Introduction
In toxicology and pharmacology, embryonic stem cell
(ESC) derived hepatocytes have been suggested as an in vitro
alternative model for toxicity and/or carcinogenicity assay testing
(1,2). Hepatic differentiation of mouse ESCs
was first demonstrated using a stepwise method, adding specific
growth factors after the formation of an embryoid body (3). The recent generation of functional
hepatocytes from ESCs using chemically defined culture conditions
has revealed similarities to in vivo hepatogenesis (4,5). The
functionality of differentiated hepatocytes is generally confirmed
by the expression of specific marker proteins, such as cytokeratins
(CK), GATA binding proteins (GATA), and α-fetoprotein (AFP).
Expression of these indicators of hepatic development can also be
used as a measure of hepatotoxicity.
ESC-derived hepatocytes and hepatic progenitors may
also serve as a model to study the induction of hepatocarcinoma, as
hepatic tumors originate from liver stem cells (6). Prior to tumor formation, exposure to
carcinogens first causes the formation of preneoplastic lesions as
an adaptive non-oncogenic response. Similar to numerous solid
tumors, hepatocarcinoma is sustained by a distinct subpopulation of
cancer stem cells (7).
Hepatocellular carcinoma (HCC) is distinguished from
normal liver or dysplastic lesions by the expression of the
neighbor of Punc E11 (Nope), a transmembrane protein in the
immunoglobulin superfamily. Nope was initially described by Salbaum
and Kappen (8) and has high
sequence homology with Punc and the axonal guidance receptors,
deleted in colorectal cancer and Neogenin (9–11).
High levels of Nope expression are noted at the time of
transformation from preneoplastic lesion to malignant HCC (12). Moreover, expression levels
progressively increase with the advance of HCC, suggesting a
potential role of Nope as a prognostic indicator.
Following our observation that diethylnitrosamine
(DEN)-induced carcinogenicity in animals varies with age, we
hypothesized that DEN-induced expression of Nope would vary with
the differentiation stage of hepatic cells. In the present study,
we investigated how sensitivity to DEN, as measured by the
expression of Nope, varies with hepatic differentiation from ESCs
to mature hepatocytes.
Materials and methods
Culture of mouse ESCs and differentiation
of hepatic lineage cells
Mouse ESCs (NVRQS-11F) were cultured using mitomycin
C-treated mouse embryonic fibroblasts as feeder cells on 0.1%
gelatin-coated dishes in Dulbecco’s modified Eagle’s medium (DMEM)
(Millipore, Billerica, MA, USA) supplemented with 15% fetal bovine
serum (Invitrogen, Rockville, MD, USA), 2 ml-glutamine (Millipore),
0.1% nonessential amino acids (Invitrogen), 1%
penicillin-streptomycin (Millipore), and 10 ng/ml mouse leukemia
inhibitory factor (Millipore). To differentiate ESCs into hepatic
lineage cells (HPCs) in vitro, defined culture media were
supplemented with rmHGF (Invitrogen), dimethyl sulfoxide (DMSO) and
sodium butyrate (Sigma-Aldrich, St. Louis, MO, USA). Subsequently,
mEGF (Invitrogen), oncostatin M, dexamethasone, nicotinamide and
ascorbic acid (Sigma-Aldrich) were added to differentiate HCs from
HPCs.
DEN treatment of ESCs, HPCs and HCs
ESCs (day 0), HPCs (day 22 of differentiation) and
HCs (day 40 of differentiation) were treated with four
concentrations of DEN (0, 1, 5 and 15 mM) for 24 h.
Nope and E-cadherin mRNA expression
RNA was isolated from cultured cells using an RNeasy
Mini Kit (Qiagen, Valencia, CA, USA), dissolved in
diethylpyrocarbonate (DEPC)-treated distilled water and stored at
−80°C until use. RNA concentrations were measured
spectrophotometrically. Nope and GAPDH mRNA expression was
determined by relative quantitative real-time PCR in 96-well
optical plates using an ABI StepOnePlus™ Real-Time PCR
System (Applied Biosystems, Foster City, CA, USA). The primers are
listed in Table I. The expression
levels of the target genes were normalized to mouse GAPDH mRNA and
are expressed as the relative expression. Gene expression was
normalized according to the cycle number at which the fluorescence
signal of the target product was detectable (threshold cycle, Ct)
to provide ΔCt. The expression of the genes relative to a reference
was calculated as 2−ΔΔCt, where ΔΔCt refers to the
difference between the ΔCt values of the test group and the
reference.
 | Table IMurine oligonucleotide primer
sequences used for quantitative RT-PCR. |
Table I
Murine oligonucleotide primer
sequences used for quantitative RT-PCR.
