Introduction
Glioblastoma is one of the most malignant and
aggressive cerebral gliomas. The strong aggression always leads to
diffuse invasion of glioblastoma cells into adjacent normal brain
tissue and migration to a considerable distance from the primary
tumor area (1,2). Despite the advances in surgery and
radiotherapy for glioblastoma, it is still difficult to remove all
tumor tissues without severe damage to the brain, and avoid
vulnerability of healthy brain tissue to radiotherapy, resulting in
unsatisfactory therapeutic efficacy and poor prognosis of
glioblastoma (3). Thus, it is
necessary to further investigate the molecular mechanism regulating
the aggression of glioblastoma cells. Increasing evidence suggests
the microenvironment in solid tumors plays a vital role in
migration and invasion. Hypoxia is the common characteristic of
tumor microenvironment, and also the major stimulator of migration
and invasion in tumors (4,5). It is well documented that hypoxia
modifies cellular activities via stabilizing hypoxia-inducible
factor-1α (HIF-1α). Under normoxia, O2-dependent
enzymatic hydroxylation at P402 and/or P564 leads to ubiquitination
and proteasomal degradation of HIF-1α. However, under hypoxia in
tumor microenvironment, HIF-1α remains unhydroxylated and stable
against proteasomal degradation (6,7). As a
transcription factor, HIF-1α promotes the adaptation of tumor cells
to hypoxia through upregulating the expression of genes related to
angiogenesis, glycolysis and cellular mobility, such as VEGF, GLUT1
and MMPs (4,8,9). In
glioblastoma, HIF-1α is often detected predominantly in hypoxic
regions and in tumor cells infiltrating the normal brain tissue
(10). This suggests the possible
role of HIF-1α in the aggressiveness of glioblastoma.
Mitochondria play an essential function in cells
through the production of energy and the ability to regulate
intracellular Ca2+. As such, they are involved in a
variety of cellular processes, including survival, proliferation
and apoptosis (11–13). These functions are crucial for
cancer cells, since cancer is characterized as a disease of
inappropriate cell proliferation and dysregulated cell cycle
control. On the other hand, mitochondria are also dynamic
organelles and move through the cell with frequent fission and
fusion events. The dynamic balance of fission and fusion events is
important to maintain the normal shape, structure and function of
mitochondria (14). It has been
demonstrated that the dynamic mitochondrial morphology corresponds
to the metabolic status of cells, and unbalanced mitochondrial
fission and fusion events potentially contribute to tumorigenesis
(15). In addition, some highly
conserved dynamin-related GTPases are identified as the mediator of
mitochondrial dynamics. Dynamin-related protein 1 (Drp1) is
involved in the process of mitochondrial fission, while mitofusins
and OPA1 are required for mitochondrial fusion in mammalian cells
(14,15). Recent studies demonstrated the
involvement of Drp1 in the development of lung and breast cancer
and neuroblastoma (16–18). Inhibition of Drp1 efficiently
reduced cancer cell growth and enhanced spontaneous apoptosis in
several types of cancer, including lung, breast, colon and cervical
cancer (16,19–21).
Thus, Drp1 is considered as a potential therapeutic target of
cancer in the future. In addition, a recent study demonstrated the
role of Drp1 in the migration and invasion of breast cancer cells.
The high expression of Drp1 leads to more fragmented mitochondria
and redistribution of mitochondria to lamellipodial regions.
Drp1-dependent mitochondrial fission promotes lamellipodia
formation at the leading edge of breast cancer cells, which is
critical for their migration and invasion (17). However, whether Drp1 contributes to
the migration of human glioblastoma cells under hypoxia remains
unknown.
In the present study, we first examined the effect
of hypoxia on the transcription and expression of Drp1 in
glioblastoma U251 cells. It was found that hypoxia upregulated the
transcription and expression of Drp1, and consequently increased
mitochondrial fission. The migration of U251 cells was evaluated by
scratch and Transwell assay. Consistent with previous studies
(5), CoCl2 and hypoxic
incubation promoted the migration of U251 cells. To test the
involvement of Drp1 in hypoxia-induced migration, the effect of
Mdivi-1, a Drp1 inhibitor, on the migration of U251 cells was
examined. Results showed that Mdivi-1 efficiently attenuated
hypoxia-induced migration of U251 cells. To demonstrate the direct
role of Drp1 in hypoxia-induced migration of U251, we established
three U251 stable cell lines expressing GFP, GFP-Drp1 and dominant
negative GFP-Drp1-K38A. As expected, exogenously expressed GFP-Drp1
enhanced hypoxia-induced migration, while expression of dominant
negative GFP-Drp1-K38A inhibited the migration of U251.
Collectively, our data demonstrate for the first time that
mitochondrial fission protein Drp1 is involved in hypoxia-induced
migration of human glioblastoma U251 cells. Inhibition of
Drp1-dependent mitochondrial fission may be a potential strategy
for prevention and therapy of glioblastoma in the future.
Materials and methods
Materials
Fetal bovine serum (FBS), Dulbecco’s modified
Eagle’s medium (DMEM), trypsin and the solution of
penicillin-streptomycin (P/S) were purchased from Gibco.
CoCl2, G418 and Drp1 inhibitor Mdivi-1 were purchased
from Sigma. Transfection kits including Lipofectamine 2000 and
Opti-MEM were from Invitrogen. Dimethyl sulfoxide (DMSO) was
purchased from Solarbio (Beijing, China). Primers for qRT-PCR were
synthesized by Sangon Biotech Co. (Shanghai, China). PrimeScript™
RT reagent kit with gDNA Eraser and SYBR® Premix Ex Taq™ II were
from Takara. The RNA simple total RNA kit was from Tiangen
(Beijing, China). Amersham ECL prime western blotting detection
reagents were from GE Healthcare.
Cell culture, scratch and Transwell
assay
Human U251 glioblastoma cell line was obtained from
the American Type Culture Collection (ATCC). U251 cells were grown
in a DMEM medium supplemented with 10% FBS and 100 μg/ml P/S in a
humidified incubator at 37°C with an atmosphere containing 5%
CO2. For hypoxia treatment, the cells were incubated in
hypoxia (1% O2, 5% CO2 and 94% N2)
or treated with 100 μM CoCl2. The scratch and Transwell
assays were carried out as previously described (22). In the scratch assay, the cells
firstly were seeded on 35 mm dishes and grown in growth medium.
Treatment with 100 μM CoCl2 was used to mimic hypoxia in
U251 cells. Briefly, a scratch was made in monolayer of cells with
a 200-μl pipette tip. Then, the cells were washed twice with PBS to
remove the suspended cells and incubated in DMEM without supplement
of FBS and P/S, or DMEM containing 5 μM Mdivi-1 or DMSO. The
migration status was photographed by bright-field microscopy at
different time points after scratch. Transwell assay was performed
with Transwell chamber (Corning). In brief, 1.2×104
cells were seeded into the upper chamber in 200 μl of serum-free
medium, while the bottom of the chamber was incubated with 500 μl
of complete medium containing 10% FBS and 1% P/S. After 6 h of
migration, the cells on the top surface of the insert were gently
removed with a cotton swab. The migrated cells on the lower surface
were fixed with 4% paraformaldehyde and stained with crystal violet
for 30 min, and counted under a microscope. All assays were
independently repeated at least in triplicate.
