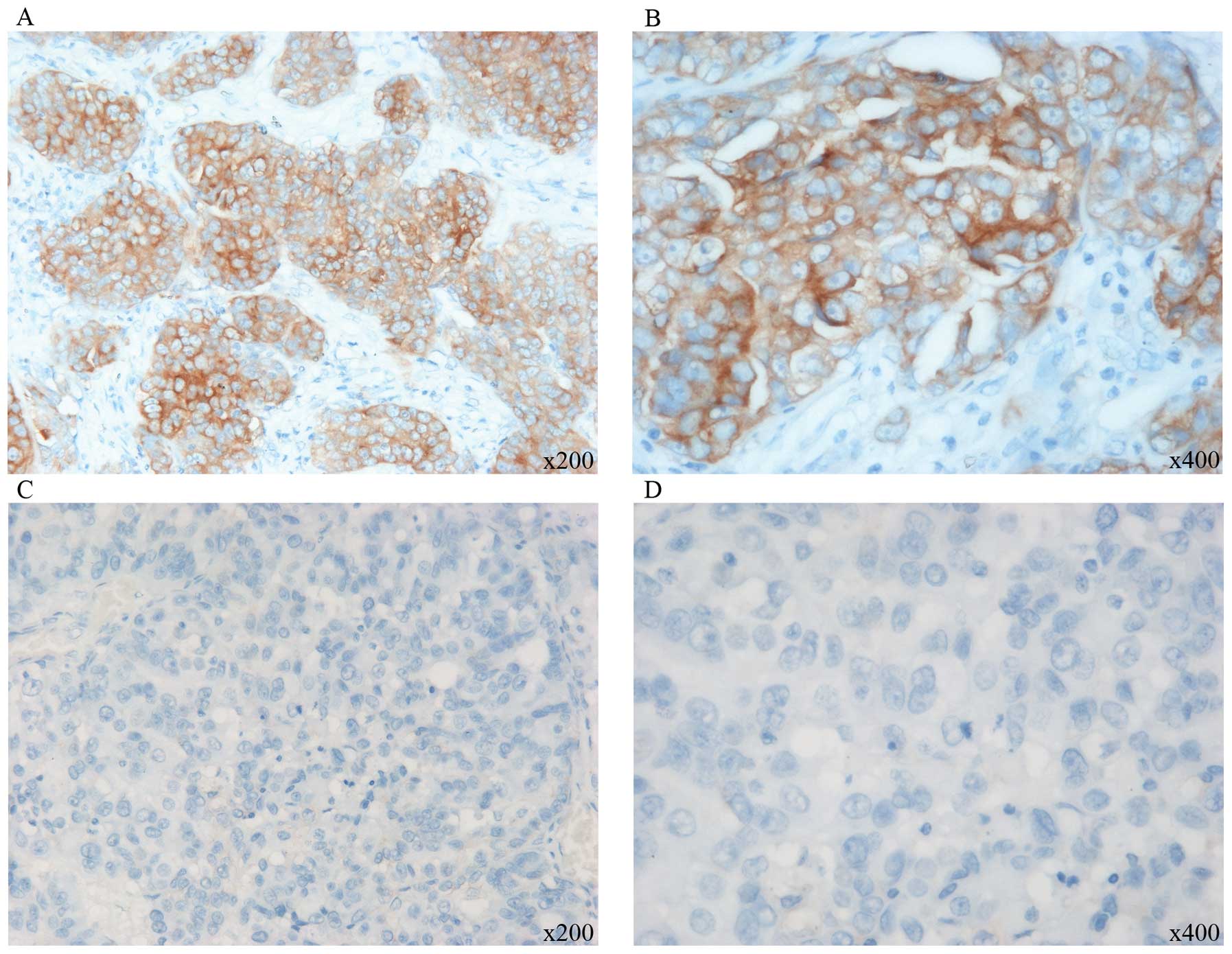
P=0.000). Since the non-canonical Wnt5a pathway may induce PKCα
activation (9,11), the expression of PKCα in ovarian
cancer and its relationship with Wnt5a was determined. PKCα was
expressed via a cytoplasmic staining pattern (Fig. 2). Of the 79 ovarian cancer cases, 35
(44.3%) exhibited positive PKCα expression and 44 (55.6%) exhibited
negative expression. The correlation of Wnt5a and PKCα expression
was analyzed, and Wnt5a staining was found to be significantly
correlated with PKCα (Table II,
P=0.000). The results indicate that Wnt5a expression is associated
with VM and may trigger PKCα pathway activation.
