Introduction
Esophageal cancer is one of the most common human
malignant tumors in the digestive system; more than 200,000
individuals die from esophageal cancer each year worldwide, while
more than 150,000 individuals die of esophageal cancer each year in
China (1). Thus, esophageal cancer
is a serious threat to human health. Current methods of early
diagnosis and treatment are not satisfactory. The most common
pathological type of esophageal cancer is squamous cell carcinoma
(ESCC), particularly in China. In recent years, with the continuous
development of molecular biology, genetics, epigenetics, immunology
and histochemistry, scientists have gradually started to reveal the
genetic and molecular processes underlying the development of
esophageal cancer and their clinical application.
The important role of histone methylation
modification and its relationship with DNA methylation have
received much attention from researchers. SET and MYND
domain-containing protein 3 (SMYD3) is a type of histone
methyltransferase. Recent studies have shown that SMYD3 is
extensively expressed in human hepatocellular carcinoma, colon,
breast, cervical cancer and other malignant tumors, where it is
involved in proliferation, apoptosis, invasion and metastasis.
However, the expression of SMYD3 in ESCC has not been reported. The
relatively recent technology of RNA interference (RNAi) has been
widely used in research related to gene expression in tumor cells,
and is expected to be developed as a new tumor therapy. If SMYD3
expression is associated with ESCC, such as in tumor cell
proliferation, invasion or other malignant cell biologic behaviors,
then SMYD3 would offer a new diagnostic and therapeutic target for
ESCC. Its downregulation by RNAi would represent a novel ESCC
therapy. The present study aimed to evaluate the biological role of
SMYD3 in ESCC and to clarify the relationship between SMYD3 and the
important anti-oncogene retinoblastoma protein-interacting zinc
finger gene 1 (RIZ1) in ESCC.
Materials and methods
Ethics statement
The research was conducted in accordance with the
Declaration of Helsinki. All experimental protocols were approved
by the Ethics Committee for the Use of Human Subjects of Tianjin
Medical University General Hospital. Patients provided written
consent to participate in this study, and this consent was also
approved by the Ethics Committee for the Use of Human Subjects of
Tianjin Medical University General Hospital.
Cell culture and tissues
Human ESCC cell line TE13 was purchased from ATCC
(Rockville, MD, USA) and cultured in RPMI-1640 (HEPES 4.76 g;
NaCO3 2.0 g; RPMI-1640 10.4 g; ddH2O 1,000
ml) media supplemented with 10% fetal calf serum, 2 mM 1X
L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Life
Technologies, Carlsbad, CA, USA). Cells were maintained at 37°C in
a humidified atmosphere with 5% CO2.
Carcinoma and distal ending normal tissues (>5 cm
from the tumor) were obtained in our department during surgical
excision from 11 patients with ESCC. All specimens were placed in
liquid nitrogen immediately after resection and stored at −80°C
until RNA or genomic DNA (gDNA) extraction. No patient had received
chemotherapy or radiation therapy before surgery. All patients were
confirmed to have ESCC by pathological tests.
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections of ESCC
and distal ending normal tissues were subjected to immunostaining
with a rabbit polyclonal antibody against SMYD3. Briefly, 5-μm
thick tissue sections were deparaffinized, rehydrated and subjected
to antigen retrieval by boiling in sodium citrate buffer (10 mM, pH
6.0). The sections were incubated at 4°C overnight with SMYD3
antibody AP1202c (1:75 dilution; Abgent, San Diego, CA, USA) and
then stained with 3,3′-diaminobenzidine. After visualization of
immunoreactivity, the sections were counterstained with hematoxylin
and mounted. Adjacent non-cancerous esophageal tissues were used as
internal positive controls.
The stains were graded as follows: i) positive when
immunoreactivity was equivalent to that observed in normal
esophageal squamous cells or had moderately decreased; and ii)
negative when immunoreactivity was weak or absent. Under a
high-resolution optical microscope (SP200), four different fields
of view were selected randomly, and the percentage of positive
cells among the total cells was calculated. If the positive cell
fraction was <10%, it was scored as 1; if the fraction was
>10% but ≤50%, it was scored as 2; if the fraction was >50%
but ≤75%, it was scored as 3; and if the fraction was >75%, it
was scored as 4. Different degrees of pigmentation were also
scored: negative as 1; weak pigmentation as 2; moderate
pigmentation as 3; and strong pigmentation as 4. By combining the
above two indices, the final results became: ≤4 as (−), >4 but
≤8 as (+), >8 but ≤12 as (++), >12 but ≤16 as (+++) and
>16 as (++++).
