Introduction
Heat shock protein 20 (HSPB6) is a member of the
small HSP family (HSPB) and is ubiquitously expressed in many
tissues including liver (1,2). HSP20 has a variety of functions in
addition to a molecular chaperoning function. We previously showed
that HSP20 acts as an extracellular inhibitor of human platelet
aggregation induced by thrombin or botrocetin (3,4).
Additionally, it has been reported that HSP20 acts in processes
ranging from insulin resistance to prevention of vasospasms, to
airway smooth muscle relaxation, and it has also been demonstrated
to have a protective function in the heart (5–8).
However, the exact roles of HSP20 (HSPB6) remain to be
elucidated.
Human hepatocellular carcinoma (HCC) is the fifth
most common cancer worldwide, and is the third leading cause of
cancer-related mortality (9). Even
after resection of the primary HCC, recurrence frequently develops.
The survival rate of HCC is 30–40% at five years post-surgery. A
significant number of the molecular events altered in HCC
progression, compromise the balance between survival and apoptotic
signals in the tumor cells. We previously reported that HSP20
protein levels in HCC inversely correlate with the TNM stage
(10). In our previous studies on
HCC (11,12), we demonstrated that the HSP20
protein directly interacts with phosphoinositide 3-kinase (PI3K)
which activates AKT, a major mediator of cell survival, and
suppresses its activity resulting in reducing the cell
proliferation (11,12).
Accumulating evidence suggests that apoptosis is
important in hepatocarcinogenesis, from the initial genotoxic
insult (initiation), through the clonal expansion from a
premalignant to a tumorous lesion (promotion) and finally to the
progression of tumor cell growth by further clonal expansion
(13). Caspases, a family of
cysteine proteases, are central regulators of apoptosis (14). Caspases hydrolyze peptide bonds
after certain aspartic acid residues in the substrate. Caspases are
initially produced as inactive form, procaspases, and require
cleavage for activation. Caspase-3 has a critical role for
apoptosis, and subsequently the activated caspase-3 cleaves many
key proteins, such as the nuclear enzyme poly (ADP-ribose)
polymerase (PARP) (15). Since PARP
is involved in DNA repair and helps cells to maintain their
viability, the cleavage of PARP leads to apoptosis (15,16).
Upstream of the caspase pathways, mitochondria play a pivotal role
in apoptosis, inducing cytochrome c release, which
subsequently activates caspases (13). In the mitochondrial-mediated
regulation of apoptosis, particularly the Bcl-2 family of proteins,
which include the members of both pro- and anti-apoptotic effects,
act as important regulators (17).
The balance between pro- and anti-apoptogenic Bcl-2 family member
activities and their interactions plays central roles in the
mitochondrial-mediated apoptosis pathway. In response to
mitochondrial pathway stimulation, processing of caspases is
induced. An imbalance in the pro- and anti-apoptotic members of the
Bcl-2 family has been observed in HCC (17). Bcl-xL is overexpressed, whereas
pro-apoptotic members of the family, such as Bax, are downregulated
in HCC (17). However, the
relationships between HSP20 and apoptosis in HCC remain to be
elucidated. The aim of the present study was to clarify the effect
of HSP20 protein expression on apoptosis in human HCC. We herein
demonstrated that HSP20 directly interacts with Bax and activates
caspase cascade in human HCC cells.
Materials and methods
Materials
HSP20 antibodies were purchased from Enzo Life
Sciences Inc. (Farmingdale, NY, USA). Antibodies against caspase-3,
cleaved caspase-3, caspase-7, cleaved caspase-7, cleaved PARP, Bad,
Bcl-2, Bcl-xL, Bax and peroxidase-conjugated anti-rabbit-IgG
(conformation specific) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies
and normal rabbit IgG were purchased from Santa Cruz Biotechnology
Inc. (Santa Cruz, CA, USA). Wild-type human HSP20 cDNA (clone ID
6074542), which was obtained from Open Biosystems, Inc.
(Huntsville, AL, USA), was subcloned into the eukaryotic expression
vector, pcDNA 3.1(+), as previously described (11). The eukaryotic expression vector,
pcDNA 3.1(+) and Dynabeads protein A were purchased from Life
Technologies Corp. (Carlsbad, CA, USA). The BCA protein assay kit
was obtained from Thermo Fisher Scientific Inc. (Waltham, MA,
USA).