Quantitative real-time PCR
Total RNA was extracted from cells cultured in DMEM
or DMEM containing 100 μM CoCl2 using an RNAsimple Total
RNA kit (Tiangen). Total RNA was reverse-transcribed using
PrimeScript™ RT reagent kit with gDNA Eraser. Quantitative RT-PCR
was performed using SYBR® Premix Ex Taq™ II and a 7500
Real-Time PCR System (Applied Biosystems). Relative quantification
of gene expression was calculated using the formula:
2−ΔΔCt, ΔΔCt = (Cttarget gene −
CtGAPDH)hypoxia −
(Cttarget gene −
CtGAPDH)normoxia. The qRT-PCR of Drp1
was performed using a sense primer, 5′-TG AAGGATGTCATGTCGGACC-3′
and an antisense primer, 5′-GTTGAGGACGTTGACTTGGCT-3′. The primers
for GAPDH were: 5′-CAGGGCTGCTTTTAACTCTGGT-3′ (sense), and
5′-GATTTTGGAGGGATCTCGCT-3′ (antisense). Three independent
experiments for each condition were carried out.
Western blot analysis
The U251 cells or the three U251 stable cell lines
expressing GFP, GFP-Drp1 or GFP-Drp1-K38A after hypoxia treatment
were harvested and lysed. The cell lysates were subjected to 8%
SDS-PAGE gel electrophoresis. After electrophoresis, the proteins
were transferred onto polyvinylidene difluoride (PVDF) membrane
(Millipore, USA). The membrane was blocked with 5% skim milk in
TBST buffer for 1 h at room temperature. Then, the PVDF membranes
were immunoblotted with mouse anti-Drp1 antibody (BD Biosciences)
at a dilution of 1:1,000. After three washes with TBST, the
membranes were further incubated with an HRP-conjugated relative
secondary antibody (at a dilution of 1:2,000) for 2 h at room
temperature. Chemiluminescence assay were performed with Amersham
ECL prime western blotting detection reagents, and the
immunobloting signal was detected using Molecular
Imager® ChemiDocT XRS+ System
(Bio-Rad).
Mitochondrial imaging
As previously described (32), pDsRed2-Mito was transfected into
U251 cells to label mitochondria with Lipofectamine 2000.
Twenty-four hours after transfection, mitochondrial morphology was
observed with an inverted fluorescence microscope (Axiovert 200;
Carl Zeiss Inc.) with excitation at 545 nm. After 6 h hypoxia
treatment, U251 cells were fixed with 4% PFA, and mitochondrial
morphology was detected again under fluorescence microscope after
hypoxic treatment for 12 h. To examine the role of Drp1 on
hypoxia-induced mitochondrial morphology, cells were pretreated
with 5 μM Mdivi-1 1 h prior to hypoxia.
Establishment of U251 stable cell lines
expressing GFP, GFP-DRP1 and GFP-Drp1-K38A
Firstly, the minimum lethal concentration of G418
was determined as previously described (22). Briefly, U251 cells were seeded in
12-well plates at a density of 5×104. The cells were
cultured in growth medium containing 0–1,100 μg/μl of G418 for 12
days. Growth medium containing different concentrations of G418 was
exchanged every 3–4 days. The survival state of cells was monitored
every 24 h under a bright-field microscope. The minimum
concentration of G418 that induced complete cell death in 12-well
plates was the minimum lethal concentration of G418 to U251 cells.
GFP, GFP-Drp1 and GFP-Drp1-K38A plasmids were transfected into U251
cells with Lipofectamine 2000 according to the manufacturer’s
instructions. Forty-eight hours after transfection, the cells were
selected with a higher concentration than minimum lethal
concentration of G418. The selected colonies with GFP fluorescence
were picked up and passaged. The cell lines stably expressing GFP,
GFP-Drp1 or GFP-Drp1-K38A were further confirmed by fluorescence
microscopy and western blotting.
MTT assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was applied to assess the proliferation of three U251 cell
lines according to the manufacturer’s instructions. Approximately,
5×103 cells were seeded in 96-well tissue culture plates
and cultured in growth medium. The proliferation of U251 cells was
detected after the cells cultured at 37°C, 5% CO2 for
12, 24, 48 and 72 h. Twenty microliters of the MTT solution was
added to each well (5 mg/ml, 0.5% MTT) and the cells continued to
culture for 4 h. After the incubation, the supernatant was
discarded. DMSO (100 μl) was added to each well, and the culture
plate was shaken at low-speed for 10 min until the crystal
dissolved completely. The color intensity was measured
spectrophotometrically using a microplate reader (Thermo
Multiskan® FC) at 570 nm. All assays were performed at
least three times.
Statistical analysis
The quantitative data are shown as the means ± SD.
Data were analyzed using either Student’s t-test to compare two
conditions or ANOVA followed by planned comparisons of multiple
conditions, and p<0.05 was considered to indicate a
statistically significant difference.