Design and construction of the expression
plasmid of SMYD3-shRNA; plasmid transformation, extraction,
identification and transfection of cells
For the knockdown of SMYD3 expression, according to
the design principles of shRNA, specific target sequences from the
SMYD3 mRNA (NM_022743.1) coding sequence were identified (Table I). Target oligonucleotide sequences
were designed and synthesized into eukaryotic expression plasmids
named 1241-1244. The Guangzhou Synthetic Genes Company synthesized
the above materials.
 | Table ISMYD3 interference target
sequence. |
Table I
SMYD3 interference target
sequence.
| Clone name | Symbol | Location | Length | Target sequences |
|---|
|
HSH017124-1-CH1(OS238397) | SMYD3 | 290 | 19 |
gatggagcaccttcagaat |
|
HSH017124-2-CH1(OS238398) | SMYD3 | 658 | 19 |
catctgctacctggatatg |
|
HSH017124-3-CH1(OS238399) | SMYD3 | 714 | 19 |
accagtactgctttgaatg |
|
HSH017124-4-CH1(OS238400) | SMYD3 | 763 | 19 |
ggatgctgatatgctaact |
Briefly, 2×105 TE13 cells/well in 6-well
plates were transfected with 4 μg of specific or control shRNA
using Lipofectamine 2000™ (Life Technologies). After incubation for
the designated time intervals, the cells were prepared for
real-time PCR and western blotting.
RNA extraction and reverse transcription
reaction
Total RNA from cells and tissues was isolated using
the TRIzol reagent (Life Technologies), according to the
manufacturer’s recommendations. Cellular RNA was isolated from
5×106 to 1×107 cells by 1 ml TRIzol
decomposition, and tissues samples were ground into a fine powder
using a mortar and pestle, and then incubated in TRIzol solution
(100 g/l) for 15 min. One fifth of the volume of chloroform was
then added. After vigorous agitation and standing for 5 min, the
inorganic phase was separated by centrifugation at 12,000 × g for
15 min at 4°C. RNA was then precipitated in the presence of an
equal volume of isopropanol and centrifuged at 12,000 × g for 10
min at 4°C. RNA pellets were washed with 1 ml 75% ice-cold ethanol
[diethylpyrocarbonate (DEPC)-treated], centrifuged at 8,000 × g for
5 min at 4°C and then dissolved in DEPC-treated H2O. A
UV spectrophotometer (Beckman Coulter, Brea, CA, USA; absorbance at
260 and 280 nm) and 1.2% denaturing agarose gels were used to
determine the concentration and quality of the total RNA. For
quantitative real-time PCR analysis, 2 μg of RNA was reverse
transcribed using reverse transcriptase M-MLV, ribonuclease
inhibitor and dNTP mixture (all from Takara, Japan), according to
the manufacturer’s protocol. The cDNA templates were then subjected
to PCR amplification.
RT-PCR
Two microliters of cDNA from the TE13 cell lines was
used as the template to amplify specific fragments in a 25 μl
reaction mixture, under the following conditions: denaturation at
94°C for 3 min; 30 cycles at 94°C for 30 sec, at 55°C for 30 sec,
at 72°C for 60 sec; and then extension at 72°C for 10 min. As a
control for cDNA integrity, GAPDH expression was also analyzed. The
primer (10 μM) sets were: SMYD3 forward, 5′-AACGGCTTCCCGATATCA-3′
and reverse, 5′-ATCACTTGAACCCCTCTGA-3′; GAPDH forward,
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse, 5′-GGGTGGAATCATATTGGAAC-3′.
The SMYD3 primer set yielded a band of 169 bp and the GAPDH primer
set yielded a band of 152 bp. Five microliters of the RT-PCR
reaction product was analyzed by electrophoresis on a 1% agarose
gel, and the electrophoresis images were scanned using a UV
spectrophotometer (Beckman Coulter).