| Gene name | Accession number | Primer sequence | Product length
(bp) |
|---|
| Nope | NM_020043 |
5′-CCTGGTATATGACGCCATAA-3′
5′-GAGTGGACAATGACCTCAG-3′ | 90 |
| AFP | NM_007423 |
5′-TTGTGTATAAGGAATGAAGCAAG-3′
5′-CCTGTTGGAATACGAAGAGTT-3′ | 75 |
| E-cadherin | NM_009864 |
5′-AACTGGCTGGAGATTAACC-3′
5′-CTGTGGCGATGATGAGAG-3′ | 115 |
| GAPDH | NM_008084 |
5′-GAAACCTGCCAAGTATGATG-3′
5′-GGAGTTGCTGTTGAAGTC-3′ | 121 |
Immunofluorescence staining of Nope and
E-cadherin
Cells were cultured at a low density on coverslips
and fixed in 4% paraformaldehyde for 15 min at room temperature
(RT). The medium was removed by aspiration, and cells were washed
twice with 1× phosphate-buffered saline (PBS; pH 7.4; Invitrogen),
fixed in 4% paraformaldehyde (Sigma-Aldrich) for 5 min, and rinsed
twice with 1× PBS. After cell permeabilization in 1× PBS and 0.1%
Triton X-100 (PT) for 10 min, proteins were blocked with 4% normal
goat serum in PT and incubated with anti-Nope (1:100 dilution;
R&D Systems, Minneapolis, MN, USA). Hepatic lineage cells were
incubated in anti-E-cadherin (1:100 dilution; Abcam, Cambridge, MA,
USA) for 1 h at RT. Cells were then incubated with
Alexa488-conjugated anti-rabbit IgG and Alexa597-conjugated
anti-mouse IgG/IgM (Invitrogen) for 1 h at RT and counterstained
with Hoechst 33258. After three washes with PBS, cells were mounted
in Fluorescence Mounting Medium (Dako, Glostrup, Denmark).
Immunofluorescence was detected under a confocal microscope
(LSM-700; Carl Zeiss, Thornwood, NY, USA).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5 (GraphPad Software, La Jolla, CA, USA). All data were
analyzed using the Mann-Whitney U test and are expressed as the
mean ± SD of at least three independent experiments performed in
triplicate or quadruplicate. P-values <0.05 were considered to
indicate statistically significant results.
Results
Nope, E-cadherin and AFP mRNA
expression
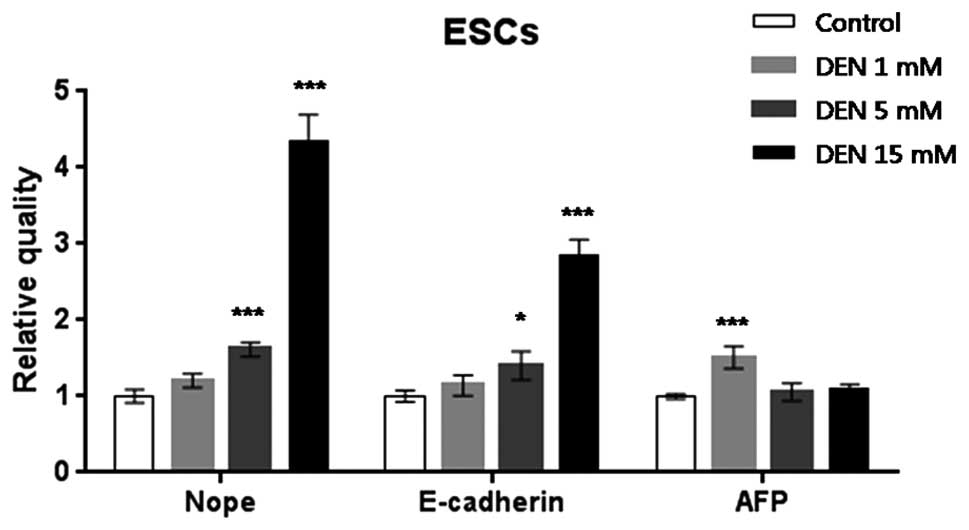
ESCs treated with 5 or 15 mM DEN expressed 1.5- or
4.5-fold greater levels of Nope, respectively, when compared with
normal ESCs (P<0.001). Expression level of E-cadherin increased
significantly by 3- and 1.3-fold in the ESCs treated with 5 or 15
mM DEN (P<0.05 and P<0.001, respectively). AFP mRNA
expression increased significantly 1.5-fold in the ESCs treated
with 1 mM DEN (P<0.001), as compared to the control ESCs
(Fig. 1).