Cell culture
Human HCC-derived HuH7 cells were obtained from the
Health Science Research Resources Bank (Tokyo, Japan). The HuH7
cells were maintained in RPMI-1640 medium (Sigma-Aldrich Corp., St.
Louis, MO, USA) supplemented with 1% fetal calf serum (FCS; Hyclone
Corp., Logan, UT, USA).
Stable transfections
To analyze caspase activity, the stably
HSP20-overexpressing HuH7 cells and the control empty
vector-transfected HuH7 cells were used. These cells were
established as described previously, by means of Tet-Off™ gene
expression systems (Clontech Laboratories Inc., Palo Alto, CA, USA)
according to the manufacturer’s instructions (11). Induction of HSP20 protein expression
in the HSP20-overexpressing cells can be controlled by the presence
of doxycycline (Sigma-Aldrich). The HSP20-overexpressing cells and
the control cells were maintained in RPMI-1640 supplemented with 1%
FCS, 200 μg/ml G418 (Invitrogen), 100 μg/ml hygromycin B (Merck
KGaA, Darmstadt, Germany) and 1 μg/ml doxycycline. For western
blotting, both cells were cultured under serum-starvation for the
indicated days.
Transient transfections
The transiently HSP20-overexpressing HuH7 cells and
the control empty vector-transfected HuH7 cells were used for
immunoprecipitation as previously described (12). For transient transfections, the HuH7
cells were cultured in 90 mm diameter dishes (1×106
cells/dish) and were transfected with 4 μg of the wild-type HSP20
plasmid or the control empty pcDNA 3.1(+) vector using the
UniFector transfection reagent (B-Bridge International, Mountain
View, CA, USA) in 4 ml of RPMI-1640 medium without FCS. One day
after transfection, the medium was changed to 6 ml of RPMI-1640
medium with 1% FCS. The cells were then cultured for another 24
h.
Protein preparation
For coimmunoprecipitation, the transfected cells
were lysed in ice-cold TNE lysis buffer [10 mM Tris-HCl, pH 7.8, 1%
Nonidet P-40, 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1 mM
sodium fluoride, 1 mM sodium vanadate and protease inhibitor
cocktail (Roche Diagnostics K.K.)]. The lysates were then
centrifuged at 10,000 × g at 4°C for 30 min, and the supernatant
was collected as TNE-soluble proteins, as previously described
(12). For the western blot
analysis, the serum-starved cells were lysed, homogenized and
sonicated in lysis buffer, as previously described (11).
Coimmunoprecipitation
Coimmunoprecipitation was performed as previously
described (12). The indicated
antibodies were added to the TNE-soluble proteins, and the mixture
was shaken gently overnight at 4°C, followed by the addition of 50
μl of Dynabeads protein A and incubation for a further 1 h with
continuous mixing. Protein immunocomplexes were isolated with the
use of a magnetic particle concentrator (6-tube magnetic separation
rack; New England BioLabs Inc., Ipswich, MA, USA). The
immunoprecipitated proteins and TNE-soluble proteins (for analysis
total protein) were resuspended in the loading buffer for sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE),
heated at 95°C for 5 min, and analyzed by western blot
analysis.
Western blot analysis
Western blot analysis was performed as previously
described (10). Briefly, SDS-PAGE
was performed by the method described by Laemmli (18). The proteins in the gel were
transferred onto polyvinylidene fluoride (PVDF) membranes, which
were then blocked with 5% fat-free dry milk in phosphate-buffered
saline (PBS) with 0.1% Tween-20 for 1 h before incubation with the
indicated primary antibodies. Peroxidase-labeled anti-rabbit IgG
antibodies were used as secondary antibodies. The peroxidase
activity on the PVDF membranes was visualized on X-ray film by
means of an ECL western blotting detection system (GE Healthcare,
Waukesha, WI, USA) as described in the manufacturer’s protocol.