Results
Hypoxia upregulates the transcription and
expression of Drp1 and stimulates mitochondrial fission in
glioblastoma U251 cells
A recent study found Drp1-dependent mitochondrial
fission could enhance the migration and invasion of breast cancer
cells through modifying lamellipodial formation (17). On the other hand, CoCl2
is a common chemical HIF-1α activator (23,24,36),
and HIF-1α promotes the adaption of cancer cells to hypoxia through
upregulating some genes related to cellular mobility, angiogenesis
and glycolysis (4,5). In the present research, we
investigated whether Drp1 is also involved in hypoxia-induced
migration of human glioblastoma U251 cells. To test the hypothesis,
the mRNA expression of Drp1 in U251 cells after CoCl2
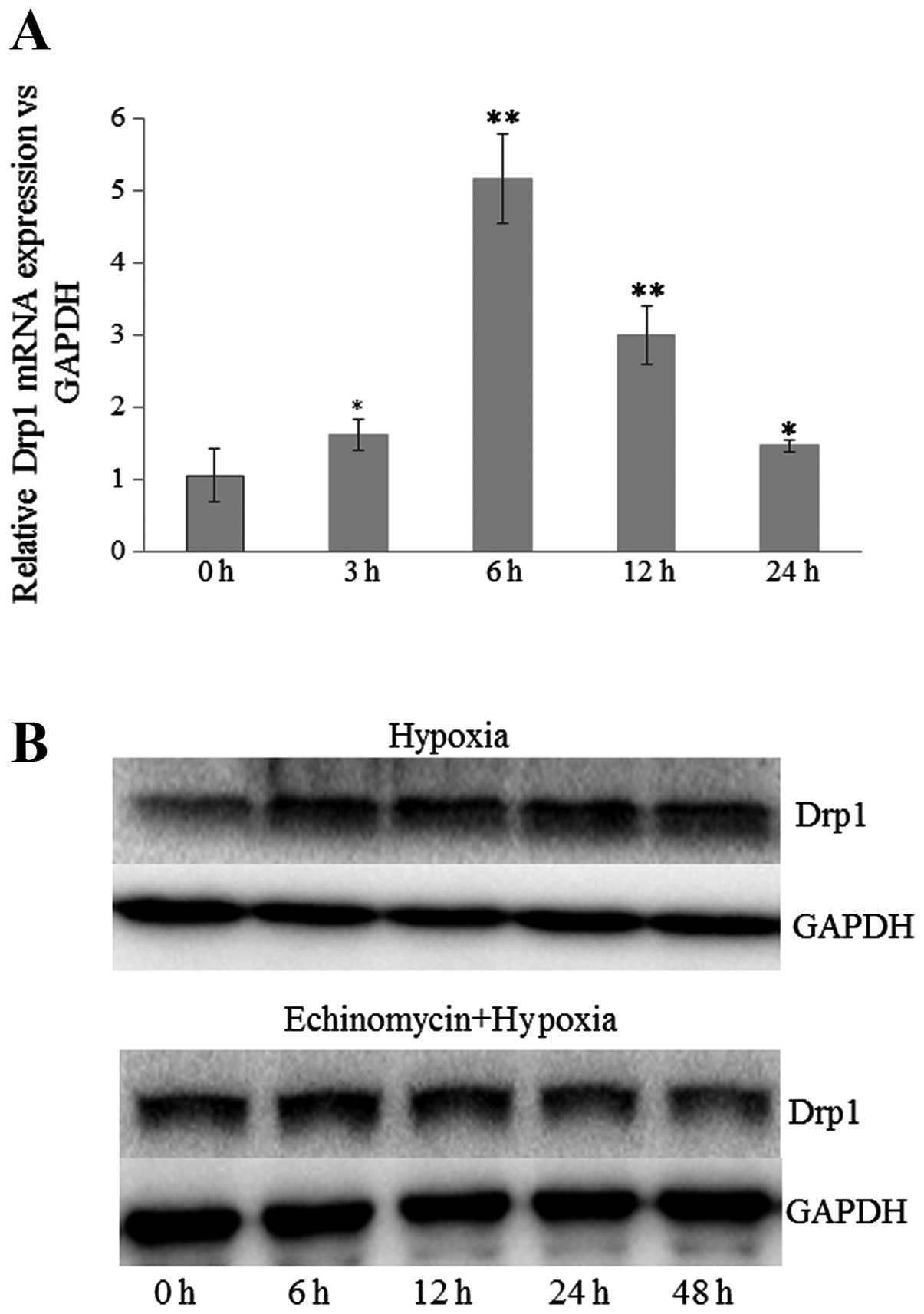
treatment was examined by quantitative RT-PCR. As shown in Fig. 1A, the mRNA expression of Drp1 was
significantly increased from 3 h post-treatment. The transcription
level of Drp1 was increased ~5-fold at 6 h post-treatment with
CoCl2. In addition, the protein level of Drp1 after
hypoxia treatment (1% O2, 5% CO2 and 94%
N2) was also examined by western blot assay. Consistent
with the results of mRNA expression, the expression of Drp1 in U251
cells was also upregulated by hypoxia treatment. Notably,
pretreatment with inhibitor of HIF-1α, echinomycin, blocked
hypoxia-induced expression of Drp1 (Fig. 1B). Drp1 is a large GTPase mediating
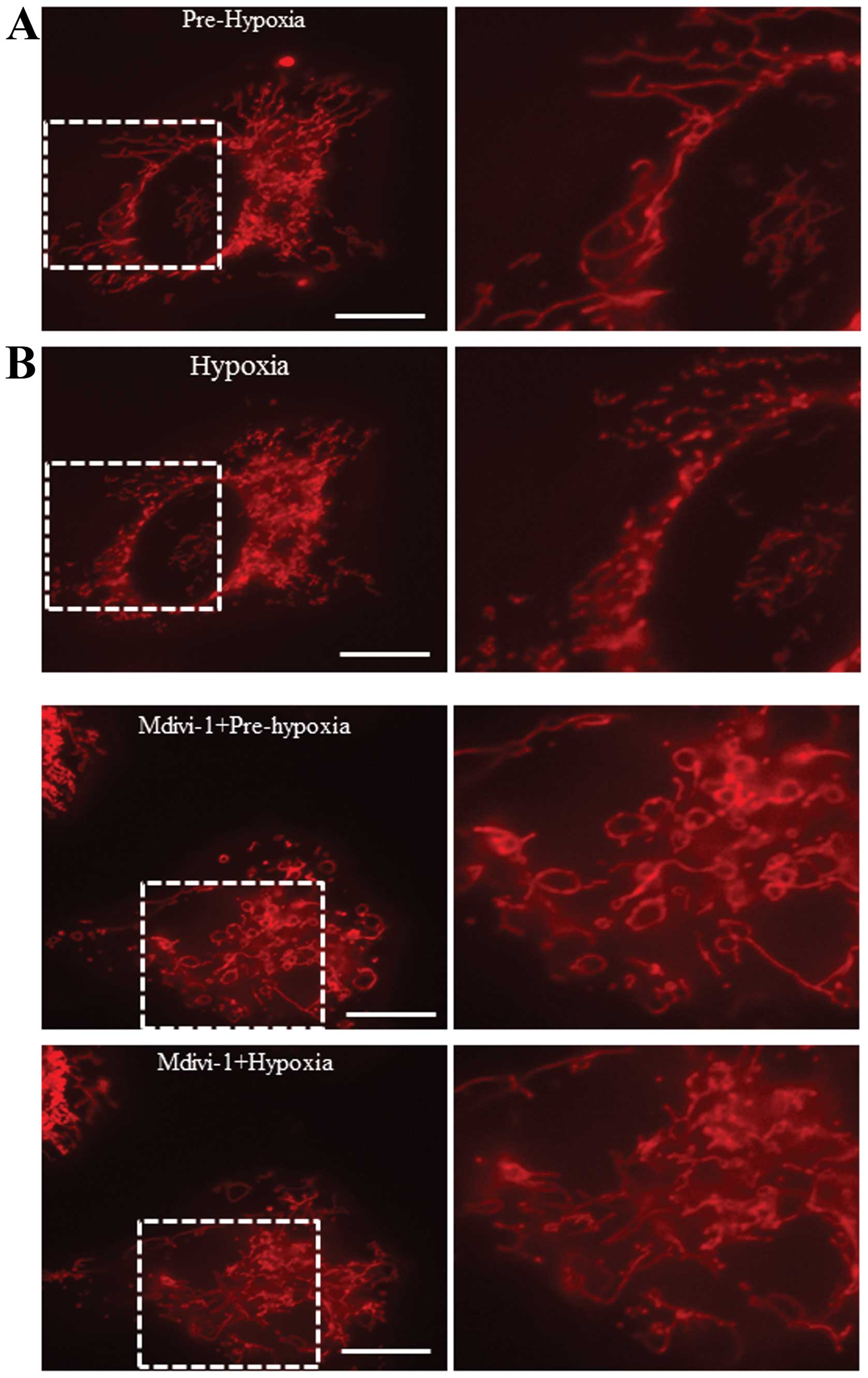
mitochondrial fission. Thus, mitochondrial morphology was examined
to evaluate the function of high expressed Drp1 under hypoxia. As
shown in Fig. 2, mitochondrial
fission was increased after hypoxia treatment, while pretreatment
with Drp1 inhibitor Mdivi-1 efficiently attenuated hypoxia-induced
mitochondrial fission. These results suggest that hypoxia may
upregulate the transcription and expression of Drp1 through
activation of HIF-1α and subsequently enhance mitochondrial
fission.