 | Table IICorrelation of Wnt5a expression with
VM and PKCα in 79 epithelial ovarian cancer cases. |
Table II
Correlation of Wnt5a expression with
VM and PKCα in 79 epithelial ovarian cancer cases.
| | Wnt5a
expression | | |
|---|
| |
| | |
|---|
| Variables | Cases n=79 | Negative n (%) | Positive n (%) | χ2 | P-value |
|---|
| VM | | | | | 0.000a |
| Negative | 56 | 46 (82.1) | 10 (17.9) | 16.906 | |
| Positive | 23 | 8 (34.8) | 15 (65.2) | | |
| PKCα | | | | | 0.000a |
| Negative | 44 | 40 (90.1) | 4 (9.1) | 23.356 | |
| Positive | 35 | 14 (40.0) | 21 (60.0) | | |
Wnt5a enhances the vasculogenic capacity
of ovarian cancer cells in vitro
SKOV3 and OVCAR3 cells were used in this study to
verify the association of Wnt5a in ovarian cancer cells with VM
in vitro. The endogenous expression of Wnt5a in the cell
line SKOV3 was significantly lower than that in the cell line
OVCAR3. Thus, SKOV3 cells were infected with the Wnt5a plasmid and
OVCAR3 cells with the shWnt5a plasmid. Stably transfected cells
were used in the following study.
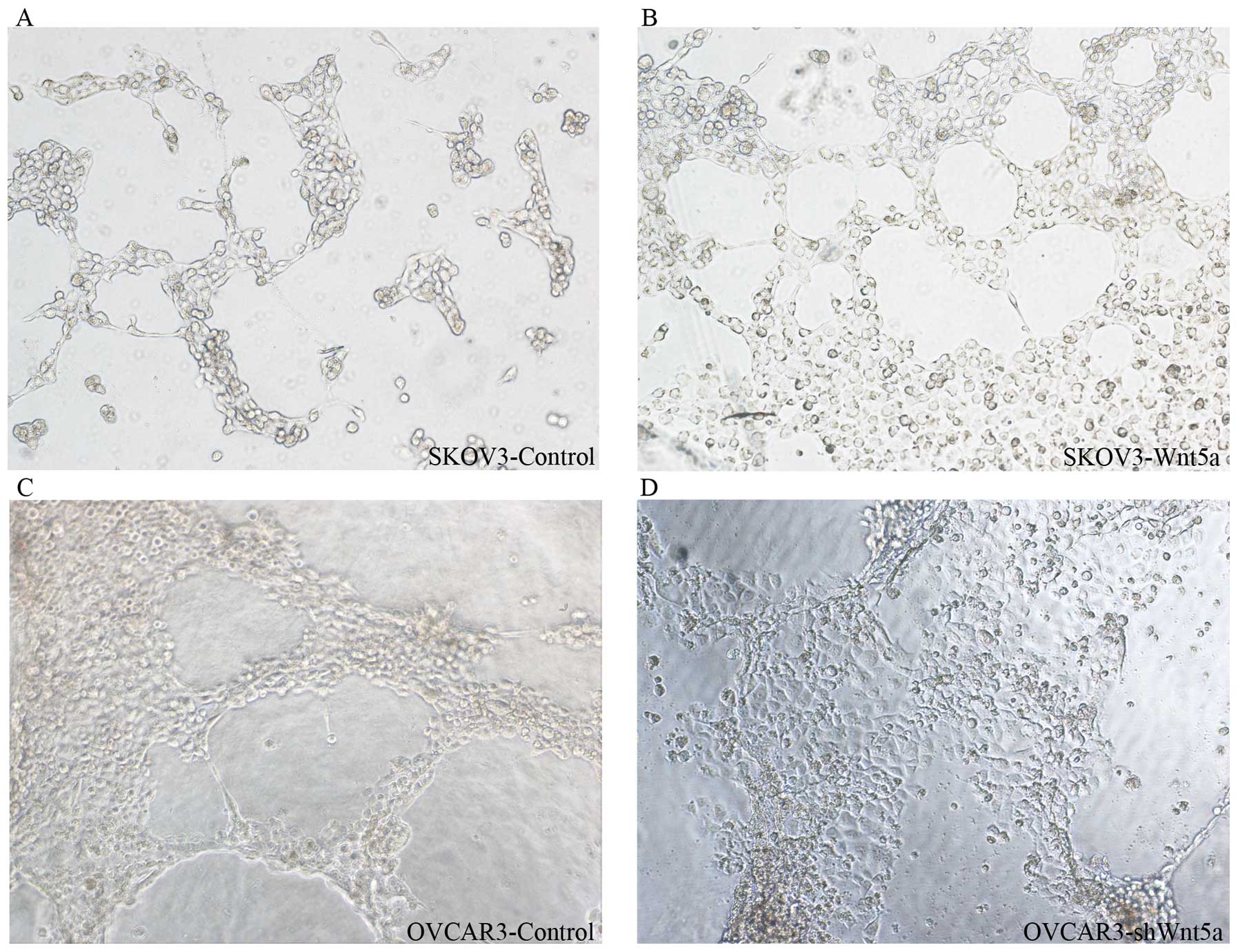
Three-dimensional culture is recognized as an
important method by which to evaluate VM in vitro. Thus, the
well-established Matrigel culture was used to investigate
vasculogenic capacity in ovarian cancer cell lines. OVCAR3, a
poorly differentiated ovarian cancer line, displayed higher
vasculogenic capacity than SKOV3, a well-differentiated cell line
(Fig. 3). However, OVCAR3 cells
transfected with Wnt5a shRNA displayed low vasculogenic capacity
(Fig. 3), and SKOV3 cells
transfected with Wnt5a cDNA exhibited high vasculogenic capacity
(Fig. 3). This result indicates
that Wnt5a can enhance the vasculogenic capacity of ovarian cancer
cells in vitro.