Results
Increased cleavage of caspase-3 and
caspase-7 by HSP20 overexpression in HCC cells
In our previous studies (10–12),
we showed that the HSP20 protein is expressed in the tumor tissue
of human HCC, although the expression level is lower than in
non-tumor tissues. However, the HSP20 protein is not expressed in
human HCC cell lines. Therefore, we transfected wild-type HSP20
cDNA into HuH7 cells, a HCC-derived cell line, to make them express
the HSP20 protein, and then analyzed its function. We first
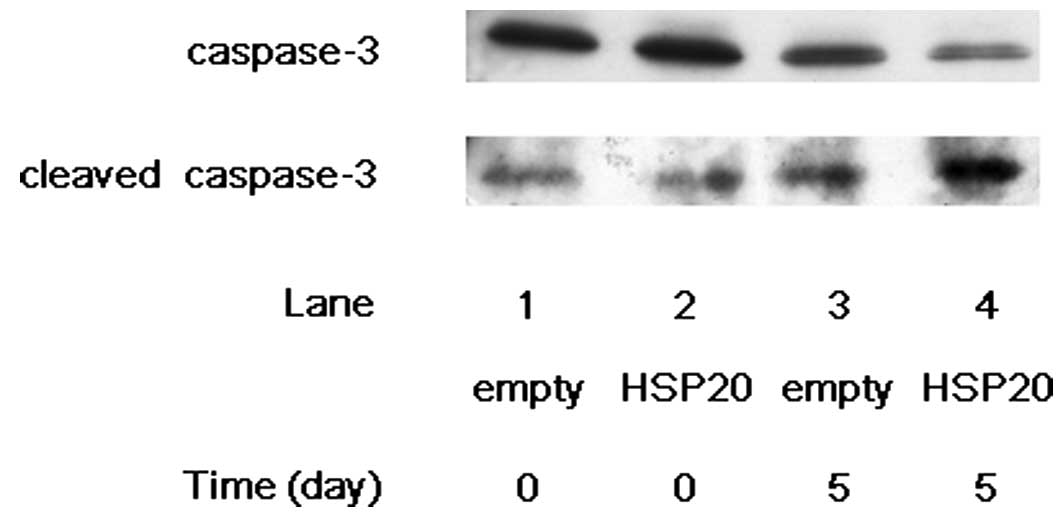
examined the effect of HSP20 expression on the cleavage of
caspase-3 in the HSP20-overexpressing HCC cells. After 5 days of
incubation without FCS, the level of cleaved caspase-3 markedly
increased in the HSP20-overexpressing HuH7 cells compared with that
in the empty vector-transfected cells (Fig. 1, lane 4 compared with lane 3). On
the other hand, the level of caspase-3 was decreased by HSP20
overexpression on day 5 (Fig. 1,
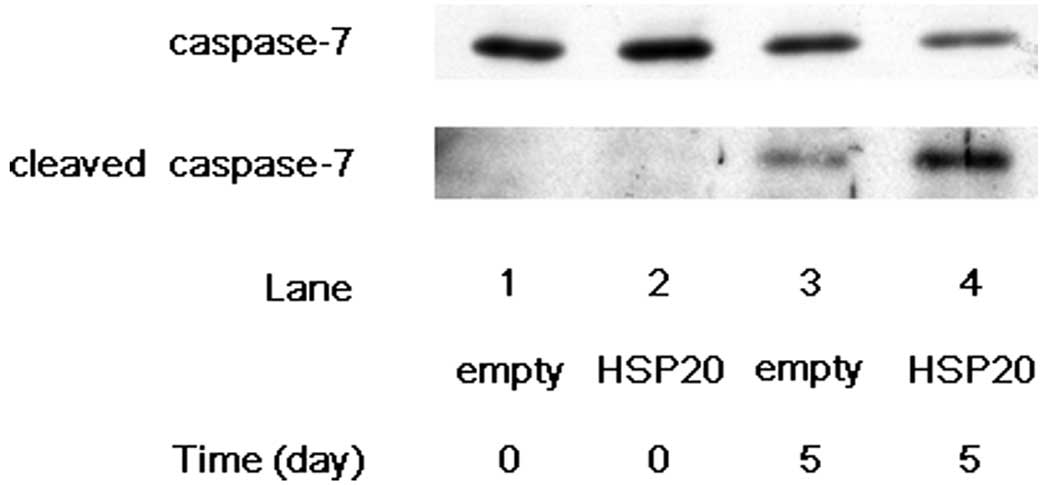
lane 4 compared with lane 3). We next examined the effect of HSP20
expression on the cleavage of caspase-7 in the HSP20-overexpressing
HCC cells. After 5 days of incubation without FCS, the expression
level of cleaved caspase-7 showed marked increase in the
HSP20-overexpressing cells compared with that in the empty
vector-transfected cells (Fig. 2,
lane 4 compared with lane 3), while the level of caspase-7 was
decreased by HSP20 overexpression at day 5 (Fig. 2, lane 4 compared with lane 3). These
findings suggest that the HSP20 protein plays a role activating the
cascade of caspases in the HCC cells.
