Introduction
Esophageal squamous cell carcinoma (ESCC) is an
aggressive disease characterized by high mortality rates.
Epidemiological studies indicate that ESCC is the fifth most common
cause of cancer-related mortality in men (1). Despite significant improvements in
diagnosis and treatment including surgery, chemotherapy and
radiotherapy, patient prognosis remains poor, with a 5-year
survival rate of 15–34% (2–4). Systemic chemotherapy with cisplatin
and 5-fluorouracil (5-FU) is the most commonly used treatment
regimen for advanced ESCC. However, response rates are low at
15–45% and median survival is generally <8 months (5,6).
Therefore, novel therapeutic agents are urgently needed to improve
clinical outcomes.
Epidermal growth factor receptor (EGFR) is the most
extensively studied of the ErbB receptors in relation to cancer.
EGFR is overexpressed in 60–70% of ESCC cases and EGFR gene
amplification was detected in approximately 28% of tumors by
fluorescence in situ hybridization (7). Activated EGFR signals via the AKT, ERK
and RAS pathways and has an essential role in the control of many
fundamental cellular processes. As such, the inhibition of EGFR
signaling has emerged as an important antitumor treatment strategy.
Cetuximab (also known as Erbitux or C225) is a recombinant chimeric
human-murine monoclonal antibody and is among the most promising
and clinically effective of these agents (8–10).
Cetuximab binds EGFR with high affinity and prevents receptor
activation, thereby suppressing proliferation and angiogenesis and
promoting antibody-dependent cellular toxicity (11). When administered in conjunction with
the platinum-based chemotherapy drug cisplatin, treatment with
cetuximab resulted in a longer overall survival time in patients
with recurrent or metastatic head and neck SCC (HNSCC) (12). However, despite several clinical
trials for cetuximab and cisplatin, the precise role of these
agents in the regulation of the EGFR signaling pathway is
unclear.
The present study investigated the molecular
mechanisms of cetuximab and cisplatin in two ESCC cell lines in the
regulation of EGF signaling and assessed the potential for
combination therapy in vitro and in vivo.
Materials and methods
Reagents and antibodies
The humanized mouse anti-human EGFR antibody
cetuximab was purchased from Merck (Dietikon, Switzerland) and
cisplatin was obtained from Dong-A PharmTech Co., Ltd. (Seoul,
Korea). Primary antibodies against EGFR, phosphorylated p-EGFR,
AKT, p-AKT, ERK and p-ERK were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA) and Cell Signaling Technology
(Boston, MA, USA). Horseradish peroxidase (HRP)-conjugated
secondary antibody was from Santa Cruz Biotechnology.
Cell culture
Human ESCC cell lines TE-1, -2, -4, -5, -6, -8, -9,
-10, -11, -14 and -15 were obtained from RIKEN (Tsukuba, Japan) and
the human primary esophageal epithelial cell line Het-1A was
purchased from American Type Culture Collection (Manassas, VA,
USA). Cells were cultured in RPMI-1640 medium supplemented with 10%
(v/v) fetal bovine serum (FBS) and antibiotics (100 U/ml penicillin
and 100 μg/ml streptomycin) at 37°C under 5% CO2 and 95%
air.
Cell viability assay
Cells were seeded at 4×103/well in
96-well plates in 0.1 ml medium for 24 h before treatment. Cells
were exposed to a range of concentrations of cetuximab, cisplatin,
or both in the presence of 0.5% FBS. At the indicated time points,
CellTiter 96 AQueous (MTS) solution (Promega, Madison, WI, USA) in
serum-free medium was added to each well. After 2 h, the colored
MTS product in the supernatant was measured using a microplate
reader (BioTek, Winooski, USA) at 490 nm absorbance.
Western blot analysis
Protein was extracted from cells and frozen tissues
using RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA)
containing protease inhibitors (Roche, Basel, Switzerland). Lysates
containing equivalent amounts of protein were resolved by 10%
SDS-PAGE. Samples were transferred to nitrocellulose membranes,
which were blocked in 5% skim milk in TBS containing 0.1% Tween-20.
