Introduction
Acquisition of apoptosis resistance is a hallmark of
cancer cells and limits the treatment options in cancer patients
(1,2). Therefore, co-treatment with several
chemotherapy agents has been considered in order to reduce the
incidence of acquired apoptosis resistance in cancer cells
(3). TRAIL is considered to be a
promising anticancer agent due to its ability to selectively induce
apoptosis in cancer cells; Walczak et al reported that
injection of TRAIL into mouse xenografts transplanted with human
tumor cells resulted in growth inhibition of the transplanted
tumors. In contrast, no TRAIL-induced toxicity was observed in
normal tissue cells such as hepatocytes (4).
Binding of TRAIL to DR4 or DR5 is known to initiate
apoptosis through the formation of death-inducing signaling
complexes (DISCs) via the recruitment of FAS-associated protein and
caspase-8. Through the formation of the DISC complex, proteolytic
cleavage and activation of caspase-3 occur, eventually resulting in
the biochemical and morphological hallmarks of apoptosis. However,
depending on the cell type and external stimuli, caspase-8 also
activates caspase-9 through the cleavage of pro-apoptotic proteins,
such as BH3-interacting domain death agonist. Subsequently,
pro-apoptotic molecules such as cytochrome c are released
from the mitochondria to amplify the apoptotic response by
mediating the simultaneous activation of the extrinsic and
intrinsic apoptotic pathways (5).
However, other decoy receptors can inhibit TRAIL-induced apoptosis
by competing with DR4 or DR5, while dysfunction of DISC components
such as fas-associated protein with death domain (FADD), caspase-8
and cellular FLICE-like inhibitory protein (cFLIP), and mutational
inactivation of pro-apototic genes Bax or Bak can also lead to
TRAIL resistance (6). One strategy
that has been considered for overcoming TRAIL resistance is the
combination of different chemotherapeutic agents with TRAIL. The
combinatorial approach of treatment with various genotoxic drugs,
such as 5-flurouracil and cisplatin, in the presence of TRAIL has
been successful in animal models in vivo, and clinical
trials in humans are currently underway (7).
We recently demonstrated that TOR signaling pathway
regulator-like (TIPRL) is highly upregulated in hepatocellular
carcinoma (HCC) cells and contributes to TRAIL resistance by
forming the complex MKK7/PP2Ac/TIPRL, which blocks the
phosphorylation of JNK, thus inhibiting the apoptosis cascade
induced by TRAIL. In support of this, the combination of TIPRL
knockdown in the presence of TRAIL sensitizes cancer cells to
TRAIL-induced apoptosis via sustained MKK7 phosphorylation and JNK
activation (8). In the present
study, we aimed to identify compounds that inhibit the interaction
between MKK7/TIPRL and could thus be used as TRAIL sensitizers.
Using an ELISA system which detects MKK7 and TIPRL interaction
in vitro, we determined that Tussilago farfara L.
(TF; commonly known as coltsfoot) specifically inhibited the
TIPRL-MKK7 interaction. This inhibition led to an
apoptotic/cytotoxic effect on Huh7 cells after co-treatment with TF
and TRAIL.
Tussilago farfara L. belongs to the family
Asteraceae, which is known to have antioxidant, anti-inflammatory
effects (9), antimicrobial activity
and an α-glucosidase inhibitory effect (10). In vitro studies indicate that
TF has significant antiproliferative activity in cancer cells
(11). In a study by Park et
al, sesquiterpenoids isolated from TF inhibited the growth of
HCC HepG2 cells via inactivation of acyl CoA: diacylglycerol
acyltransferase (DGAT) (11).
However, the function of TF as a TRAIL sensitizer has never been
demonstrated. Therefore, in this study, we sought to investigate
the role of TF in TRAIL-induced apoptosis and to evaluate it as a
novel strategy to overcome the resistance of cancer cells to
apoptosis.
Materials and methods
Preparation of a methanol fraction of
Tussilago farfara L. herb
TF from leaves and stems (International Biological
Material Research Center, Daejeon, Korea) with voucher no.
FBM026-99 (9) was extracted with
methanol for three days at room temperature. Concentrated and
lyophilized TF was dissolved in dimethyl sulfoxide (DMSO) (stock
200 mg/ml); the final concentration of DMSO in the culture medium
was controlled at 0.1% (v/v).
High-performance liquid chromatography
(HPLC) analysis
HPLC profiles of TF were analyzed using a photodiode
array system (PDA) as a detector under the following conditions:
column, BEH 1.7 μM (2.1×100 mm); mobile phase, solvent A
H2O + 0.1% formic acid and solvent B acetonitrile (ACN)
+ 0.1% formic acid; the flow rate, 0.4 ml/min, oven temperature
35°C; injection volume, 2 μl. The solution was filtered and
analyzed by HPLC in a similar way.