Increased cleavage of PARP by HSP20
overexpression in HCC cells
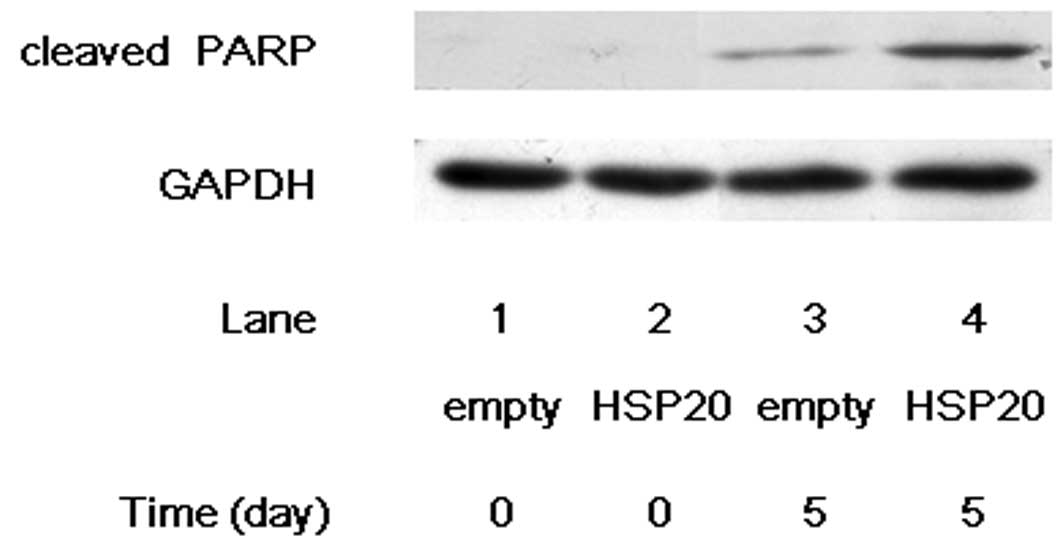
PARP, which helps cells to maintain their viability,
is a main cleavage target of caspase-3, and cleaved PARP induces
apoptosis, indicating that cleaved PARP is observed in the cells
undergoing apoptosis (15,16). After 5 days of incubation without
FCS, the cleavage of PARP markedly increased in the
HSP20-overexpressing HuH7 cells compared with that in the empty
vector-transfected cells (Fig. 3,
lane 4 compared with lane 3), suggesting that HSP20 induces the
caspase cascade which leads to apoptosis.
HSP20 directly interacts with Bax among
the Bcl-2 family proteins
Among several apoptotic pathways, mitochondria are
key participants (14). The
mitochondrial pathway is coupled to the activation of caspase-3 and
caspase-7. It is well known that the Bcl-2 family proteins are
critical death regulators for mitochondria-mediated apoptosis
(17). Therefore, we next examined
whether HSP20 interacts with the Bcl-2 family proteins, Bad, Bcl-2,
Bcl-xL and Bax in the HCC cells. Bad, Bcl-2, Bcl-xL and Bax
proteins were expressed in both the empty vector-transfected and
HSP20-overexpressing HuH7 cells (Fig.
4). However, HSP20 protein in the HSP20-overexpressing cells
was not coimmunoprecipitated with Bad, Bcl-2 or Bcl-xL proteins
(Fig. 4A–C). On the other hand, as
shown in Fig. 4D, the HSP20 protein
in the HSP20-overexpressing cells was markedly coimmunoprecipitated
with Bax (Fig. 4D, lane 2 in
comparison with lane 1). We confirmed that the HSP20 protein was
not coimmunoprecipitated with normal rabbit IgG (Fig. 4D). These results suggest that the
HSP20 protein directly interacts with the Bax protein but not with
the Bad, Bcl-2 and Bcl-xL proteins in the HCC cells.