Western blotting
Tissues from each group were homogenized in RIPA
buffer (50 mmol/l Tris-HCl, pH 7.4; 150 mmol/l NaCl; 1% Nonidet
P-40; 0.5% sodium deoxycholate; 0.1% SDS; 1 mmol/l EDTA; 1 mmol/l
PMSF; 1 mg/ml aprotinin). With 2 min fully cracking the cell then
14,000 × g centrifugation for 5 min, the supernatant was collected
and the protein concentrations were determined using a
bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL,
USA). Subsequently, 30 μg of whole-cell lysate was separated on an
8% SDS-PAGE gel, transferred to nitrocellulose (NC) membranes
(Amersham Biosciences, Piscataway, NJ, USA) and immunoblotted with
the following antibodies: anti-β-actin, control; primary
antibodies, 1:2,000 dilution; secondary antibodies goat anti-mouse,
1:5,000 dilution (all from Abcam, UK). A PowerLook scanner (UMAX,
Taiwan) was used to analyze the films and ImageQuant software (GE,
USA) quantified the band intensities. The control samples (TE13
cells; TE-13 cells transfected with the blank plasmid) were treated
in the same manner. The relative expression of SMYD3 = the grey
value of SMYD3 protein/grey value of β-actin.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Some TE13 cells, which are in the
logarithmic-increase period, were selected and inoculated on
96-well plates with each well having 0.8×104/ml (200
μl). The original culture medium was discarded and the cells were
transfected when the cell density reached approximately 70–80% and
divided into groups (blank, negative control and SMYD3-shRNA) as
described above. Each group was assigned 8 wells as a parallel
experiment. After 48 h, one of the 96-well plates was examined
according to following steps: adding 20 μl MTT solution (5 g/l),
removing the upper clear part after 5 h at 37°C, adding 150 μl
dimethylsulfoxide (Newprobe, Beijing, China) and vibrating at low
speed for 10 min to thoroughly dissolve the crystals, measuring ray
density at a wavelength of 492 nm using a microplate reader, and
using the blank cells as zero for the calculation of the
