Introduction
The MAP kinase pathway has been known for a long
time to be involved in mediating cell proliferation in response to
growth signals. However, BRAF mutations were identified in melanoma
cell lines and primary tumour samples only 10 years ago using a
genome-wide screening method (1).
More than 75 somatic BRAF mutations have been described in melanoma
(2). Most of these mutations cause
uncontrolled activation of the RAF kinase and of the downstream
pathway, resulting in cell proliferation and survival.
This discovery has enhanced the research in
developing effective target therapies for metastatic melanoma. In a
phase III trial comparing vemurafenib to dacarbazine in 675
patients with metastatic melanoma previously untreated, the authors
showed an improvement in overall survival (OS) and progression-free
survival (PFS) in patients treated with the BRAF inhibitor
(3). More recently, another BRAF
inhibitor, dabrafenib, has also shown promising results for
metastatic melanoma including patients with brain metastases
(4,5). The combined treatment of dabrafenib
with a MEK inhibitor (trametinib) improves the PFS when compared to
dabrafenib alone (6).
However, the relationship between BRAF mutations and
the patient clinical profile is still under question. In a
meta-analysis published in 2011, the occurrence of the BRAF
mutation was associated with a histological superficial spreading
melanoma (SSM) subtype and was significantly higher in melanomas
located on the trunk than on the face or scalp (7). In another cohort study, a truncal or
lower extremity location of the melanoma, younger age and low solar
elastosis were independently associated with BRAF mutations
(8). A recent retrospective study
showed that BRAF mutation correlated with younger age, a higher
number of melanocytic naevi and a melanoma location in intermittent
UV-exposed skin (9). The link
between the BRAF mutation and a poorer outcome remains unclear with
contradictory results (10–15).
The aims of the present study were to define the
clinical profile of BRAF-mutated patients and to determine the
correlation between the BRAF mutation and survival.
Patients and methods
Patient selection
All consecutive inpatients with melanoma [American
Joint Committee on Cancer (AJCC) stage I, II, III or IV (16)] seen in our Unit between January 2011
and June 2012 were included. Mucous and ocular melanomas were not
excluded. Considering that melanoma is a cancer with a high risk of
recurrence, BRAF screening was systematically performed in the
primary melanoma, if available, and in the metastases at the
Department of Biochemistry. Informed consent was obtained from the
study participants who allowed access to their data for scientific
objectives, with approval by the local ethics committees. This
cohort study focused on clinical correlations and survival in the
whole population. Data concerning a part of this series (i.e. the
74 patients with multiple BRAF testing) have been published
elsewhere (17).
Data concerning patients were retrospectively
collected from clinical notes and the local database in regards to
gender, age, and for the primary melanoma: location,
histopathological subtype, Breslow thickness, ulceration; for lymph
node melanoma: type of sample (biopsy or lymph node excision),
number of invaded lymph nodes and capsular breaking; for all
melanomas: date of primary melanoma, date and location of
progression, treatments received, date of death. For patients with
unknown primary melanoma, the date of diagnosis was defined as the
date of the first biopsy confirming the presence of melanoma cells.
Concerning the location of the primary melanoma, two groups were
defined: the ‘sun-exposed’ location group (including face or scalp,
hands, forearms and legs) and the ‘non-sun-exposed’ location group
(including trunk, arms, thighs and feet). Progression of the
disease was defined as a change in melanoma stage, i.e. for primary
melanoma (stage I or II), appearance of a lymph node, cutaneous or
visceral metastasis and for stage III melanoma, recurrence of lymph
node or cutaneous metastasis or appearance of visceral metastasis
(stage IV). As the present study did not aim to consider the
therapeutic effects of the treatments, the progression of stage IV
patients as recently updated by the RECIST (Response Evaluation
Criteria in Solid Tumors) working group was not considered
(18). The OS was defined from the
date of primary melanoma diagnosis to the date of death.
Tumour samples
Serial sections were cut from each paraffin block
and placed on glass slides. The first section was stained for
histopathological examination. The 2–5 subsequent sections were
processed for DNA extraction. To enrich the analysed specimen with
tumour cells, tumour areas highlighted by a pathologist were
macrodissected.
DNA extraction
DNA was extracted after paraffin removal and
macrodissection using the Forensic kit and an iPrep system
according to the manufacturer’s recommendations (Invitrogen, Life
Technologies SAS, Villebon sur Yvette, France). The DNA
concentration was quantified by spectrophotometry (NanoDrop ND-100
instrument; Thermo Fisher Scientific, Waltham, MA, USA) and
normalised to 5 ng/μl.
