Introduction
Squamous cell carcinoma of head and neck (SCCHN), a
malignant tumor of epithelial origin, represents >90% of all
head and neck cancers (1). In
SCCHN, the 5-year survival rate is only 30–40%. The mechanisms
which lead to the high level of malignancy, particularly metastasis
to lymph node and low survival rate, are not completely understood,
despite substantial improvements in the diagnosis and local
management of, and chemotherapy for, SCCHN (2).
Chemokines are a superfamily of small
chemoattractant cytokines that mediate their effects by binding to
G-protein-coupled receptors. Chemokines are classified into 4
highly conserved groups: CXC, CC, C and CX3C, based on the position
of the first two cysteines adjacent to the amino terminus. The CC
chemokine receptor 7 (CCR7) plays a central role in regulating
migration of lymphocytes to lymph nodes, such as in mature
dendritic cells (DCs) (3), T and B
cells (4); flow of calcium ions,
changes of cytoskeletal structure, cell cycle and cell metabolism.
Since both metastasis and normal migration of leukocytes involve
site-directed movement across vascular barriers, non-small cell
lung cancer (5,6), gastric (7) and colorectal carcinoma (8) and SCCHN (1,2,9,10)
cells also use chemokine-mediated mechanisms during the metastatic
process (11). However, the
signaling mechanisms mediated by CCR7 and induced by CCL19 have yet
to be elucidated in SCCHN cells.
The matrix metalloproteinases (MMPs) are a family of
zinc-dependent proteases that are responsible for proteolytic
degradation of specific extracellular matrix (ECM) components
(12). MMP-9 is one of them, known
as gelatinase or type IV collagenase. They are able to degradate IV
collagen, which is associated with invasion and metastasis of
tumor. Many researchers report high expression of MMP-9 in several
types of migration and invasion cancers, such as SCCHN (13), prostate cancer (14), Hodgkin’s lymphoma (15), papillary thyroid carcinoma (16) and brain tumor (17). Thus, MMP-9 is considered the maker
of metastasis and invasion of SCCHN to malignant tumor.
Recently, a new role was described for the chemokine
family: signaling via chemokine receptors can modulate tumor cell
expression of MMP-9 which can then facilitate adhesion of cancer
cells to and/or invasion through ECM. CCR7-induced MMP-9 expression
is an important regulatory factor (18). The expression of MMP-9 was enhanced
in a variety of malignant tumors, cultured tumor cells and oncogene
transformed cells. In vitro migration assay confirmed that
the high invasive ability of tumor is associated with the high
expression of MMP-9 (19–21).
In the present study, we showed for the first time a
direct relationship between CCR7 and MMP-9 in SCCHN cells. We also
identified a novel mechanism for CCR7-induced invasion and
migration, mediated by regulating MMP-9 in SCCHN.
Materials and methods
SCC cell lines in the head and neck
Metastatic SCCHN cell line PCI-37B, which strongly
expresses CCR7, was supplied by the University of Pittsburgh Cancer
Institute, USA. PCI-37B were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA). DMEM was
supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA,
USA), 100 U/ml penicillin G and 100 U/ml streptomycin.
Antibodies and reagents
CCL19 and CCR7-specific monoclonal antibody (mouse
anti-human CCR7 antibody), MMP-2 and -9, bovine serum albumin (BSA)
and fibronectin (FN), were purchased from R&D Systems
(Minneapolis, MN, USA). SB-3CT (the inhibitor of MMP-9), and
dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO,
USA). Matrigel™ Basement Membrane Matrix was purchased from BD
Biosciences Pharmingen (Rockville, MD, USA).
Cell chemotaxis assay
The chemotaxis was assayed in Transwell filter
insert chambers (10-μm pore size; Corning Costar) as previously
described (25). CCL19 (final
density, 500 ng/ml) was placed in the lower wells. PCI-37B cells
treated with or without SB-3CT (inhibition of MMP-9 ) and anti-CCR7
mAb in different concentrations or for different times, at 37°C in
5% CO2, were removed from the culture flasks and added
to the upper chamber. After 24 h at 37°C, cells on the upper
surface of the Transwell membrane were wiped off gently with cotton
swabs. The lower surface of the filters was fixed with methanol and
stained with hematoxylin. Cells that migrated to the lower surface
were counted under a microscope (Nikon TE2000-S Eclipse; Nikon,
Tokyo, Japan) at ×200 magnification. For each experimental
condition, 4–5 wells were analyzed in parallel.
Cell migration assay
The methods were the same as for the chemotaxis
assay, the changes are cells were seeded onto the Matrigel-coated
filter and incubated in serum-free medium with 500 ng/ml CCL19 for
36 h. Non-invading cells on the upper side of the filters were
removed with a moistened cotton swab. Cells that penetrated the
membrane were fixed with ice-cold methanol, stained with 0.5%
crystal violet, photographed and counted under the microscope. To
assess cellular migration potential, the protocol described above
was used, except that Matrigel was omitted. For each experimental
condition, 4–5 wells were analyzed in parallel.
