Introduction
Pancreatic cancer is the fourth leading cause of
cancer-related mortality in the US. Metastasis is the cause of
pancreatic cancer fatality. Approximately 80% of patients are
diagnosed with pancreatic cancer at a locally advanced or
metastatic stage (1,2). An approach that inhibits the
metastatic, invasive or cell migratory abilities of this cancer may
facilitate the development of an effective strategy for changing
the natural progress of this malignancy and producing marked
improvements in patient survival rates.
A common feature of the environment is the presence
of hypoxic areas within the tumor mass that develop when the high
proliferation rate of tumor cells outstrips vasculature
development. Hypoxia may be associated with the generation of a
more invasive phenotype of tumor cells and tumor cell dissemination
(3–5). Hypoxia-inducible factor-1α (HIF-1α) is
a central transcription factor that mediates hypoxia responsive
genes and has been widely accepted to play a critical role in tumor
invasion, metastasis, due to its increased cell survival,
angiogenesis and cell migration and invasion. However, HIF-1α has
broad influence on tumor biology and its new roles in the malignant
progression are still under investigation.
Hypoxia commonly results in autophagy which is
involved in the process whereby cells deliver their own protein and
organelle to lysosomes for degradation (6,7).
Autophagy is an evolutionarily conserved catabolic pathway and
facilitates the removal of ROS-altered mitochondria, and reduces
oxidative stress (8). It is well
known that upregulation of autophagy during hypoxia can favor tumor
cell survival and growth.
Epithelial-to-mesenchymal transition (EMT) phenomena
endow epithelial cells with enhanced migratory and invasive
potential and, as such, have been implicated in many physiological
and pathological processes requiring cell migration (9,10). In
cancer, EMT is a key process that contributes to cancer metastasis
and is marked by a loss of epithelial features (E-cadherin) and an
upregulation of mesenchymal properties (Vimentin and N-cadherin).
Recent studies have implicated EMT as a process that is associated
with cancer stem cells (CSCs) (11,12).
It is now evident that sustained metastatic growth requires the
dissemination of CSCs from the primary tumor followed by their
re-establishment in a secondary site.
In the present study, we examined the effect of
intermittent hypoxia on cell migration and expression of
CSC-related markers in pancreatic carcinoma cells under
intermittent hypoxic conditions including LC3-II and Beclin,
HIF-1α, E-cadherin, Vimentin and N-cadherin. We also investigated
the role of HIF-1α in the regulation of autophagy. Finally, we
examined the effect of HIF-1a induced autophagy on the expression
of CSC-related markers including E-cadherin, Vimentin and
N-cadherin.
Materials and methods
Cell culture
Panc-1 and BxPC3 were obtained from the Cell Bank of
China Academy of Sciences (Shanghai, China). They were maintained
in Dulbecco’s modified Eagle’s medium (DMEM)-F12 supplemented with
10% fetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 100
U/ml penicillin and 100 U/ml streptomycin. To propagate the
CSC-like fraction of the pancreatic cancer cells, culture
conditions favoring proliferation of undifferentiated cells were
used. We cultured the cells in serum-free DMEM-F12 medium
containing insulin (Gibco), EGF and FGF (PeproTech, Rocky Hill, NJ,
USA), B-27 (Gibco), in low-attachment dishes (Corning, Corning, NY,
USA). Cells were passaged with 0.25% trypsin/EDTA every 3 days.
Transwell assay
Cell migration assay was performed in Boyden chamber
using 8-μm pore size filters. Briefly, 1×104 viable
cells suspended in serum-free DMEM-F12 were allowed to migrate for
12 h toward DMEM-12 containing 10% FBS under the intermittent
hypoxia and normoxia conditions, respectively. At the end of the
assay, cells in the upper chamber and on the upper filter surface
were removed, whereas cells on the lower filter surface were fixed
with ethanol and stained with Giemsa. The number of migrating cells
was determined by counting cells in 10 random fields at ×200
magnification. All experiments were performed in triplicates.
Intermittent hypoxia and normoxia
environmental exposure
The bulk and migrated pancreatic cancer cells were
exposed to 5 cycles of hypoxia and normoxia. Each cycle consisted
of a period of 12 h in hypoxia followed by 12 h recovery under
normoxic conditions. The medium was changed during the
re-oxygenation period. For hypoxia induction, cells were cultured
in hypoxia chambers (Sanyo; containing 1% O2, 5%
CO2, 94% N2). Nitrogen gas was supplied to
the chambers to induce a controlled reduced percentage of oxygen.
For normoxia, cells were cultured in incubators containing 5%
CO2 and ~20% O2.
siRNA knockdown of HIF-1α gene and
chemical treatment
Pancreatic cancer cells were seeded in 12-well
plates. When the cell density reached 50% confluence, two
experiment conditions were established: i) the cells were treated
with increasing concentrations of deferoxamine CoCl2 (0,
200 and 400 μM; Sigma, United Kingdom) for 48 h; ii) the cells were
transfected with either 40 nmol/l control siRNA or HIF-1α-specific
siRNA (Suzhou Ribo Life Science Co., Ltd.). Transfections were
carried out according to the manufacturer’s instructions. Then, the
cells were put in intermittent hypoxic and normoxic conditions,
respectively. For the autophagy inhibition experiment, the cells
were treated with 30 μl of 3-methyladenine (3-MA) at the
concentration of 10 mM and continually cultured for 24 h.