Membranes were probed overnight at 4°C with primary antibodies
against EGFR (1:3,000), p-EGFR (1:1,000), AKT, p-AKT (both
1:1,000), ERK (1:1,000) and p-ERK (1:1,000); the following day,
HRP-conjugated secondary antibody (1:5,000) was applied for 1 h at
room temperature. Protein was visualized using the enhanced
chemiluminescence detection system (Thermo Fisher Scientific).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tumor tissue
samples were sectioned at a thickness of 4 μm and mounted on glass
slides. Endogenous peroxidase was blocked by treating sections with
0.3% H2O2 for 30 min. Antigen retrieval was
performed in a steamer with citrate buffer for 30 min.
Immunostaining was performed using a Lab Vision Autostainer (Thermo
Scientific, Bremen, Germany) and primary antibodies against EGFR
(1:500) and Ki67 (1:300), followed by treatment with the Lab Vision
HRP polymer detection system (Thermo Scientific) according to the
manufacturer’s protocol. The TUNEL reaction was performed according
to the manufacturer’s instructions. Stained sections were
visualized using a light microscope (Olympus, Tokyo, Japan).
Subcutaneous xenograft model
Animals were housed and treated in accordance with
institutional guidelines for animal care and use. Female athymic
mice (BALB/c-nu/nu; 5–6 weeks old; 17–23 g) were injected