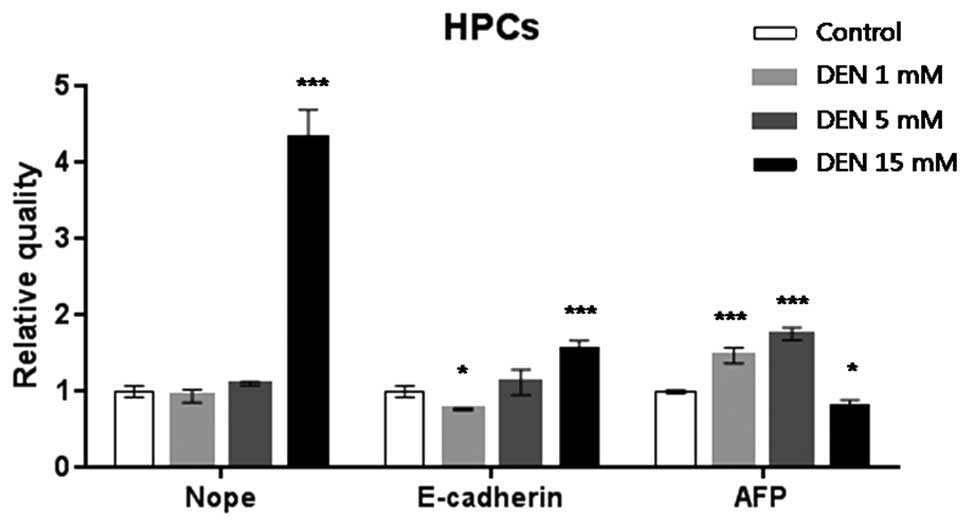
HPCs treated with 15 mM DEN expressed 4.5-fold
higher levels of Nope expression compared with the normal ESCs
(P<0.001). Expression of E-cadherin significantly increased
1.5-fold in the ESCs treated with DEN 15 mM (P<0.001). AFP mRNA
expression increased significantly 1.5- and 2-fold in the HPCs
treated with 1 and 5 mM DEN (P<0.001), respectively, relative to
the control HPCs (Fig. 2).
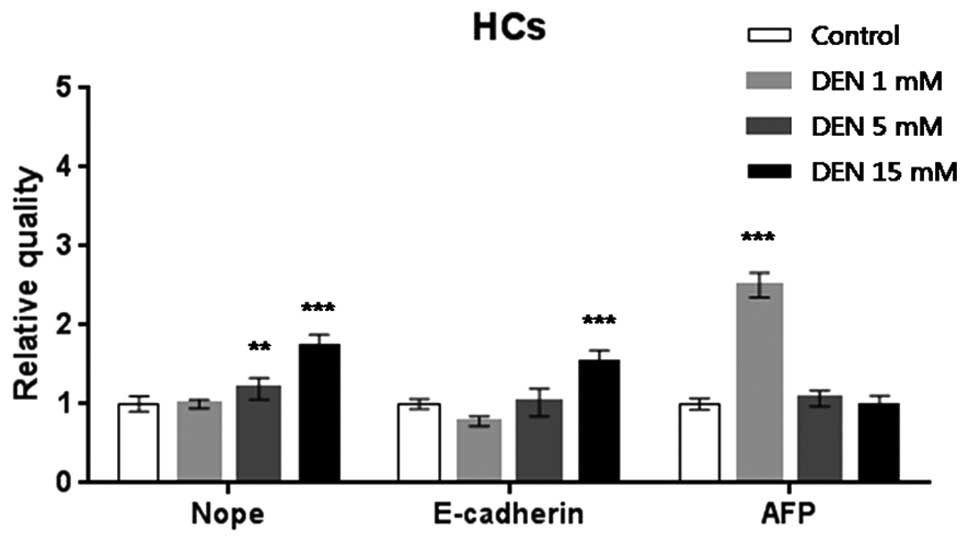
HCs treated with 5 or 15 mM DEN expressed 1.2- or
1.5-fold higher levels of Nope relative to the untreated HCs
(P<0.01, P<0.001, respectively). Expression of E-cadherin
increased by 3- and 1.3-fold in the HCs treated with 5 and 15 mM
DEN, respectively (P<0.001). AFP mRNA expression increased
significantly by 2.2-fold in the HCs treated with 1 mM DEN
(P<0.001) as compared to the control HCs (Fig. 3).
Expression of Nope and E-cadherin increased in a
dose-dependent manner with the concentration of DEN in the cultured
hepatic lineage cells. However, AFP mRNA expression increased only
at lower concentrations of DEN (1 or 5 mM).