restraining rates of cell proliferation. Inhibition ratio of cell
proliferation = [(comparison group A492 - blank group A492) −
(experiment group A492 - blank group A492)]/(comparison group
A492-experiment group A492) × 100%.
Statistical methods
SPSS 19.0 statistical software was used for
statistical analysis. Fisher’s exact test was used to analyze the
positive rate between two groups. All values are expressed as the
means ± standard deviation (SD). For more than one group, the mean
was compared using one-way ANOVA analysis of variance. Means of
more than two groups were compared using the least squares
difference (LSD) method. Differences with a P-value <0.05 were
considered to indicate a statistically significant result.
Results
SMYD3 is significantly expressed in ESCC
tissues
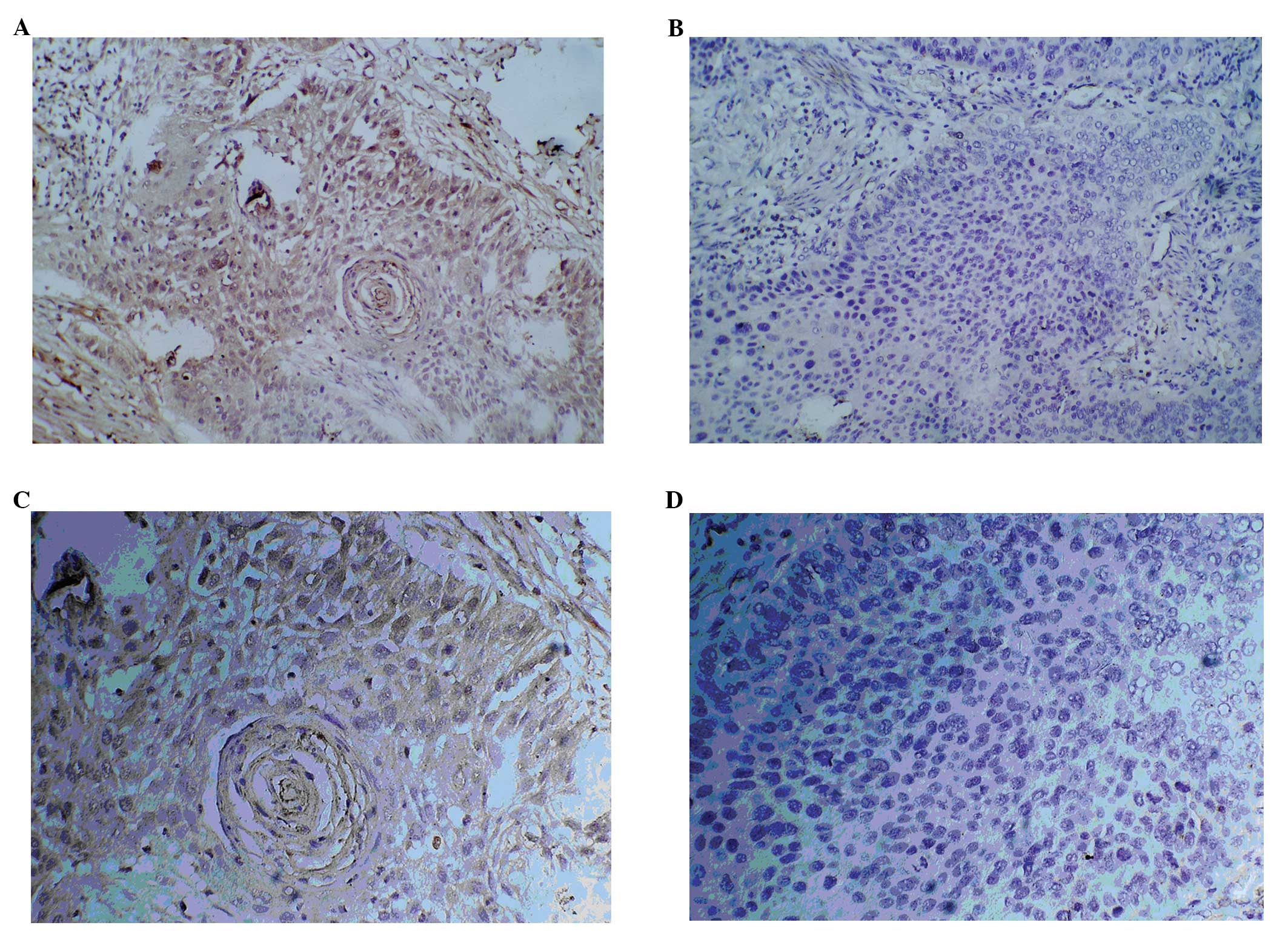
Fig. 1 shows the
immunohistochemical staining for SMYD3 using a rabbit anti-SMYD3
antibody. In the ESCC tissue samples (Fig. 1A and C), SMYD3 staining was
concentrated in the submucosa, mainly in the esophageal tissue cell
cytoplasm. In the normal esophageal tissues, even at ×200
magnification, little or no SMYD3 staining was visible. A
semi-quantitative method was used to count the SMYD3-positive cells
to calculate the number of positive cells between the two groups.
The results are shown in Table II.
Histopathological biopsies from 11 cases of esophageal cancer
showed an SMYD3-positive expression rate of 72.7% (8/11). In the
normal esophageal tissues, the SMYD3-positive rate was 18.2% (2/11)
(Fisher’s exact test, P=0.03; Mann-Whitney U rank sum test,
P<0.05).
 | Table IISMYD3 protein expression in ESCC and
adjacent non-cancerous tissues by immunohistochemical
detection. |
Table II
SMYD3 protein expression in ESCC and
adjacent non-cancerous tissues by immunohistochemical
detection.
| Positive | | | | |
|---|
|
| | | | |
|---|
| Group | ++++ | +++ | ++ | + | Total | Negative | Total samples | Positive rate
(%) |
|---|
| Cancer | 0 | 2 | 4 | 2 | 8 | 3 | 11 | 72.7 |
| Adjacent
non-cancerous | 0 | 0 | 0 | 2 | 2 | 9 | 11 | 18.2 |