Western blotting
PCI-37B cells were treated with and without the
inhibitors SB-3CT and CCR7 mAb, followed by CCL19. Whole cells were
harvested in lysis buffer, containing sodium diphosphate, sodium
trivanadium oxygen, Tris hydrochloric acid, 1% Triton X-100,
protease and phosphatase inhibitors. Then, lysates were centrifuged
at 4°C, 14,000 rpm, for 30 min after sonication for 3 sec. Protein
concentration of the lysate was determined by Bio-Rad protein assay
dye reagent (Bio-Rad Laboratories, Richmond, CA, USA). Proteins
were size-fractionated on 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose
filters by semi-dry blotting. The filter was blocked in
phosphate-buffered saline (PBS) containing 1% skim milk, 0.1%
Triton X-100, sodium chloride and Tris [Tris
(hydroxymethyl)aminomethane] overnight at 4°C. The membrane was
incubated with 1/1,000 diluted rabbit antibody SB-3CT (inhibition
of MMP-9) for 30 min at room temperature, and incubated with
horseradish peroxidase-conjugated secondary antibodies (goat
anti-rabbit; Sigma). Immune complexes were visualized using
enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech,
Piscataway, NJ, USA). Quantification of the signals was carried out
by scanning densitometry using FluorChem software (version 2.0).
β-actin (1:1,000) served as the internal control. For each
experimental condition, 4–5 wells were analyzed in parallel.
Actin polymerization assay
PCI-37B cells pretreated with or without CCR7 mAb
and MMP-9 inhibitor SB-3CT were fixed, permeabilized and stained
with TRITC-labeled phalloidin. Following labeling, the samples were
washed three times for 10 min each in PBS to remove the
unincorporated label. F-actin distribution following CCL19
stimulation was evaluated by confocal laser scanning microscope
(CLSM Leica SP2, Germany).
Immunohistochemical analysis
Seventy-eight specimens of SCCHN tumors with the
adjacent metastatic (or normal) lymph nodes and 30 specimens of