subcutaneously with TE-8 cells (1.5×107) in the right
flank. Tumors were allowed to grow to a volume of approximately 100
mm3, at which time mice were randomly assigned to one of
four groups (10 animals each). One group received intraperitoneal
(i.p.) injections of cisplatin at doses of 40 μg/head per injection
three times a week. A second group was administered cetuximab
intravenously at 0.5 mg/head per week. In the third group, both
cisplatin and cetuximab were administered using the same schedule
for each drug as for single treatments. The fourth group, the
control group, received i.p. injections of saline. The tumor volume
and weight of each animal were assessed every other day for 4
weeks. Tumor volumes (V) were estimated using the formula: V =
length × width2 / 2.
Statistical analysis
Data were obtained from at least three independent
experiments and are expressed as mean ± standard deviation. Mean
differences were analyzed using the Student’s t-test. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Expression and phosphorylation status of
EGFR, AKT and ERK in ESCC cell lines
The expression of EGFR, p-EGFR, AKT, p-AKT, ERK,
p-ERK was examined in 10 ESCC and control Het-1A cell lines by
western blotting (Fig. 1). TE-8 and
-11 cells showed the highest expression of EGFR and p-EGFR, whereas
activated EGFR (i.e., p-EGFR) levels were low in the other cell
lines, including TE-4. Levels of p-AKT and p-ERK did not differ
significantly between cell lines. Two cell lines, TE-4 and -8, were
selected for subsequent experiments to examine the correlation
between EGFR and p-EGFR expression and combined drug treatment.
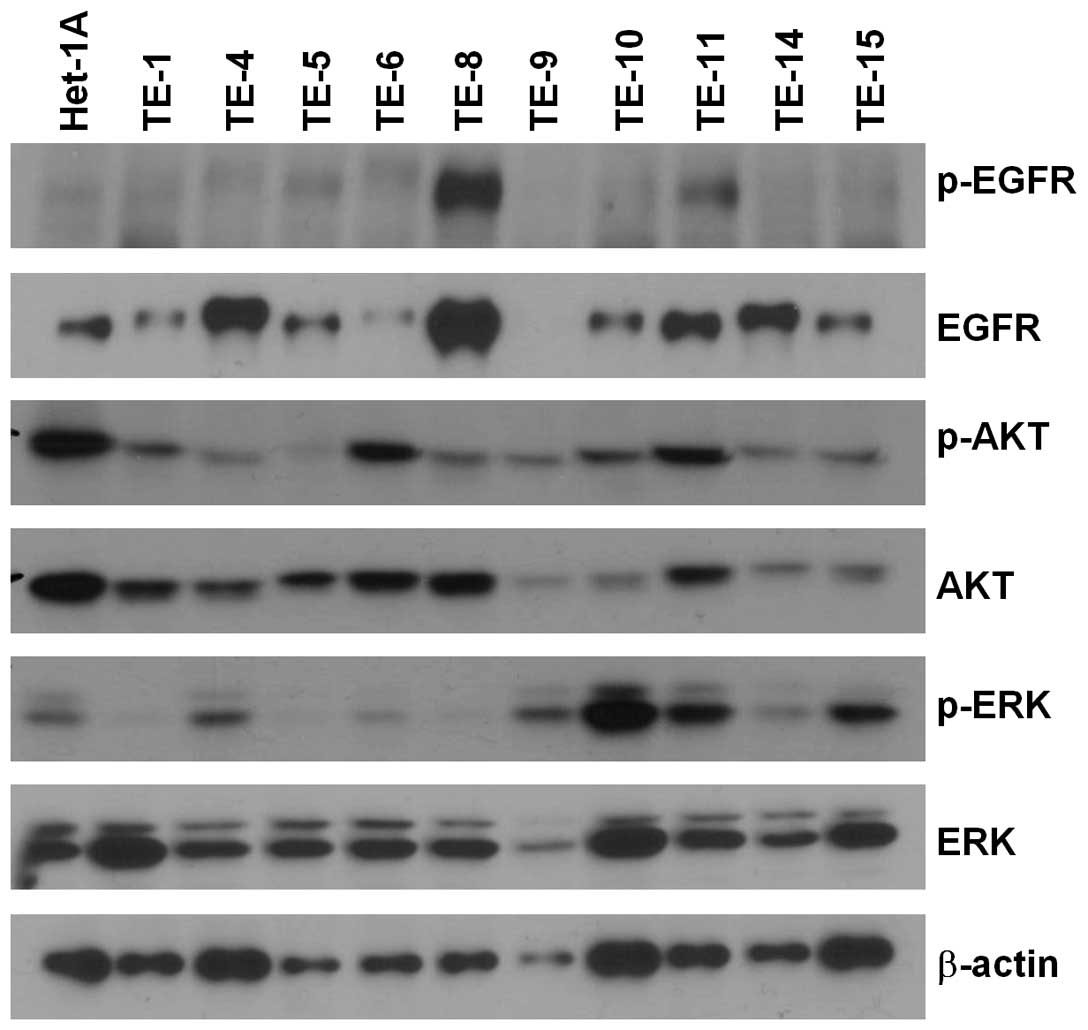 | Figure 1Relative levels of EGFR, p-EGFR, AKT,
p-AKT, ERK and p-ERK expression in ESCC cell lines (TE-1, -2, -4,
-5, -6, -8, -9, -10, -11, -14 and -15) and a normal esophageal
epithelial cell line (Het-1A), as determined by western blot
analysis. β-actin was used as a loading control. |
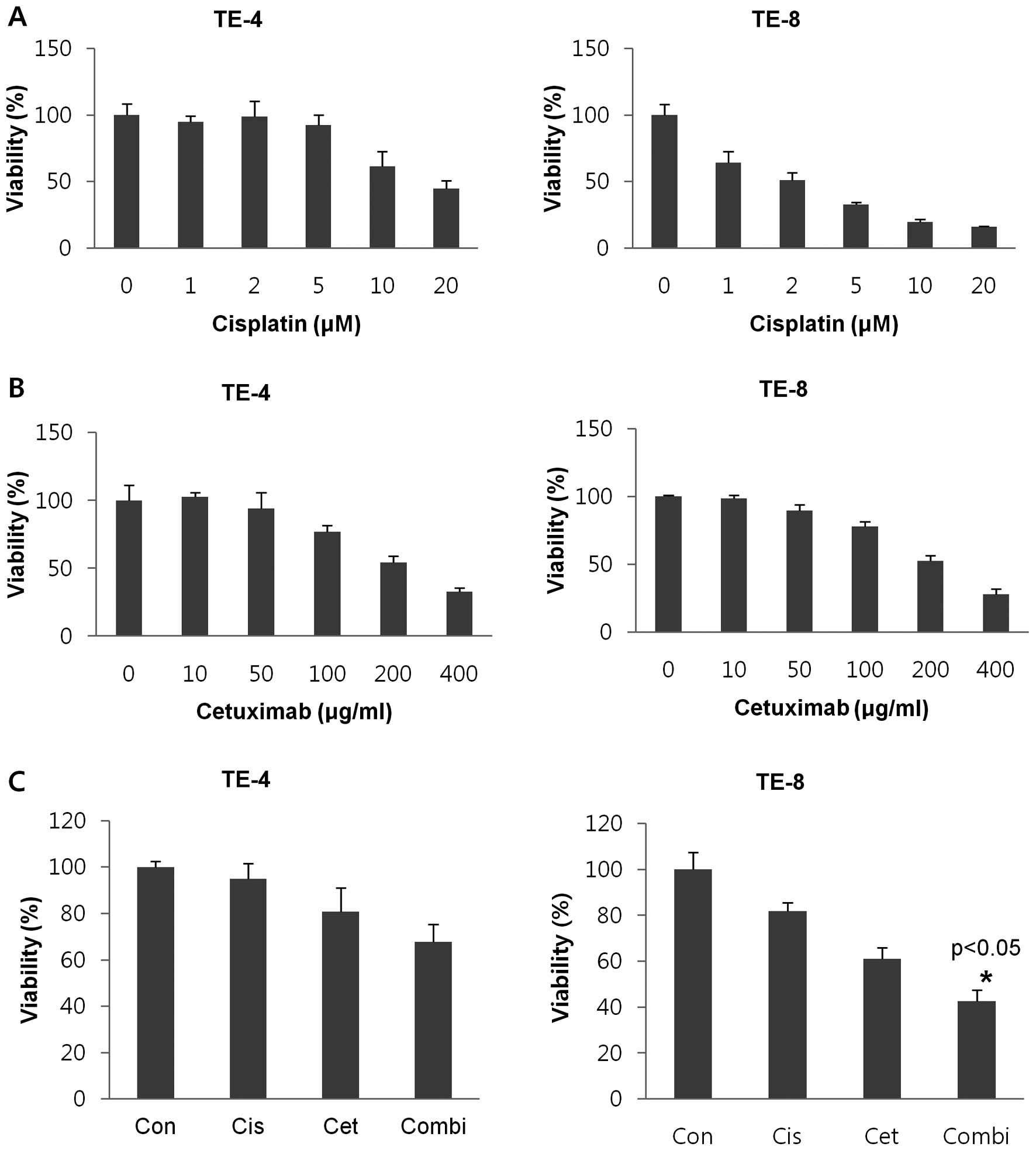
Cisplatin-induced cytotoxicity is
enhanced by cetuximab in EGFR-activated cells
The effects of cisplatin and cetuximab administered
in isolation or in combination were assessed in two ESCC cell
lines, TE-4 and -8, expressing low and high endogenous levels of
EGFR, respectively. A range of concentrations of both agents were
tested (cisplatin: 0, 1, 2, 5, 10 and 20 μM for 3 days; cetuximab:
0, 10, 50, 100, 200 and 400 μg/ml for 7 days). TE-8 had greater
sensitivity to cisplatin than TE-4 cells, with IC50
values of 2.06 and 16.79, respectively (Fig. 2A). There was no difference in
sensitivity to cetuximab between the two cell lines
(IC50 = 232.65 in TE-4 vs. 230.35 in TE-8) (Fig. 2B). Cell viability was examined in