Detection of BRAF V600 mutations
Detection of the most frequent BRAF mutations was
performed by allele-specific amplification as previously described
(19) with minor modifications. Two
forward primers with variations in their 3′ nucleotides to be
specific either for the wild-type (V600; AG GTGATTTTGGTCTAGCTACAGT)
or the mutated variant (600E; AGGTGATTTTGGTCTAGCTACAGA), and one
common reverse primer (AS; ATGGATCCAGACAACTGT TCAAAC) were
designed. The sequence-specific forward and reverse primers were
then combined in ‘Primer mix V’ (primers V600 and AS) and ‘Primer
mix E’ (primers 600E and AS). The amplification conditions were
optimised for the RotorGene 3000 instrument (Qiagen, Courtaboeuf,
Ozyme, Saint Quentin en Yvelines, France). For each sample, the Ct
value was determined for the V600 (control) PCR and for the 600E
(mutation-specific) PCR, and the difference was calculated (ΔCt).
The lower was the amount of mutated DNA in the sample, the higher
was the ΔCt value. Serial dilutions of mutant DNA (Colo205 cell
line) in a background of wt DNA allowed determination of a cut-off
value of 5. Thus, samples with ΔCt <5 were considered as
positive for the p.V600E mutation.
This assay can detect (but not distinguish) the
V600E, V600K and V600D mutations, but not the V600R mutation.
Therefore, each sample was further analysed by conventional Sanger
DNA sequencing using BRAF 15S (TCATAATGCT TGCTCTGATAGGA) and BRAF
15AS (GGCCAAAAATT TAATCAGTGGA) primers for both amplification and
sequencing.
Assay performances
Our assay sensitivity was determined using serial
dilutions of mutated DNA (Colo205 cell line) in wild-type human
genomic DNA (Invitrogen). The allele-specific amplification
sensitivity was 5% for the V600E mutation, and DNA sequencing
allowed detection of a BRAF V600 alteration when it was present in
at least 10% of cells. In light of these results and to avoid
false-negative results, we considered that a minimum of 10% of
tumour cells had to be present in the sample. If no alteration was
found in a sample presenting <10% of tumour cells, we concluded
that the test was not contributive.
Our laboratory has been involved in external quality
schemes organized in Western France in 2011 and more recently in
the National External Quality Control Scheme organized by the
French National Cancer Institute. We obtained the expected results
for all the samples tested using our procedures.
Statistical analysis
Overall and progression-free survivals were
determined for the 278 patients and the 91 primary melanomas. As
mucous melanomas are currently considered apart from the other
primary melanomas, they were not included in the statistical
analysis focusing on primary melanomas. Clinicopathological
characteristics were tested for their association with the BRAF
mutation using the Fisher’s exact test or Pearson’s χ2
test. Log-rank tests and Cox models were used for survival
analyses. As vemurafenib has been demonstrated to improve survival,
the survival analysis was completed with a censored analysis of the
patients treated with this drug at the first day of treatment. For
all analyses, two-tailed P<0.05 was set to indicate statistical
significance. Statistical analyses were performed using the R2.15.1
statistical software version.