Western blot analysis
Protein concentrations were determined by the BCA
method. Cell lysates were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene fluoride membranes (Merck Millipore, USA). Membranes
were blocked with 5% (w/v) bovine serum albumin (BSA) in TBST for 1
h at room temperature and incubated overnight with primary
antibodies at 4°C. They were subsequently incubated with
horseradish peroxidase-conjugated secondary antibodies for 2 h at
room temperature. Cell results were normalized to β-actin as
appropriate. The immunoreactive bands were detected by
chemiluminescence (ECL Plus; Merck Millipore) and relevant blots
were quantified by densitometry using Lane 1D software.
Immunoblotting with primary antibodies was as follows: rabbit
anti-human-E-ca, rabbit anti-human-Vimentin, rabbit
anti-human-N-ca, rabbit anti-human-Oct-4, mouse anti-human-Sox-2,
rabbit anti-human-HIF-1α, rabbit anti-human-LC3 and rabbit
anti-human-MMP-9. All antibodies were obtained from Cell Signaling
Technology, Inc. (Boston, MA, USA). Anti-β-actin was obtained from
Abcam (Cambridge, MA, USA). The secondary antibody preparations
either anti-rabbit or anti-mouse were purchased from Boster
Biotechnology Company (Wuhan, China).
Real-time PCR
Real-time quantitative PCR was carried out with
SYBR-Green qPCR SuperMix using the CFX-96 system (both from
Bio-Rad, Hercules, CA, USA). Total cellular RNA was isolated using
TRIzol reagent, and cDNA was synthesized from 1 μg of total RNA
using oligo(dT) and murine Moloney leukemia virus reverse
transcriptase (Toyobo, Japan). Relative expression levels of the
genes were calculated using the 2−ΔΔCT method.
Flow cytometry analysis
To quantify the stem-like cells of the migrated
pancreatic cancer cells under intermittent hypoxic and normoxic
conditions, we measured the expression of the stem-related
molecular marker CD133 using anti-CD133-PE (Miltenyi Biotec Ltd.,
Surrey, UK). Cells (5×106) were harvested, disaggregated
to a single cell suspension and stained with mouse anti-human CD133
PE. The antibody was incubated for 30 min at 4°C in the dark.
Following incubation, the samples were washed with PBS and analyzed
by FACSAria II (Becton-Dickinson, USA).
Statistical analysis
The significance of differences between groups was
analyzed using the Student’s t-test, one-way or two-way ANOVA.
Values of P<0.05 were considered to indicate a statistically
significant difference. All experiments were performed at least in
triplicate.
Results
Intermittent hypoxia increases the in
vitro migration potential and promotes EMT in pancreatic cancer
cells
It was reported that hypoxia is strongly associated
with tumor metastasis. Firstly, we evaluated the migration ability
of pancreatic cancer cells under intermittent hypoxic conditions.
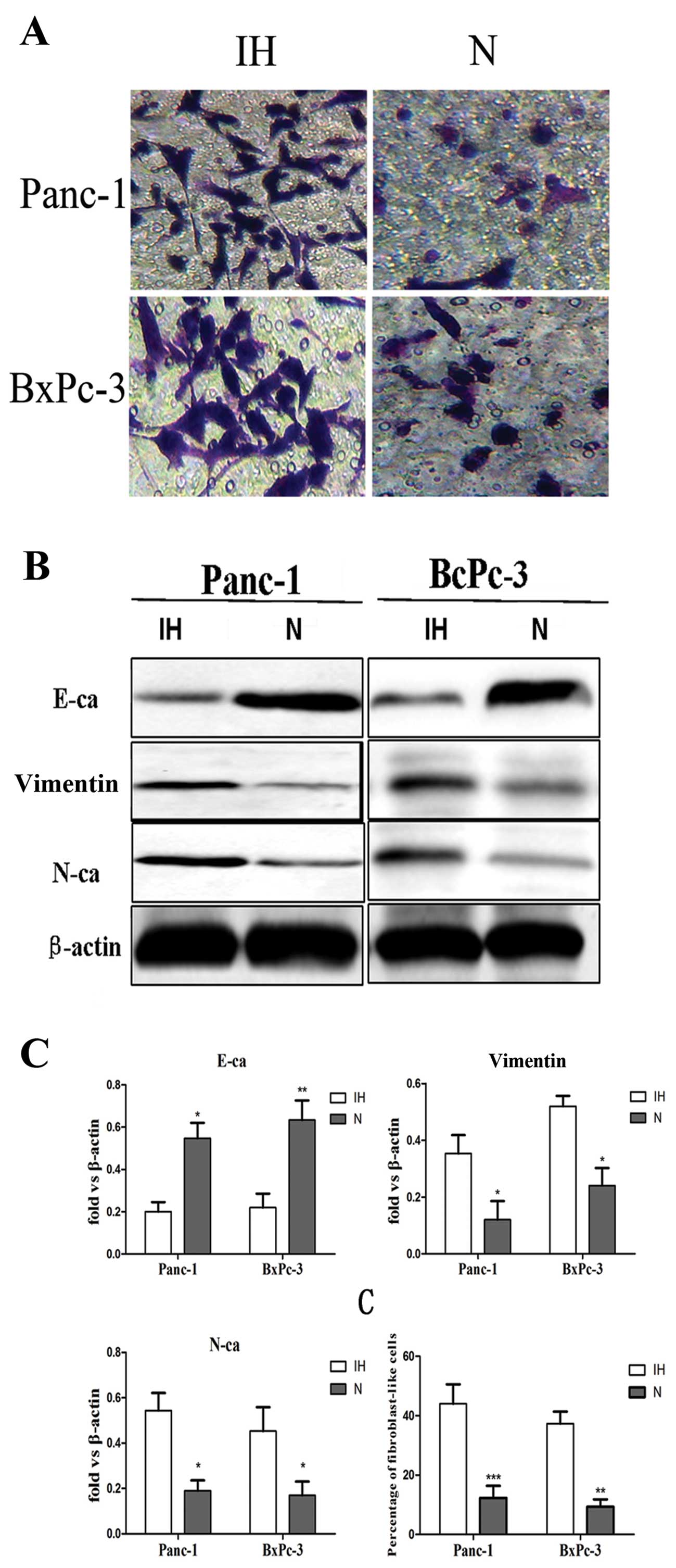
Transwell assay results revealed that the number of Panc-1 and
BxPC-3 cells under intermittent hypoxic conditions that passed
through the membrane was 4-fold higher than the number of cells
under normoxic conditions (Fig.