Immunofluorescence staining of Nope and
E-cadherin
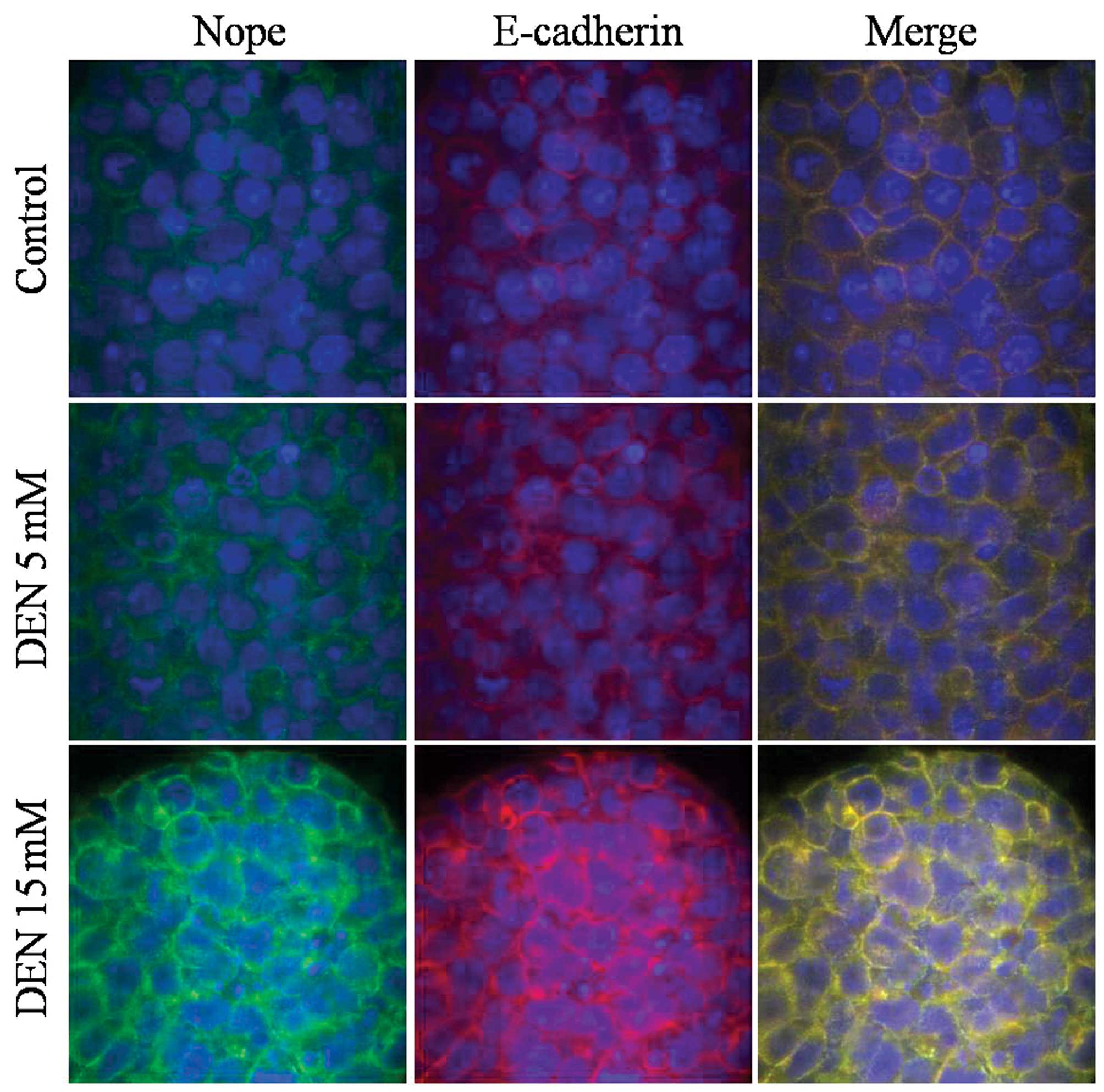
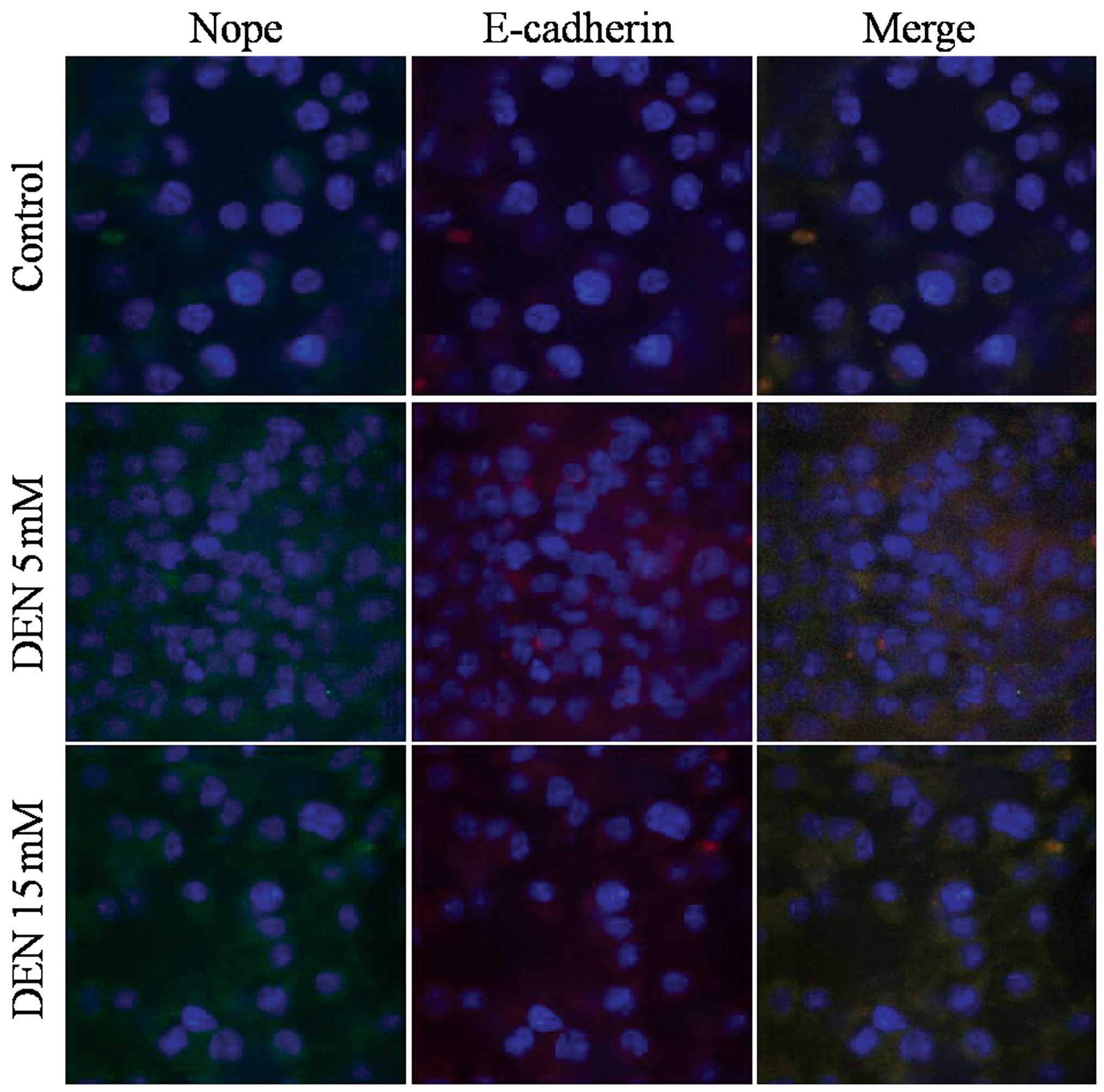
Immunofluorescence staining for Nope revealed
membranous expression in hepatic lineage cells derived from mouse
ESCs, as well as the co-expression of E-cadherin. Merged images
indicate that all cells were positive for both markers.