| F-value | 6.3 | | | | | | | |
| P-value | 0.03 | | | | | | | |
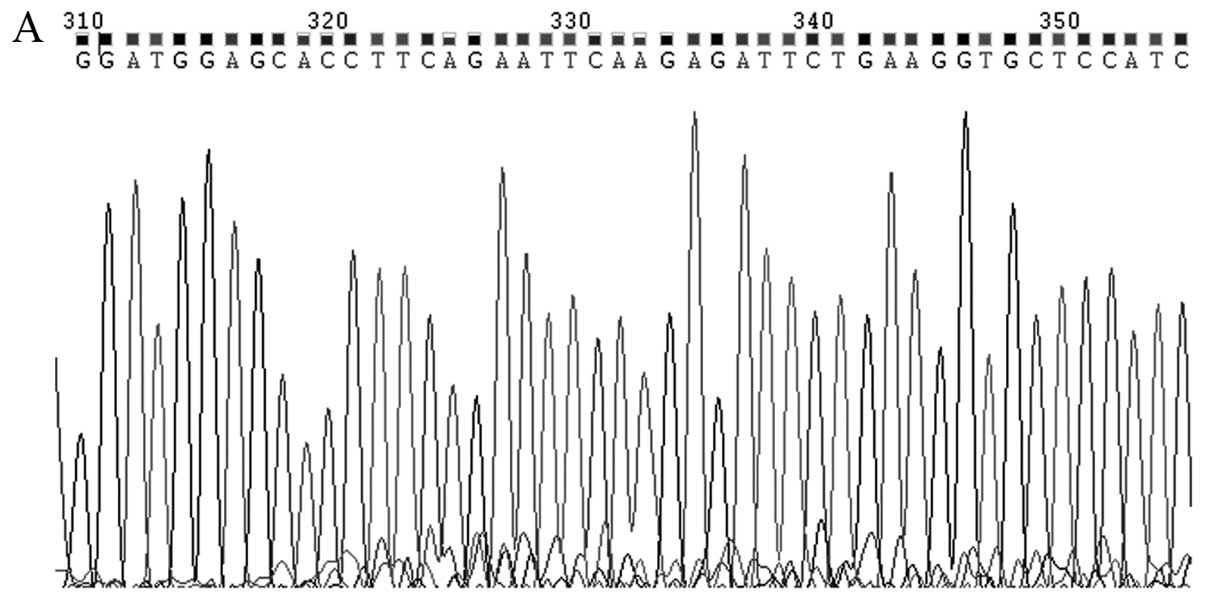
Testing shRNA constructs for their
ability to suppress SMYD3 mRNA expression
Four shRNA plasmids were constructed and tested for
their ability to suppress SMYD3 mRNA expression. The sequences of
the four shRNAs in plasmids SMYD3-shRNA-1241 - SMYD3-1244 are shown
in Fig. 2. The four plasmids were
transfected into TE13 ESCC cells, and RT-PCR was used to determine
the quantities of SMYD3 mRNA following treatment with each shRNA.
Fig. 3 shows that all four shRNAs
significantly reduced the expression of SMYD3 mRNA relative to the
blank control (P<0.05). For constructs 1241-1244, the inhibition
rates were 29.9, 52.2, 38.4 and 38.3%, respectively. The
performance of construct 1242 was the best (52.2%) and the
differences were statistically significant between 1242 and the
other groups (P<0.05). Among constructs 1241, 1243 and 1244,
there was no statistically significant difference in their
performances (P>0.05). The expression of GAPDH mRNA was not
significantly different between the groups (P>0.05). Therefore,
construct 1242 was used for subsequent experiments.
Effect of suppression of SMYD3 on the
expression of RIZ1 mRNA
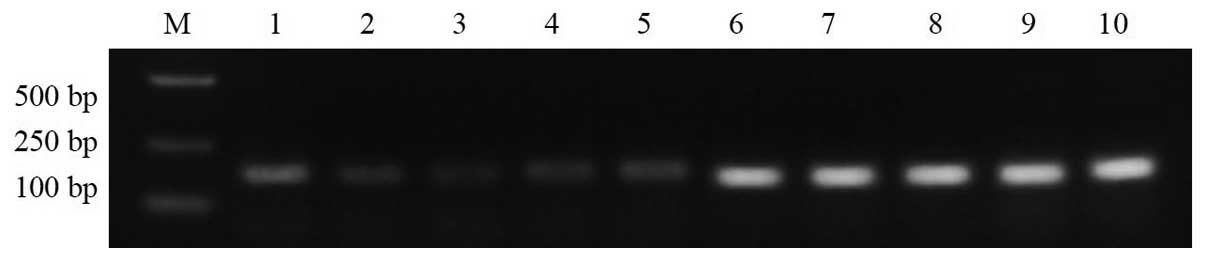
TE13 cells transfected with shRNA construct 1242
were subjected to RT-PCR to determine the levels of SMYD3, RIZ1 and
GAPDH (control) (Fig. 4). The
expression of SMYD3 was significantly reduced relative to the blank
group and the negative control group (P<0.05). SMYD3 expression
was not significantly different between the negative control group
and the blank group (P>0.05) (Fig.
4A and B). RIZ1 mRNA expression was significantly increased in
the cells transfected with SMYD3-shRNA-1242, relative to the blank
group and negative control group (P<0.05) (Fig. 4A and B). RIZ1 expression in the
negative control group and the blank group was not significantly
different (P>0.05). Among all three groups, the expression of
GAPDH mRNA was not significantly different (P>0.05).