1A), suggesting that intermittent hypoxia promotes the invasive
activity of pancreatic carcinoma cells. Secondly, to illustrate
whether initiation of EMT was induced by intermittent hypoxia, we
evaluated the expression level of EMT-related markers altered in
the cells. The results demonstrated that expression of E-cadherin
was significantly reduced in Panc-1 and PC-3 cells. In contrast,
expression of Vimentin and N-cadherin were significantly increased
in these cells (Fig. 1B).
Furthermore, the percentage of fibroblast-like cells of Panc-1 and
BxPC-3 cells under hypoxic conditions was higher than the cells
cultured under normoxic conditions which often show an
epithelial-like appearance (Fig.
1C).
Intermittent hypoxia maintains stem cell
properties and the expression of CSC markers in pancreatic
carcinoma cells
It is widely accepted that CSCs are highly
associated with tumor growth, invasion and metastasis, and are
commonly considered one of the major causes of tumor recurrence and
relapse. The surface markers CD133 have been well defined for
isolating and identifying CSCs from pancreatic cancer cells
(13). The migrated Panc-1 cells
were digested and re-cultured in the serum-free medium (SFM) under
the intermittent hypoxic and normoxic conditions, respectively. The
total Panc-1 cells cultured in normal medium (DMEM-F12 plus 10%
FBS) under the normoxic conditions were used as the control group.
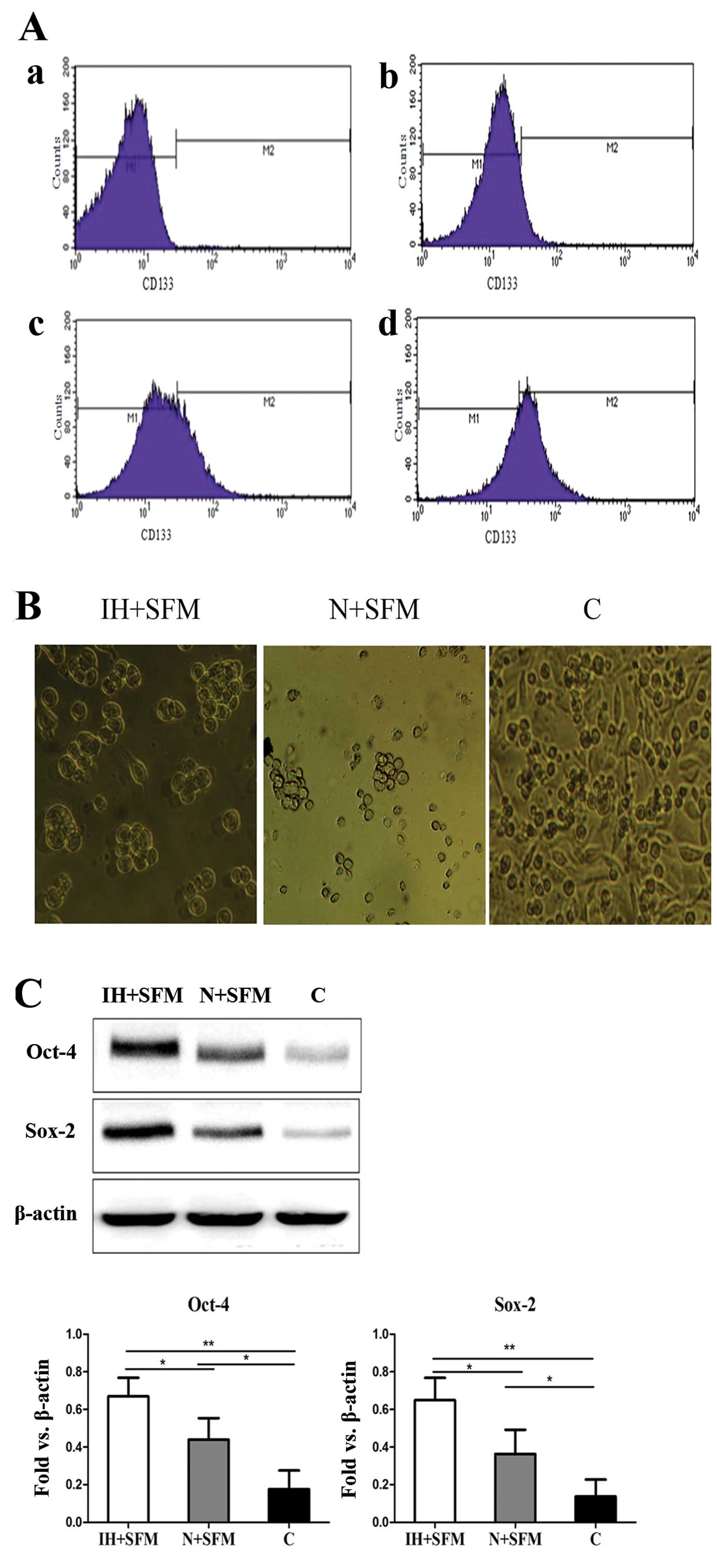
Flow cytometry analysis assay was used to quantify the
CD133+ subpopulation. Cell quantification indicated that
65±5% of the migrated cells cultured in the serum-free medium under
the intermittent hypoxic conditions were positive for CD133,
whereas 30±3% of the migrated cells cultured in the serum-free
medium under normoxic conditions were positive for CD133 and 10±3%
for the control group (Fig. 2A). We
next evaluated the role of intermittent hypoxia in the self-renewal
capacity of the migrated cells using the sphere-formation assay. It
successfully obtained the percentage of the spheres in the