Effect of Drp1 inhibitor Mdivi-1 on
hypoxia-induced migration of U251 cells
Hypoxia is an important factor to stimulate
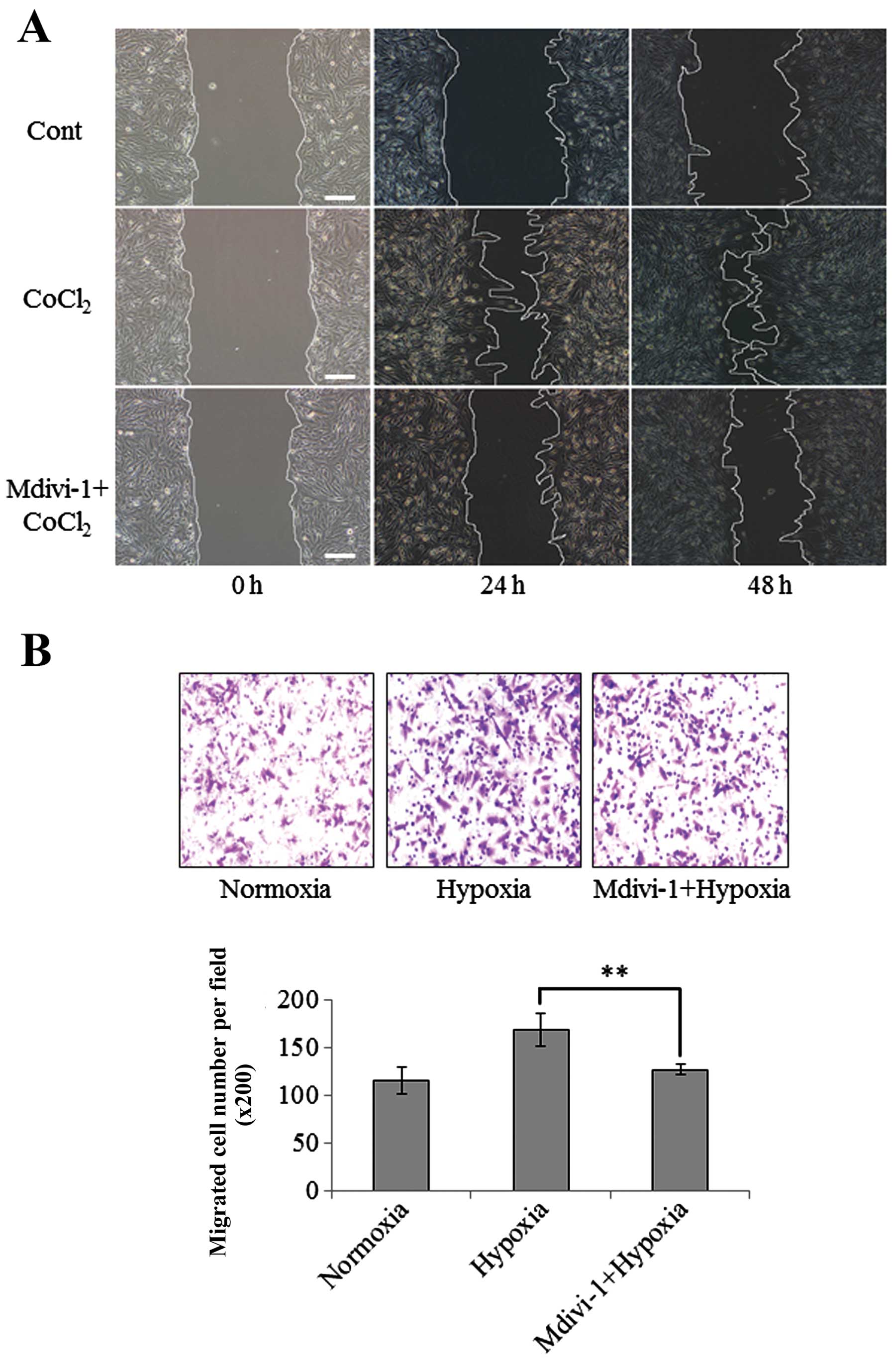
migration of cancer cells. Firstly, the migration of U251 cells
under normoxia and hypoxia was evaluated by scratch and Transwell
assays. In scratch assay, CoCl2 treatment was used to
mimic the hypoxia. Consistent with previous studies (5), both scratch and Transwell assays
indicated that hypoxia significantly enhanced the migration of
U251, shown in Fig. 3. To
investigate the role of Drp1 in hypoxia-induced migration of U251
cells, the effect of Mdivi-1 was evaluated. Mdivi-1 is the first
selective inhibitor of Drp1, which inhibits Drp1 self-assembly and
attenuates mitochondrial fission (25). Mdivi-1 has been applied to prevent
mitochondrial fission in a number of disease models (26,27).
Results showed pretreatment with 5 μM of Mdivi-1 significantly
attenuated hypoxia-induced migration of U251 cells, shown in
Fig. 3. However, recent studies
suggest that Mdivi-1 could potentially have off-target effects on
preventing mitochondrial outer membrane permeabilization (28). Therefore, further investigations are
required to directly demonstrate the involvement of Drp1 in
hypoxia-induced migration of U251 cells.
Establishment of the U251 cell lines
stably expressing GFP, GFP-Drp1 or GFP-Drp1-K38A
The dominant negative mutation of K38A in Drp1 has
been well identified, and Drp1-K38A is a frequently used tool to
investigate the direct role of Drp1 in cellular activities
(29,30). To explore the direct involvement of
Drp1 in hypoxia-induced migration, three U251 cell lines stably
expressing GFP, GFP-Drp1 or GFP-Drp1-K38A were established as
previously described (22).