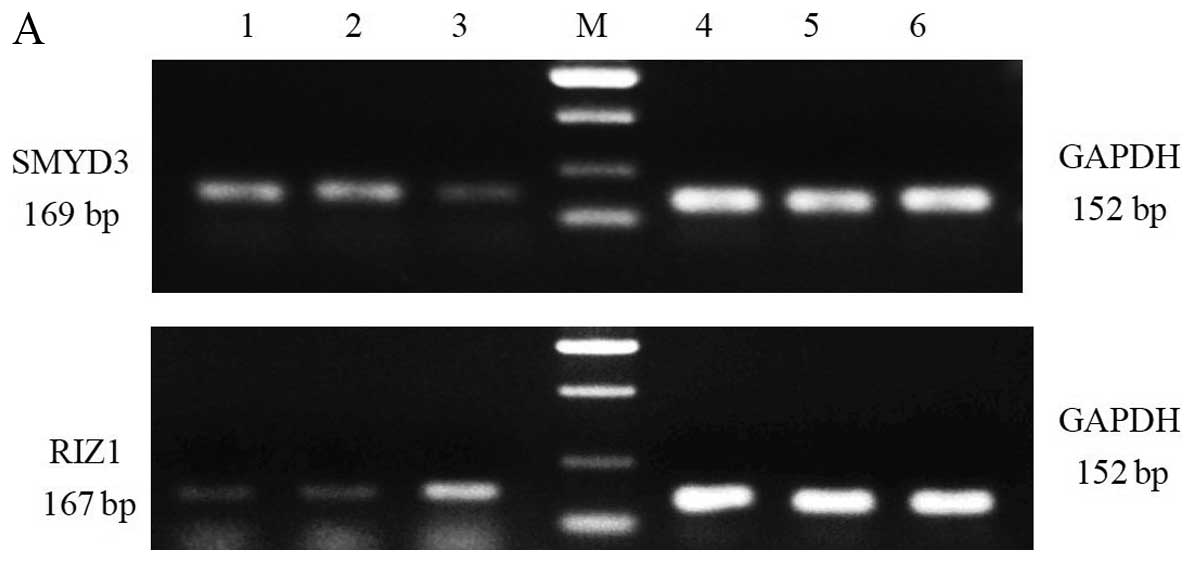 | Figure 4RNA interference of SMYD3 increases
the expression of RIZ1 mRNA. (A) Upper panel: lanes 1 and 2, SMYD3
expression in the blank and negative controls; lane 3, SMYD3
expression following treatment with SMYD3-shRNA; M, DNA markers,
from top to bottom 750, 500, 200 and 100 bp; lanes 4–6, GAPDH mRNA
expression in the cells of lanes 1–3. Lower panel: lanes 1 and 2,
RIZ1 expression in the blank and negative controls; lane 3, RIZ1
expression following treatment with SMYD3-shRNA; M, DNA markers,
from top to bottom 750, 500, 200 and 100 bp; lanes 4–6, GAPDH mRNA
expression in the cells of lanes 1–3. (B) Graph generated from the
RT-PCR analyses in A. SMYD3, SET and MYND domain-containing protein
3; RIZ1, retinoblastoma protein-interacting zinc finger gene 1. |
Effect of suppression of SMYD3 on the
expression of RIZ1 protein
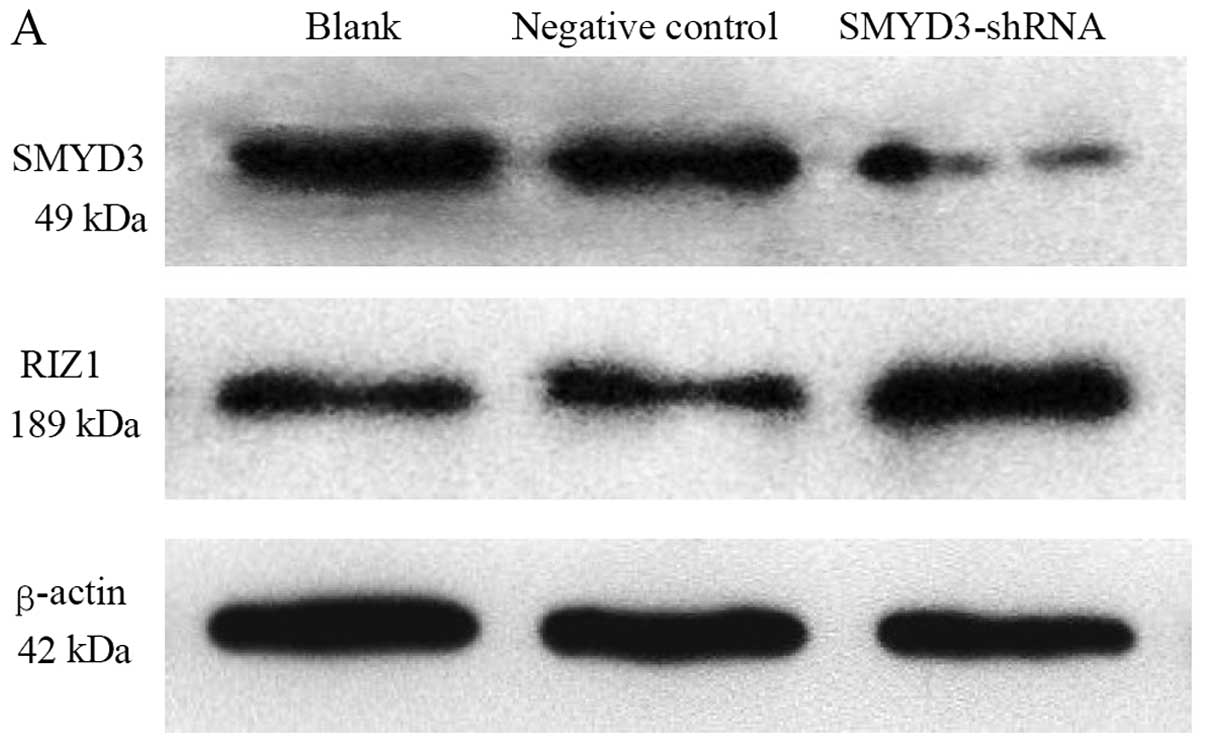
Western blotting of TE13 cells transfected with
shRNA construct 1242 was used to determine the effect of
suppressing SMYD3 expression on the levels of the RIZ1 protein.
Fig. 5A shows that compared with
the blank control and negative control, the levels of the SMYD3
protein were obviously reduced in the shRNA-treated cells. The
levels of RIZ1 were obviously increased in the shRNA-treated cells.