The cellular distribution of Nope was also compared
to that of E-cadherin induced by exposure to DEN. Nope was
specifically detected at the cell surface in cells treated with
high doses of DEN in the ESCs and HPCs.
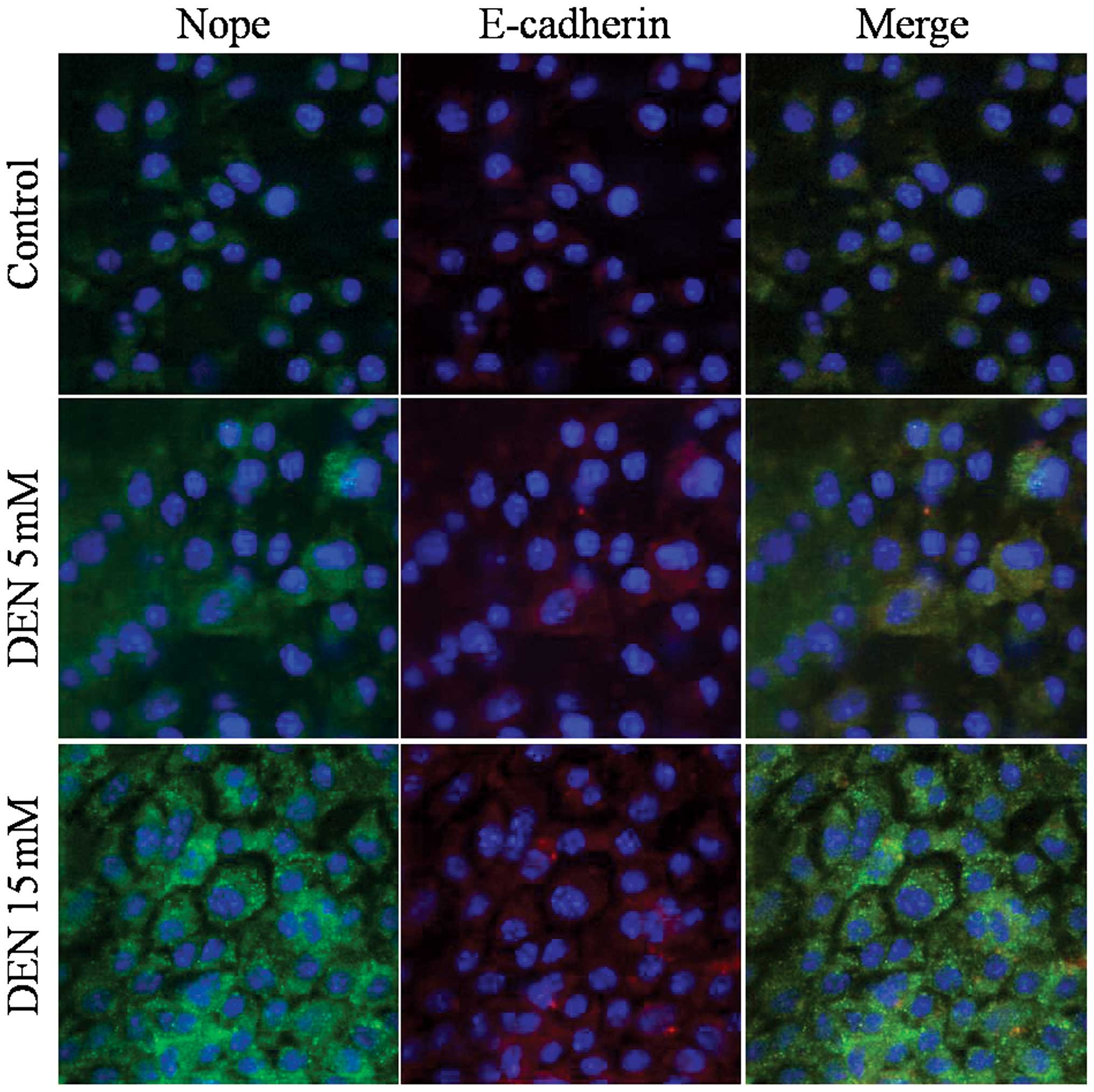
DEN treatment increased Nope and E-cadherin
expression in ESCs, HPCs and HCs (Figs.