Wnt5a enhances EMT in ovarian cancer
cells in vitro
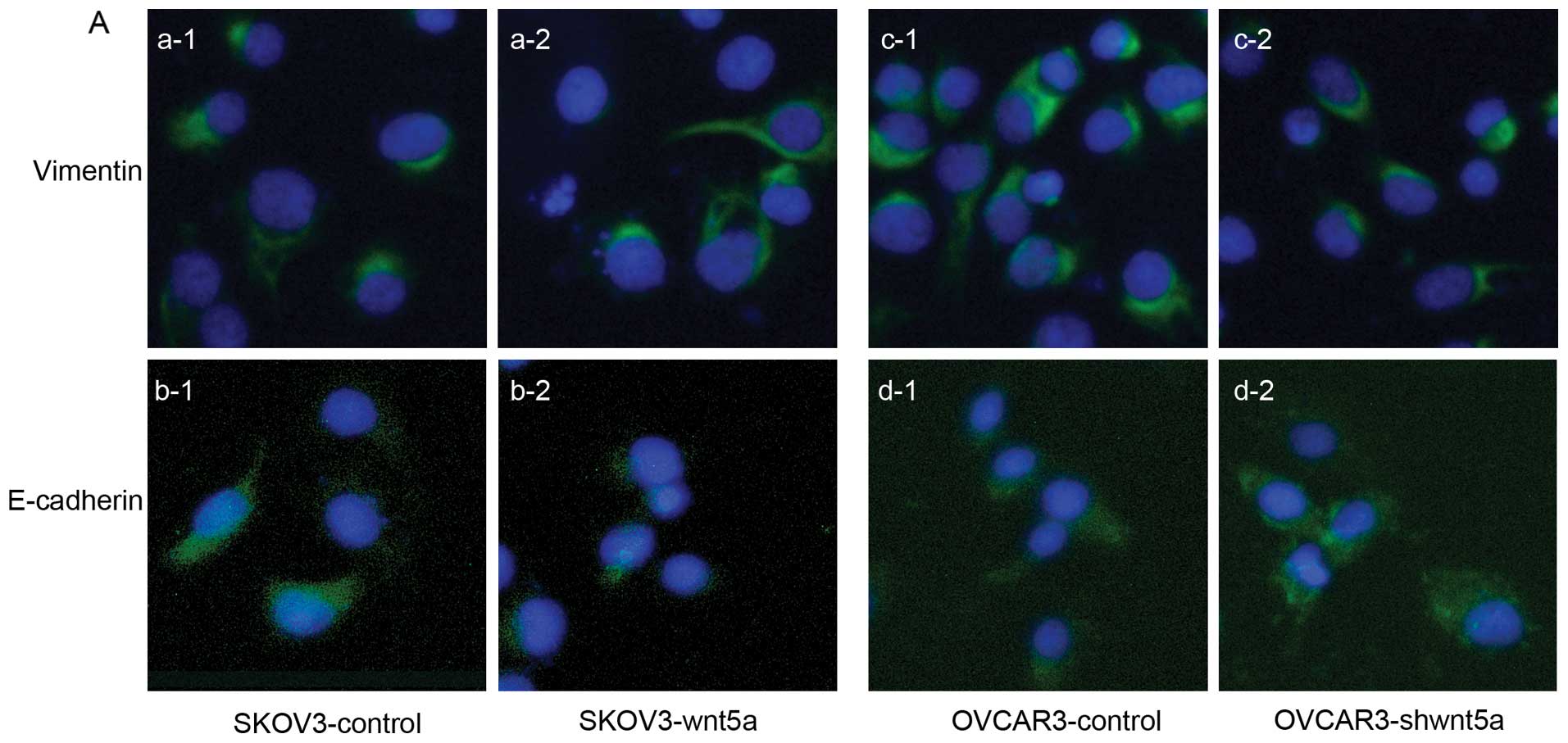
Our previous studies demonstrated that EMT is a
critical step in VM. E-cadherin and vimentin are recognized as
EMT-associated proteins (3,5). Immunofluorescence was performed to
investigate the changes in the expression levels of these two
proteins after in vitro Wnt5a transfection. The result
revealed that vimentin expression was increased and E-cadherin
expression was decreased in the SKOV3 cells after Wnt5a
upregulation (Fig. 4A). By
contrast, vimentin expression was decreased and E-cadherin
expression was increased in OVCAR3 cells after shWnt5a transfection
(Fig. 4A). We also detected the
protein expression levels of vimentin and E-cadherin in the two
ovarian cancer cell lines. Western blot analysis confirmed the
results of the immunofluorescence analysis (Fig. 4B). The results were statistically
significant (P<0.05). These findings indicate that Wnt5a can