The levels of β-actin appeared similar across all samples. The band
intensities were scanned and quantified to permit a statistical
analysis of the protein expression data (Fig. 5B). The difference between the SMYD3
protein expression in the experimental group was significant
compared with both controls (P<0.05). The controls were not
significantly different to each other (P>0.05). The difference
between the RIZ1 protein expression in the experimental group was
significant compared with both controls (P<0.05). The controls
were not significantly different to each other (P>0.05). β-actin
expression in all three groups was not significantly different
(P>0.05).
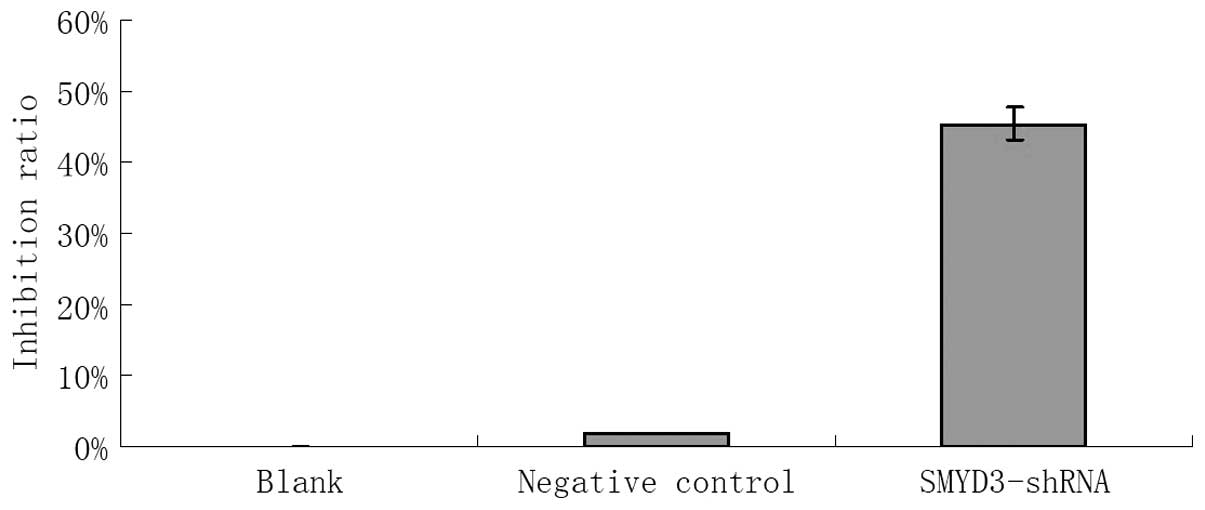
Effect of suppression of SMYD3 expression
on the proliferation of TE13 ESCC cells
TE13 cells transfected with shRNA-1242, blank
control cells and negative control cells were cultured for 48 h
in vitro and then subjected to the MTT test to assay the
inhibition of their proliferative ability. Cells transfected with
the shRNA showed an inhibition of their proliferative ability of
45.41%, whereas the negative control’s inhibition rate was 1.86%
(P<0.05). Compared with the blank group, the negative control
group showed no significant inhibition of proliferation (P>0.05)
(Fig. 6).