normal human oral mucosal tissue were obtained from the Head and
Neck Tumor Center, School of Stomatology, China Medical University.
All the specimens were obtained with the consent of the patients
before surgery and in accordance with the Health Insurance
Portability. The classification of SCCHN, including primary tumors
(T), regional lymph nodes (N), distant metastasis (M) and stage
grouping, was determined according to the rules of the
International Union Against Cancer (UICC) for Head and Neck Cancer
[tumor-node-metastasis (TNM) classification, 1997).
Immunohistochemical staining used conventional horseradish
peroxidase immunohistochemical staining methods. In brief, 5-μm
sections of the specimens were deparaffinized and hydrated with
0.6% H2O2 in methanol to inhibit endogenous
peroxidase, antigen retrieval was performed and then incubated with
normal blocking serum for 10 min. Then, the sections were incubated
with primary antibodies (1:100):CCR7-specific monoclonal antibody
and MMP-9 overnight at 4°C. Immunodetection was performed using
peroxidase labeled secondary antibody (R&D Systems) and
diaminobenzidine for visualization. All sections were
counterstained with hematoxylin (Sigma). Negative controls included
omission of the primary antibody. The cell morphology was analyzed
by microscopy (Nikon Eclipse 80i; Nikon, Tokyo, Japan) at ×100–400
magnification. According to the percentage of positive tumor cells,
all cells were scored as negative (−, <10% or no staining); weak
positive (+, 11–50%); positive (++, 51–75%); or strongly positive
(+++, >75%).
Results
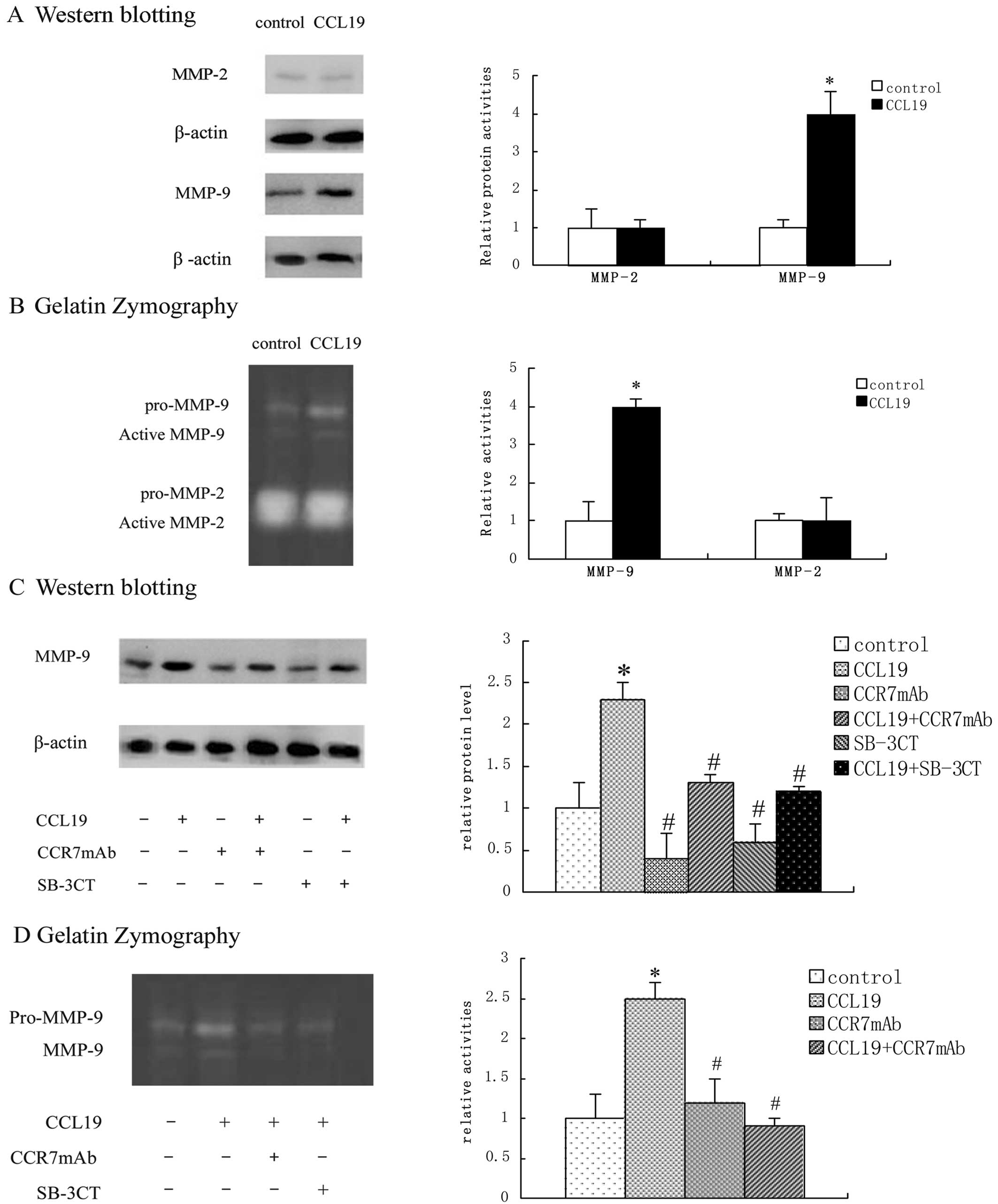
CCL19 induces MMP-9 high expression and
enhances its activity
MMP-2 and -9 are well-documented ECM-degrading
enzymes whose activities are associated with SCCHN tumor
invasiveness (8). To investigate
whether MMP-2 and -9 play a role in the CCL19-stimulated cell
invasion, MMP-2 and -9 protein and enzymatic activities were
measured by western blotting and gelatin zymography. As shown, the