enhance EMT in ovarian cancer cells in vitro.
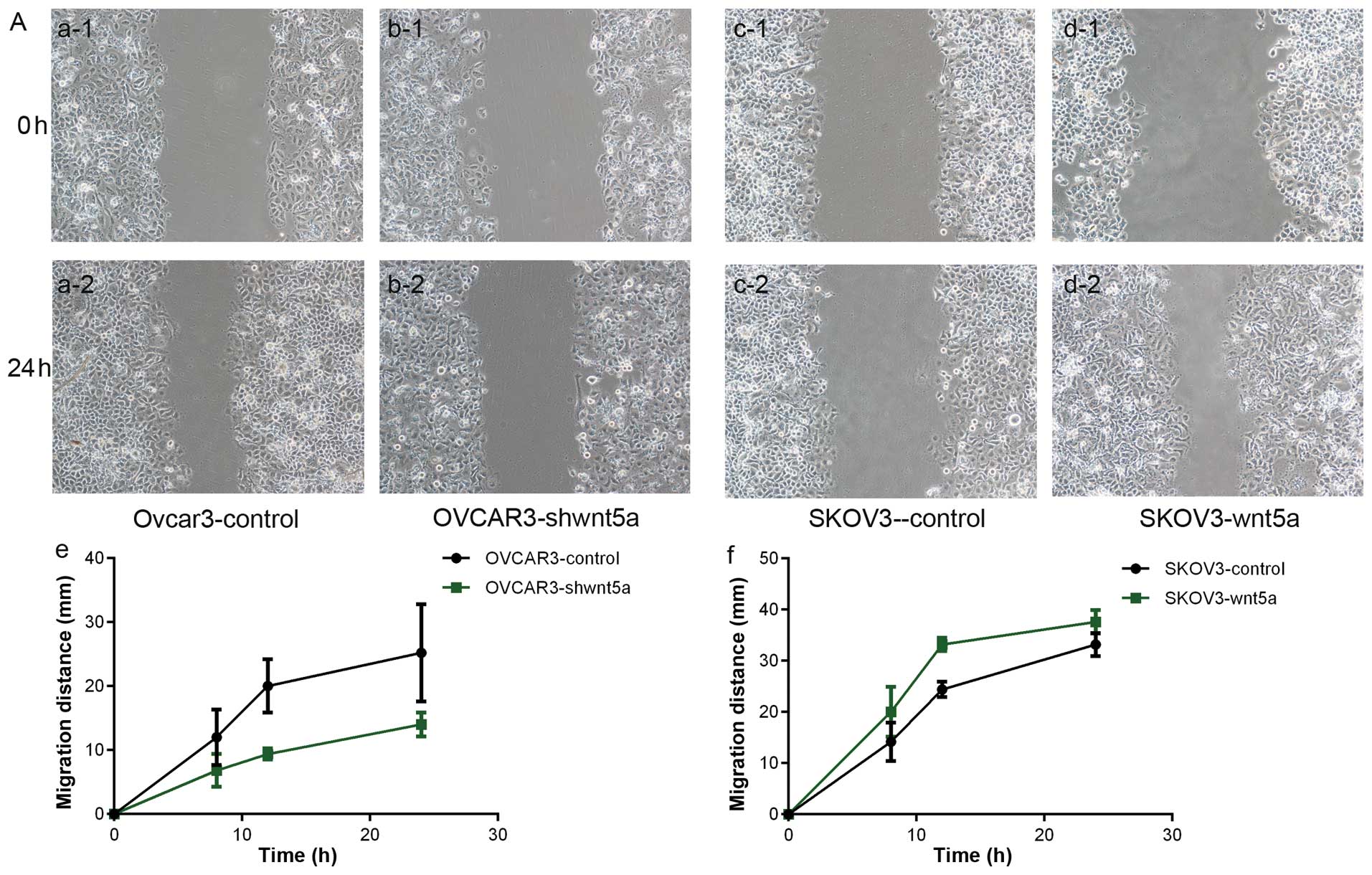
Wnt5a increases the motility and
invasiveness of ovarian cancer cells in vitro
Cell motility is closely related to tumor
metastasis, and Wnt5a was found to be positively correlated with
melanoma motility and invasiveness (9–11). The
effect of Wnt5a on the motility and invasiveness of ovarian cancer
cells in vitro was investigated. Cell migration was
evaluated by a wound healing assay, also known as the ‘scratch’
assay, and cell invasion was examined by Transwell assay. SKOV3
cells were transfected with the Wnt5a plasmid and OVCAR3 cells were
transfected with the shWnt5a plasmid based on endogenous Wnt5a
expression. The results showed that Wnt5a overexpression enhanced
cell migration and invasiveness in the SKOV3 cells following
transfection with Wnt5a (Fig. 5,
P<0.05). These findings corresponded with the weakened motility