Reagents
Antibodies against phospho(p)-MKK7 (Ser271/Thr261),
MKK7, p-JNK (Thr183/Tyr185), JNK, caspase-8, caspase-9, caspase-3
and PARP were purchased from Cell Signaling Technology (Beverly,
MA, USA); anti-cytochrome c antibody was purchased from BD
Pharmingen (San Diego, CA, USA). Antibodies against glutathione
S-transferase, hemagglutinin, GAPDH and HRP-conjugated secondary
antibodies were obtained from AbFrontier Co. (Seoul, Korea).
Z-VAD-fmk and SP600125 were purchased from Calbiochem (Darmstadt,
Germany). The human TRAIL was a kind gift from Dr C. Choi (KAIST,
Korea) and was also purchased from Apotech (Chemin Des Croisettes,
Switzerland).
Cell culture
Huh7 and 293T cells and human aortic endothelial
cells (HAECs) (American Type Culture Collection, Manassas, VA, USA)
were maintained in DMEM and EBM-2, respectively, supplemented with
10% FBS and 1% penicillin/streptomycin in a humidified atmosphere
in a 5% CO2 incubator at 37°C.
Cell viability assay
Cell viability was assessed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma Chemical Co., St. Louis, MO, USA) assay. The cells were
seeded at a density of 1×104 cells/well and were treated
with TF and/or TRAIL. After 24 h, MTT (2 mg/ml) was added to each
well, and the absorbance was measured using a microplate reader
(Bio-Rad, Hercules, CA, USA) at 570 nm. The cell viability was
calculated as a percentage of viable cells in drug-treated group
vs. the untreated control by the following equation: Cell viability
(%) = [OD (drug) - OD (blank)]/[OD (control) - OD (blank)] ×
100.
Crystal violet cell growth assays
The cells were fixed and stained with crystal violet
solution [40% ethanol, 60% phosphate-buffered saline (PBS) and 0.5%
crystal violet]. After 20 min, 1 ml of 10% acetic acid was added to
each well, and the absorbance was read at 590 nm.
Cell cycle analysis
The cells were fixed in 70% ethanol at −20°C and
treated with 10 mg/ml RNase A for 1 h at 37°C. Then the pellets
were suspended in 1 ml of propidium iodide (PI) solution [50 μg/ml
PI, 1 mg/ml RNase A and 0.1% (w/v) Triton X-100] in 3.8 mM sodium
citrate. Samples were analyzed using CellQuest Software (BD
Biosciences, San Jose, CA, USA).
Caspase-3 activity assay
Caspase-3 activity was measured using the Caspase-3
Luminescent assay kit (Promega, Madison, WI, USA) according to the
manufacturer’s instructions. Caspase-3 activity was measured using
a Luminescence Plate Reader (Molecular Devices Co., Sunnyvale, CA,
USA).
Measurement of mitochondrial membrane
potential (MMP)
The cells were stained with tetramethylrhodamine
(TMRE) (Molecular Probes, Eugene, OR, USA), for 30 min at 37°C. MMP
was determined by flow cytometry using CellQuest software.
ELISA assay
The full-length cDNAs of human TIPRL and MKK7 were
provided by the Korea Human Gene Bank (Korea Research Institute of
Biosciences and Biotechnology, Korea). TIPRL and MKK7 were
subcloned into the PCGN vector (W. Herr, Cold Spring Harbor
Laboratory), and pEBG containing GST tag (kindly provided by Y.
Liu, NIA, National Institutes of Health) vector in order to
construct the HA-tagged and the GST-tagged plasmid, respectively.
Purified TIPRL was conjugated with HRP. To screen natural products
in a high-throughput system (HTS), an ELISA system that detects the
TIPRL and MKK7 interaction was constructed. Briefly, 96-well ELISA
plates were coated with GST-MKK7 dissolved in PBS-T buffer
(Tween-20 0.05% and BSA 0.5%), followed by the addition of 100 μl
of TIPRL-HRP dissolved in PBS-T buffer. After 1 h, 100 μl of TMB
solution was added to each well, and the absorbance was read at 450
nm.
GST-pull down assay
293T cells were transfected with expression vector
constructs using TurboFect transfection reagent (Thermo Scientific,
Lafayette, CO, USA), lysed in RIPA buffer (50 mM Tris-HCl, 150 mM
NaCl, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 1mM
Na3VO4, 1 mM NaF and 1X protease inhibitor
cocktail) containing complete protease inhibitor cocktail (Roche
Applied Science, Indianapolis, IN, USA). Whole-cell extract was
incubated with Glutathione Sepharose 4B (GE Healthcare, Uppsala,
Sweden), and the eluted GST-tagged proteins were subjected to
western blotting using the indicated antibodies.
Western blotting
The cells were lysed in lysis buffer (50 mM
Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM
EDTA, 1 mM Na3VO4, 1 mM NaF and 1X protease
inhibitor cocktail) and spun down at 14,000 × g for 20 min at 4°C.
The protein samples were subjected to western blotting with the
indicated antibodies.
Subcellular fractionation
The cells were harvested in ice-cold lysis buffer
(20 mM HEPES, 2 mM EDTA, 0.1 mM PMSF, 10 μg/ml of pepstatin A and
leupeptin), homogenized and centrifuged for 10 min at 3,500 × g.