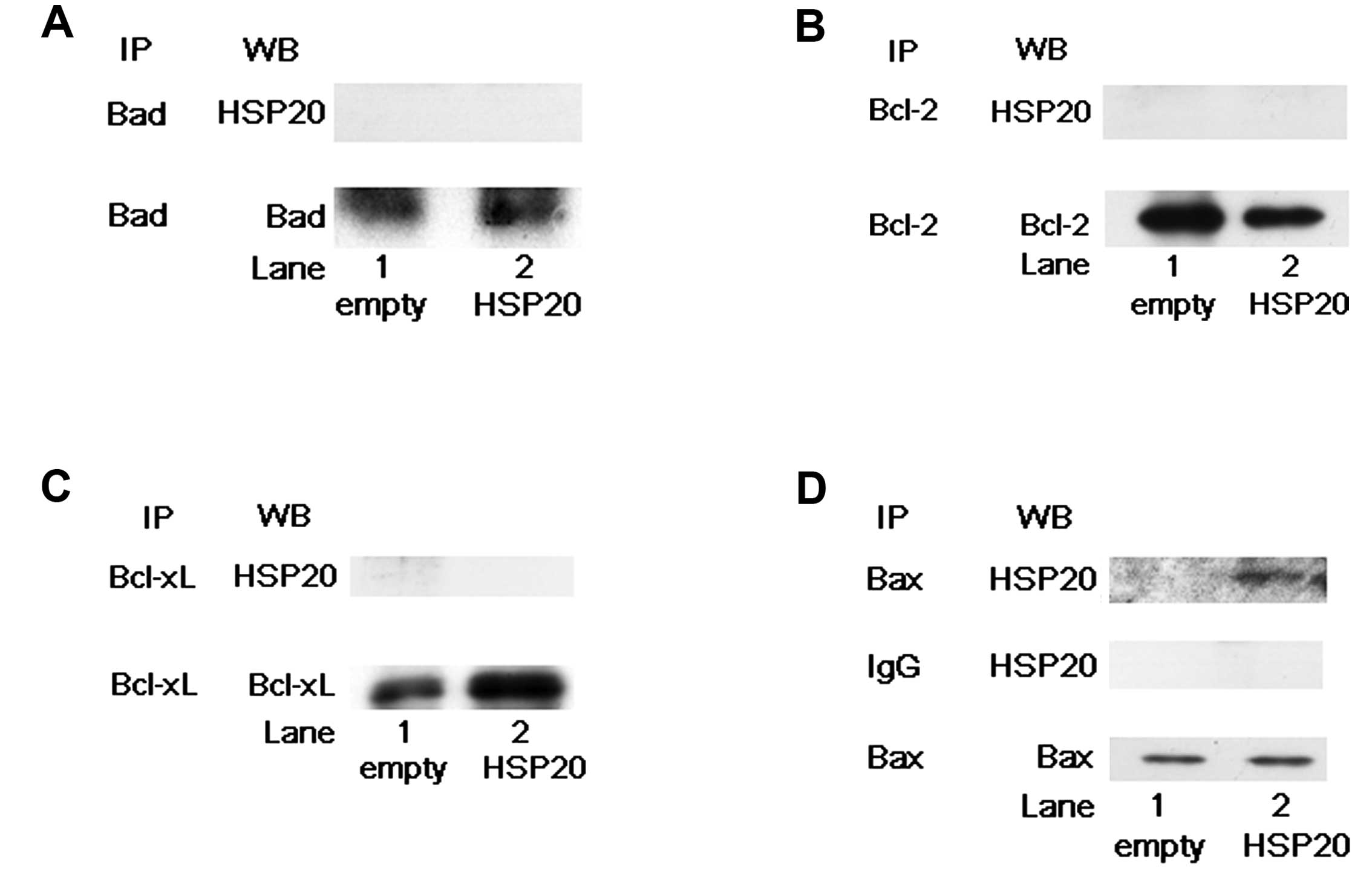 | Figure 4HSP20 does not directly interact with
Bad, Bcl-2 or Bcl-xL, but it does with Bax. (A) The empty
vector-transfected (empty, lane 1) and HSP20-overexpressing (HSP20,
lane 2) HuH7 cell lysates were immunoprecipitated with Bad
antibodies followed by western blotting (WB) using HSP20
antibodies. Immunoprecipitation (IP) of Bad proteins in the cells
was confirmed by WB using Bad antibodies. (B) The empty
vector-transfected (empty, lane 1) and HSP20-overexpressing (HSP20,
lane 2) HuH7 cell lysates were immunoprecipitated with Bcl-2
antibodies, followed by WB using HSP20 antibodies. IP of Bcl-2
proteins in the cells was confirmed by WB using Bcl-2 antibodies.