4–6). Furthermore, in ESCs
treated with DEN, Nope expression not only increased but followed
the same cell membrane pattern as E-cadherin (Fig. 4). During differentiation into HPCs
and HCs, Nope and E-cadherin expression decreased, while DEN
treatment reversed this trend (Figs.
5 and 6).
Discussion
Given that no effective treatment exists for HCC,
and upon diagnosis, most patients with advanced disease have a
remaining life-span of 4–6 months, the early detection of HCC is
crucial (13). Serum AFP
concentrations are often elevated in patients with HCCs, but can
only be detected at advanced stages (14).
AFP, first described by Abelev et al in 1960,
is the most intensively investigated tumor marker for HCC. AFP is
an oncofetal glycoprotein with a molecular weight of 70 kDa and
unknown function (15). It is
physiologically expressed in fetal liver and pathologically
increased in the serum of many patients with HCC, but its
sensitivity and specificity are limited (60–70 and 80–90%,
respectively) (16,17). For many years, AFP was the only
marker available to identify HCC development in patients at high
risk. The combination with ultrasound improves the detection rate
of AFP, but a high percentage of patients with HCC do not present
with elevated AFP levels and vice versa. Although recent clinical
trials indicate that AFP is more sensitive in detecting HCC than
other newly identified markers, current guidelines no longer
recommend AFP for surveillance due to its unreliability (11).
Marquardt et al reported the potential of
Nope as an oncofetal surface marker for murine and human HCC, and
provided evidence for its specific expression in hepatoma cell
lines and primary HCC. Nope expression was selectively detected in
HCC specimens but not in normal liver or dysplastic lesions, and
increases in Nope expression coincided with the transformation of
preneoplastic lesions to malignant HCC. Nope was found to be
expressed at higher levels than AFP, and was 100% sensitive and
specific for HCC in the selected mouse model (12).
In the present study, expression of Nope was
independent of AFP expression in the DEN-treated hepatic lineage
cells from mouse ESCs. Although Nope expression increased with
greater concentrations of the carcinogen, AFP expression remained
low following treatment with lower concentrations of the
carcinogen. Nope was expressed at higher levels than AFP in the
hepatic lineage cells derived from mouse ESCs. Several studies have
not been able to detect increased AFP in dysplastic lesions and
early HCC; AFP expression appears to be specifically expressed by
advanced HCC (18–20). Thus, promising markers for the
specific detection of preneoplastic and early HCC are needed. Nope
seems to be a promising marker for specific, early detection of HCC
that could complement other markers.
Although initial studies to identify and enrich for
hepatoblasts or endodermal cells in the fetal liver relied on
combinations of known stem cell surface markers expressed by many
different cell types within the liver (21,22),
more recent studies have shown that Liv2 (23), δ-like 1 homolog (Dlk1)
(Drosophila) (24), and
E-cadherin (25,26) are specific cell surface markers of
fetal liver stem/progenitor cells (FLSPCs) within the developing
liver.
In the present study, we detected high expression
levels of Nope in DEN-treated hepatic lineage cells. DEN, a
well-known hepatocarcinogen, is widely used in mouse liver cancer
models (27–32). Expression of E-cadherin and AFP was
also highly elevated in DEN-treated cells as compared to untreated
cells. When the concentration of DEN was varied (1, 5 and 15 mM),
the mRNA expression of Nope and E-cadherin increased in a
dose-dependent manner. In contrast, mRNA expression of AFP did not
change at the highest concentration of DEN. Nope expression
patterns changed as cells differentiated into more defined hepatic
cell types. Furthermore, Nope-positive cells also expressed
E-cadherin, and merged images revealed that Nope was expressed only
in E-cadherin-positive cells, indicating that all Nope-positive
cells were of epithelial origin.
Clinically established screening methods for HCC
such as ultrasound and elevated serum levels of AFP have slightly
improved the accuracy of prognosis, but their sensitivity and
specificity are still limited, particularly at the early stages of
tumor development (7,33,34).