Results
Patients
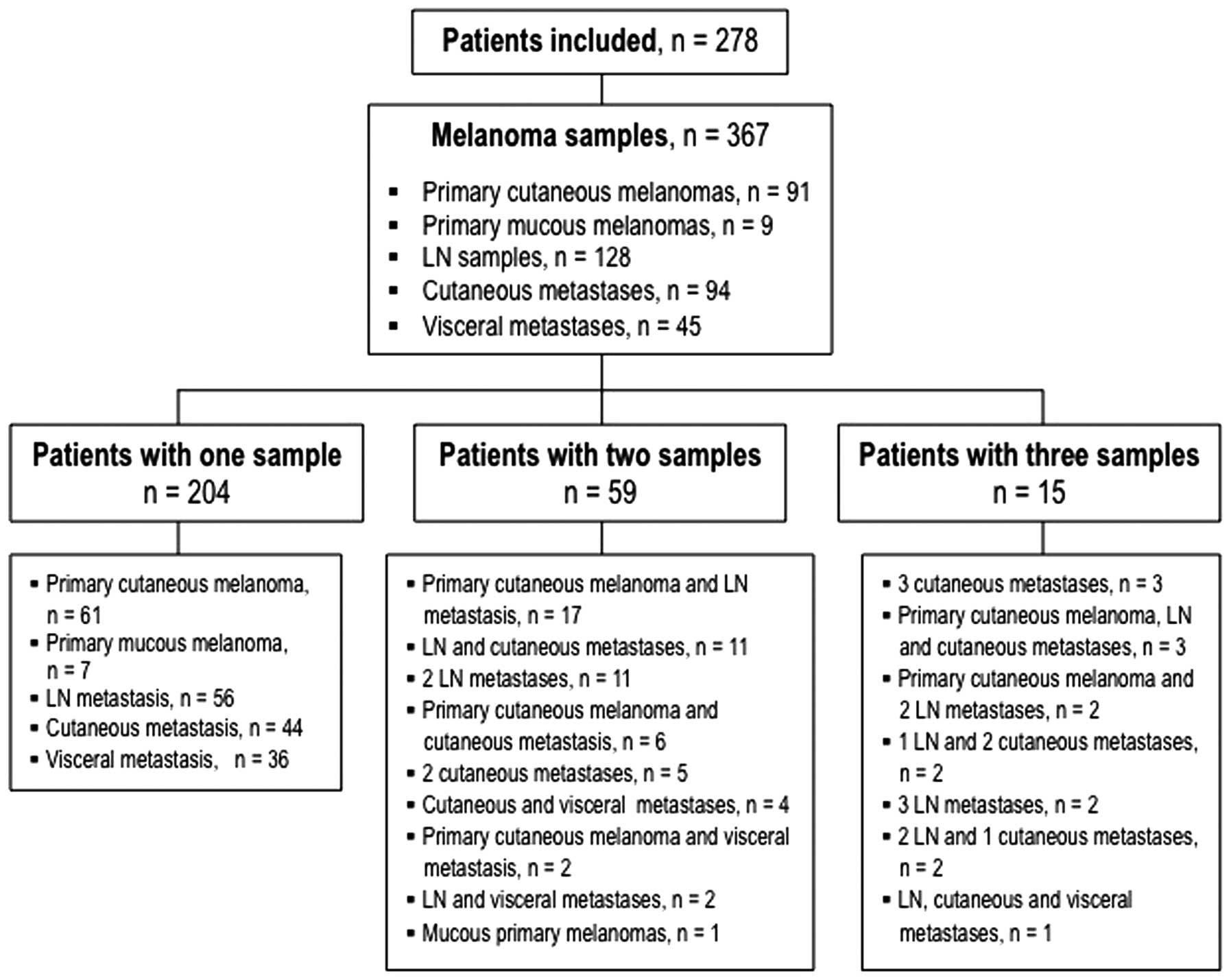
A total of 367 melanoma samples from 278 patients
(138 females and 140 males) were included (Fig. 1). Among the 367 samples, 91 were
primary cutaneous melanomas, 9 mucous melanomas, 128 lymph node
metastases, 94 cutaneous metastases and 45 visceral metastases. The
BRAF mutation was found in 152 samples (41.4%) corresponding to 114
patients (41%). Seven samples were V600K-mutated (2%), 2 were V600R
(0.6%) and all the others had the V600E mutation. The
characteristics of these 278 patients are summarised in Table I. BRAF-mutated patients were
significantly younger than the BRAF wild-type patients
(P<0.001). No difference was noted in regard to the gender
distribution between the BRAF-mutated and BRAF wild-type patients.
The median follow-up of all patients was 24.8 months, and the
median follow-up of patients with stage III was 14.5 months.
 | Table ICharacteristics of the 278 patients
tested for BRAF mutation. |
Table I
Characteristics of the 278 patients
tested for BRAF mutation.
| BRAF-mutated patients
(n=114) n (%) | BRAF-wild type
patients (n=164) n (%) | OR | 95% CI | P-value |
|---|
| Gender |
| Female | 51 (45) | 87 (53) | 1.4 | 0.83–2.32 | 0.182 |
| Male | 63 (55) | 77 (47) | | | |
| Age (years) |
| Mean age | 55.4 | 66.5 | | | <0.001 |
| <55 | 51 (45) | 36 (22) | 0.35 | 0.2–0.61 | <0.001 |
| ≥55 | 63 (55) | 128 (78) | | | |
In the overall population (all melanoma stages), the
DFS was not significantly different from the diagnosis of primary
melanoma to the first recurrence between BRAF-mutated and BRAF
wild-type patients (P=0.716) and in the multivariate analysis, no
variable (gender, age and treatments) was significantly different.