(C) The empty vector-transfected (empty, lane 1) and
HSP20-overexpressing (HSP20, lane 2) HuH7 cell lysates were
immunoprecipitated with Bcl-xL antibodies, followed by WB using
HSP20 antibodies. IP of Bcl-xL proteins in the cells was confirmed
by WB using Bcl-xL antibodies. (D) The empty vector-transfected
(empty, lane 1) and HSP20-overexpressing (HSP20, lane 2) HuH7 cell
lysates were immunoprecipitated with Bax antibodies and normal
rabbit IgG, followed by WB using HSP20 antibodies. IP of Bax
proteins in the cells with Bax antibodies was confirmed by WB using
Bax antibodies. |
Discussion
We have previously shown that HSP20 suppresses HCC
cell growth by downregulation of proliferation signals via the AKT
and mitogen-activated protein kinase pathways (11,12).
Cell growth is affected by both the survival and apoptosis signals.
Therefore, it led us to consider the relationship between HSP20 and
apoptosis in HCC. In the present study, we demonstrated that
caspase cascade, such as caspase-3 and caspase-7, the central
regulatory system of apoptosis signals is activated in HSP20
protein-overexpressing human HCC cells compared with that in the
control HCC cells. In addition, we showed that the level of cleaved
PARP is increased in the HSP20-overexpressing HuH7 cells. It is
firmly established that PARP is involved in DNA repair and
maintains cell viability (15,16).
The cleavage of PARP facilitates cellular disassembly, serving as a
marker of cells undergoing apoptosis. Based on our findings, it is
most likely that expression of HSP20 protein might suppress HCC
cell growth via both the downregulation of cell proliferation
signals and the activation of apoptosis pathway.
We next demonstrated that the HSP20 protein directly
interacts with Bax but not with Bad, Bcl-2 or Bcl-xL among the
Bcl-2 family proteins in the HCC cells. The Bcl-2 family consists
of pro-apoptotic members, such as Bad and Bax, and anti-apoptotic
members, such as Bcl-2 and Bcl-xL (17). Regarding the Bcl-2 family proteins
in HCC, it has been reported that Bcl-xL, an anti-apoptotic member,
is overexpressed whereas Bax, a pro-apoptotic member, is
downregulated (17). The activities
of the Bcl-2 family members are affected by the dimerization of
these proteins, and mutant forms of Bcl-2 that fail to
heterodimerize with Bax reportedly lose their ability to protect
cells from apoptosis (13). Bax
alone has been shown to be sufficient for induction of apoptosis.
It is generally recognized that the Bcl-2 family proteins act as
regulators for the mitochondria-mediated apoptosis, coupling to the
activation of caspase-3 and caspase-7 (13). Thus, it is probable that HSP20
interfere Bcl-2 binding to Bax protein, and exert the effects to
the mitochondria-caspase signals to induce apoptosis in the HCC
cells. Activated AKT reportedly phosphorylates and inhibits Bax,
and, as a result, prevents apoptosis (13). We have previously shown that HSP20
directly interacts with PI3K and inhibits AKT pathway activation in
the HCC cells (12). Therefore,
suppression of AKT activities by HSP20 protein in the HCC cells
might affect not only cell proliferation but also apoptosis in
HCC.
In normal mouse heart, overexpressed HSP20
reportedly interacts with the Bax protein and protects the heart
against ischemia/reperfusion injury (19). It has also been reported that acute
expression of HSP20 in rat cardiomyocytes is protective against
apoptosis (20). However, the exact
mechanism of HSP20 underlying apoptosis of HCC remains to be
clarified. Further investigations are necessary to elucidate the
detailed role of HSP20.
In conclusion, our findings strongly suggest that
HSP20 directly interacts with Bax and activates caspase cascade,
resulting in the induction of apoptosis in HCC.
Acknowledgements
The authors thank Yumiko Kurokawa for her technical
assistance. This study was supported in part by a Grant-in-Aid for
Scientific Research (22590726) from the Ministry of Education,
Science, Sports, and Culture of Japan.
References
|
1
|
Mymrikov EV, Seit-Nebi AS and Gusev NB:
Large potentials of small heat shock proteins. Physiol Rev.