intermittent hypoxic conditions was much higher than the cells
under the normoxic conditions. The migrated cells cultured in the
serum-free medium under normoxic conditions formed only small
irregular aggregates. Also, the total cells cultured in the normal
medium under normoxic conditions were grown in the adherent way and
no sphere was found (Fig. 2B). The
western bolt assay was conducted to examine the effect of
intermittent hypoxia on CSC signature proteins in the migrated
cells. It was clearly found that expression of Oct-4 and Sox-2 was
upregulated in intermittent hypoxia conditioned tumor cells
(Fig. 2C). Similar results were
acquired in the BxPC-3 cell line (data not shown). These data
indicate that the migrated pancreatic cancer cell lines with stem
cell-like properties were enriched and maintained under
intermittent hypoxic conditions.
Intermittent hypoxia upregulates the
expression level of HIF-1α and induces autophagy in pancreatic
cancer cells
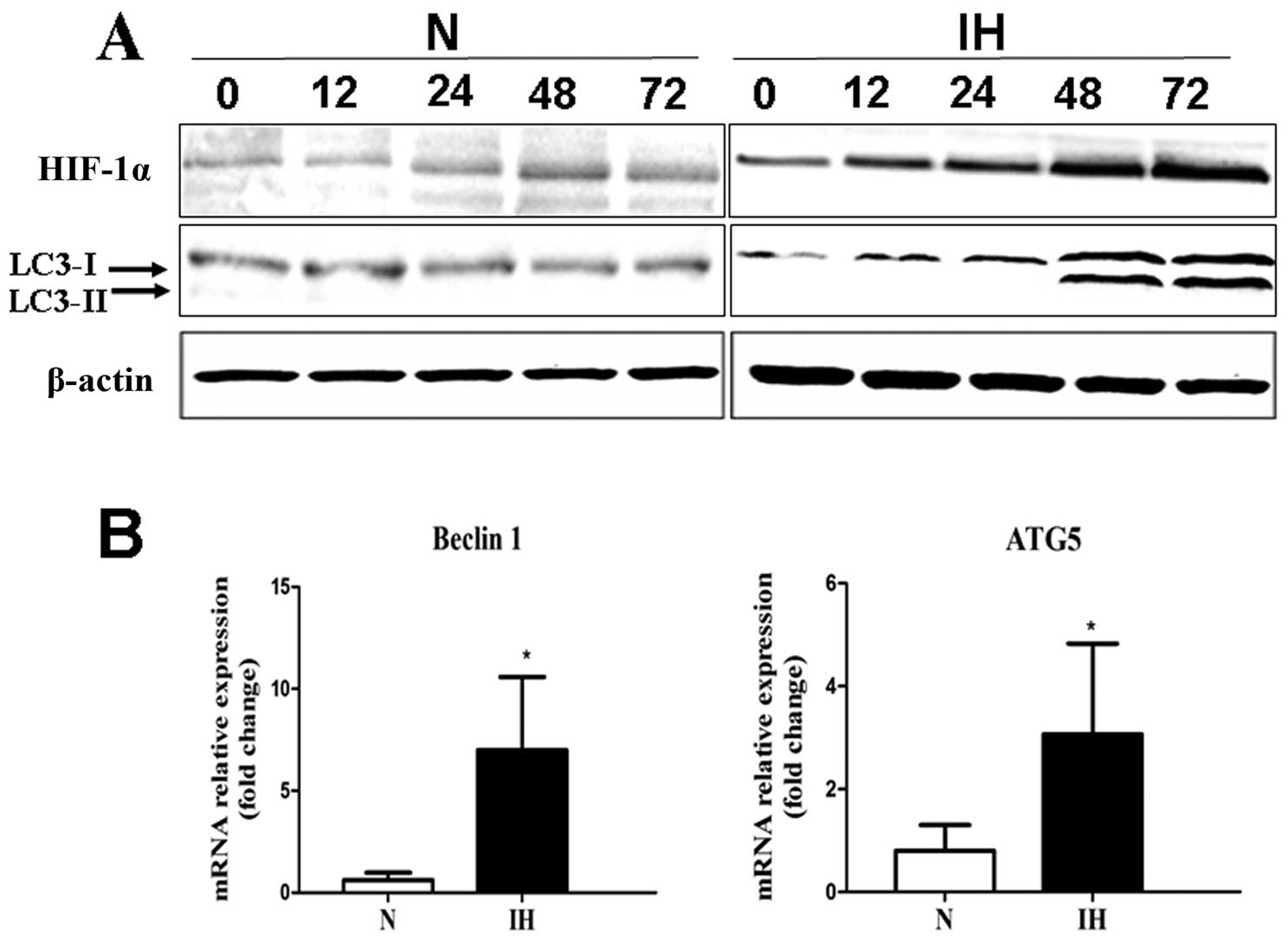
The migrated Panc-1 cells were cultured under
intermittent hypoxic conditions for various time periods (0, 12,
24, 48 and 72 h). The results confirmed that HIF-1α was rapidly
induced in migrated cells under intermittent hypoxic conditions by
western blotting. Also, HIF-1α protein level increased in a
time-dependent manner (Fig. 3A). To
assess whether intermittent hypoxia affects the level of autophagy,
we evaluated mRNA expression of Beclin-1, ATG5 in pancreatic cancer
cells under intermittent hypoxic conditions by RT-PCR. The
pancreatic cancer cells cultured under normoxic conditions were
used as control. Beclin-1 and ATG5 are all involved in
autophagosome formation. Higher expression of Beclin-1 and ATG5 was
detected by RT-PCR in the intermittent hypoxia group (Fig. 3B). LC3 was used as a measure of
downstream autophagy activation. Both cell lines under intermittent
hypoxic conditions showed enhanced expression of LC3-II protein and
expressed a higher LC3-II/LC3-I ratio compared to the cells under
the normoxic conditions (Fig.