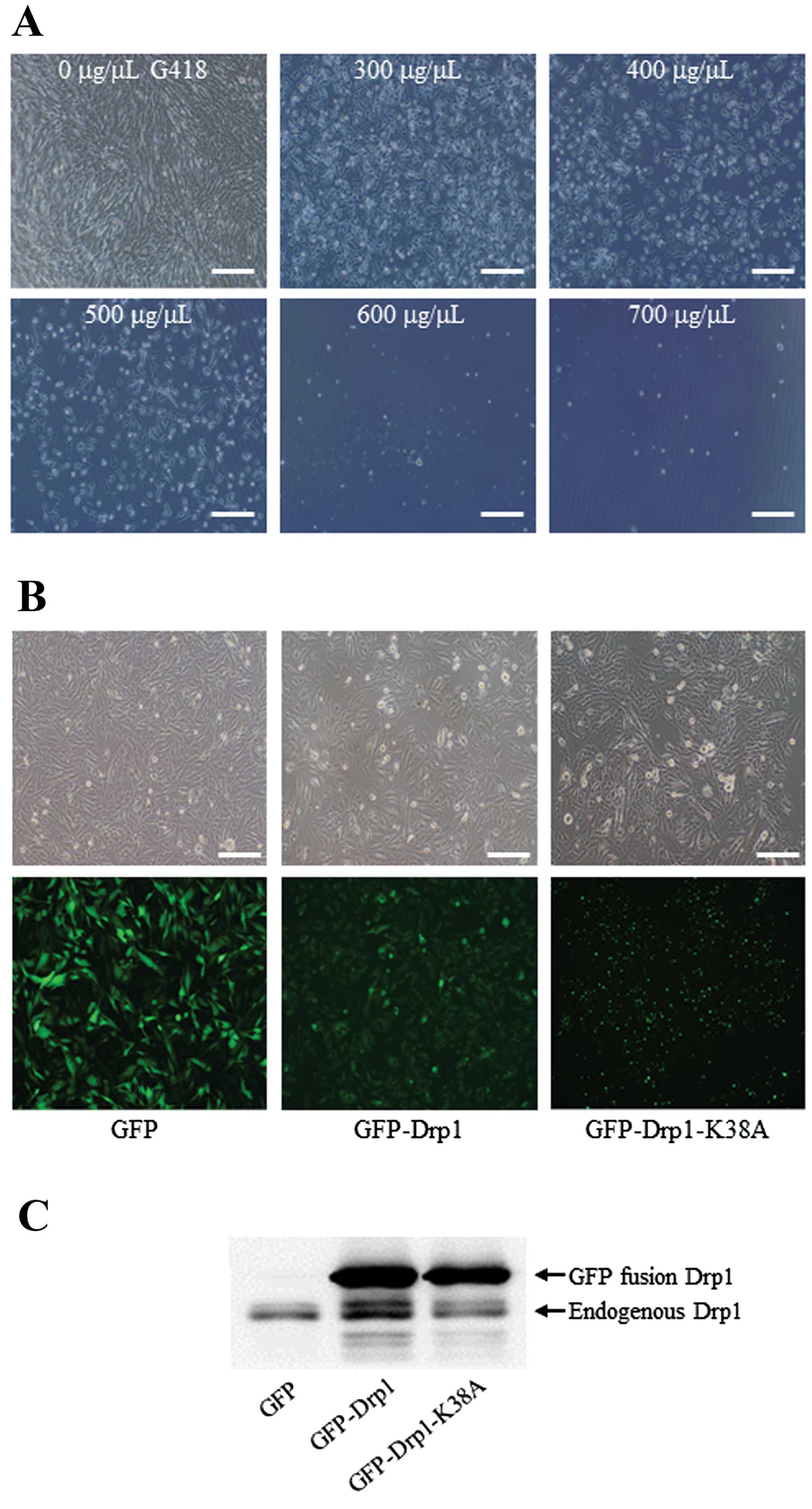
Firstly, the minimum lethal concentration of G418 to U251 cells was
determined as 600 μg/μl, as shown in Fig. 4A. Thus, 700 μg/μl of G418 was used
for selection of U251 stable cell lines. The stable cell colonies
were confirmed by fluorescence microscopy and western blot assay.
As shown in Fig. 4B, GFP
fluorescence was clearly visualized in three cell lines. Consistent
with a previous report (29),
GFP-Drp1 K38A accumulated into large aggregates and punctate foci,
while GFP and GFP-Drp1 were mostly diffused in cytoplasm. In
addition, the results of western blotting showed the endogenously
and exogenously expressed Drp1 in three stable cell lines. As shown
in Fig. 4C, several bands were
detected in GFP-Drp1 and GFP-Drp1 K38A cell lines. The upper band
was GFP fused Drp1 or Drp1 K38A, and the lower bands was endogenous
Drp1. The results of fluorescence microscopy and western blotting
indicated that three U251 cell lines stably expressing GFP,
GFP-Drp1 and GFP-Drp1 K38A were successfully established.
Effect of exogenously expressed Drp1 and
Drp1-K38A on hypoxia-induced migration of U251 cells
The result of the scratch assay may be influenced by
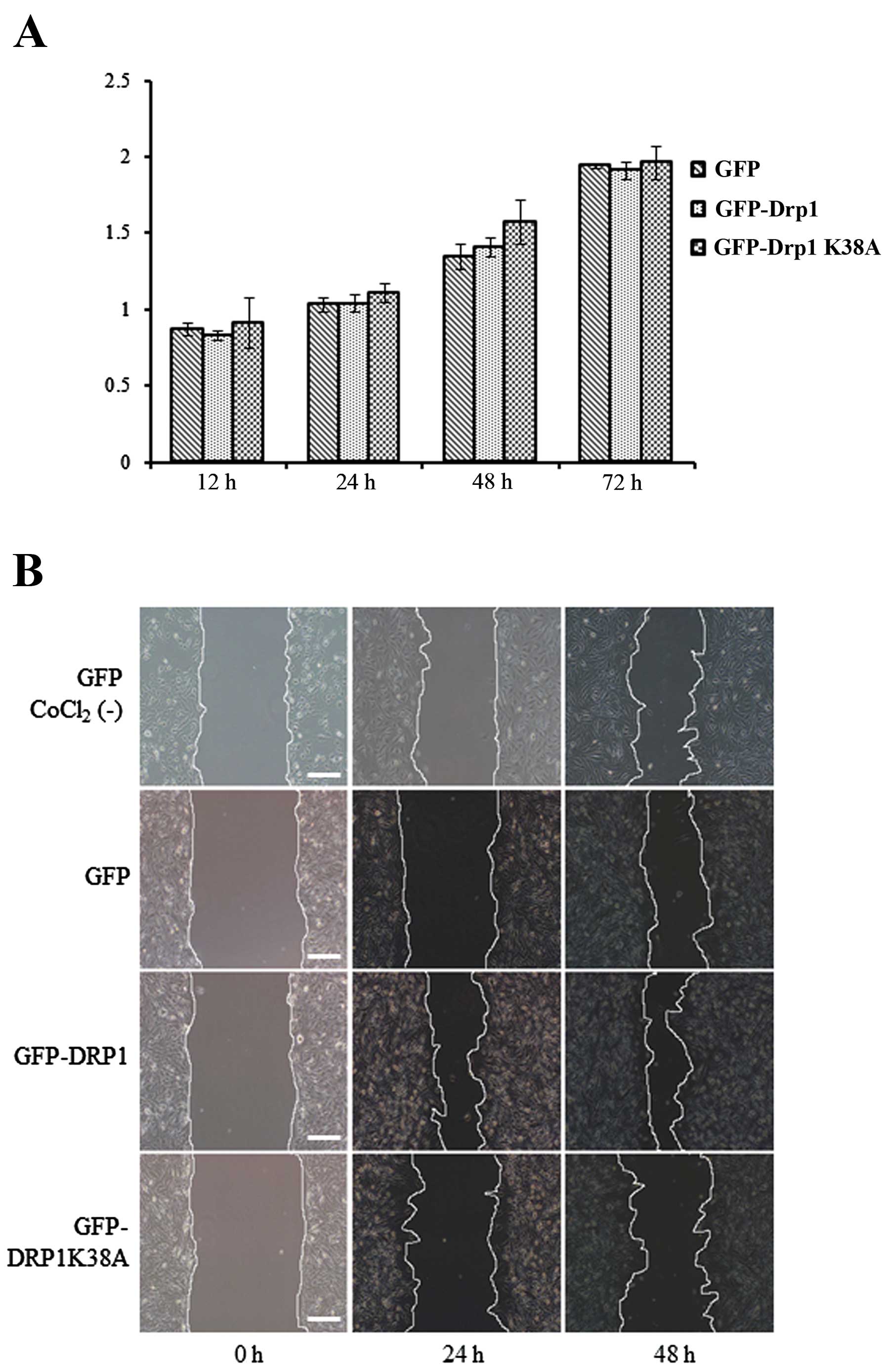
cell proliferation. Thus, we examined the effect of exogenously
expressed Drp1 and Drp1-K38A on cell proliferation by MTT assay,
prior to evaluation of the migration of three cell lines. As shown
in Fig. 5A, MTT assay indicated
that exogenously expressed Drp1 or Drp1 K38A had no significant
effect on cell proliferation. To elucidate the role of Drp1 in