expression and activity of MMP-2 were not significantly altered by
CCL19. In contrast to MMP-9, both expression and activity of MMP-9
were found to be markedly elevated after CCL19 treatment (Fig. 1A and B). Thus, we continued
researching MMP-9. We found that the expression and activity of
MMP-9 were not only elevated after CCL19 treatment but were also
diminished after CCR7 mAb treatment, which suggested that this was
induced by CCR7 activation. At the same time, the role of CCR7 in
MMP-9 activation was also blocked by SB-3CT indicating that CCR7
can activate MMP-9 (Fig. 1C and D).
The expression and the activation of MMP-9 were determined by
western blot analysis and gelatin zymography, quantified by
computer-aided densitometry.
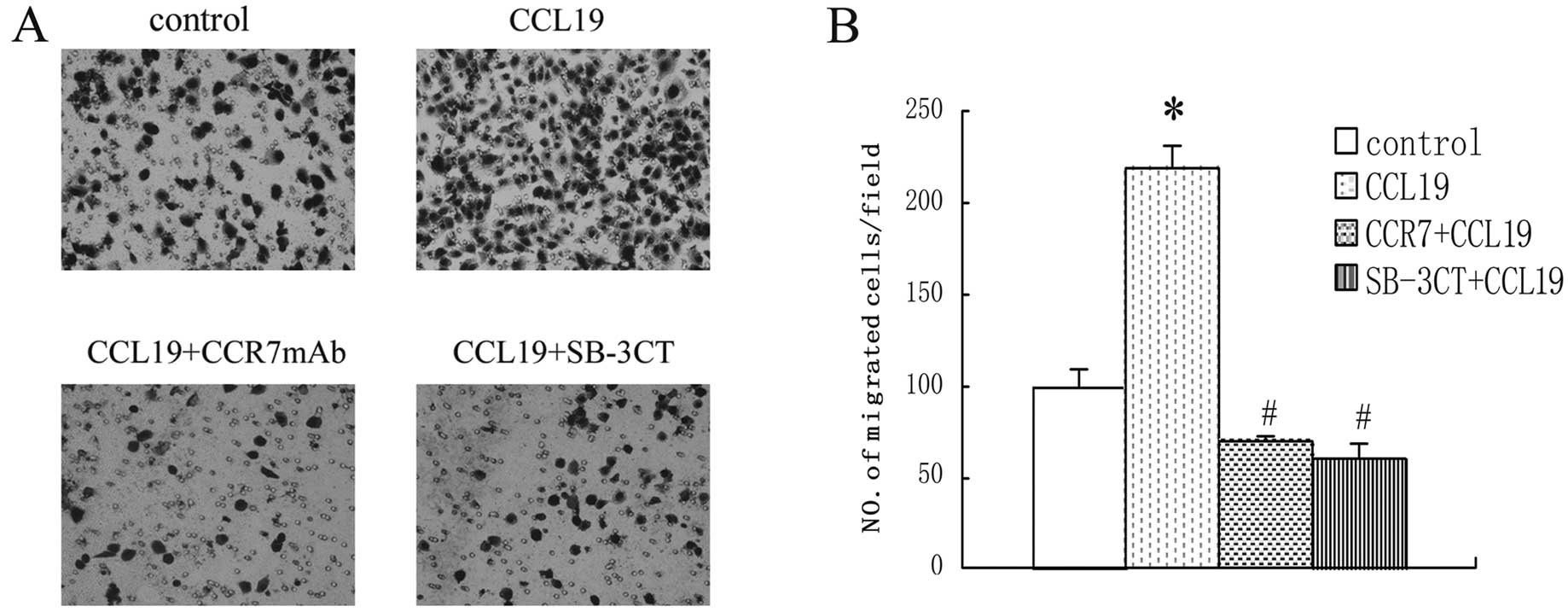
CCL19 induces the migration of PCI-37B
cells and SB-3CT blocks it
We treated the cells in the absence or presence of
SB-3CT, then conditioned medium of CCL19 was placed on the lower
part of a Transwell unit and PCI-37B cells were added to the upper
part in the absence or presence of SB-3CT. As shown in Fig. 2, CCL19 significantly enhanced the
migration ability of PCI-37B cells that were specifically blocked
by the SB-3CT. The inhibitive ability became stronger with the
SB-3CT increasing density.
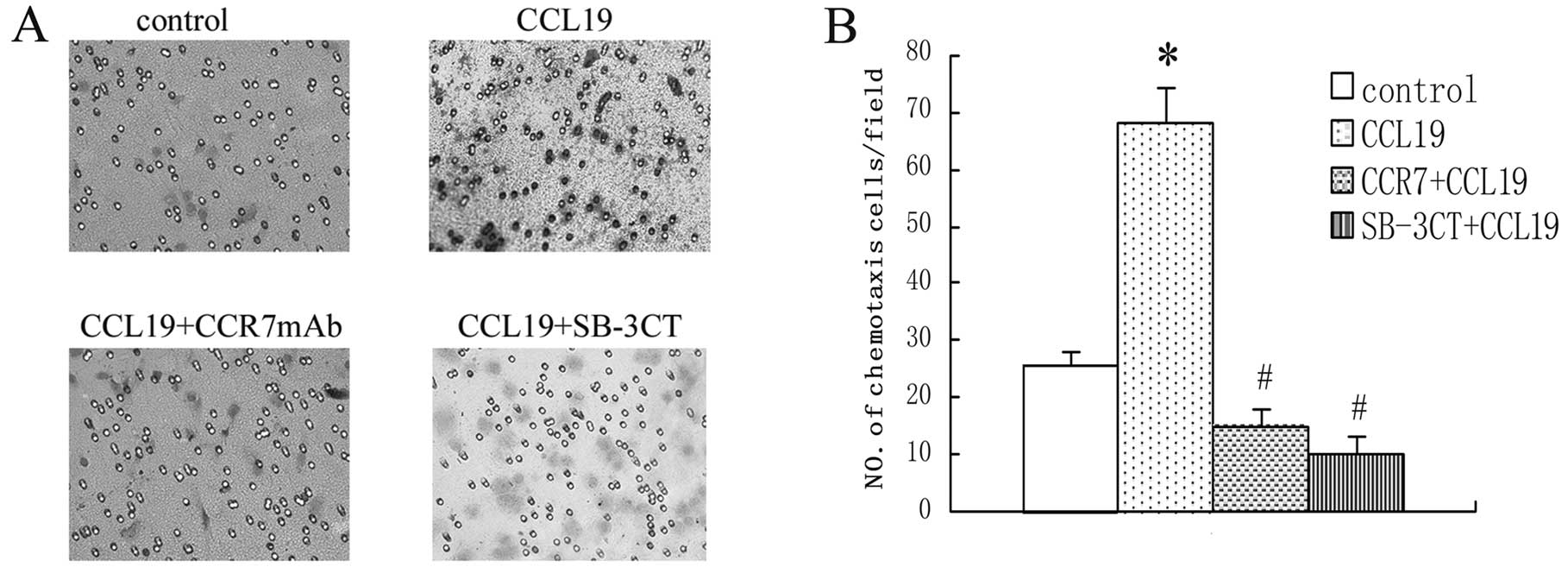
CCL19 activates the chemotaxis ability
and SB-3CT abolishes it in PCI-37B
To investigate the correlation between the
chemotaxis ability and the activities of CCR7 and MMP-9 in the
metastatic SCCHN cell line, we separated the cell line into many
teams and analyzed their chemotaxis ability in vitro in
response to the respective chemokine ligand CCL19, SB-3CT and CCR7
mAb. These experiments showed that CCL19 enhanced chemotaxis of
SCCHN significantly as compared with background control levels
established with media alone. The SB-3CT and anti-CCR7 mAb
significantly blocked CCL19-induced cell chemotaxis, as shown in
Fig. 3.