The supernatant was collected for the cytosol fraction.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD) of three independent experiments. Statistical
significance was verified by a Student’s t-test using SigmaPlot
software (Systat Software Inc., San Jose, CA, USA).
Results
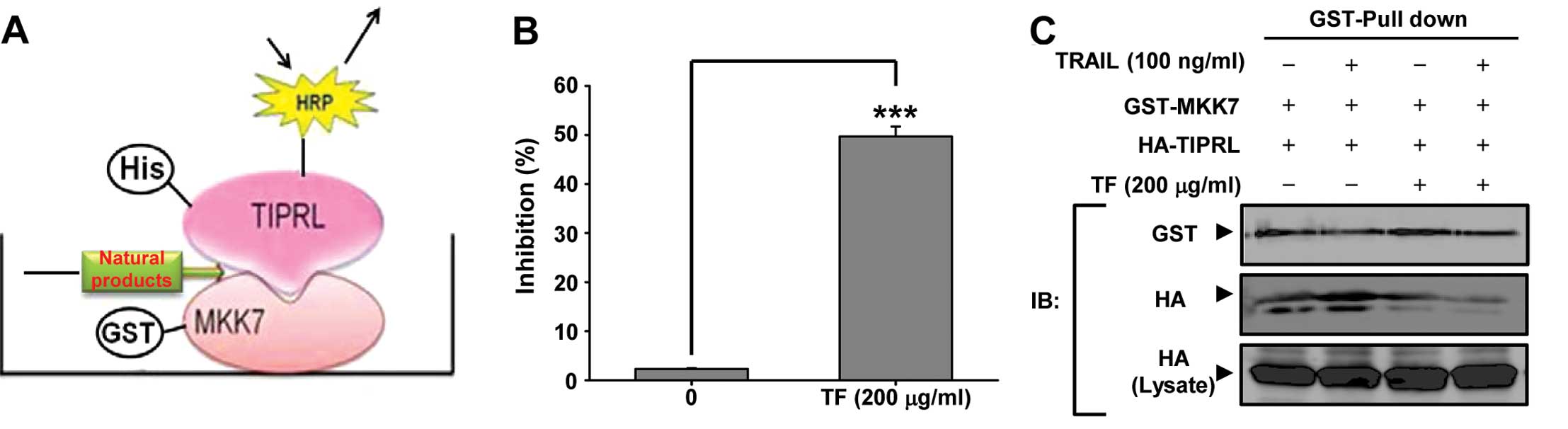
TF specifically inhibits the MKK7-TIPRL
interaction
To screen out bioactive natural products that could
potentially abolish TRAIL resistance, we constructed an ELISA
system that specially detects the MKK7-TIPRL interaction (Fig. 1A). GST-MKK7 was coated on a
polyvinyl chloride microtiter plate and was then exposed to react
with His-TIPRL-conjugated with HRP. For the quantification of
inhibition of the MKK7-TIPRL interaction, natural products were
introduced before the addition of TIPRL. Among 500 natural products
in our library (data not shown), TF showed 50% inhibition of the
interaction between MKK7 and TIPRL (Fig. 1B). To further validate the
inhibition of the MKK7/TIPRL interaction by TF, GST pull down assay
was conducted (Fig. 1C). The
MKK7-TIPRL interaction was significantly decreased by TF in the
presence of TRAIL compared with either TRAIL or TF alone,
supporting the possibility that TF specifically inhibits the
MKK7-TIPRL interaction.
The HPLC profile of the TF extract was examined
using photodiode array detector (PDA) (12). Given that the retention times and UV
spectra in TF were identical to those of commercially purchased
standards, our HPLC data showed that major constituents of the TF
extract were phenolic compounds such as chlorogenic acid,
quercertin 3-O-glucoside 7-O-rhamnoside,
quercetin-3-O-glucopyranoside and astragalin (Fig. 1D). We postulated that the phenolic
compounds in TF inhibit the interaction of TIPRL-MKK7 upon TRAIL
treatment.