cells treated with either or both agents. A dose-dependent, overall
decrease in cell viability was observed; combined treatment with
cisplatin and cetuximab had an additive cytotoxic effect compared
to single treatments (P<0.05) in the EGFR-overexpressing TE-8
but not in TE-4 cells (Fig.
2C).
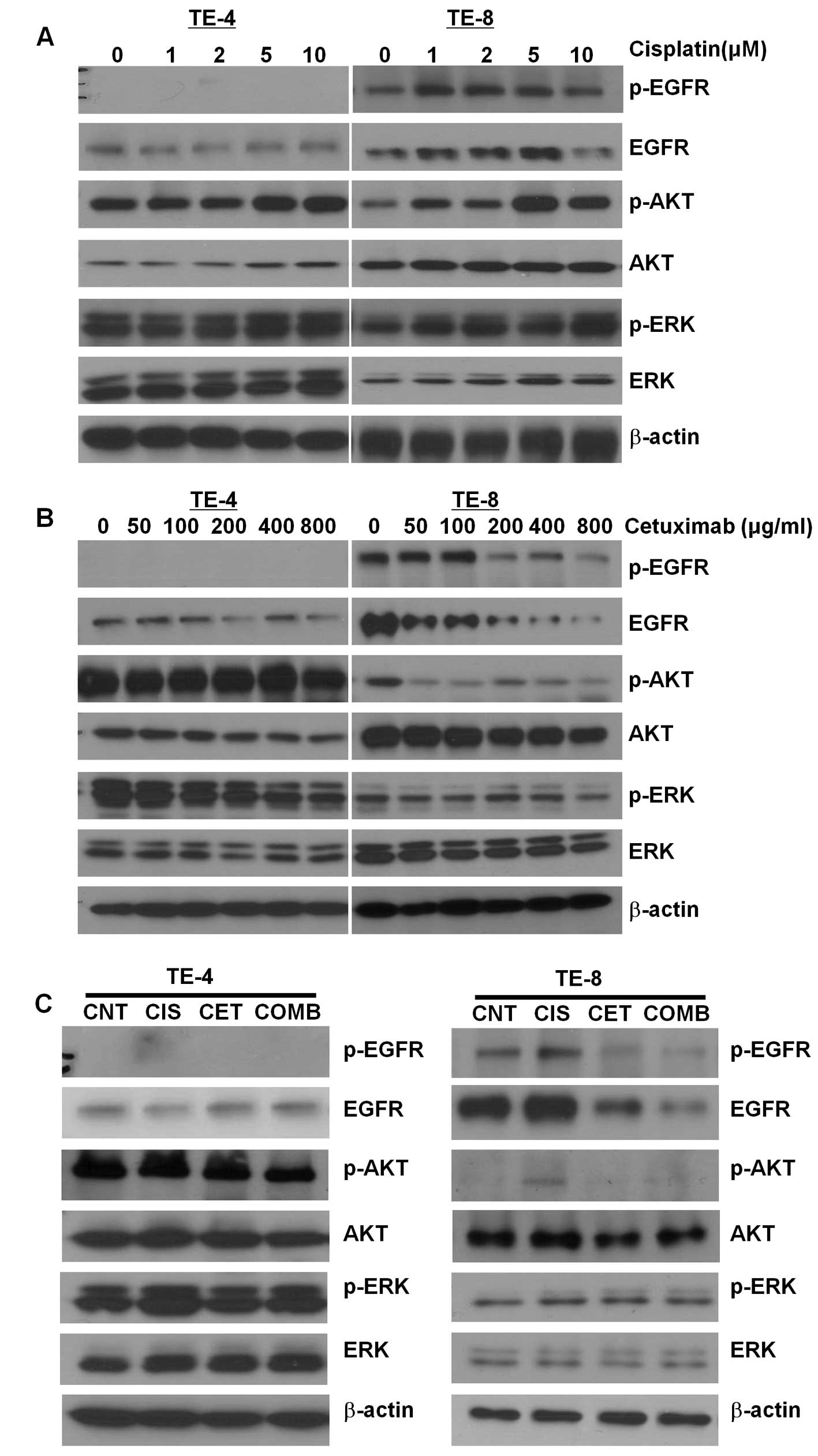
Activation of EGF signaling by cisplatin
in EGFR-expressing cells
To determine the mechanism underlying cisplatin- and
cetuximab-induced inhibition of ESCC cell growth, the expression
and phosphorylation status of EGFR and AKT were examined in TE-4
and -8 cells by western blotting. Expression of EGFR, p-EGFR and
p-AKT was upregulated by cisplatin treatment in a dose-dependent
manner in TE-8 but not TE-4 cells (Fig.
3A), indicating an activation of EGF signaling. In contrast,
treatment of TE-8 cells with cetuximab led to a dose-dependent
decrease in EGFR, p-EGFR and p-AKT expression (Fig. 3B). The cisplatin-induced increases
in EGFR, p-EGFR and p-AKT expression in TE-8 cells were abrogated
in the presence of cetuximab (Fig.
2C). In TE-4 cells, cisplatin had no effect on EGFR, p-EGFR and
p-AKT levels; treatment with cetuximab, or a combination of both
agents, led to a decrease in p-AKT expression but had no effect on
the expression or phosphorylation of EGFR.