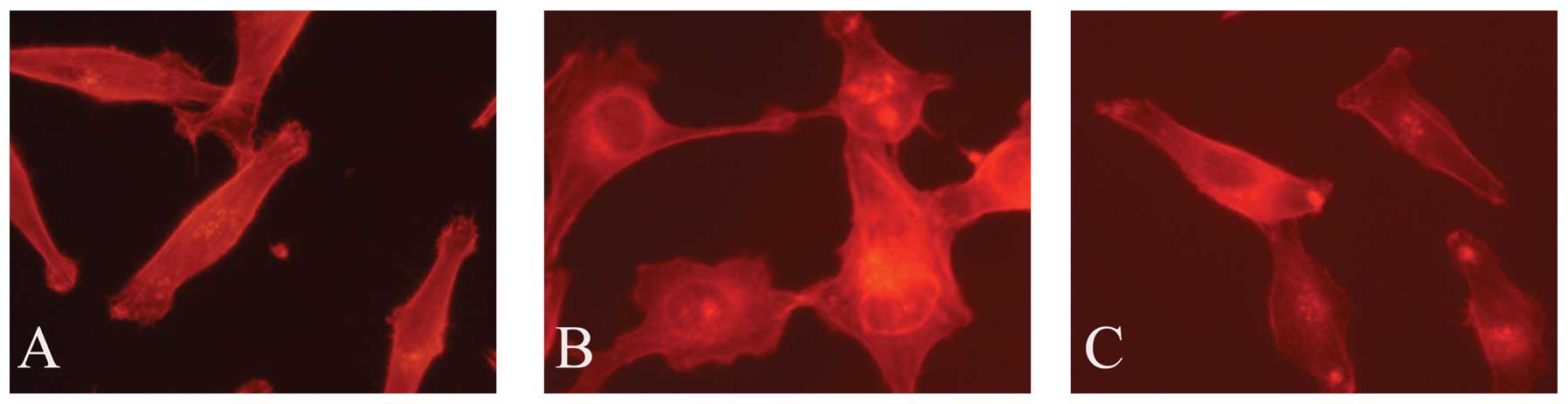
CCR7 induces F-actin rearrangement
Cell motility involves regulation of the actin
cytoskeleton and the actin-severing protein cofilin regulates actin
organization. We found that CCR7 activation leads F-actin
polymerization and pseudopodia formation. In untreated cells, we
observed a scattered distribution of F-actin (Fig. 5). In the cells treated with CCL19,
F-actin arrays and pseudopodia were formatted, while these effects
were blocked by SB-3CT. We therefore consider that the actin
cytoskeletal rearrangement induced by CCL19 requires MMP-9.
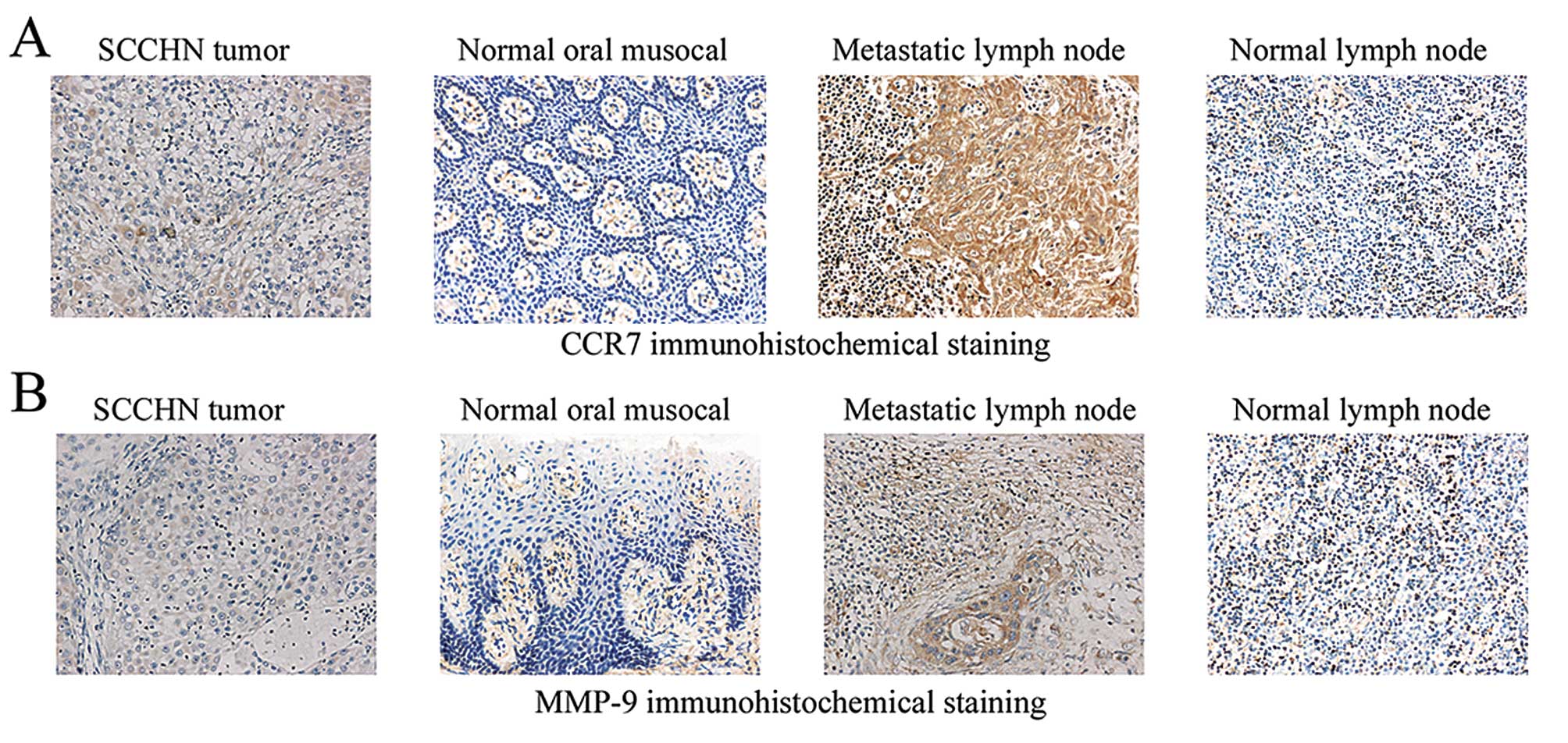
CCR7 and MMP-9 expressed by
immunohistochemical staining have significant positive correlation
in tumor tissues and metastatic lymph nodes
Using immunohistochemistry, we investigated the
location of CCR7 and MMP-9 in SCCHN tumor tissues, metastatic lymph
nodes, normal lymph nodes and oral mucosal tissues. CCR7 and MMP-9
were found in the cell membrane and cytoplasm, mainly expressed in
the surrounding of stroma in tumor cells and metastatic lymph node
cells. Analyzing the sections of CCR7 and MMP-9 staining in normal
lymph nodes and oral mucosal tissues, we observed that the number
of stained cells was low and that they were not expressed (Fig. 5 and Table I). The expression levels of CCR7 and
MMP-9 were both significantly correlated with cervical lymph node
metastasis and SCCHN clinical stage (P<0.05). In addition, the
T3/T4 tumor size also appeared to express high levels of MMP-9
(P<0.05). However, there were no significant differences between
CCR7 or MMP-9 expression and age or gender (P>0.05). A moderate
correlation was observed between CCR7 and MMP-9 expression in SCCHN
tumor tissues (P<0.05) and metastatic lymph nodes (P<0.05),
but there was no significant correlation between normal lymph nodes