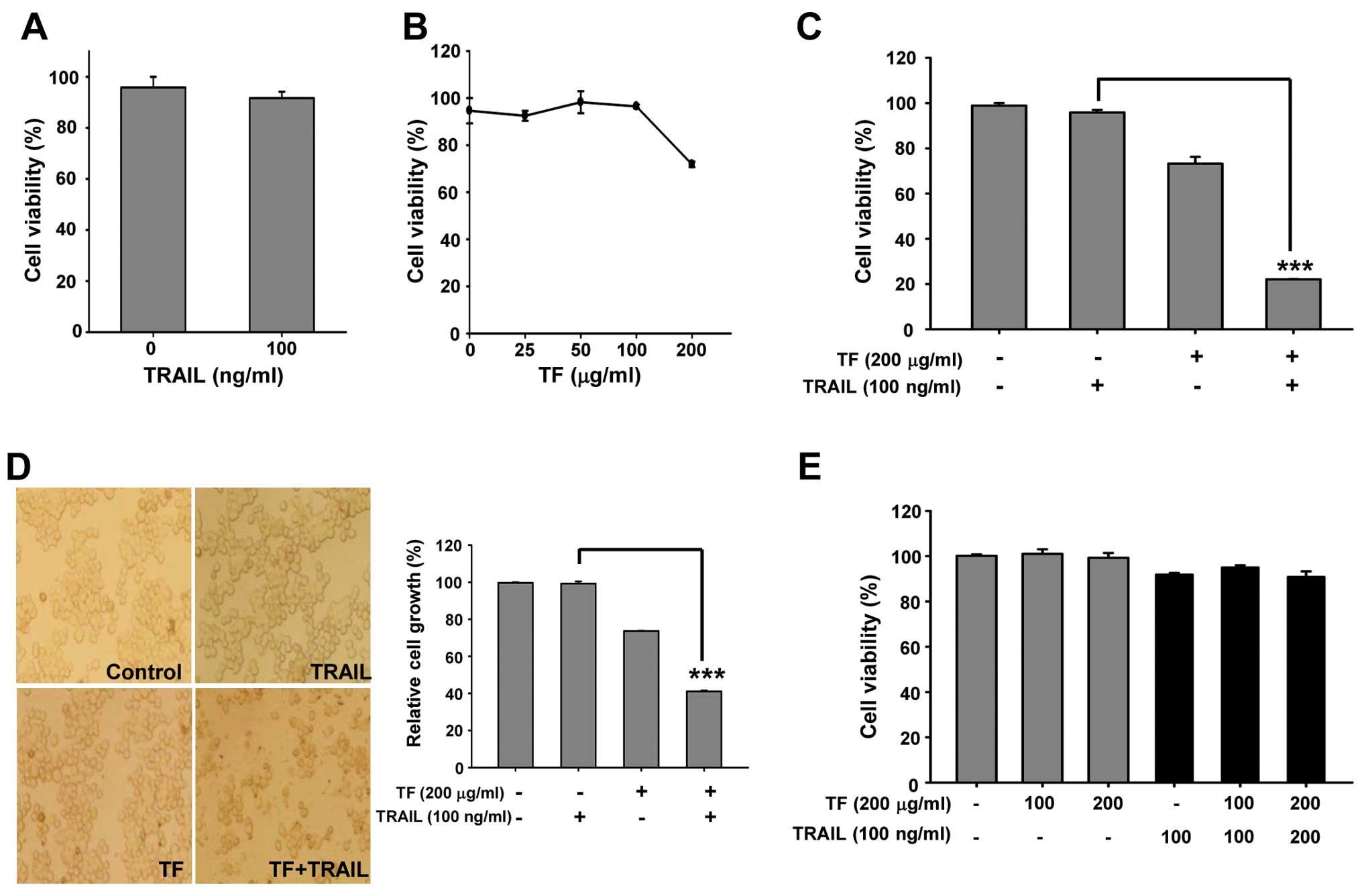
Augmentation of TRAIL-induced
cytotoxicity by TF in Huh7 cells
Next, we examined whether TF enhances the
sensitivity of Huh7 cells to TRAIL. Fig. 2A shows that 100 ng/ml of TRAIL did
not affect the viability of Huh7 cells. In addition, when Huh7
cells were treated with 200 μg/ml TF alone for 24 h, 73% cell
viability was observed (Fig. 2B),
implying that Huh7 cells are resistant to TRAIL and TF has low
cytotoxicity. However, the combination of TF and TRAIL treatment
decreased cell viability to 25% (Fig.
2C), and colony formation assays using crystal violet confirmed
that TF exhibited cytotoxicity in the presence of TRAIL (Fig. 2D). However, HAECs, normal
endothelial cells, were not harmed by TF or TRAIL alone, or by the
combination of TF and TRAIL (Fig.
2E). These findings indicate that TF can be a novel sensitizer
of apoptosis induction in TRAIL-resistant Huh7 cells.