3A).
Higher level autophagy is associated with
upregulated HIF-1α
It was confirmed that HIF-1 plays a critical role in
the cellular transcriptional response to hypoxia. Having
demonstrated that the level of autophagy and HIF-1α was elevated
together under the intermittent hypoxic conditions, we further
investigated the association between HIF-1α induction and autophagy
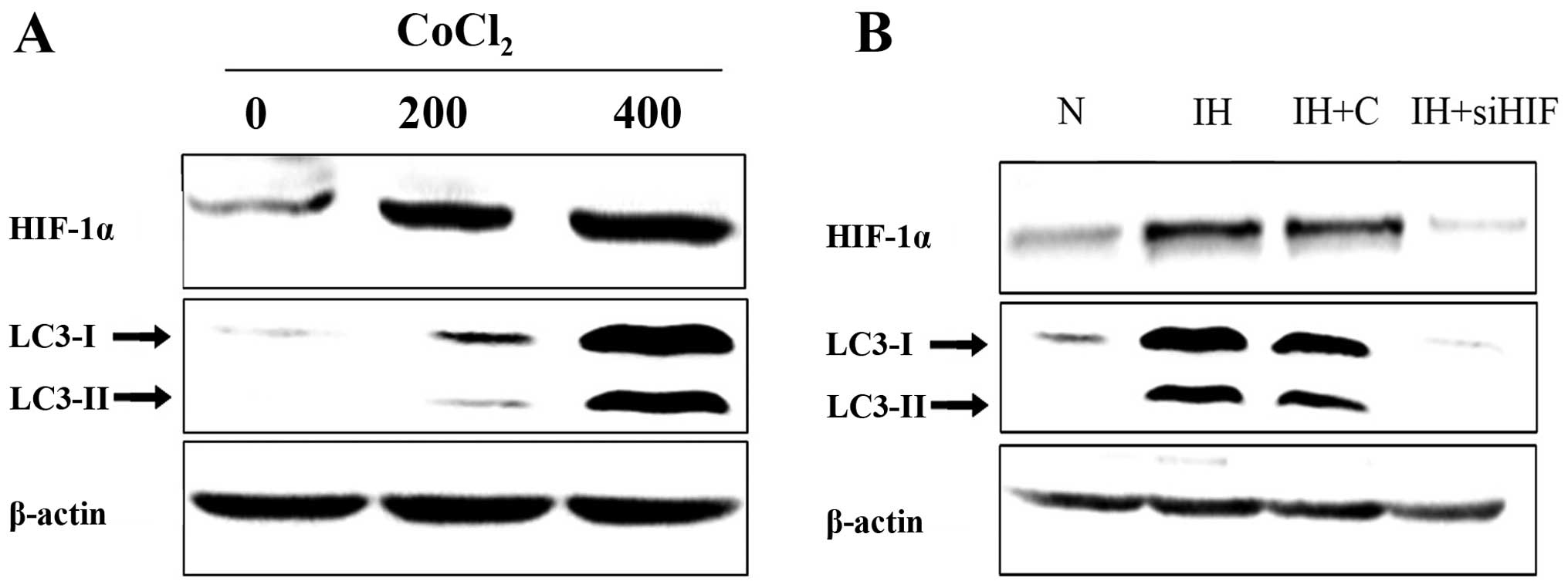
upregulation. To examine whether HIF-1α mediates hypoxia-induced
autophagy enhancement, pancreatic cancer cells were treated with
increasing concentrations of Cobalt chloride (CoCl2,
hypoxia surrogates) for 48 h. Western blot assay was employed to
assess the protein level changes. Results confirmed that
CoCl2 treatment induced HIF-1α and autophagy-related
protein expression level upregulation. The change occurred in a
dose-dependent manner (Fig. 4A).
Furthermore, suppression of HIF-1α expression by siRNA in
pancreatic cancer cells abolished hypoxia-induced autophagy
upregulation (Fig. 4B).