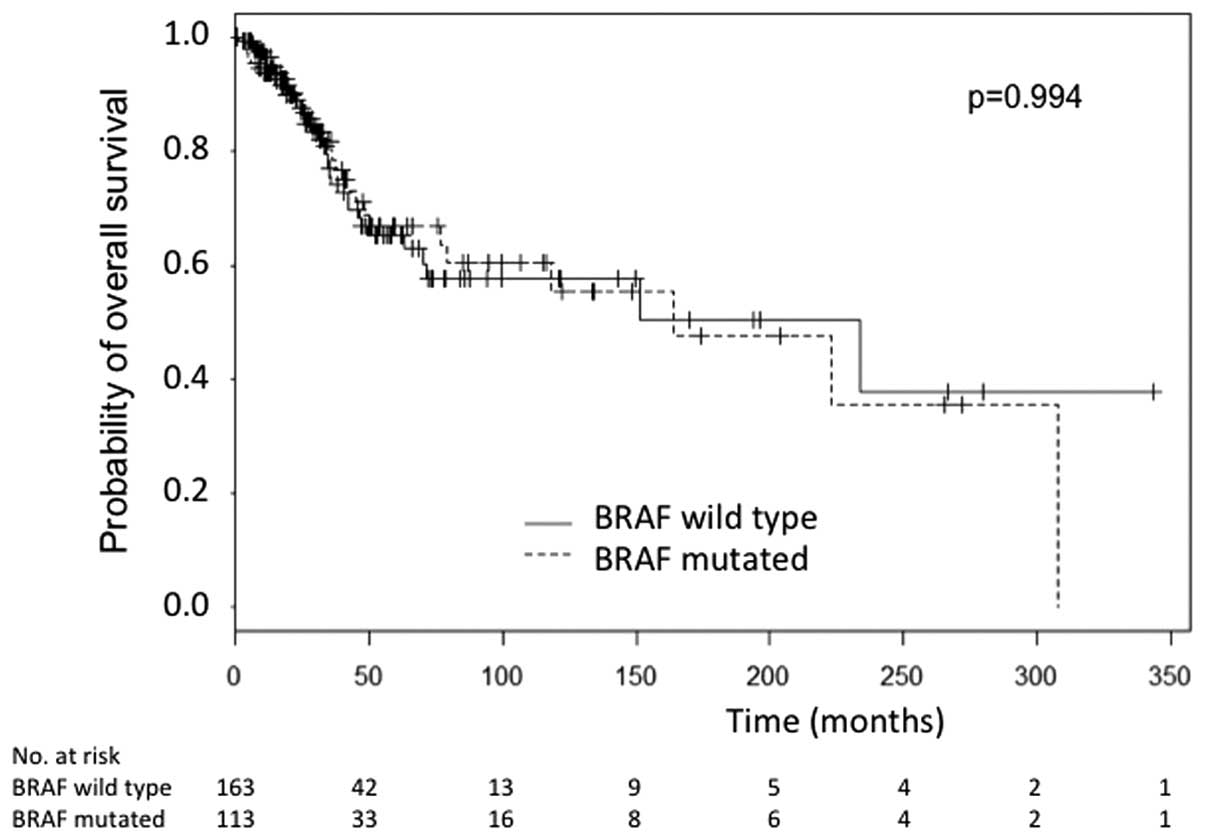
The OS was not significantly different between the BRAF-mutated and
BRAF wild-type patients (P=0.994) (Fig.
2). Forty-six patients received a BRAF inhibitor, 4 received a
MEK inhibitor, 31 received ipilimumab and 95 received chemotherapy.
After censoring patients treated with BRAF inhibitors, the OS and
DFS remained not significantly different (P=0.0502 and P=0.679,
respectively).
Discordant results
Among the 74 patients with multiple BRAF analyses
(59 patients with 2 samples and 15 with 3 samples), 10 had
discordant results in their BRAF status (8 patients with 2 samples
and 2 patients with 3 samples). Among these 10 patients, 1 had a
synchronous discordant result with a lymph-node metastasis BRAF
wild-type and a cutaneous metastasis V600E BRAF mutation (17).