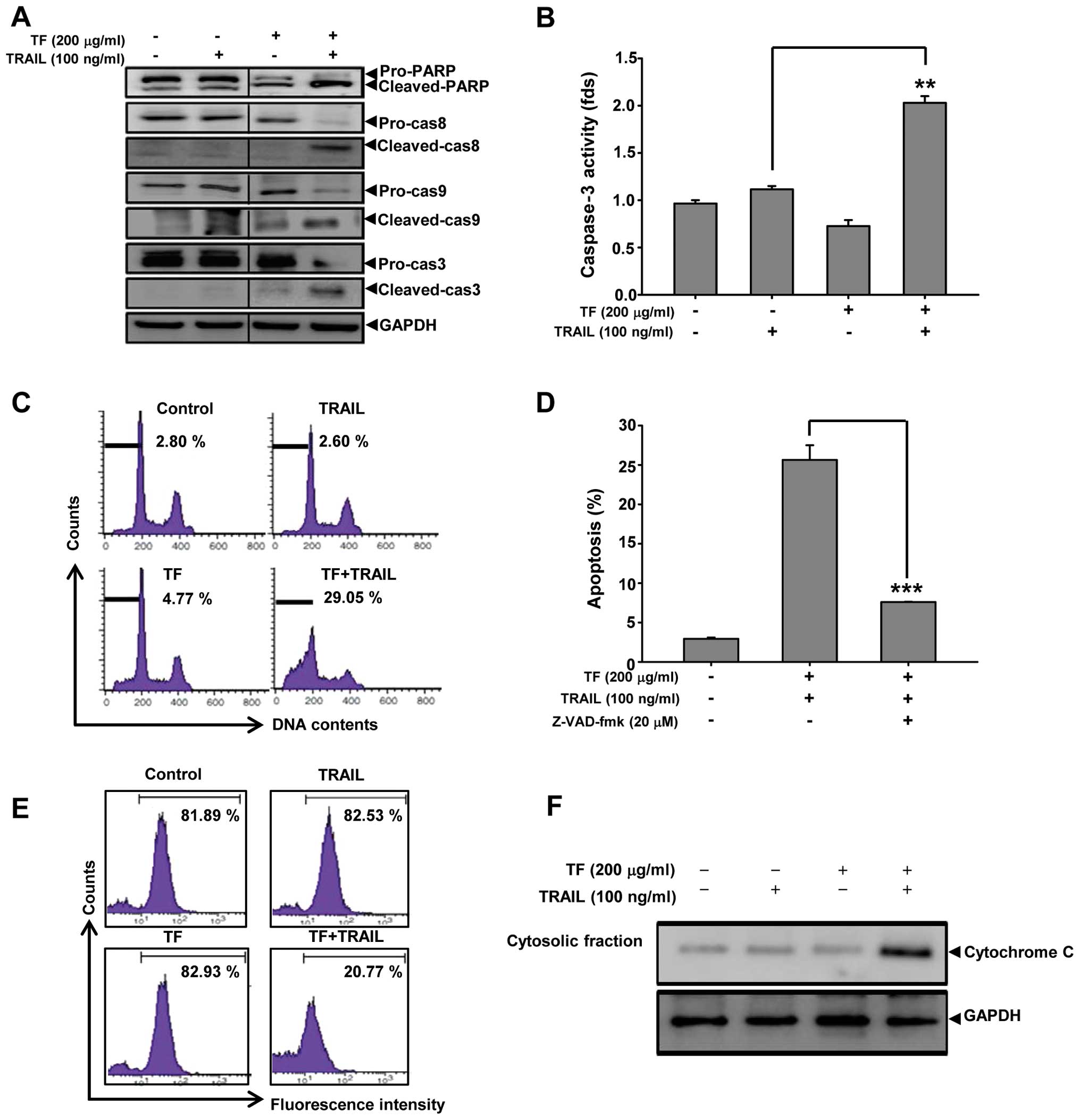
Activation of caspase-dependent apoptosis
in Huh7 cells treated with TF and TRAIL
To investigate the mechanism involved in
TF-TRAIL-induced apoptosis in Huh7 cells, immunoblot analysis was
performed using anti-caspase-8, anti-caspase-9, anti-caspase-3 and
anti-PARP antibodies. Co-treatment of TF with TRAIL induced the
activation of caspase-8, caspase-9, caspase-3 and PARP (Fig. 3A). When Huh7 cells were treated with
TF (200 μg/ml) and/or TRAIL (100 ng/ml), caspase-3 activity was
significantly enhanced in the cells co-treated with TF and TRAIL
compared to cells treated with TF or TRAIL only (Fig. 3B). In addition, cell cycle analysis
showed that sub-G1 DNA contents increased from 2.60 (TRAIL only) to
29.05% (TF/TRAIL treatment). Notably, TF increased the sub-G1
percentage to 27.05% in the presence of TRAIL, which was suppressed
to nearly 7.64% with pretreatment with the pan-caspase inhibitor
z-VAD-fmk for 1 h (Fig. 3C and D).
These results imply that TF sensitizes Huh7 cells to TRAIL-induced
cell death through the activation of the apoptotic machinery.
Next, we evaluated the MMP in Huh7 cells treated
with TF and TRAIL using TMRE solution as a fluorescent
potential-dependent indicator, and observed that the MMP of cells
that were co-treated with TF and TRAIL was 20.77%. In contrast, the
MMP of cells treated with 200 μg/ml TF and 100 ng/ml TRAIL was
82.93 and 82.53%, respectively, implying that TF/TRAIL-induced
apoptosis is mediated through the mitochondrial/extrinsic apoptotic
pathway (Fig. 3E). To further
confirm the TF/TRAIL-induced apoptosis via mitochondria, we
assessed the level of cytochrome c in the cytoplasmic
fraction of Huh7 cells co-treated with TF and TRAIL, and found
upregulation of cytochrome c in the Huh7 cells co-treated
with TF and TRAIL compared to cells treated with TF or TRAIL alone
(Fig. 3F). Collectively, the
combined treatment of TF and TRAIL induced apoptosis through the
intrinsic/extrinsic pathway.