Hypoxia-induced EMT phenotype is mediated
by HIF-1a and autophagy
Given that intermittent hypoxia can promote the
migration ability of pancreatic cancer cells and induce EMT, we
next investigated whether HIF-1α-induced autophagy has an effect on
invasion and EMT in pancreatic cancer cells. Under intermittent
hypoxic conditions, we carried out the knockdown of HIF-1α
expression in pancreatic cancer cells by siRNA and inhibition of
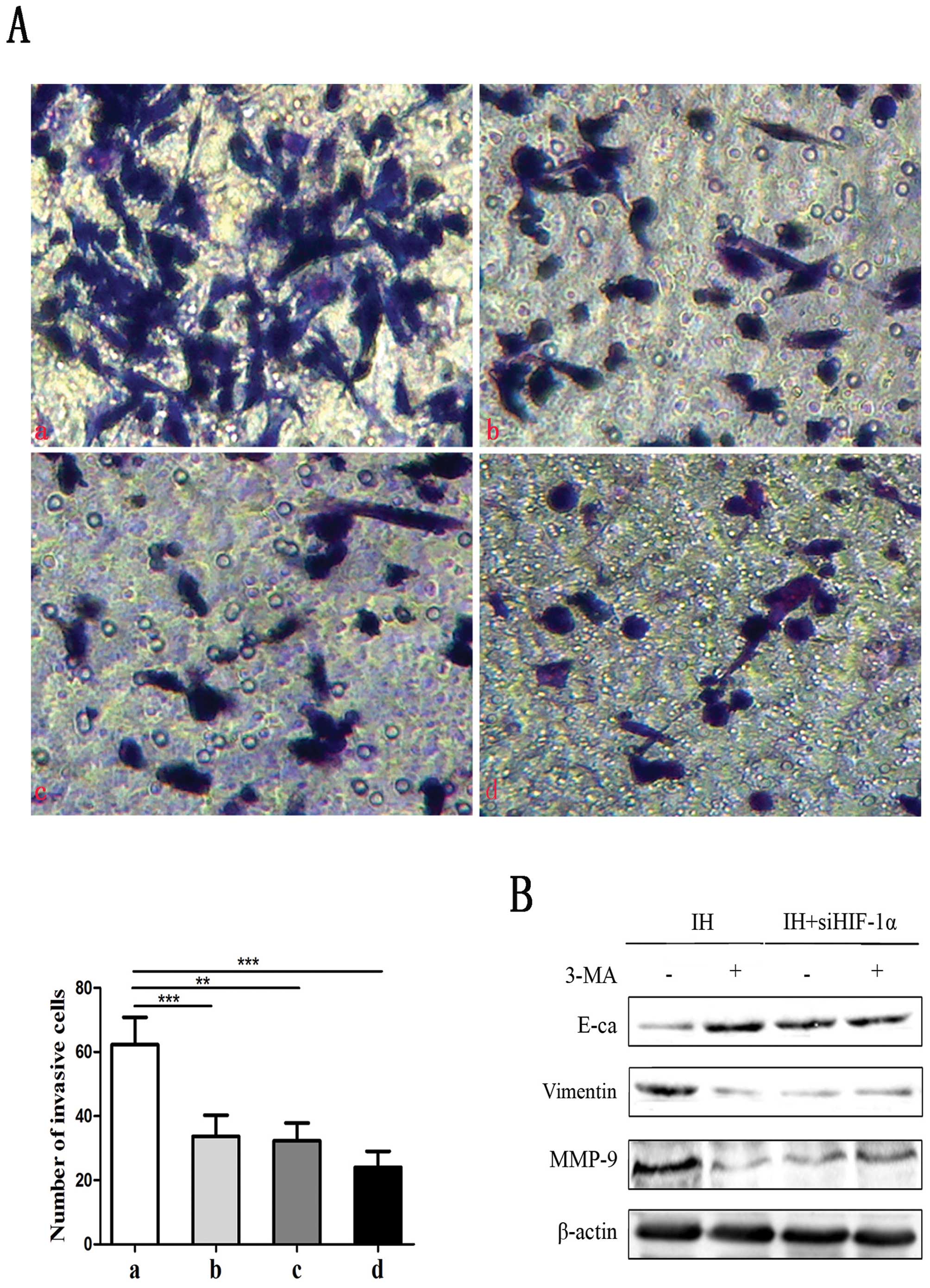
autophagy with 3-MA (the inhibitor of autophagy). As shown in
Fig. 5A, HIF-1α siRNA with or
without 3-MA treatment inhibited the capacity of intermittent
hypoxic-induced invasion of both Panc-1 and BxPC-3 cells by in
vitro invasion assay. Results demonstrated that the percentage
of the cells whose morphology appeared to change from a
fibroblast-like to an epithelial-like appearance was decreased
after the inhibition. Also, the expression level of E-cadherin was
upregulated, and Vimentin and MMP-9 protein levels were
downregulated (Fig. 5B). These data
suggest that HIF-1α-induced autophagy promotes hypoxia-induced cell
migration and invasion of human pancreatic cancer cells.
Discussion
Pancreatic carcinoma remains one of the most
aggressive diseases with only a modest improvement in 5-year
survival rates with new and improved cancer therapies. Most deaths
due to pancreatic cancer are correlated with metastasis of the
primary tumor rather than development of the primary tumor, yet our
understanding of this complex problem remains limited. As such,
gaining an understanding of pancreatic cancer metastasis could
considerably assist in selecting the appropriate therapy strategy
and may increase patient survival rates.