Primary melanomas (AJCC 2009 stage I and
II)
A total of 91 primary melanomas were screened for
their BRAF status (Table II). No
patient had two primary melanomas. For three samples, the diagnosis
was confirmed with a skin biopsy, with no histopathological subtype
and Breslow thickness data.
 | Table IIClinical and histopathological
characteristics of the 91 cutaneous primary melanoma samples tested
for BRAF mutation. |
Table II
Clinical and histopathological
characteristics of the 91 cutaneous primary melanoma samples tested
for BRAF mutation.
| BRAF-mutated samples
(n=37) n (%) | BRAF-wild-type
samples (n=54) n (%) | OR | 95% CI | P-value |
|---|
| Location of primary
melanoma |
| Face and scalp | 4 (11) | 15 (28) | | | 0.0668 |
| Upper
extremities | 6 (16) | 11 (20.5) | | | 0.7855 |
| Trunk | 14 (38) | 10 (18.5) | | | 0.053 |
| Lower
extremities | 13 (35) | 18 (33) | | | 1 |
| Age at diagnosis of
primary melanoma |
| Mean age
(years) | 53.78 | 68.14 | | | <0.001 |
| UV-exposed location
of primary melanoma |
| Yes | 10 (27) | 29 (54) | 0.3 | 0.1–0.8 | 0.0099 |
| No | 27 (73) | 25 (46) | | | |
| Ulceration |
| Absent | 19 (51) | 18 (34) | | | |
| Present | 15 (41) | 25 (46) | | | 0.256 |
| Unknown | 3 (8) | 11 (20) | | | |
| Histological
subtype |
| SSM | 20 (54) | 13 (24) | | | 0.005a |
| Nodular | 9 (24) | 15 (28) | | | 0.63b |
| Acral
lentiginous | 3 (8) | 11 (20.5) | | | |
| Lentigo
maligna | 0 (0) | 3 (5.5) | | | |
| Desmoplastic | 0 (0) | 2 (3.5) | | | |
| Unknown | 5 (14) | 10 (18.5) | | | |
| Breslow thickness
(mm) |
| Mean
thickness | 3.3 | 4.74 | | | 0.0399 |
| <1.5 | 4 (11) | 4 (7) | | | |
| ≥1.5 | 31 (84) | 46 (86) | | | 0.71 |
| Unknown | 2 (5) | 4 (7) | | | |
| AJCC stage |
| I | 16 (43) | 8 (14) | | | |
| II | 20 (54) | 44 (82.5 | 0.2 | 0.1–0.7 | 0.0036 |
| Unknown (diagnosis
on biopsy) | 1 (3) | 2 (3.5) | | | |
| Progression |
| To stage III | 13 (35) | 19 (35) | | | 0.4479 |
| To stage IV | 5 (14) | 3 (5.5) | | | |
| No
progression | 19 (51) | 32 (59.5) | | | |
Patients with a BRAF mutation were significantly
younger at the time of diagnosis (mean age, 53.78 vs. 68.14 years;
P<0.001). BRAF-mutated melanomas were significantly more often
located on a non-UV exposed area (P=0.0099), had a thinner Breslow
thickness (P=0.0399) and were significantly more often of SSM
subtype (P=0.005). The location of the primary melanoma on the
trunk almost reached significance for BRAF-mutated melanomas (38
vs. 18.5%; P=0.053). In addition, no significant difference was
found in regards to the DFS between mutated and non-mutated primary
melanomas (P=0.84). Among the prognostic factors in the univariate
analysis, the SSM subtype (P=0.15), thinner Breslow thickness
(P<0.001), the absence of ulceration (P=0.0016) and AJCC stage I
(P=0.001) correlated with a better DFS. The OS was not
significantly different between the primary mutated and the
wild-type melanomas (P=0.96) whereas a better OS was correlated
with the absence of ulceration (P=0.012) and the histological
subtype (P<0.001).