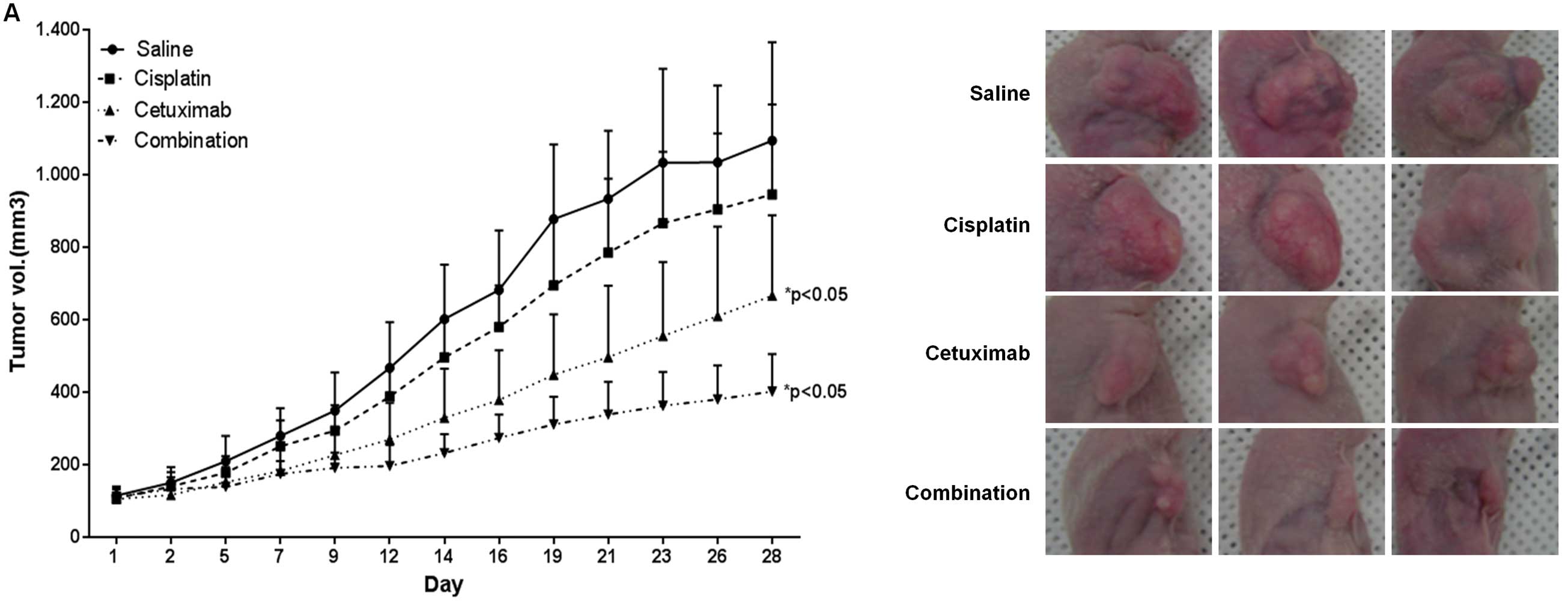
Antitumorigenic effects of cisplatin
combined with cetuximab in an ESCC xenograft model
The effects of cisplatin and cetuximab on tumor
growth were investigated in an ESCC mouse model. The volume of TE-8
cell-derived tumors was reduced by 13.5 and 39.1% upon treatment
with cisplatin and cetuximab, respectively, compared to
saline-treated controls (Fig. 4A),
demonstrating that cetuximab has an anti-tumorigenic effect in
vivo. Combined treatment with cisplatin and cetuximab decreased
tumor volume by 63.2%, suggesting that the treatment of cetuximab
and cisplatin as a combination therapy may be promising in a TE-8
cell-derived ESCC tumor model.
EGFR expression and phosphorylation were examined in
tumor tissue samples by western blotting (Fig. 4B). Consistent with in vitro
observations, EGFR and p-EGFR levels were higher in tumors from the
cisplatin treatment group, while levels were downregulated in the
combination treatment group, effects that were confirmed by
immunohistochemistry (Fig. 4C).
EGFR expression was reduced in necrotic areas of cetuximab- or
combination-treated tumor tissue samples. Moreover, the
Ki67-expressing proliferative fraction was decreased after
treatment with cisplatin (by 29% vs. control; P<0.001),
cetuximab (by 38% vs. control; P<0.001), or both (by 66% vs.
control; P<0.001). No differences in the fraction of apoptotic
cells were observed between the various treatment groups.
Discussion
Esophageal cancer is one of the leading causes of
cancer mortality worldwide and is the fifth most common cause of
cancer-related mortality in men (1). Approximately half of patients
diagnosed with esophageal cancer present with overt metastatic
disease and chemotherapy is the treatment of choice for advanced
stages. ESCC patients often receive a combination of drugs such as
cisplatin, 5-FU, etoposide and paclitaxel (13). Despite the widespread use of
combination chemotherapy, there is no solid evidence for
significant improvements in overall survival rate using this
strategy. Previous studies have examined the effects of treatment
using a combination of an anti-EGFR antibody (e.g., cetuximab) and
a conventional drug (e.g., cisplatin) on cell lines derived from
colon cancer, non-small cell lung cancer and cervical cancer
(14,15). However, little is known about the
effects of this combination of agents in ESCC cells.