Moreover, clinically established tumor markers fail to detect up to
one-third of HCC cases. In addition to high sensitivity and
reliable detection of early stages in carcinogenesis, the
expression of ideal tumor markers should be limited in normal
tissue and correlate with disease stage. Apart from AFP, other
biomarkers, including AFP-L3, Golgi protein 73, and des-γ-carboxy
prothrombin (DCP), have been identified, and some show promising
results for both screening and the evaluation of prognosis
(35–38). However, none fulfills all the
aforementioned criteria, although combinations of several markers
might increase diagnostic power and prognostic reliability.
Therefore, the identification of additional, more reliable tumor
markers is particularly relevant (16,17).
The expression of other biomarkers in HCC samples
has been shown to generally be lower than that of Nope, and does
not reach statistical significance in comparison to AFP. Notably, a
previous study suggested that the expression levels of AFP and
GPC-3 are regulated by similar mechanisms (39).
DEN-treated cells were stained positive for Nope,
and Nope was specifically detected at the cell membrane during the
early stages of hepatocarcinogenesis by confocal microscopy.
Nope-positive cells co-expressed epithelial-specific E-cadherin.
Nope was significantly overexpressed in DEN-treated hepatic lineage
cells compared to untreated cells.
In conclusion, we identified that Nope may have
prognostic significance during early hepatocarcinogenesis. These
results indicate that Nope is a sensitive and specific marker for
the early stages of hepatocarcinogenesis, and may have a superior
detection rate compared to AFP. Nope is a novel oncofetal surface
marker for preneoplastic stages in which the commonly used marker
AFP is not yet overexpressed. This study contributes to cancer
research by examining Nope expression during the early stages of
hepatocarcinogenesis. Further investigations will concentrate on
the functional and prognostic significance of Nope following
treatment with other carcinogens.
Acknowledgements
We would like to thank Dr. Hwan-Goo Kang (Toxicology
and Residue Chemistry Division, Animal, Plant and Fisheries
Quarantine and Inspection Agency, MIFAFF) for his help and
comments. This study was supported by the Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Education, Science and Technology
(2012-0002704).
References
|
1
|
Greenhough S, Medine CN and Hay DC:
Pluripotent stem cell derived hepatocyte like cells and their
potential in toxicity screening. Toxicology. 278:250–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okura H, Komoda H, Saga A, et al:
Properties of hepatocyte-like cell clusters from human adipose
tissue-derived mesenchymal stem cells. Tissue Engineering Part C
Methods. 16:761–770. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamazaki T, Iiboshi Y, Oka M, et al:
Hepatic maturation in differentiating embryonic stem cells in
vitro. FEBS Lett. 497:15–19. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Touboul T, Hannan NR, Corbineau S, et al:
Generation of functional hepatocytes from human embryonic stem
cells under chemically defined conditions that recapitulate liver
development. Hepatology. 51:1754–1765. 2010. View Article : Google Scholar
|
|
5
|
Rambhatla L, Chiu C-P, Kundu P, Peng Y and
Carpenter MK: Generation of hepatocyte-like cells from human
embryonic stem cells. Cell Transplant. 12:1–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abelev GI, Perova SD, Khramkova NI,
Postnikova ZA and Irlin IS: Production of embryonal alpha-globulin
by transplantable mouse hepatomas. Transplantation. 1:174–180.
1963. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
8
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schievenbusch S, Schrammel T, Goeser T and
Nierhoff D: Neighbor of Punc E11 in the Mdr2-/- mouse
model: Novel marker of stem/progenitor cells in regenerating adult
liver. Hepatology. Wiley-Blackwell; MA: pp. 962A. 2011
|
|
10
|
Schievenbusch S, Sauer E, Curth HM, et al:
Neighbor of Punc E 11: expression pattern of the new hepatic
stem/progenitor cell marker during murine liver development. Stem
Cells. 21:2656–2666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salbaum JM and Kappen C: Cloning and
expression of nope, a new mouse gene of the immunoglobulin
superfamily related to guidance receptors. Genomics. 64:15–23.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marquardt JU, Quasdorff M, Varnholt H, et
al: Neighbor of Punc E11, a novel oncofetal marker for
hepatocellular carcinoma. Int J Cancer. 128:2353–2363. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He G, Dhar D, Nakagawa H, et al:
Identification of liver cancer progenitors whose malignant
progression depends on autocrine IL-6 signaling. Cell. 155:384–396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato Y, Nakata K, Kato Y, et al: Early
recognition of hepatocellular carcinoma based on altered profiles
of alpha-fetoprotein. N Engl J Med. 328:1802–1806. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sell S and Dunsford H: Evidence for the
stem cell origin of hepatocellular carcinoma and
cholangiocarcinoma. Am J Pathol. 134:13471989.PubMed/NCBI
|
|
16
|
Gebo KA, Chander G, Jenckes MW, et al:
Screening tests for hepatocellular carcinoma in patients with
chronic hepatitis C: a systematic review. Hepatology. 36:S84–S92.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gupta S, Bent S and Kohlwes J: Test
characteristics of α-fetoprotein for detecting hepatocellular
carcinoma in patients with hepatitis C: A systematic review and
critical analysis. Ann Intern Med. 139:46–50. 2003.