Invaded locoregional lymph nodes (AJCC
2009 stage IIIB and IIIC)
One hundred and twenty-eight macroscopic lymph node
samples were available (Table
III). No difference in the BRAF status was noted between the
number of invaded lymph nodes and the presence or absence of
capsular breaking. The mean disease-free interval (DFI) between the
primary melanoma and the lymph node metastasis was not different
between the mutated and non-mutated patients (37.2 and 31.7 months,
respectively; P=0.60).
 | Table IIICharacteristics of the 128 lymph node
samples tested for BRAF mutation. |
Table III
Characteristics of the 128 lymph node
samples tested for BRAF mutation.
| BRAF-mutated LN
samples (n=58) n (%) | BRAF-wild-type LN
samples (n=70) n (%) | OR | 95% CI | P-value |
|---|
| Type of sample |
| LN biopsy | 28 (48) | 27 (39) | | | |
| LN dissection | 29 (50) | 42 (60) | 0.67 | 0.3–1.4 | 0.2833 |
| NA | 1 (2) | 1 (1) | | | |
| Number of LN
invaded (for LN dissection) | 29 | 42 | | | |
| 1 | 10 (34) | 22 (52) | | | |
| >1 | 19 (66) | 17 (40) | | | 0.8028 |
| NA | 0 (0) | 3 (8) | | | |
| Capsular breaking
(for LN dissection) | 29 | 42 | | | |
| Yes | 17 (59) | 22 (52) | | | |
| No | 11 (38) | 15 (36) | 1.05 | 0.3–3.3 | 1 |
| NA | 1 (3) | 5 (12) | | | |
Cutaneous and visceral metastases (AJCC
2009 stage IV)
BRAF screening was performed in 94 cutaneous
metastases and 45 visceral metastases (21 lung biopsies, 12 liver
samples, 5 intestine samples, 2 bone biopsies and 1 cerebellum
sample). Patients with BRAF-mutated metastases were significantly
younger at diagnosis of the first metastasis (P<0.001). The BRAF
mutation was found more frequently in visceral than in cutaneous
metastases. The mean DFI between the primary and visceral or
cutaneous metastases was not significantly different depending on
the BRAF status (P=0.58).
Discussion
To the best of our knowledge, this is the largest
study of BRAF screening in primary and metastatic melanomas with a
follow-up until advanced stages. We included 278 European patients
with 367 melanoma samples. The BRAF mutation was found in 41.4% of
samples, a result similar to the proportion of BRAF mutations
reported by Lee et al (7) in
the largest meta-analysis performed to date.
Four retrospective cohort studies of BRAF screening
in melanoma patients have previously been reported (8–10,20).
Our study has some critical strengths and originalities: a high
number of patients, a significant proportion of patients with
several samples (74/278, 27%), patients with melanoma of all stages
(from AJCC I to IV) and a correlation with the classical prognostic
factors in melanoma (including Breslow thickness and ulceration).
Moreover, we determined the BRAF status for each patient using the
primary melanoma itself, permitting the confirmation of a direct
correlation between BRAF status and the clinicopathological type of
the primary melanoma. Indeed, in other previous studies, the BRAF
status used for determining this correlation was established using
a metastasis sample [88% of the cases in the study by Long et
al (10)].
BRAF-mutated patients were significantly younger at
the time of diagnosis of the primary melanoma and metastasis. In
the meta-analysis published in 2011, the age was not identified as
a factor linked to the BRAF status but patients were separated into
two groups (older or younger than 50 years of age) (7). However, many other publications have
since reported a link between a younger age of patients at
diagnosis of the primary melanoma and the BRAF mutation (8,10) as
well as in metastatic patients (20). According to our results and as
suggested in a previous report by Viros et al (11), the correct ‘cut-off age’ appears to
be 55 years. Moreover, there was no link between the occurrence of
the BRAF mutation and gender, which is in accordance with other
previously published results (7,9,10).
According to our results, BRAF-mutated patients had
the same OS and DFS than the BRAF wild-type patients. Concerning
the survival analysis, two points must be considered. First of all,
it is noteworthy to remember that our population was enrolled
before the authorization of the temporary use of vemurafenib in
France; thus, the entire population of BRAF-mutated patients
included in the present study did not receive this drug. Moreover,
to avoid the bias of vemurafenib effect on survival, treated
patients were censored at the first day of treatment, which did not
change the results of the survival analysis. The second aspect to
consider is the retrospective design of the study that selected
long survivors, i.e. patients with a better prognosis. We completed
with a stratified analysis depending on the date of the melanoma
sample (before or after January 1 2011), but the OS and DFS between
BRAF-mutated patients and BRAF wild-type patients remained not
significantly different.
In the present study, 91 primary melanoma samples
were included to directly correlate the patient clinicopathological
characteristics with the BRAF status of the primary melanoma while
other more advanced melanoma samples (lymph nodes or metastases)
were not considered, contrary to all the other studies published to
date. Thus, the present study rules out the potential bias observed
in previous studies when considering the BRAF status of metastases.