Involvement of MKK7/JNK activation in
TF/TRAIL-induced apoptosis
Previously, we reported that activation of MKK7/JNK
is involved in TRAIL-induced apoptosis in HCCs after TIPRL
knockdown (8). Based on our
results, we examined the involvement of MKK7/JNK activation in
TF/TRAIL-induced apoptosis, and found an increase in the
phosphorylation status of MKK7/JNK in cells co-treated with TF and
TRAIL (Fig. 4A). Furthermore, as
shown in Fig. 4B, pretreatment of
Huh7 cells with SP600125, a JNK-specific inhibitor, led to
reduction in the apoptotic portion and cleaved PARP following
TF/TRAIL treatment. Our results strongly imply that combined TF and
TRAIL treatment induced the apoptosis of Huh7 cells through the
activation of MKK7/JNK.
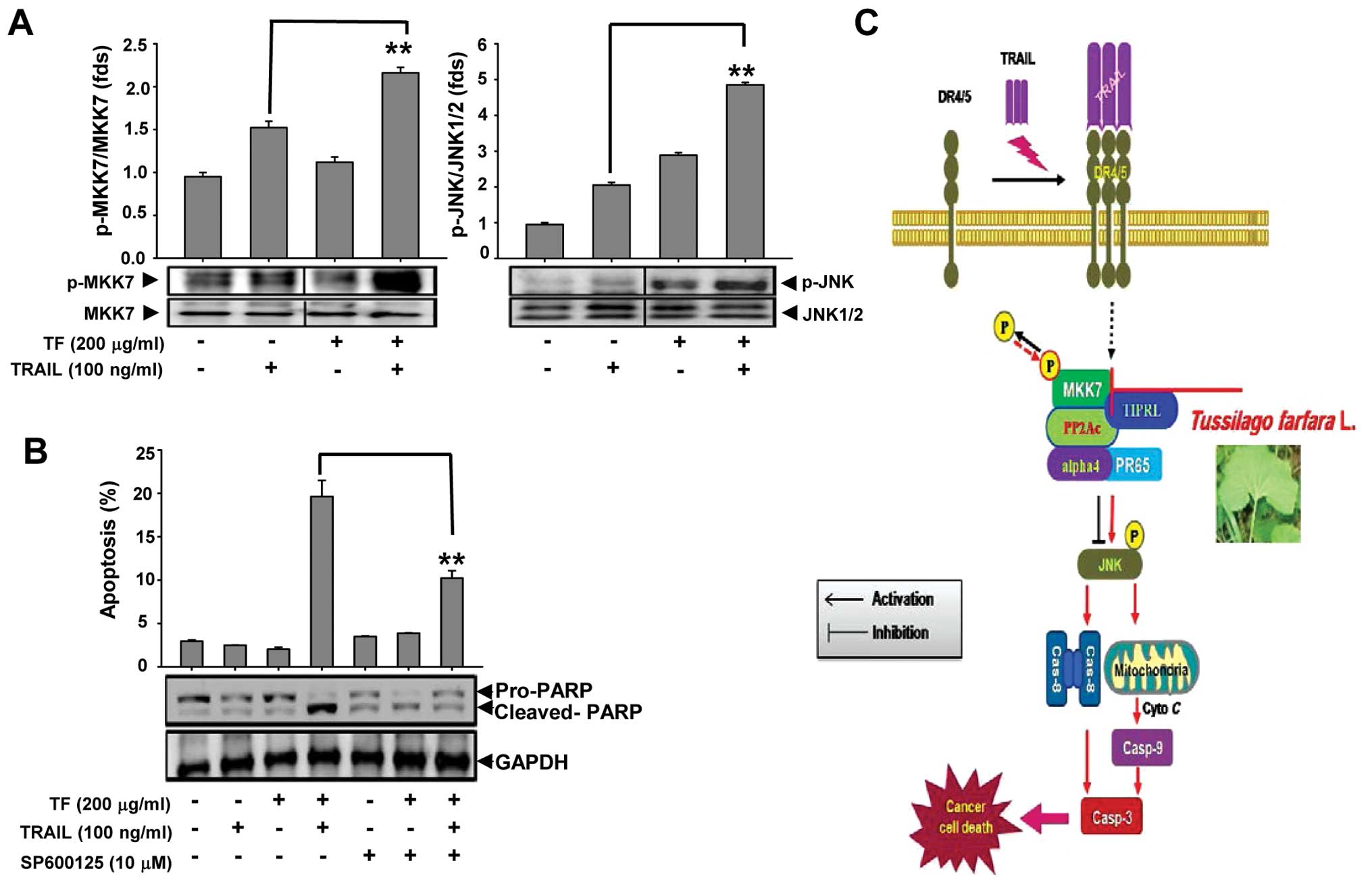 | Figure 4Involvement of MKK7/JNK in
TF/TRAIL-induced apoptosis. Cells were treated with TF (200 μg/ml)
and/or TRAIL (100 ng/ml) for 24 h. (A) The cell lysates were
subjected to western blot analysis using the indicated antibodies.