(P>0.05) and normal oral mucosal tissues (P>0.05).
 | Table ICorrelations between MMP-9 expression
and clinicopathological factors of SCCHN. |
Table I
Correlations between MMP-9 expression
and clinicopathological factors of SCCHN.
| Clinicopathological
characteristics | No. of cases | CCR7 | Statistical
analysis | MMP-9 | Statistical
analysis |
|---|
|
|
|---|
| + - +++ | − | + - +++ | − |
|---|
| Age (years) |
| ≥60 | 40 | 28 | 12 |
χ2=0.023 | 21 | 19 |
χ2=0.060 |
| <60 | 38 | 26 | 12 | | 21 | 17 | |
| Gender |
| Male | 50 | 31 | 19 |
χ2=0.177 | 30 | 20 |
χ2=1.336 |
| Female | 28 | 16 | 12 | | 13 | 15 | |
| Tumor size |
| T1,T2 | 64 | 37 | 27 |
χ2=2.091 | 22 | 42 |
χ2=6.519a |
| T3,T4 | 14 | 11 | 3 | | 10 | 4 | |
| Clinical stage |
| I, II | 37 | 15 | 22 |
χ2=13.113a | 9 | 28 |
χ2=18.562a |
| III, IV | 41 | 33 | 8 | | 30 | 11 | |
| Nodal metastasis |
| Yes | 36 | 28 | 8 |
χ2=9.770a | 19 | 17 |
χ2=11.375a |
| No | 42 | 18 | 24 | | 7 | 35 | |
Discussion
CCR7 has been shown to interact with chemokines
(CCL19, CCL21) and to modulate tumor cell migration, invasion and
proliferation of metastatic squamous cell carcinoma of the head and
neck (SCCHN) (9,10,24).
However, the mechanisms of chemotaxis and migration and the
signaling pathway involved remain poorly understood. We
demonstrated that CCR7 regulates cell chemotaxis and migration via
MMP-9 in metastatic SCCHN.
Remodeling of the extracellular matrix (ECM), which
occurs during many physiological and pathological processes, is one
of the requisite events of cellular invasion. MMPs can degrade
almost all ECM proteins in the destruction of tumor cell invasion
(12). MMP-9 is a well-documented
ECM-degrading enzyme whose activities are associated with SCCHN
tumor invasion (26). Regulating
the activity of MMP-9 modulates the degradation of the ECM
components which in turn alter cellular invasion, expression and
activation.