and invasiveness in the OVCAR3 cells transfected with shWnt5a
(Fig. 5, P<0.05). Thus, Wnt5a
can enhance the motility and invasiveness of ovarian cancer
cells.
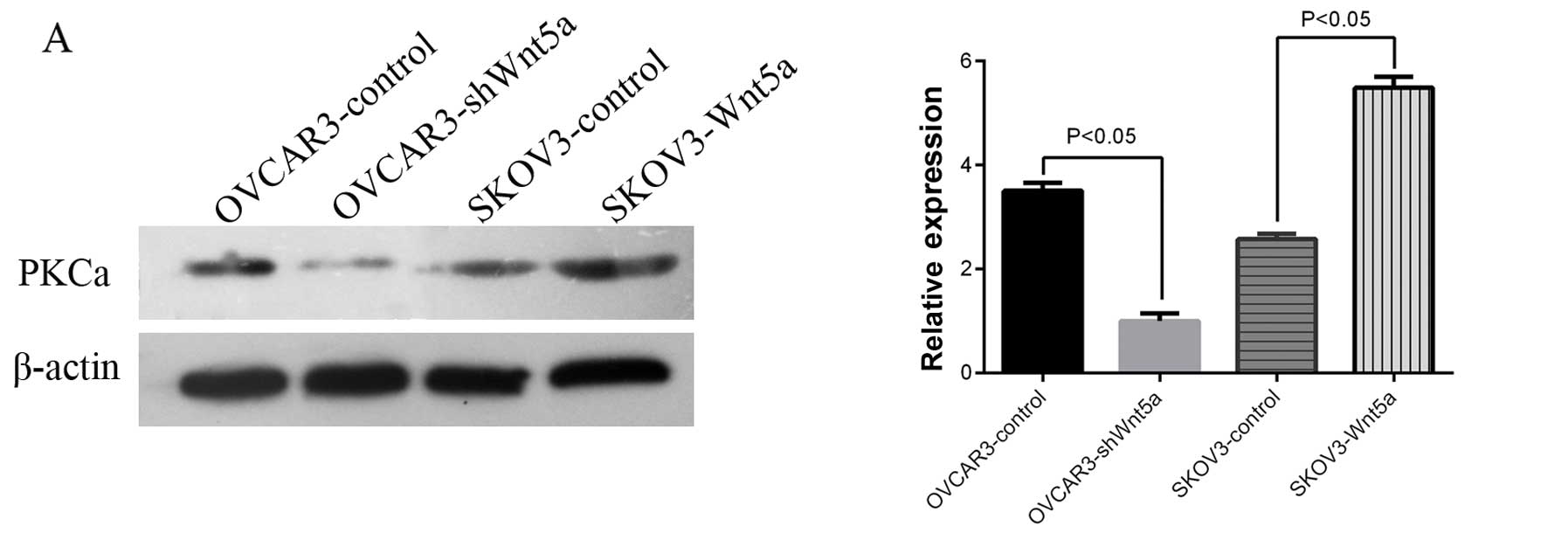
Wnt5a promotes ovarian cancer EMT and VM
via the PKCα pathway in vitro
The non-canonical pathway signaled by Wnt5a is
involved in PKC activation. Dissanayake et al (9), and Weeraratna et al (10) demonstrated the importance of PKC
signaling in melanoma metastasis. Therefore, we hypothesized that
PKC is critical to the effect of Wnt5a on ovarian cancer cells. The
effect of Wnt5a expression on PKCα expression was investigated via
western blot analysis. The results showed that changes in Wnt5a
expression coincided with changes in PKCα expression (Fig. 6A, P<0.05). The addition of a PKCα
inhibitor (1 μM) to SKOV3-Wnt5a cells significantly reduced cell
motility (Fig. 6B, P<0.05). This
result indicates that PKCα is critical to the effect of Wnt5a on
ovarian cancer cells in vitro.