We demonstrated that 13.5% of our patients had discordant results
for the BRAF mutation between primary and metastatic biopsies. This
could explain the differences between our results and those
previously published.
The present study suggests that BRAF-mutated primary
melanomas are more often located on non-sun-exposed locations
(corresponding to trunk, arms, thighs and feet), are more likely of
SSM subtype with a thin Breslow thickness, but are not linked to
ulceration. These results are in agreement with previous
publications (7,8). The location of BRAF-mutated primary
melanomas on the trunk almost reached significance, maybe due to a
lack of statistical strength. BRAF-mutated patients were younger at
the time of the first metastasis. However, the presence of a BRAF
mutation in the primary melanoma was not associated with a worse
survival or a more rapid progression. Five previous studies showed
no link between the presence of the BRAF mutation in primary
melanomas and prognosis (9,12,13,15,21).
Concerning stage III, we studied the BRAF status of
128 macroscopic lymph node samples, which represents the largest
study of BRAF screening on melanoma-invaded lymph nodes reported.
No correlation was found between BRAF status and tumour burden
represented by the number of invaded lymph nodes which is in
accordance with the literature (22). Furthermore, the rapidness of lymph
node relapse was not dependent of the BRAF status. This has been
reported for the interval between primary melanoma and distant
metastasis but never between primary melanoma and lymph node
metastasis (10).
Concerning the metastatic stage, the BRAF mutation
was more frequent in visceral than in cutaneous metastases. This
shows the importance of verifying the BRAF status in a visceral
metastasis when negative results are obtained in a cutaneous
metastasis for a given patient. We confirmed that the DFI between
the primary and visceral metastases (including cutaneous) was
similar in mutated and non-mutated patients as already reported by
Long et al (10).
Another strength of the present study was the
significant proportion of patients with several samples (74/278,
27%), allowing a comparison of the evolution of the BRAF status for
a given patient with disease progression. Notably, discrepancies
were found in the mutation pattern among multiple samples in 10
patients (13.5%), similarly to what was previously observed by
Colombino et al (23). We
noted for the first time that this discrepancy can be observed in 2
different synchronous metastases in a same patient. Our hypothesis
is based on the fact that different melanoma cell sub-clones can be
present in the same tumour (24–26).
This reinforces the importance of determining the BRAF status of a
patient with metastatic melanoma at each step of disease
progression.
In conclusion, we report here data on the BRAF
mutation status in a large cohort of 278 European patients, and for
the first time on the evolution of the BRAF status in a given
patient from the primary to the metastatic stage, highlighting
discrepancies according to stage. From our data, we conclude that
BRAF-mutated patients are younger at the time of the primary
melanoma and the first diagnosis of metastasis but these patients
have the same OS than BRAF wild-type patients.
Acknowledgements
The authors gratefully acknowledge Aurore Foureau
(CENGEPS network) for her helpful assistance in collecting the data
and the pathologists from the Department of Pathology, Nantes
University Hospital, for their contribution to the sample
preparation prior to molecular testing. The Department of
Biochemistry is designated and funded by the French National Cancer
Institute (INCa) to perform BRAF testing of melanoma patients.
Abbreviations:
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
DFI
|
disease-free interval
|
References
|
1
|
Davies H, Bignell GR, Cox C, et al:
Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002.
|
|
2
|
Arkenau HT, Kefford R and Long GV:
Targeting BRAF for patients with melanoma. Br J Cancer.
104:392–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chapman PB, Hauschild A, Robert C, et al:
Improved survival with vemurafenib in melanoma with BRAF V600E
mutation. N Engl J Med. 364:2507–2516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hauschild A, Grob JJ, Demidov LV, et al:
Dabrafenib in BRAF-mutated metastatic melanoma: a
multicentre, open-label, phase 3 randomised controlled trial.
Lancet. 380:358–365. 2012.PubMed/NCBI
|
|
5
|
Long GV, Trefzer U, Davies MA, et al:
Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant
melanoma metastatic to the brain (BREAK-MB): a multicentre,
open-label, phase 2 trial. Lancet Oncol. 13:1087–1095. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flaherty KT, Infante JR, Daud A, et al:
Combined BRAF and MEK inhibition in melanoma with BRAF V600
mutations. N Engl J Med. 367:1694–1703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JH, Choi JW and Kim YS: Frequencies of
BRAF and NRAS mutations are different in histological
types and sites of origin of cutaneous melanoma: a meta-analysis.