|
|
18
|
Marrero JA and Feng Z: Alpha-fetoprotein
in early hepatocellular carcinoma. Gastroenterology. 138:400–401.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Forner A, Reig M and Bruix J:
α-fetoprotein for hepatocellular carcinoma diagnosis: the demise of
a brilliant star. Gastroenterology. 137:26–29. 2009.
|
|
20
|
Marrero JA, Feng Z, Wang Y, et al:
Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound
alpha-fetoprotein in early hepatocellular carcinoma.
Gastroenterology. 137:110–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kubota H and Reid LM: Clonogenic
hepatoblasts, common precursors for hepatocytic and biliary
lineages, are lacking classical major histocompatibility complex
class I antigen. Proc Natl Acad Sci USA. 97:12132–12137. 2000.
View Article : Google Scholar
|
|
22
|
Suzuki A, Zheng YW, Kondo R, et al:
Flow-cytometric separation and enrichment of hepatic progenitor
cells in the developing mouse liver. Hepatology. 32:1230–1239.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Watanabe T, Nakagawa K, Ohata S, et al:
SEK1/MKK4-mediated SAPK/JNK signaling participates in embryonic
hepatoblast proliferation via a pathway different from
NF-kappaB-induced anti-apoptosis. Dev Biol. 250:332–347. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tanimizu N, Nishikawa M, Saito H,
Tsujimura T and Miyajima A: Isolation of hepatoblasts based on the
expression of Dlk/Pref-1. J Cell Sci. 116:1775–1786. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nierhoff D, Ogawa A, Oertel M, Chen YQ and
Shafritz DA: Purification and characterization of mouse fetal liver
epithelial cells with high in vivo repopulation capacity.
Hepatology. 42:130–139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nitou M, Sugiyama Y, Ishikawa K and
Shiojiri N: Purification of fetal mouse hepatoblasts by magnetic
beads coated with monoclonal anti-e-cadherin antibodies and their
in vitro culture. Exp Cell Res. 279:330–343. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rabes H: Development and growth of early
preneoplastic lesions induced in the liver by chemical carcinogens.
J Cancer Res Clin Oncol. 106:85–92. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang JS, Kang HG, Park YI, et al:
Expression of epithelial cell adhesion molecule and proliferating
cell nuclear antigen in diethylnitrosamine induced
hepatocarcinogenesis in mice. Exp Ther Med. 5:138–142.
2013.PubMed/NCBI
|
|
29
|
Kang JS: Expression of epithelial cell
adhesion molecule in early phase of hepatocarcinogenesis of mice
treated with diethylnitrosamine. J Biomed Res. 13:243–247.
2012.
|
|
30
|
Fausto N and Campbell JS: Mouse models of
hepatocellular carcinoma. Seminars in Liver Disease. Thieme Medical
Publishers; pp. 087–098. 2010, View Article : Google Scholar
|
|
31
|
Kang JS, Wanibuchi H, Morimura K, Gonzalez
FJ and Fukushima S: Role of CYP2E1 in diethylnitrosamine-induced
hepatocarcinogenesis in vivo. Cancer Res. 67:11141–11146. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bannasch P: Sequential cellular changes
during chemical carcinogenesis. J Cancer Res Clin Oncol. 108:11–22.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang BH, Yang BH and Tang ZY: Randomized
controlled trial of screening for hepatocellular carcinoma. J
Cancer Res Clin Oncol. 130:417–422. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song BC, Chung YH, Kim JA, et al:
Transforming growth factor-β1 as a useful serologic marker of small
hepatocellular carcinoma. Cancer. 94:175–180. 2002.
|
|
35
|
Li D, Mallory T and Satomura S: AFP-L3: a
new generation of tumor marker for hepatocellular carcinoma. Clin
Chim Acta. 313:15–19. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marrero JA, Su GL, Wei W, et al: Des-gamma
carboxyprothrombin can differentiate hepatocellular carcinoma from
nonmalignant chronic liver disease in American patients.
Hepatology. 37:1114–1121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Capurro M, Wanless IR, Sherman M, et al:
Glypican-3: a novel serum and histochemical marker for
hepatocellular carcinoma. Gastroenterology. 125:89–97. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marrero JA, Romano PR, Nikolaeva O, et al:
GP73, a resident Golgi glycoprotein, is a novel serum marker for
hepatocellular carcinoma. J Hepatol. 43:1007–1012. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morford LA, Davis C, Jin L, Dobierzewska
A, Peterson ML and Spear BT: The oncofetal gene glypican 3 is
regulated in the postnatal liver by zinc fingers and homeoboxes 2
and in the regenerating liver by alpha-fetoprotein regulator 2.
Hepatology. 46:1541–1547. 2007. View Article : Google Scholar : PubMed/NCBI
|