91:1123–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kato K, Goto S, Inaguma Y, Hasegawa K,
Morishita R and Asano T: Purification and characterization of
20-kDa protein that is highly homologous to alpha B crystallin. J
Biol Chem. 269:15302–15309. 1994.PubMed/NCBI
|
|
3
|
Matsuno H, Kozawa O, Niwa M, Usui A, Ito
H, Uematsu T and Kato K: A heat shock-related protein, p20, plays
an inhibitory role in platelet activation. FEBS Lett. 429:327–329.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kozawa O, Matsuno H, Niwa M, Hatakeyama D,
Oiso Y, Kato K and Uematsu T: HSP20, low-molecular-weight heat
shock-related protein, acts extracellularly as a regulator of
platelet functions: a novel defense mechanism. Life Sci.
72:113–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Xu A, Ye J, Kraegen EW, Tse CA and
Cooper GJ: Alteration in phosphorylation of P20 is associated with
insulin resistance. Diabetes. 50:1821–1827. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flynn CR, Brophy CM, Furnish EJ,
Komalavilas P, Tessier D, Thresher J and Joshi L: Transduction of
phosphorylated heat shock-related protein 20, HSP20, prevents
vasospasm of human umbilical artery smooth muscle. J Appl Physiol.
98:1836–1845. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Komalavilas P, Penn RB, Flynn CR, Thresher
J, Lopes LB, Furnish EJ, Guo M, Pallero MA, Murphy-Ullrich JE and
Brophy CM: The small heat shock-related protein, HSP20, is a
cAMP-dependent protein kinase substrate that is involved in airway
smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol.
294:L69–L78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan GC and Kranias EG: Small heat shock
protein 20 (HspB6) in cardiac hypertrophy and failure. J Mol Cell
Cardiol. 51:574–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aravalli RN, Cressman ENK and Steer CJ:
Cellular and molecular mechanisms of hepatocellular carcinoma: an
update. Arch Toxicol. 87:227–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Noda T, Kumada T, Takai S,
Matsushima-Nishiwaki R, Yoshimi N, Yasuda E, Kato K, Toyoda H,
Kaneoka Y, Yamaguchi A and Kozawa O: Expression levels of heat
shock protein 20 decrease in parallel with tumor progression in
patients with hepatocellular carcinoma. Oncol Rep. 17:1309–1314.
2007.PubMed/NCBI
|
|
11
|
Matsushima-Nishiwaki R, Adachi S, Yoshioka
T, Yasuda E, Yamagishi Y, Matsuura J, Muko M, Iwamura R, Noda T,
Toyoda H, Kaneoka Y, Okano Y, Kumada T and Kozawa O: Suppression by
heat shock protein 20 of hepatocellular carcinoma cell
proliferation via inhibition of the mitogen-activated protein
kinases and AKT pathways. J Cell Biochem. 112:3430–3439. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsushima-Nishiwaki R, Kumada T, Nagasawa
T, Suzuki M, Yasuda E, Okuda S, Maeda A, Kaneoka Y, Toyoda H and
Kozawa O: Direct association of heat shock protein 20 (HSPB6) with
phosphoinositide 3-kinase (PI3K) in human hepatocellular carcinoma:
Regulation of the PI3K activity. PLoS One. 8:e784402013. View Article : Google Scholar
|
|
13
|
Kanzler S and Galle PR: Apoptosis and the
liver. Semin Cancer Biol. 10:173–184. 2000. View Article : Google Scholar
|
|
14
|
Mcllwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013.PubMed/NCBI
|
|
15
|
Decker P and Muller S: Modulating poly
(ADP-ribose) polymerase activity: potential for the prevention and
therapy of pathogenic situations involving DNA damage and oxidative
stress. Curr Pharm Biotechnol. 3:275–283. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krishnakumar R and Kraus WL: The PARP side
of the nucleus: molecular actions, physiological outcomes, and
clinical targets. Mol Cell. 39:8–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fabregat I, Roncero C and Fernández M:
Survival and apoptosis: a dysregulated balance in liver cancer.
Liver Int. 27:155–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P,
Wang Y, Jones WK, Chu G and Kranias EG: Novel cardioprotective role
of a small heat-shock protein, Hsp20, against ischemia/reperfusion
injury. Circulation. 111:1792–1799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan GC, Chu G, Mitton B, Song Q, Yuan Q
and Kranias EG: Small heat-shock protein Hsp20 phosphorylation
inhibits beta-agonist-induced cardiac apoptosis. Circ Res.
94:1474–1482. 2004. View Article : Google Scholar : PubMed/NCBI
|