Results from recent clinical trials using a
combination of cetuximab, cisplatin, irinotecan and radiotherapy to
treat ESCC patients revealed significant adverse side-effects, such
as diarrhea and dehydration, which were indicators of increased
toxicity, without parallel increases in treatment efficacy
(16,17). Similarly, SCOPE1 trials in patients
in the UK did not show any benefits to adding cetuximab to a
standard chemo-/radiotherapy treatment regimen. However, in a
multicenter phase II trial in Chinese patients with non-resectable,
locally advanced ESCC, it was found that cetuximab can be safely
used concurrently with chemo- and radiotherapy and may actually
increase the clinical response rate (18,19).
The reasons for these conflicting results of cetuximab combination
therapy remain unclear, but recent data point to the influence of
mutations in, or amplification of, specific genes; for instance,
combination therapy had different survival outcomes for patients
depending on the presence of EGFR or MET gene amplifications, or
mutations in EGFR, KRAS, or PI3CA (20,21).
The results of the present study, showing that the combined
treatment effects are predominantly observed in an ESCC cell line
overexpressing EGFR, support the theory that the choice and
efficacy of treatment methods depend on the specific genotype of
each patient.
The results of this study also underscore the
complexity of the effects produced by combined treatment with
cetuximab and cisplatin. For instance, the cytotoxic effects of the
two agents used in combination were additive in TE-8 cells
(Fig. 2), suggesting that it is
promising as a treatment strategy. However, cisplatin-induced
increases in EGF signaling were reversed by cetuximab treatment
(Fig. 3). Given the role of
phosphorylated EGFR in stimulating proliferation of some cancer
cell types, this antagonistic interaction could be advantageous in
ESCC treatment. Further studies on the downstream effects of EGF
signaling in ESCC cells and a closer examination of the genotypic
differences between different ESCC cell lines that could explain
the differential responses to combined drug treatment, are required
for the development of personalized and effective treatment
regimens that are tailored to individual patients.
Acknowledgements
This study was supported by grants from the
Radiological Translational Research Program (RTR) of the Korea
Institute of Radiological and Medical Sciences (50455-2013).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Darling G: The role of lymphadenectomy in
esophageal cancer. J Surg Oncol. 99:189–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kranzfelder M, Schuster T, Geinitz H,
Friess H and Buchler P: Meta-analysis of neoadjuvant treatment
modalities and definitive non-surgical therapy for oesophageal
squamous cell cancer. Br J Surg. 98:768–783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chiu PW, Chan AC, Leung SF, Leong HT,
Kwong KH, Li MK, Au-Yeung AC, Chung SC and Ng EK: Multicenter
prospective randomized trial comparing standard esophagectomy with
chemoradiotherapy for treatment of squamous esophageal cancer:
early results from the Chinese University Research Group for
Esophageal Cancer (CURE). J Gastrointest Surg. 9:794–802. 2005.
View Article : Google Scholar
|
|
5
|
Bleiberg H, Conroy T, Paillot B, Lacave
AJ, Blijham G, Jacob JH, Bedenne L, Namer M, De Besi P, Gay F,
Collette L and Sahmoud T: Randomised phase II study of cisplatin
and 5-fluorouracil (5-FU) versus cisplatin alone in advanced
squamous cell oesophageal cancer. Eur J Cancer. 33:1216–1220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polee MB, Kok TC, Siersema PD, Tilanus HW,
Splinter TA, Stoter G and Van der Gaast A: Phase II study of the
combination cisplatin, etoposide, 5-fluorouracil and folinic acid
in patients with advanced squamous cell carcinoma of the esophagus.
Anticancer Drugs. 12:513–517. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanawa M, Suzuki S, Dobashi Y, Yamane T,
Kono K, Enomoto N and Ooi A: EGFR protein overexpression and gene
amplification in squamous cell carcinomas of the esophagus. Int J
Cancer. 118:1173–1180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mendelsohn J and Baselga J: The EGF
receptor family as targets for cancer therapy. Oncogene.