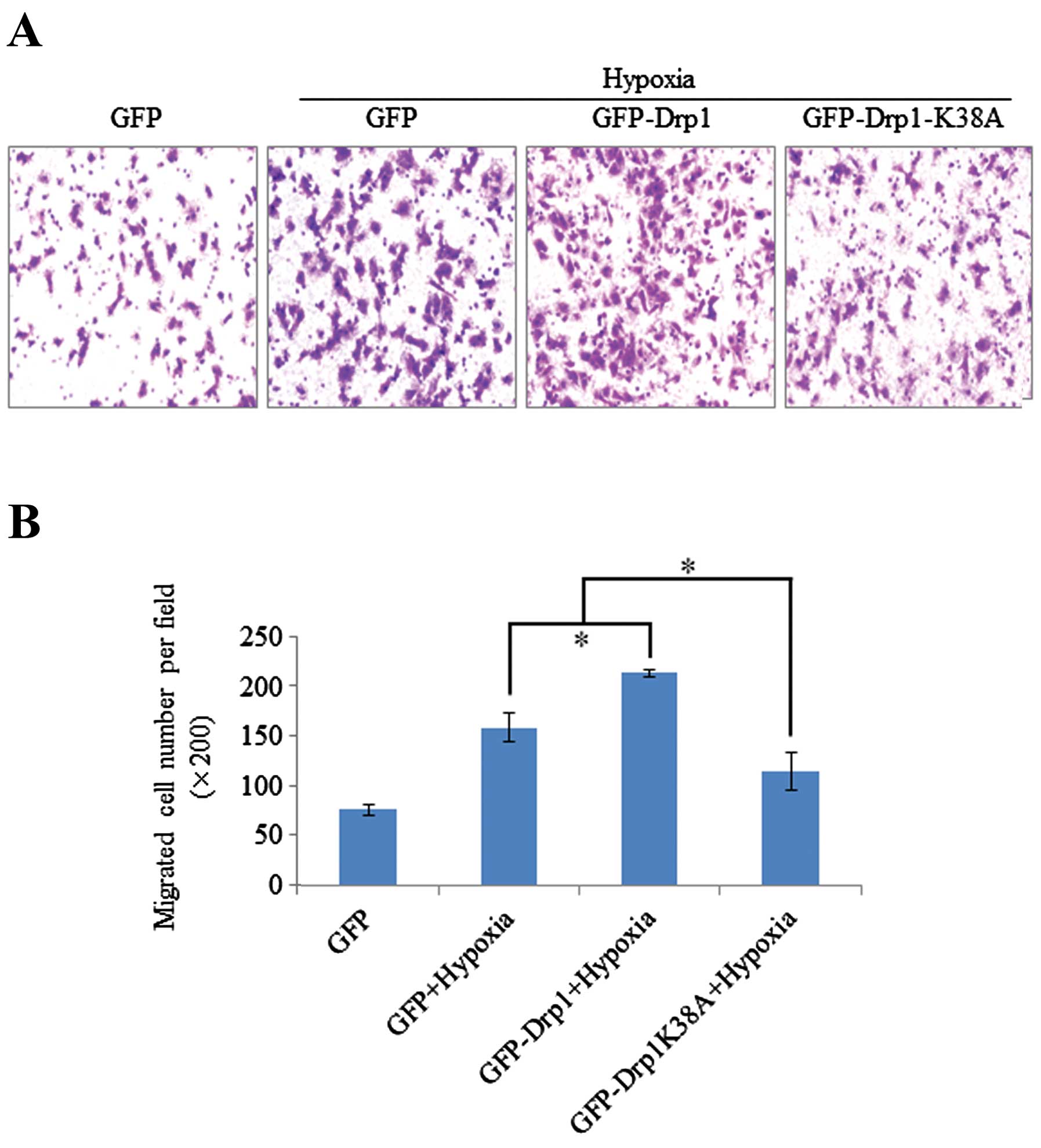
hypoxia-induced migration, the migratory activity of three stable
cell lines expressing GFP, GFP-Drp1 or GFP-Drp1 K38A was evaluated
after CoCl2 or hypoxia treatment. Scratch and Transwell
assay showed that exogenously expressed Drp1-K38A efficiently
attenuated the migration of U251 cells, while exogenously expressed
Drp1 enhanced the migratory activity of U251 cells under hypoxia
(Figs. 5B and 6). Collectively, these results demonstrate
the involvement of Drp1 in hypoxia-induced migration of human
glioblastoma U251 cells.
Discussion
Cancer cells are characterized by their uncontrolled
high proliferation. The rapid proliferation always results in a
hypoxic microenvironment in the central region of solid tumors. It
has been well documented that the hypoxic microenvironment in
tumors plays an important role in migration and invasion of cancer
cells (4,5). HIF-1α, a hypoxia-inducible factor, is
the vital transcription factor activated by hypoxia, which promotes
the adaptation of cancer cells to the hypoxic microenvironment
through upregulating gene expression related to metabolism,
cellular mobility and angiogenesis, such as GLUT1, MMPs and VEGF
(4,8,9). On
the other hand, mitochondrial functions are crucial for cancer
survival, proliferation and metastasis through regulation of ATP
production, Ca2+ homeostasis, cell cycle and apoptosis
(11–13). Mitochondria are also dynamic
organelles in cells with frequent fission and fusion processes
(14). Recent studies have provided
insight into the involvement of Drp1-dependent mitochondrial
fission in cancer cells (15–20).