Bar graphs represent the relative level of phospho(p)-MKK7/MKK7
(left panel) and p-JNK/JNK (right panel) (**p<0.01,
significant difference between cells treated with TF/TRAIL and
TRAIL). (B) The cells were pre-treated with SP600125 for 1 h and
were then treated with TF and/or TRAIL for an additional 24 h. The
bar graphs represent the percentages of the sub-G1 apoptotic DNA
fraction (**p<0.01, significant difference between
the apoptotic portion of cells treated with TF/TRAIL/SP600125 and
with TF/TRAIL only). (C) In TRAIL-resistant cancer, the
dephosphorylation of MKK7 by PP2Ac via TIPRL leads to the
inhibition of MKK7/JNK, the caspase cascade and apoptosis,
eventually contributing to TRAIL resistance in cancer cells.
However, TF interferes with the MKK7-TIPRL interaction,
subsequently inducing phosphorylation/activation of MKK7/JNK, and
finally activating the caspase cascade including extrinsic and
intrinsic pathways and cell death. MKK7, mitogen protein kinase
kinase 7; JNK, c-Jun N-terminal kinase; TF, Tussilago
farfara L.; TIPRL, TOR signaling pathway regulator-like
protein. |
Discussion
Molecular targeted therapies against cancer cells
are currently being studied with the hope of reducing side-effects
and increasing target specificity for cancer patients (13,14).
We previously reported that the TIPRL protein contributes to TRAIL
resistance of HCC cells by interacting with MKK7, and that
knockdown of TIPRL by siRNA and TRAIL treatment leads to apoptosis
via activation of MKK7 and its downstream JNK (8). In the present study, we aimed to
identify natural compounds which overcome TRAIL resistance by
inhibition of the MKK7 and TIPRL interaction. This is the first
report suggesting Tussilage farfara L. (TF) as a novel TRAIL
sensitizer that induces apoptosis by inhibiting the MKK7-TIPRL
interaction.
To identify bioactive natural products that inhibit
the interaction between MKK7 and TIPRL, we constructed an ELISA
system with GST-MKK7 and His-TIPRL recombinant proteins, and
selected TF as the most potent inhibitor of the MKK7/TIPRL
interaction among 500 natural products. We further confirmed the
inhibition of MKK7-TIPRL interaction by TF via a GST-pull down
assay, which revealed that TF specifically inhibited the MKK7-TIPRL
interaction in the presence of TRAIL (Fig. 1). TF, which is also known as
coltsfoot, is a herbal remedy currently used as an antioxidant and
anti-inflammatory agent (15). Many
chemicals have been reported as constituents in TF, including
phenolic acids, such as ferulic, p-hydroxybenzoic, caffeic, and
caffeotartric acids, and flavonoids, such as quercetin, kaempferol,
quercetin-3-arabinoside, kaempferol-3-flucosides,
kaempferol-3-arabonsides and quercetin glucoside (16). Our PDA study also determined that TF
contains flavonoid-related compounds such as chlorogenic acid,
quercertin 3-O-glucoside 7-O-rhamnoside,
quercetin-3-O-glucopyranoside and astragalin. Given reports
that flavonoids exert antitumor effects on several types of cancers
such as ovarian, breast, cervical, pancreatic and prostate cancer
(17), we hypothesized that these
flavonoid-related compounds contained in TF may have a role in
overcoming TRAIL resistance by inhibition of the MKK7-TIPRL
interaction.
As it is well known that HCC shows resistance to
TRAIL treatment (18,19), we examined the viability of Huh7
cells following treatment with a high concentration of TRAIL (100
ng/ml), and observed resistance of Huh7 cells to TRAIL. However, TF
caused synergistic cytotoxicity in Huh7 cells co-treated with
TRAIL. Song et al reported that TIPRL knockdown induces
TRAIL-induced cell death via a caspase/mitochondrial-dependent
pathway (8). Similarly in the
present study, the combined treatment of TF with TRAIL induced the
activation of caspase-9, caspase-8, caspase-3 and PARP and enhanced
the sub-G1 apoptotic population. These results were confirmed by
the finding that TF/TRAIL-induced apoptosis was reduced by
pretreatment with z-VAD-fmk. MMP and cytochrome c levels
were also increased, supporting induction of apoptosis through the
instrinsic pathway. In addition, co-treatment of TF and TRAIL also
induced the phosphorylation of MKK7/JNK, which was suppressed by
pretreatment with SP600125. These results strongly indicate that TF
augments TRAIL-induced apoptosis via activation of MKK7/JNK in Huh7
cells, and that it could be used as a TRAIL sensitizer.
Resistance of apoptosis is one of the hallmarks of
cancer and is a major obstacle that needs to be overcome for the
successful application of cancer therapies. Resistance to
TRAIL-induced apoptosis is one of the main mechanisms of apoptosis
resistance. We identified TF as a potential TRAIL sensitizer among
500 natural products, and we elucidated the molecular mechanism of
TF/TRAIL-induced apoptosis in Huh7 cells (Fig. 4C). However, further studies are
warranted to identify the specific chemical component in TF that is
responsible for the inhibition of MKK7-TIPRL interaction and to
validate these results as well as the safety of TF in in
vivo models.