19:6550–6565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vermorken JB, Trigo J, Hitt R, Koralewski
P, Diaz-Rubio E, Rolland F, Knecht R, Amellal N, Schueler A and
Baselga J: Open-label, uncontrolled, multicenter phase II study to
evaluate the efficacy and toxicity of cetuximab as a single agent
in patients with recurrent and/or metastatic squamous cell
carcinoma of the head and neck who failed to respond to
platinum-based therapy. J Clin Oncol. 25:2171–2177. 2007.
|
|
10
|
Sobrero AF, Maurel J, Fehrenbacher L,
Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C,
Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kroning H,
Luppi G, Kisker O, Zubel A, Langer C, Kopit J and Burris HA III:
EPIC: phase III trial of cetuximab plus irinotecan after
fluoropyrimidine and oxaliplatin failure in patients with
metastatic colorectal cancer. J Clin Oncol. 26:2311–2319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vincenzi B, Schiavon G, Silletta M,
Santini D and Tonini G: The biological properties of cetuximab.
Crit Rev Oncol Hematol. 68:93–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C,
Schueler A, Amellal N and Hitt R: Platinum-based chemotherapy plus
cetuximab in head and neck cancer. N Engl J Med. 359:1116–1127.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ilson DH: Esophageal cancer chemotherapy:
recent advances. Gastrointest Cancer Res. 2:85–92. 2008.
|
|
14
|
Cascone T, Morelli MP, Morgillo F, Kim WY,
Rodolico G, Pepe S, Tortora G, Berrino L, Lee HY, Heymach JV and
Ciardiello F: Synergistic anti-proliferative and pro-apoptotic
activity of combined therapy with bortezomib, a proteasome
inhibitor, with anti-epidermal growth factor receptor (EGFR) drugs
in human cancer cells. J Cell Physiol. 216:698–707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meira DD, de Almeida VH, Mororo JS,
Nobrega I, Bardella L, Silva RL, Albano RM and Ferreira CG:
Combination of cetuximab with chemoradiation, trastuzumab or MAPK
inhibitors: mechanisms of sensitisation of cervical cancer cells.
Br J Cancer. 101:782–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tomblyn MB, Goldman BH, Thomas CR Jr,
Benedetti JK, Lenz HJ, Mehta V, Beeker T, Gold PJ, Abbruzzese JL
and Blanke CD; SWOG GI Committee. Cetuximab plus cisplatin,
irinotecan, and thoracic radiotherapy as definitive treatment for
locally advanced, unresectable esophageal cancer: a phase-II study
of the SWOG (S0414). J Thorac Oncol. 7:906–912. 2012. View Article : Google Scholar
|
|
17
|
Lee MS, Mamon HJ, Hong TS, Choi NC, Fidias
PM, Kwak EL, Meyerhardt JA, Ryan DP, Bueno R, Donahue DM, Jaklitsch
MT, Lanuti M, Rattner DW, Fuchs CS and Enzinger PC: Preoperative
cetuximab, irinotecan, cisplatin, and radiation therapy for
patients with locally advanced esophageal cancer. Oncologist.
18:281–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meng X, Wang J, Sun X, Wang L, Ye M, Feng
P, Zhu G, Lu Y, Han C, Zhu S, Liao Z and Yu J: Cetuximab in
combination with chemoradiotherapy in Chinese patients with
non-resectable, locally advanced esophageal squamous cell
carcinoma: a prospective, multicenter phase II trail. Radiother
Oncol. 109:275–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crosby T, Hurt CN, Falk S, Gollins S,
Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI,
Cunningham D, Blazeby J, Roy R, Maughan T and Griffiths G:
Chemoradiotherapy with or without cetuximab in patients with
oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised
trial. Lancet Oncol. 14:627–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lennerz JK, Kwak EL, Ackerman A, Michael
M, Fox SB, Bergethon K, Lauwers GY, Christensen JG, Wilner KD,
Haber DA, Salgia R, Bang YJ, Clark JW, Solomon BJ and Iafrate AJ:
MET amplification identifies a small and aggressive subgroup of
esophagogastric adenocarcinoma with evidence of responsiveness to
crizotinib. J Clin Oncol. 29:4803–4810. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwak EL, Jankowski J, Thayer SP, Lauwers
GY, Brannigan BW, Harris PL, Okimoto RA, Haserlat SM, Driscoll DR,
Ferry D, Muir B, Settleman J, Fuchs CS, Kulke MH, Ryan DP, Clark
JW, Sgroi DC, Haber DA and Bell DW: Epidermal growth factor
receptor kinase domain mutations in esophageal and pancreatic
adenocarcinomas. Clin Cancer Res. 12:4283–4287. 2006. View Article : Google Scholar : PubMed/NCBI
|