Finally, EMT-associated proteins were assessed in
the two ovarian cancer cell lines at the protein level after cells
were transfected with the Wnt5a plasmids. The findings showed that
PI3K and Snail expression increased with Wnt5a upregulation
(Fig. 6C), but not β-catenin,
indicating that Wnt5a may mediate EMT and VM in ovarian cancer
cells via PKCα.
Discussion
VM was derived from aggressive uveal melanoma
microcirculation by Maniotis et al (1). Our previous studies have shown that VM
is present in ovarian cancer and that the prognosis of patients
with VM is significantly worse than that of patients without VM
(4). EMT is pivotal in malignant
tumor progression and VM formation (12). EMT is a reversible dedifferentiation
process that converts epithelial cancer cells into dedifferentiated
cells with additional mesenchymal features. This process is
characterized by the loss of epithelial traits and the acquisition
of mesenchymal phenotypes (13).
Our laboratory reported that EMT contributes to VM formation and
that EMT-associated transcription factors are upregulated in
VM-forming tumor cells, such as twist1, DKK-1, ZEB1, β-catenin and
Snail/Slug (5,14–16).
Wnt families are crucial in tumorigenesis and chick
embryo development; these families include canonical
(β-catenin-dependent) Wnt signaling pathways and non-canonical
(β-catenin-independent) Wnt signaling pathways. Alterations that
affect Wnt pathway proteins on the cell membrane, in the cytoplasm,
and in the nucleus are involved in ovarian cancer tumorigenesis.
Wnt/β-catenin target genes regulate cell proliferation and
apoptosis, thereby mediating cancer initiation and progression. The
Wnt/β-catenin pathway is a major signaling pathway that may be
involved in EMT (17).
Research has shown that the non-canonical
(β-catenin-independent) Wnt signaling pathways are important in
embryonic development and tumor progression (6,9,10).
Wnt5a, a member of the Wnt protein family, can activate the
non-canonical Wnt signaling pathway. However, the function of Wnt5a
in tumors remains controversial. Wnt5a has been described as a
tumor suppressor in various malignancies (18,19);
however, increasing evidence suggests that it has pro-migratory and
pro-invasive effects in other tumors (19,20). A
correlation between Wnt5a expression and increased tumor
aggressiveness has been observed in human melanoma biopsies
(9,10,21),
suggesting that Wnt5a is involved in the progression of tumors to
malignant stages. We hypothesized that other factors affect the
function of Wnt5a. In the present study, the expression of Wnt5a in
79 ovarian cancers at different stages was examined by
immunohistochemistry. Wnt5a expression was found to be correlated
with tumor metastasis. The presence of VM was investigated in 79
ovarian cancers and was determined to be significantly correlated
with Wnt5a expression. The effect of Wnt5a on ovarian cancer cells
in vitro was studied by establishing models of upregulated
or downregulated Wnt5a expression. Tube formation is recognized as
a universal in vitro VM evaluation method. Wnt5a
upregulation promoted tube formation, whereas Wnt5a downregulation
inhibited it. This finding indicated the important function of
Wnt5a in VM. Our previous studies also showed that EMT is a
critical step in VM. E-cadherin and vimentin are recognized as
characteristic EMT proteins (3,5). The
results of the present study showed that the upregulation of Wnt5a
enhanced mesenchymal characteristics and increased the motility and
invasiveness of ovarian cancer cells and that Wnt5a downregulation
enhanced epithelial traits and decreased motility and invasiveness.
This finding suggests that Wnt5a is crucial for VM via EMT. These
results are consistent with findings that Wnt5a increases cell
invasiveness in vector-transfected melanoma cells constitutively
overexpressing Wnt5a (10).