Acknowledgements
This study was supported by the Mid-Career
Researcher Program through the National Research Foundation of
Korea, funded by the Ministry of Education, Science and Technology,
and by the KRIBB Research Initiative Program.
Abbreviations:
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
GST
|
glutathione S-transferase
|
|
HAECs
|
human aortic endothelial cells
|
|
HCC
|
hepatocellular carcinoma
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MKK7
|
mitogen protein kinase kinase 7
|
|
MMP
|
mitochondrial membrane potential
|
|
TIPRL
|
TOR signaling pathway regulator-like
protein
|
|
TMRE
|
tetramethylrhodamine
|
|
TF
|
Tussilago farfara L.
|
|
TRAIL
|
TNF-related apoptosis-inducing
ligand
|
|
PARP
|
poly (adenosine diphosphate-ribose)
polymerase
|
References
|
1
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
2
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: a link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang XZ, Wang J, Huang C, et al: Emodin
enhances cytotoxicity of chemotherapeutic drugs in prostate cancer
cells: the mechanisms involve ROS-mediated suppression of multidrug
resistance and hypoxia inducible factor-1. Cancer Biol Ther.
7:468–475. 2008. View Article : Google Scholar
|
|
4
|
Walczak H, Miller RE, Ariail K, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walczak H, Bouchon A, Stahl H and Krammer
PH: Tumor necrosis factor-related apoptosis-inducing ligand retains
its apoptosis-inducing capacity on Bcl-2- or
Bcl-xL-overexpressing chemotherapy-resistant tumor
cells. Cancer Res. 60:3051–3057. 2000.PubMed/NCBI
|
|
6
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hellwig CT and Rehm M: TRAIL signaling and
synergy mechanisms used in TRAIL-based combination therapies. Mol
Cancer Ther. 11:3–13. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song IS, Jun SY, Na HJ, et al: Inhibition
of MKK7-JNK by the TOR signaling pathway regulator-like protein
contributes to resistance of HCC cells to TRAIL-induced apoptosis.
Gastroenterology. 143:1341–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ravipati AS, Zhang L, Koyyalamudi SR, et
al: Antioxidant and anti-inflammatory activities of selected
Chinese medicinal plants and their relation with antioxidant
content. BMC Complement Altern Med. 12:1732012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao H, Huang YN, Gao B, Xu PY, Inagaki C
and Kawabata J: α-Glucosidase inhibitory effect by the flower buds
of Tussilago farfara L. Food Chemistry. 106:1195–1201.
2008.
|
|
11
|
Park HR, Yoo MY, Seo JH, et al:
Sesquiterpenoids isolated from the flower buds of Tussilago
farfara L. inhibit diacylglycerol acyltransferase. J Agric Food
Chem. 56:10493–10497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scordino M, Sabatino L, Traulo P, Gargano
M, Panto V and Gagliano G: HPLC-PDA/ESI-MS/MS detection of
polymethoxylated flavones in highly degraded citrus juice: a
quality control case study. Eur Food Res Technol. 232:275–280.
2011. View Article : Google Scholar
|
|
13
|
Falschlehner C, Emmerich CH, Gerlach B and
Walczak H: TRAIL signalling: decisions between life and death. Int
J Biochem Cell Biol. 39:1462–1475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin Z, McDonald ER III, Dicker DT and
El-Deiry WS: Deficient tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) death receptor transport to the
cell surface in human colon cancer cells selected for resistance to
TRAIL-induced apoptosis. J Biol Chem. 279:35829–35839. 2004.
View Article : Google Scholar
|
|
15
|
Vogl S, Picker P, Mihaly-Bison J, et al:
Ethnopharmacological in vitro studies on Austria’s folk medicine -
an unexplored lore in vitro anti-inflammatory activities of 71
Austrian traditional herbal drugs. J Ethnopharmacol. 149:750–771.
2013.
|
|
16
|
Didry N, Pinkas M and Torck M: Phenolic
components from Tussilago farfara. Ann Pharm Fr. 38:237–241.
1980.(In French).
|
|
17
|
Batra P and Sharma AK: Anti-cancer
potential of flavonoids: recent trends and future perspectives.
Biotech. 3:439–459. 2013.
|
|
18
|
Chen KF, Yeh PY, Hsu C, et al: Bortezomib
overcomes tumor necrosis factor-related apoptosis-inducing ligand
resistance in hepatocellular carcinoma cells in part through the
inhibition of the phosphatidylinositol 3-kinase/Akt pathway. J Biol
Chem. 284:11121–11133. 2009. View Article : Google Scholar
|
|
19
|
Shin EC, Seong YR, Kim CH, et al: Human
hepatocellular carcinoma cells resist to TRAIL-induced apoptosis,
and the resistance is abolished by cisplatin. Exp Mol Med.
34:114–122. 2002. View Article : Google Scholar : PubMed/NCBI
|