Drp1 is a highly conserved dynamin-related GTPase in mammalian
cells, and its GTPase activity may be regulated by some
post-transcriptional modification, such as phosphorylation,
S-nitrosylation, ubiquitination and SUMOylation (31–35).
The high expression of Drp1 was recently found in several cancers,
such as neuroblastoma, breast and lung cancer. Inhibition of Drp1
has been found to efficiently reduce cancer cell growth and enhance
spontaneous apoptosis in cancer (27).
In the present study, we explored whether Drp1 is
involved in the hypoxia-induced migration of human glioblastoma
U251 cells. It was found that hypoxia significantly promoted the
transcription and expression of Drp1, and stimulated Drp1-dependent
mitochondrial fission (Figs. 1 and
2). HIF-1α inhibitor echinomycin
efficiently blocked hypoxia-induced expression of Drp1 (Fig. 1B). Consistent with previous studies
(5), hypoxia significantly enhanced
the migratory activity of U251 cells (Fig. 3). To examine the possible role of
Drp1 in hypoxia-induced migration, pharmacological experiments were
performed using Mdivi-1, a Drp1 inhibitor, which attenuates Drp1
self-assembly. Mdivi-1 (5 μM) efficiently attenuated
hypoxia-induced mitochondrial fission and migration of U251 cells
(Figs. 2B and 3). These results suggest that hypoxia may
enhance migration of U251 cells through upregulation of Drp1 via
HIF-1α. However, the Drp1 inhibitor Mdivi-1 could have some
off-target effects, such as Drp1-independent effect on
mitochondrial outer membrane permeabilization (MOMP) (28). Changes in MOMP and other
mitochondrial dysfunctions may regulate the migration of cancer
cells via reactive oxygen species (37,38).
To elucidate the direct role of Drp1 in hypoxia-induced migration,
three U251 stable cell lines expressing GFP, GFP-Drp1 or GFP-Drp1
K38A were established. Compared with the GFP cell line, stable
expression of Drp1 or dominant negative Drp1 K38A respectively
enhanced or attenuated the migration of U251 cells under hypoxia
(Figs. 5B and 6). Therefore, the present study
demonstrated the involvement of Drp1 in hypoxia-induced migration
of human glioblastoma U251 cells. These results are consistent with
a recent study, which demonstrated the involvement of
Drp1-dependent mitochondrial fission in migration of breast cancer
cells through modifying lamellipodial formation (17). However, a major challenge for
further studies will be to identify the molecular pathway through
which Drp1 regulates hypoxia-induced migration of U251 cells.
In contrast, another recent study reported that
hypoxia induced upregulation of mitochondrial fusion protein Mfn1
in some types of cancer cells (39). The upregulation of Mfn1 and two
Bcl-2 family members, BNIP3 and BNIP3L, participates in the
formation of enlarged mitochondria under hypoxia. Hypoxic enlarged
mitochondria efficiently enhance the resistance of cancer cells to
chemotherapy. However, this phenomenon was only observed in some
specific types of cancer cell lines, such as LS174, A549, HeLa and
786-O cells, but not in PC3 (prostate cancer), MCF7 (breast
cancer), CAL33, ORL3 (head and neck) and SkMel (melanoma) cells. In
our present study, hypoxia upregulated mitochondrial fission
protein Drp1 and enhanced the migration of glioblastoma U251 cells.
The different effect of hypoxia on the expression of mitochondria
related proteins and the activity of cancer cells is possibly due
to the different cancer cell lines. It is also worth investigating
the underlying mechanism of the effect of hypoxia on different
types of cancer cells.
In conclusion, our data show for the first time that
Drp1 is involved in hypoxia-induced migration of glioblastma U251
cells. Hypoxia promotes the migratory activity of U251 cells
through the upregulation of Drp1 expression. Inhibition of Drp1
activity by Mdivi-1 or expression of dominant negative Drp1 K38A
efficiently attenuates hypoxia-induced migration of U251 cells.
Glioblastomas are highly aggressive brain tumors; prevention of
migration and invasion is critical to improve therapeutic effect
and prognosis. Thus, our data suggest that mitochondrial fission
protein Drp1 may be a potential target for the prevention and
therapy of glioblastoma in the future.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (31360241), the Postgraduate Student
Foundation for New Teacher from the Ministry of Education of China
(20123601120001), and the Foundation from the Department of
Education of Jiangxi Province (GJJ13162).
References
|
1
|
Behin A, Hoang-Xuan K, Carpentier AF and
Delattre JY: Primary brain tumours in adults. Lancet. 361:323–331.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Claes A, Idema AJ and Wesseling P: Diffuse
glioma growth: a guerilla war. Acta Neuropathol. 114:443–458. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anton K, Baehring JM and Mayer T:
Glioblastoma multiforme: overview of current treatment and future
perspectives. Hematol Oncol Clin North Am. 26:825–853. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan DA and Giaccia AJ: Hypoxia, gene
expression, and metastasis. Cancer Metastasis Rev. 26:333–339.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Liu Q, Wang F, Ling EA, Liu S,
Wang L, Yang Y, Yao L, Chen X, Wang F, Shi W, Gao M and Hao A:
Melatonin antagonizes hypoxia-mediated glioblastoma cell migration
and invasion via inhibition of HIF-1α. J Pineal Res. 55:121–130.
2013.PubMed/NCBI
|
|
6
|
Chan DA, Sutphin PD, Yen SE and Giaccia
AJ: Coordinate regulation of the oxygen-dependent degradation
domains of hypoxia-inducible factor 1α. Mol Cell Biol.
25:6415–6426. 2005.PubMed/NCBI
|
|
7
|
Chan DA, Sutphin PD, Denko NC and Giaccia
AJ: Role of prolyl hydroxylation in oncogenically stabilized
hypoxia-inducible factor-1α. J Biol Chem. 277:40112–40117.
2002.PubMed/NCBI
|
|
8
|
Krishnamachary B, Berg-Dixon S, Kelly B,
Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P
and Semenza GL: Regulation of colon carcinoma cell invasion by
hypoxia-inducible factor 1. Cancer Res. 63:1138–1143.
2003.PubMed/NCBI
|
|
9
|
Muñoz-Nájar UM, Neurath KM, Vumbaca F and
Claffey KP: Hypoxia stimulates breast carcinoma cell invasion
through MT1-MMP and MMP-2 activation. Oncogene. 25:2379–2392.