Br J Dermatol. 164:776–784. 2011.
|
|
8
|
Bauer J, Buttner P, Murali R, et al:
BRAF mutations in cutaneous melanoma are independently
associated with age, anatomic site of the primary tumor, and the
degree of solar elastosis at the primary tumor site. Pigment Cell
Melanoma Res. 24:345–351. 2011. View Article : Google Scholar
|
|
9
|
Schlaak M, Bajah A, Podewski T, et al:
Assessment of clinical parameters associated with mutational status
in metastatic malignant melanoma: a single-centre investigation of
141 patients. Br J Dermatol. 168:708–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Long GV, Menzies AM, Nagrial AM, et al:
Prognostic and clinicopathologic associations of oncogenic
BRAF in metastatic melanoma. J Clin Oncol. 29:1239–1246.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Viros A, Fridlyand J, Bauer J,
Lasithiotakis K, Garbe C, Pinkel D and Bastian BC: Improving
melanoma classification by integrating genetic and morphologic
features. PLoS Med. 5:e1202008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Houben R, Becker JC, Kappel A, Terheyden
P, Brocker EB, Goetz R and Rapp UR: Constitutive activation of the
Ras-Raf signaling pathway in metastatic melanoma is associated with
poor prognosis. J Carcinog. 3:62004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ellerhorst JA, Greene VR, Ekmekcioglu S,
et al: Clinical correlates of NRAS and BRAF mutations
in primary human melanoma. Clin Cancer Res. 17:229–235. 2011.
|
|
14
|
Safaee Ardekani G, Jafarnejad SM, Tan L,
Saeedi A and Li G: The prognostic value of BRAF mutation in
colorectal cancer and melanoma: a systematic review and
meta-analysis. PLoS One. 7:e470542012.
|
|
15
|
Nagore E, Requena C, Traves V, Guillen C,
Hayward NK, Whiteman DC and Hacker E: Prognostic value of BRAF
mutations in localized cutaneous melanoma. J Am Acad Dermatol. Jan
2–2014.(Epub ahead of print). View Article : Google Scholar
|
|
16
|
Balch CM, Gershenwald JE, Soong SJ, et al:
Final version of 2009 AJCC melanoma staging and classification. J
Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saint-Jean M, Quereux G, Nguyen JM, et al:
Is a single BRAF wild-type test sufficient to exclude melanoma
patients from vemurafenib therapy? J Invest Dermatol.
134:1468–1470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
19
|
Jarry A, Masson D, Cassagnau E, Parois S,
Laboisse C and Denis MG: Real-time allele-specific amplification
for sensitive detection of the BRAF mutation V600E. Mol Cell
Probes. 18:349–352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Menzies AM, Haydu LE, Visintin L, et al:
Distinguishing clinicopathologic features of patients with V600E
and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res.
18:3242–3249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shinozaki M, Fujimoto A, Morton DL and
Hoon DS: Incidence of BRAF oncogene mutation and clinical
relevance for primary cutaneous melanomas. Clin Cancer Res.
10:1753–1757. 2004.
|
|
22
|
Moreau S, Saiag P, Aegerter P, et al:
Prognostic value of BRAFV600 mutations in
melanoma patients after resection of metastatic lymph nodes. Ann
Surg Oncol. 19:4314–4321. 2012.
|
|
23
|
Colombino M, Capone M, Lissia A, et al:
BRAF/NRAS mutation frequencies among primary tumors and
metastases in patients with melanoma. J Clin Oncol. 30:2522–2529.
2012. View Article : Google Scholar
|
|
24
|
Lin J, Goto Y, Murata H, Sakaizawa K,
Uchiyama A, Saida T and Takata M: Polyclonality of BRAF mutations
in primary melanoma and the selection of mutant alleles during
progression. Br J Cancer. 104:464–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilmott JS, Tembe V, Howle JR, et al:
Intratumoral molecular heterogeneity in a BRAF-mutant, BRAF
inhibitor-resistant melanoma: a case illustrating the challenges
for personalized medicine. Mol Cancer Ther. 11:2704–2708. 2012.
|
|
26
|
Yancovitz M, Litterman A, Yoon J, et al:
Intra- and inter-tumor heterogeneity of
BRAFV600E mutations in primary and
metastatic melanoma. PLoS One. 7:e293362012. View Article : Google Scholar : PubMed/NCBI
|