Metastasis mainly consists of four steps; namely,
primary tumor cells enter the circulatory system, survival of
circulating tumor cells (CTCs), movement from the circulation into
a secondary tissue, and tumor growth at a secondary site (14). An emerging concept for metastasis is
that cellular plasticity is associated with EMT. EMT is defined as
a biologic process that allows a polarized epithelial cell, that
normally interacts with the basement membrane via its basal
surface, to undergo multiple biochemical changes that enable it to
assume a mesenchymal cell phenotype, which includes enhanced
migratory capacity, invasiveness, elevated resistance to apoptosis,
and greatly increased production of extracellular matrix components
(15–17). In addition to weakening cell-cell
adhesion, EMT provides tumor cells with an enhanced ability to
degrade the extracellular matrix, a property which favors cell
invasion and intravasation. Indeed, EMT can induce the expression
of proteases, such as different MMPs, that can degrade the basement
membrane (18). Although the
involvement of EMT processes in the metastatic cascade remains a
subject of debate, an increasing number of studies demonstrate
their involvement in increased cell migration and invasion. Our
results confirmed that intermittent hypoxia-induced EMT enhanced
the pancreatic cancer cell migratory capacity, illustrating the
importance of epithelial-mesenchymal plasticity as a
metastasis-promoting property. CSCs, which comprise a small
fraction of cancer cells, are believed to constitute the origin of
most human tumors (19). Several
studies also suggest that CSCs serve as the basis of metastases.
Our results demonstrated that CSCs were enriched in the migrated
pancreatic cancer cells which were EMT-induced. EMT programs may
provide a ready source of CSCs by enabling the dedifferentiation of
more epithelial cells within carcinomas.
Tumor metastasis is driven not only by the
accumulation of intrinsic alterations in malignant cells, but also
by the interactions of cancer cells with the tumor microenvironment
in which these cells are located, the niches (vascular
proliferations, hypoxia/necrosis) (20–22).
Hypoxia is a common phenomenon in malignant tumors including
pancreatic carcinoma. Hypoxia is created in a tumor when the
O2 consumption outweighs the O2 supply. Many
studies have demonstrated that hypoxia generally correlates with
tumor progression and metastasis (5,23,24).
However, the molecular mechanism of hypoxia-mediated invasion and
metastasis remains poorly understood. Several previous studies
carried out experiments by acute or chronic hypoxia, but in the
present study we used intermittent hypoxia which is characterized
by cyclic periods of hypoxia and re-oxygenation. Intermittent
hypoxia is described as more representative of the oxygen tension
of the environment in tumors than a permanent exposure to low
oxygen levels.
Tumor hypoxia has been reported to influence the
tumor progression through the EMT process by regulation of a number
of key factors. It has been observed that hypoxia-induced factor
(HIF-1α), a key effector of hypoxia, activates Twist, Snail and
SIP1 expression, thereby leading to E-cadherin repression (25–27).
In the present study, we found that the upregulated HIF-1α induced
the EMT process in pancreatic cancer cells which was consistent
with the previous study. However, the complex relationship between
HIF-1α, EMT and metastasis remains to be delineated. There are
findings that implicate the importance of hypoxia and HIF-1α in the
induction of cancer metastasis beyond angiogenesis.
Cancer cells face diverse stresses, environmental
and cellular, during every step of metastatic progression. To cope
with this, tumor cells induce adaptive pathways, such as autophagy.
Autophagy is an evolutionarily conserved catabolic pathway that
degrades long-lived cellular macromolecules and can protect cells
during various types of stress. It is involved in the process that
cells deliver their own protein and organelle to lysosomes for
degradation (28–30). A series of autophagy-related genes
(Beclin-1, ATGs) regulate the process of autophagy (31). Signaling involves the conversion of
LC3-I to LC3-II by binding to the membrane of autophagosomes after
these vacuoles are formed (32).
Indeed, autophagy has been found to be upregulated in cancer cells
during many of the principal events directing metastasis, by which
cells adapt their metabolism to the stresses induced by starvation,
hypoxia, radiation or chemotherapeutic agents, thus allowing cells
to evade apoptosis (32,33). In the present study, we found that
autophagy can be induced by HIF-1α under hypoxic stress.
Upregulated autophagy further enhanced the EMT process and the
migration ability in pancreatic cell lines. However, the function
of HIF-1α and their potential downstream targets in tumor autophagy
require further clarification.
In the present study, we reported a model of EMT and
metastasis that is generated by cooperation of tumor
microenvironment with intrinsic genetic changes within a developing
cancer cell.
Acknowledgements
This study was supported by the Natural Science
Foundation of Jiangsu Province (grant no. BK2011487), the Social
Development Foundation of Zhenjiang City (grant nos. SH2012028 and
SH2013026), the National Natural Science Foundation of China (grant
nos. 81001319 and 81370084, 81101677), the Postdoctoral foundation
of China (2012M511705, 2013T60508), the Postdoctoral foundation of
Jiangsu Province (1102129C), the Natural Science Foundation of
Colleges and Universities in Jiangsu Province (grant no.
10KJB310003), and the High-Tech of Jiangsu University (grant no.
11JDG128).