Several studies have shown that the Wnt
non-canonical pathways, such as the Wnt/Ca2+ pathway,
are involved in the regulation of tumor cell motility and migration
through PKC activation (9,10). PKCα is composed of isoforms
(11) with variable regulatory
regions and conserved catalytic domains. Several isoforms have
complex and occasionally opposite functions in the melanogenesis,
proliferation and transformation of human melanocytes. Numerous
studies have consistently shown that phorbol esters serve dual
functions as growth promoters of normal melanocyte proliferation
and as inhibitors of melanoma cell growth via the activation of
different sets of PKC isoforms (22). Other studies have demonstrated the
involvement of Wnt5a in the progression and metastasis of several
malignant diseases via PKC (9,11,23).
Wnt5a may also be involved in EMT in ovarian cancer. Thus, we
examined PKCα expression in 79 ovarian cancer cases.
Immunohistochemical staining showed that PKCα expression coincided
with Wnt5a expression. This result suggests that Wnt5a is important
in ovarian cancer via the PKC pathway. A PKCα inhibitor was used to
upregulate Wnt5a cells in vitro. The results of a clinical
study of ovarian cancer patients revealed that the effect of Wnt5a
was blocked and matched. This finding is consistent with those of
previous studies, which found that Wnt5a overexpression promotes
PKCα activation. The proinvasive effects of Wnt5a are supposedly
predominantly mediated by PKCα, and this mediation is associated
with EMT, an event related to tumor progression (24–26).
Palma-Nicolas (27)
previously demonstrated that the in vitro treatment of
retinal pigment epithelium cells with a-thrombin induces cell
proliferation through the joint activation of PKC and
mitogen-activated protein kinase pathways. Exogenously treated
Wnt5a induces the phosphorylation of PKCα, PKC-pan and PI3K/Akt,
which is a downstream modulator of PKC. This result suggests that
PKC and PI3K/Akt activation is associated with Wnt5a-mediated,
premalignant transformation (28).
In the present study, the results of the western blotting revealed
that PI3K increased with Wnt5a upregulation but that β-catenin was
not affected. This result indicates that Wnt5a may mediate EMT and
VM in ovarian cancer cells via PKCα and PI3K. However, the
mediation is β-catenin-independent. The results also showed that
Snail expression increased along with upregulated Wnt5a expression
and decreased with increased vimentin level. E-cadherin expression
was decreased in ovarian cancer cells with increased Wnt5a
expression. The transfection of vectors that overexpressed Snail
into squamous carcinoma cells has been shown to upregulate Wnt5a
expression (29), suggesting that
Wnt5a and Snail induce a positive feedback loop, as with PKC and
Wnt5a (9).
In summary, Wnt5a expression was correlated with VM
in ovarian cancer and was associated with poor patient prognosis.
Wnt5a overexpression increased tube formation, enhanced mesenchymal
characteristics, and increased the motility and invasiveness of
ovarian cancer cells. Wnt5a also promoted EMT via PKCα but did not
affect the β-catenin pathway. Wnt5a promoted ovarian cancer and
could be a novel prognostic marker of ovarian cancer in the
future.
Acknowledgements
This study was supported by grants from the Key
Project of the National Natural Science Foundation of China (no.
81230050), the National Natural Science Foundation of China (no.
81172046), the National Natural Science Foundation of China (no.
81173091), and the Key Project of the Tianjin Natural Science
Foundation (no. 12JCZDJC23600).
References
|
1
|
Maniotis AJ, Folberg R, Hess A, et al:
Vascular channel formation by human melanoma cells in vivo and in
vitro: vasculogenic mimicry. Am J Pathol. 155:739–752. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun B, Zhang S, Zhang D, et al:
Vasculogenic mimicry is associated with high tumor grade, invasion
and metastasis, and short survival in patients with hepatocellular
carcinoma. Oncol Rep. 16:693–698. 2006.PubMed/NCBI
|
|
3
|
Zhang S, Zhang D and Sun B: Vasculogenic
mimicry: current status and future prospects. Cancer Lett.
254:157–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang JY, Sun T, Zhao XL, et al: Functional
significance of VEGF-a in human ovarian carcinoma: role in
vasculogenic mimicry. Cancer Biol Ther. 7:758–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun T, Zhao N, Zhao XL, et al: Expression
and functional significance of Twist1 in hepatocellular carcinoma:
its role in vasculogenic mimicry. Hepatology. 51:545–556. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jessen JR: Noncanonical Wnt signaling in
tumor progression and metastasis. Zebrafish. 6:21–28. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qi L, Sun B, Liu Z, Li H, Gao J and Leng
X: Dickkopf-1 inhibits epithelial-mesenchymal transition of colon
cancer cells and contributes to colon cancer suppression. Cancer
Sci. 103:828–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Christian JL, McMahon JA, McMahon AP and
Moon RT: Xwnt-8, a Xenopus Wnt-1/int-1-related
gene responsive to mesoderm-inducing growth factors, may play a
role in ventral mesodermal patterning during embryogenesis.