It has been reported that CCR7 can regulate MMP-9 in
lung cancer cells, thus affecting the expression of tumor (18). Chemokine CXCL12, through its
specific receptor CXCR4, induced colon cancer metastasis of HT-29
cells by secretion of MMP-9 (22).
The interaction of CCL21/CCR7 enhances the expression and secretion
of MMP-9 in colon cancer, degradation of ECM and basement membrane,
thus promoting invasion and metastasis of colon cancer (23). We speculated that CCR7-induced MMP-9
expression is an important regulatory factor.
In the present study, our results (Fig. 1) showed that stimulation of CCL19
could also result in increased expression of MMP-9 in western blot
analysis, and the activity in gelatin zymography. Furthermore, we
used Transwell chemotaxis and migration assays to examine
CCL19-induced activation of MMP-9 that significantly enhanced the
chemotaxis (Fig. 3) and migration
(Fig. 2) index in SCCHN, and was
blocked by MMP-9 inhibitor SB-3CT, thus supporting the hypothesis
that CCL19 concentrations in the lymph nodes probably induce SCCHN
cell migration into these organs through a CCR7-mediated mechanism.
High levels of actin polymerization are required for the formation
of pseudopodia, which are needed for chemokine mediated cell
migration and invasion into surrounding tissues and efficient
metastasis formation (27). In the
present study (Fig. 4), we examined
TRITC-labeled phalloidin staining by inverted microscope, and
observed reorganization of the actin cytoskeleton of PCI-37B was
enhanced in response to CCL19, and this function was inhibited by
SB-3CT. Immunohistochemical studies confirmed the presence of CCR7
and MMP-9 in the cytoplasm and cell membrane of SCCHN tumor
tissues, metastatic lymph nodes, and all significantly correlated
with cervical lymph node metastasis and clinical stage, but in
normal lymph nodes and oral mucosal tissues they were low or
absent.
CCR7 has been reported to be a novel prediction
biomarker of metastasis in cancer. Our results showed that
stimulation of CCL19 could also result in increased chemotaxis and
migration of SCCHN cells. CCR7 induced the activation of MMP-9, and
MMP-9 interacted with its counterpart molecules. As has previously
been reported, we found that when MMP-9 was inhibited, the
CCL19-induced chemotaxis and migration of SCCHN cells were also
inhibited.
Future studies including the immunohistochemical
analysis of both CCR7 and MMP-9 may be useful for predicting lymph
node metastasis (23). Therefore,
we suggest that CCR7 activation by CCL19 via MMP-9 may promote
SCCHN cell chemotaxis, migration.
In summary, our findings emphasize the potential
role of overexpression of the CCR7 in promoting cellular migration
and matrix-degrading activities through MMP-9 in SCCHN cells. This
information provides a mechanistic rationale for the observed MMP-9
overexpression in advanced-stage SCCHN. To our knowledge, the
chemotaxis and migration of SCCHN are very complex systems. It is
therefore impossible to cure the tumor by blocking only this
pathway. However, it provides us with a novel idea for enhancing
the invasiveness of SCCHN and may contribute to the development of
improved and more specific therapeutics for the treatment of
SCCHN.
Acknowledgements
The authors acknowledge the University of Pittsburgh
Cancer Institute, USA, for supplying the cell line PCI-37B. This
study was supported by a grant from the National Natural Science
Foundation of China (no. 30672331), the Foundation of Education
Bureau of Liaoning Province, China (no. 2009A755) and the National
Science Foundation for Young Scholars of China (no. 81102058).
References
|
1
|
Yoon Y, Liang Z, Zhang X, Choe M, Zhu A,
Cho HT, Shin DM, Goodman MM, Chen ZG and Shim H: CXC chemokine
receptor-4 antagonist blocks both growth of primary tumor and
metastasis of head and neck cancer in xenograft mouse models.
Cancer Res. 67:7518–7524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Younes MN, Yigitbasi OG, Yazici YD, Jasser
SA, Bucana CD, El-Naggar AK, Mills GB and Myers JN: Effects of the
integrin-linked kinase inhibitor QLT0267 on squamous cell carcinoma
of the head and neck. Arch Otolaryngol Head Neck Surg. 133:15–23.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yanagawa Y and Onoé K: CCL19 induces rapid
dendritic extension of murine dendritic cells. Blood.
100:1948–1956. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagira M, Imai T, Yoshida R, Takagi S,
Iwasaki M, Baba M, Tabira Y, Akagi J, Nomiyama H and Yoshie O: A
lymphocyte-specific CC chemokine, secondary lymphoid tissue
chemokine (SLC), is a highly efficient chemoattractant for B cells
and activated T cells. Eur J Immunol. 28:1516–1523. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takanami I: Overexpression of CCR7 mRNA in
nonsmall cell lung cancer: correlation with lymph node metastasis.