Abbreviations:
|
CSCs
|
cancer stem cells
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
PCR
|
polymerase chain reaction
|
|
CD133
|
cluster of differentiation 133
|
|
CD44
|
cluster of differentiation 44
|
|
3-MA
|
3-methyladenine
|
|
OCT-4
|
octamer-binding transcription
factor-4
|
References
|
1
|
Prasad R and Katiyar SK: Grape seed
proanthocyanidins inhibit migration potential of pancreatic cancer
cells by promoting mesenchymal-to-epithelial transition and
targeting NF-κB. Cancer Lett. 334:118–126. 2013.PubMed/NCBI
|
|
2
|
Hafez HZ: Cutaneous pancreatic metastasis:
a case report and review of literature. Indian J Dermatol.
53:206–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilkes DM and Semenza GL: Role of
hypoxia-inducible factors in breast cancer metastasis. Future
Oncol. 9:1623–1636. 2013. View Article : Google Scholar
|
|
4
|
Abaza M and Luqmani YA: The influence of
pH and hypoxia on tumor metastasis. Exp Rev Anticancer Ther.
13:1229–1242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eisinger-Mathason TS, Zhang M, Qiu Q, et
al: Hypoxia-dependent modification of collagen networks promotes
sarcoma metastasis. Cancer Discov. 3:1190–1205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Z and Klionsky DJ: An overview of the
molecular mechanism of autophagy. Curr Top Microbiol Immunol.
335:1–32. 2009.PubMed/NCBI
|
|
7
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levine B: Eating oneself and uninvited
guests: autophagy-related pathways in cellular defense. Cell.
120:159–162. 2005.PubMed/NCBI
|
|
9
|
Iwatsuki M, Mimori K, Yokobori T, et al:
Epithelial-mesenchymal transition in cancer development and its
clinical significance. Cancer Sci. 101:293–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryu HS, Park do J, Kim HH, Kim WH and Lee
HS: Combination of epithelial-mesenchymal transition and cancer
stem cell-like phenotypes has independent prognostic value in
gastric cancer. Hum Pathol. 43:520–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonnomet A, Brysse A, Tachsidis A, et al:
Epithelial-to-mesenchymal transitions and circulating tumor cells.
J Mammary Gland Biol Neoplasia. 15:261–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee HJ, You DD, Choi DW, et al:
Significance of CD133 as a cancer stem cell marker focusing on the
tumorigenicity of pancreatic cancer cell lines. J Korean Surg Soc.
81:263–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang MH, Hsu DS, Wang HW, et al: Bmi1 is
essential in Twist1-induced epithelial-mesenchymal transition. Nat
Cell Biol. 12:982–992. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pham CG, Bubici C, Zazzeroni F, et al:
Upregulation of Twist-1 by NF-κB blocks cytotoxicity induced by
chemotherapeutic drugs. Mol Cell Biol. 27:3920–3935.
2007.PubMed/NCBI
|
|
17
|
Peyri N, Berard M, Fauvel-Lafeve F, et al:
Breast tumor cells transendothelial migration induces endothelial
cell anoikis through extracellular matrix degradation. Anticancer
Res. 29:2347–2355. 2009.
|
|
18
|
Sounni NE and Noel A: Membrane type-matrix
metalloproteinases and tumor progression. Biochimie. 87:329–342.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fessler E, Dijkgraaf FE, De Sousa E, Melo
F and Medema JP: Cancer stem cell dynamics in tumor progression and
metastasis: is the microenvironment to blame? Cancer Lett.
341:97–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Filatova A, Acker T and Garvalov BK: The
cancer stem cell niche(s): the crosstalk between glioma stem cells
and their microenvironment. Biochim Biophys Acta. 1830:2496–2508.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Christensen K, Schrøder HD and Kristensen
BW: CD133+ niches and single cells in glioblastoma have
different phenotypes. J Neurooncol. 104:129–143. 2011.
|
|
22
|
Hjelmeland AB, Wu Q, Heddleston JM, et al:
Acidic stress promotes a glioma stem cell phenotype. Cell Death
Differ. 18:829–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bar EE, Lin A, Mahairaki V, Matsui W and
Eberhart CG: Hypoxia increases the expression of stem-cell markers
and promotes clonogenicity in glioblastoma neurospheres. Am J
Pathol. 177:1491–1502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Evans SM, Judy KD, Dunphy I, et al:
Hypoxia is important in the biology and aggression of human glial
brain tumors. Clin Cancer Res. 10:8177–8184. 2004. View Article : Google Scholar
|
|
25
|
Evans AJ, Russell RC, Roche O, et al: VHL
promotes E2 box-dependent E-cadherin transcription by HIF-mediated
regulation of SIP1 and snail. Mol Cell Biol. 27:157–169. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang MH, Wu MZ, Chiou SH, et al: Direct
regulation of TWIST by HIF-1α promotes metastasis. Nat Cell Biol.
10:295–305. 2008.
|
|
27
|
Sullivan R and Graham CH: Hypoxia-driven
selection of the metastatic phenotype. Cancer Metastasis Rev.
26:319–331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Degenhardt K, Mathew R, Beaudoin B, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Macintosh RL and Ryan KM: Autophagy in
tumour cell death. Semin Cancer Biol. 23:344–351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Johansen T and Lamark T: Selective
autophagy mediated by autophagic adapter proteins. Autophagy.
7:279–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kenific CM, Thorburn A and Debnath J:
Autophagy and metastasis: another double-edged sword. Curr Opin
Cell Biol. 22:241–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lock R and Debnath J: Extracellular matrix
regulation of autophagy. Curr Opin Cell Biol. 20:583–588. 2008.
View Article : Google Scholar : PubMed/NCBI
|