Development. 111:1045–1055. 1991.
|
|
9
|
Dissanayake SK, Wade M, Johnson CE, et al:
The Wnt5A/protein kinase C pathway mediates motility in melanoma
cells via the inhibition of metastasis suppressors and initiation
of an epithelial to mesenchymal transition. J Biol Chem.
282:17259–17271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weeraratna AT, Jiang Y, Hostetter G, et
al: Wnt5a signaling directly affects cell motility and invasion of
metastatic melanoma. Cancer Cell. 1:279–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Wu J, Hong J, et al: PKC promotes
the migration of colon cancer cells by regulating the
internalization and recycling of integrin αvβ6. Cancer Lett.
311:38–47. 2011.PubMed/NCBI
|
|
12
|
Fan YL, Zheng M, Tang YL and Liang XH: A
new perspective of vasculogenic mimicry: EMT and cancer stem cells
(Review). Oncol Lett. 6:1174–1180. 2013.PubMed/NCBI
|
|
13
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Z, Sun B, Qi L, Li H, Gao J and Leng
X: Zinc finger E-box binding homeobox 1 promotes vasculogenic
mimicry in colorectal cancer through induction of
epithelial-to-mesenchymal transition. Cancer Sci. 103:813–820.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun D, Sun B, Liu T, et al: Slug promoted
vasculogenic mimicry in hepatocellular carcinoma. J Cell Mol Med.
17:1038–1047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun T, Sun BC, Zhao XL, et al: Promotion
of tumor cell metastasis and vasculogenic mimicry by way of
transcription coactivation by Bcl-2 and Twist1: a study of
hepatocellular carcinoma. Hepatology. 54:1690–1706. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: a
review. Gynecol Oncol. 131:772–779. 2013.
|
|
18
|
Blanc E, Goldschneider D, Douc-Rasy S,
Benard J and Raguénez G: Wnt-5a gene expression in malignant
human neuroblasts. Cancer Lett. 228:117–123. 2005. View Article : Google Scholar
|
|
19
|
Ripka S, König A, Buchholz M, et al: WNT5A
- target of CUTL1 and potent modulator of tumor cell migration and
invasion in pancreatic cancer. Carcinogenesis. 28:1178–1187. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taki M, Kamata N, Yokoyama K, Fujimoto R,
Tsutsumi S and Nagayama M: Down-regulation of Wnt-4 and
up-regulation of Wnt-5a expression by epithelial-mesenchymal
transition in human squamous carcinoma cells. Cancer Sci.
94:593–597. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Postovit LM, Margaryan NV, Seftor EA and
Hendrix MJ: Role of nodal signaling and the microenvironment
underlying melanoma plasticity. Pigment Cell Melanoma Res.
21:348–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oka M and Kikkawa U: Protein kinase C in
melanoma. Cancer Metastasis Rev. 24:287–300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Medrano EE: Wnt5a and PKC, a deadly
partnership involved in melanoma invasion. Pigment Cell Res.
20:258–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin Z, Zhao C, Han X and Han Y: Wnt5a
promotes ewing sarcoma cell migration through upregulating CXCR4
expression. BMC Cancer. 12:4802012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pourreyron C, Reilly L, Proby C, et al:
Wnt5a is strongly expressed at the leading edge in non-melanoma
skin cancer, forming active gradients, while canonical Wnt
signalling is repressed. PLoS One. 7:e318272012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto H, Oue N, Sato A, et al: Wnt5a
signaling is involved in the aggressiveness of prostate cancer and
expression of metalloproteinase. Oncogene. 29:2036–2046. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palma-Nicolas JP, López E and López-Colomé
AM: PKC isoenzymes differentially modulate the effect of thrombin
on MAPK-dependent RPE proliferation. Biosci Rep. 28:307–317. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Whang YM, Jo U, Sung JS, et al: Wnt5a is
associated with cigarette smoke-related lung carcinogenesis via
protein kinase C. PLoS One. 8:e530122013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharili AS, Allen S, Smith K, Price J and
McGonnell IM: Snail2 promotes osteosarcoma cell motility through
remodelling of the actin cytoskeleton and regulates tumor
development. Cancer Lett. 333:170–179. 2013. View Article : Google Scholar : PubMed/NCBI
|