Int J Cancer. 105:186–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zlotnik A: Chemokines and cancer. Int J
Cancer. 119:2026–2029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arigami T, Natsugoe S, Uenosono Y,
Yanagita S, Arima H, Hirata M, Ishigami S and Aikou T: CCR7 and
CXCR4 expression predicts lymph node status including
micrometastasis in gastric cancer. Int J Oncol. 35:19–24. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Günther K, Leier J, Henning G, Dimmler A,
Weissbach R, Hohenberger W and Förster R: Prediction of lymph node
metastasis in colorectal carcinoma by expression of chemokine
receptor CCR7. Int J Cancer. 116:726–733. 2005.PubMed/NCBI
|
|
9
|
Liu FY, Zhao ZJ, Li P, Ding X, Zong ZH and
Sun CF: Mammalian target of rapamycin (mTOR) is involved in the
survival of cells mediated by chemokine receptor 7 through PI3K/Akt
in metastatic squamous cell carcinoma of the head and neck. Br J
Oral Maxillofac Surg. 48:291–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao ZJ, Liu FY and Sun CF: Effect of
chemokine receptor 7 small interfering RNA on proliferation and
invasion of squamous cell carcinoma of head and neck. Zhonghua Kou
Qiang Yi Xue Za Zhi. 44:5–10. 2009.(In Chinese).
|
|
11
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL,
Mohar A, Verástegui E and Zlotnik A: Involvement of chemokine
receptors in breast cancer metastasis. Nature. 410:50–56.
2001.PubMed/NCBI
|
|
12
|
Corcoran ML, Hewitt RE, Kleiner DE Jr and
Stetler-Stevenson WG: MMP-2: expression, activation and inhibition.
Enzyme Protein. 49:7–19. 1996.
|
|
13
|
Hong SD, Hong SP, Lee JI and Lim CY:
Expression of matrix metalloproteinase-2 and -9 in oral squamous
cell carcinomas with regard to the metastatic potential. Oral
Oncol. 36:207–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du B, Wang P, Juo X and Du B: Expression
of membrane type 1-matrix metalloproteinase in laryngeal carcinoma.
Pathol Oncol Res. 5:214–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Flavell JR, Baumforth KR, Williams DM, et
al: Expression of the matrix metalloproteinase 9 in Hodgkin’s
disease is independent of EBV status. Mol Pathol. 53:145–149.
2000.
|
|
16
|
Maruyama S, Kawata R, Shimada T, et al:
Study of matrix metalloproteinase-2 and -9 in thyroid papillary
cancer. Nihon Jibiinkoka Gakkai Kaiho. 103:499–505. 2000.(In
Japanese).
|
|
17
|
Jäälinojä J, Herva R, Korpela M, et al:
Matrix metalloproteinase 2 (MMP-2) immunoreactive protein is
associated with poor grade and survival in brain neoplasms. J
Neurooncol. 46:81–90. 2000.PubMed/NCBI
|
|
18
|
Li Y, Liu W, Fang L, Nan J, Zhang Z and
Zhou Q: Chemokine receptor 7 induces metastasis of NSCLC via
upregulating MMP-9 expression. Zhongguo Fei Ai Za Zhi.
13:1016–1020. 2010.(In Chinese).
|
|
19
|
Sato H and Seiki M: Regulatory mechanism
of 92 kDa type IV collagenase gene expression which is associated
with invasiveness of tumor cells. Oncogene. 8:395–405.
1993.PubMed/NCBI
|
|
20
|
Strup-Perrot C, Vozenin-Brotons MC,
Vandamme M, et al: Expression and activation of MMP -2, -3, -9, -14
are induced in rat colon after abdominal X-irradiation. Scand J
Gastroenterol. 41:60–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogata Y, Matono K, Nakajima M, et al:
Efficacy of the MMP inhibitor MMI270 against lung metastasis
following removal of orthotopically transplanted human colon cancer
in rat. Int J Cancer. 118:215–221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brand S, Dambacher J, Beigel F, et al:
CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells
and modulate cancer cell migration, invasion and MMP-9 activation.
Exp Cell Res. 310:117–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun RH, Wang GB, Li J and Cui J: Role of
CCL21/CCR7 in invasion of colorectal carcinoma cell line SW480. Ai
Zheng. 28:708–713. 2009.(In Chinese).
|
|
24
|
Wang J, Zhang X, Thomas SM, Grandis JR,
Wells A, Chen ZG and Ferris RL: Chemokine receptor 7 activates
phosphoinositide-3 kinase-mediated invasive and prosurvival
pathways in head and neck cancer cells independent of EGFR.
Oncogene. 24:5897–5904. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou HY, Wan KF, Ip CK, Wong CK, Mak NK,
Lo KW and Wong AS: Hepatocyte growth factor enhances proteolysis
and invasiveness of human nasopharyngeal cancer cells through
activation of PI3K and JNK. FEBS Lett. 582:3415–3422. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurahara S, Shinohara M, lkebe T, et al:
Expression of MMPS, MT-MMP, and TIMPs in squamous cell carcinoma of
the oral cavity: correlations with tumor invasion and metastasis.
Head Neck. 21:627–638. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li P, Zhao ZJ, Liu FY, Sun LY, Ding X,
Zhang WZ, Shang DH and Sun CF: The chemokine receptor 7 regulates
cell adhesion and migration via β1 integrin in metastatic squamous
cell carcinoma of the head and neck. Oncol Rep. 24:989–995.
2010.
|