2006.PubMed/NCBI
|
|
10
|
Zagzag D, Zhong H, Scalzitti JM, Laughner
E, Simons JW and Semenza GL: Expression of hypoxia-inducible factor
1α in brain tumors: association with angiogenesis, invasion, and
progression. Cancer. 88:2606–2618. 2000.
|
|
11
|
Oakes SA and Korsmeyer SJ: Untangling the
web: mitochondrial fission and apoptosis. Dev Cell. 7:460–462.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szabadkai G, Simoni AM, Chami M,
Wieckowski MR, Youle RJ and Rizzuto R: Drp-1-dependent division of
the mitochondrial network blocks intraorganellar Ca2+
waves and protects against Ca2+-mediated apoptosis. Mol
Cell. 16:59–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaw JM and Nunnari J: Mitochondrial
dynamics and division in budding yeast. Trends Cell Biol.
12:178–184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan DC: Mitochondrial fusion and fission
in mammals. Annu Rev Cell Dev Biol. 22:79–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grandemange S, Herzig S and Martinou JC:
Mitochondrial dynamics and cancer. Semin Cancer Biol. 19:50–56.
2009. View Article : Google Scholar
|
|
16
|
Rehman J, Zhang HJ, Toth PT, Zhang Y,
Marsboom G, Hong Z, Salgia R, Husain AN, Wietholt C and Archer SL:
Inhibition of mitochondrial fission prevents cell cycle progression
in lung cancer. FASEB J. 26:2175–2186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao J, Zhang J, Yu M, Xie Y, Huang Y,
Wolff DW, Abel PW and Tu Y: Mitochondrial dynamics regulates
migration and invasion of breast cancer cells. Oncogene.
32:4814–4824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hagenbuchner J, Kuznetsov AV, Obexer P and
Ausserlechner MJ: BIRC5/Survivin enhances aerobic glycolysis and
drug resistance by altered regulation of the mitochondrial
fusion/fission machinery. Oncogene. 32:4748–4757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian W, Choi S, Gibson GA, Watkins SC,
Bakkenist CJ and Van Houten B: Mitochondrial hyperfusion induced by
loss of the fission protein Drp1 causes ATM-dependent G2/M arrest
and aneuploidy through DNA replication stress. J Cell Sci.
125:5745–5757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inoue-Yamauchi A and Oda H: Depletion of
mitochondrial fission factor DRP1 causes increased apoptosis in
human colon cancer cells. Biochem Biophys Res Commun. 421:81–85.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Parone PA, Da Cruz S, Tondera D,
Mattenberger Y, James DI, Maechler P, Barja F and Martinou JC:
Preventing mitochondrial fission impairs mitochondrial function and
leads to loss of mitochondrial DNA. PLoS One. 3:e32572008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu D, Wu J, Xu L, Zhang R and Chen L: A
method for the establishment of a cell line with stable expression
of the GFP-LC3 reporter protein. Mol Med Rep. 6:783–786.
2012.PubMed/NCBI
|
|
23
|
Marsboom G, Toth PT, Ryan JJ, et al:
Dynamin-related protein 1-mediated mitochondrial mitotic fission
permits hyperproliferation of vascular smooth muscle cells and
offers a novel therapeutic target in pulmonary hypertension. Circ
Res. 110:1484–1497. 2012. View Article : Google Scholar
|
|
24
|
Wang GL and Semenza GL: Desferrioxamine
induces erythropoietin gene expression and hypoxia-inducible factor
1 DNA-binding activity: implications for models of hypoxia signal
transduction. Blood. 82:3610–3615. 1993.PubMed/NCBI
|
|
25
|
Cassidy-Stone A, Chipuk JE, Ingerman E,
Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR
and Nunnari J: Chemical inhibition of the mitochondrial division
dynamin reveals its role in Bax/Bak-dependent mitochondrial outer
membrane permeabilization. Dev Cell. 14:193–204. 2008. View Article : Google Scholar
|
|
26
|
Lackner LL and Nunnari J: Small molecule
inhibitors of mitochondrial division: tools that translate basic
biological research into medicine. Chem Biol. 17:578–583. 2010.
View Article : Google Scholar
|
|
27
|
Qian W, Wang J and Van Houten B: The role
of dynamin-related protein 1 in cancer growth: a promising
therapeutic target? Expert Opin Ther Targets. 17:997–1001. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kushnareva Y, Andreyev AY, Kuwana T and
Newmeyer DD: Bax activation initiates the assembly of a multimeric
catalyst that facilitates Bax pore formation in mitochondrial outer
membranes. PLoS Biol. 10:e10013942012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pitts KR, Yoon Y, Krueger EW and McNiven
MA: The dynamin-like protein DLP1 is essential for normal
distribution and morphology of the endoplasmic reticulum and
mitochondria in mammalian cells. Mol Biol Cell. 10:4403–4417. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ong SB, Subrayan S, Lim SY, Yellon DM,
Davidson SM and Hausenloy DJ: Inhibiting mitochondrial fission
protects the heart against ischemia/reperfusion injury.
Circulation. 121:2012–2022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taguchi N, Ishihara N, Jofuku A, Oka T and
Mihara K: Mitotic phosphorylation of dynamin-related GTPase Drp1
participates in mitochondrial fission. J Biol Chem.
282:11521–11529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y,
Tomizawa K, Nairn AC, Takei K, Matsui H and Matsushita M: CaM
kinase Iα-induced phosphorylation of Drp1 regulates mitochondrial
morphology. J Cell Biol. 182:573–585. 2008.
|
|
33
|
Cho DH, Nakamura T, Fang J, Cieplak P,
Godzik A, Gu Z and Lipton SA: S-nitrosylation of Drp1 mediates
β-amyloid-related mitochondrial fission and neuronal injury.
Science. 324:102–105. 2009.
|
|
34
|
Wang H, Song P, Du L, Tian W, Yue W, Liu
M, Li D, Wang B, Zhu Y, Cao C, Zhou J and Chen Q: Parkin
ubiquitinates Drp1 for proteasome-dependent degradation:
implication of dysregulated mitochondrial dynamics in Parkinson
disease. J Biol Chem. 286:11649–11658. 2011. View Article : Google Scholar
|
|
35
|
Harder Z, Zunino R and McBride H: Sumo1
conjugates mitochondrial substrates and participates in
mitochondrial fission. Curr Biol. 14:340–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Piret JP, Mottet D, Raes M and Michiels C:
CoCl2, a chemical inducer of hypoxia-inducible factor-1,
and hypoxia reduce apoptotic cell death in hepatoma cell line
HepG2. Ann NY Acad Sci. 973:443–447. 2002.PubMed/NCBI
|
|
37
|
Ma J, Zhang Q, Chen S, Fang B, Yang Q,
Chen C, Miele L, Sarkar FH, Xia J and Wang Z: Mitochondrial
dysfunction promotes breast cancer cell migration and invasion
through HIF1α accumulation via increased production of reactive
oxygen species. PLoS One. 8:e694852013.PubMed/NCBI
|
|
38
|
Tochhawng L, Deng S, Pervaiz S and Yap CT:
Redox regulation of cancer cell migration and invasion.
Mitochondrion. 13:246–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chiche J, Rouleau M, Gounon P, et al:
Hypoxic enlarged mitochondria protect cancer cells from apoptotic
stimuli. J Cell Physiol. 222:648–657. 2010.PubMed/NCBI
|