Discussion
In 2004, Hamamoto et al (2) and Sims and Reinberg (3) were the first to identify the histone
methyltransferase SET and MYND domain containing 3 (SMYD3) in human
hepatocellular carcinoma and colon cancer cells using a cDNA chip
and semi-quantitative RT-PCR. SMYD3 was found to methylate
chromosomal histone H3K4 twice (dimethylated form) or three times
(trimethylated form), which altered the expression of 80 candidate
genes, of which 61 were upregulated and 19 were downregulated.
Among these genes are cancer-associated genes, such as
tumor-suppressor genes, signal transduction genes and cell cycle
regulation genes, which can promote cell adhesion, proliferation,
invasion and inhibit tumor cell apoptosis. The SMYD3 gene is
located on human chromosome 1q44 and comprises 12 exons. The SMYD3
mRNA (GenBank login number: NM_022743) comprises 1,477 bp, encoding
a 369-amino acid protein. The SMYD3 protein contains two important
domains. Firstly, the myeloid translocation protein 8, Nervy, DEAF1
(MYND) zinc finger domain, located at amino acid 1-28 at the
N-terminus, can specifically combine with gene sequences
5′-CCCTCC-3′ or 5′-GGAGGG-3′ in promoter regions, promoting the SET
domain methylation function and influencing gene transcription.
Secondly, at position 89-180 is the Su(var)3–9, enhancer-of-zeste
trithorax (SET) lysine methyltransferase domain, comprising the
methylation transferase function, which can specifically methylate
chromosomal histone H3K4, which loosens and opens the spatial
structure of the chromosome (4–7).
In the present study, we used immunohistochemistry
to detect SMYD3 protein expression in ESCC and corresponding normal
esophageal tissues and showed that SMYD3 expression was
significantly higher in the ESCC group than in the corresponding
normal esophageal tissues. The results suggest that high expression
of SMYD3 is related to the occurrence of ESCC. Although further
study of the role and mechanism of SMYD3 in the development of ESCC
is required, it is likely to lead to new methods of early diagnosis
of esophageal cancer and to the development of new antitumor drugs
or gene therapy. To investigate the effect of suppressing SMYD3
expression, we produced and tested an RNA interference plasmid
SMYD3-shRNA-1242 in TE13 cells. Using semi-quantitative RT-PCR and
western blotting, we observed that the levels of SMYD3 mRNA and
protein in the interference group were lower than these levels in
the negative control and blank control groups. The two control
groups showed no significant differences, and the expression level
of the β-actin internal reference was similar among all three
groups, Thus, RNAi technology could successfully and efficiently
reduce SMYD3 gene expression in ESCC cells, representing an SMYD3
downregulation model for subsequent research. Furthermore, the MTT
test showed that the cell proliferation in the experimental group
was significantly reduced (P<0.001) compared with the negative
control and blank control groups. This result strongly suggests
that the SMYD3 gene is a key link in the process of cell
proliferation in ESCC, where it likely exerts its effect through
its transcriptional regulatory activity, directly or indirectly
changing the expression of proto-oncogenes (e.g., c-met, c-jun and
Wnt10B), signal transduction-related genes (e.g., GnRF2 and RAB40C)
or cell cycle regulatory genes (e.g., CDK2 and DNA topoisomerase
II-β). However, the specific molecular mechanism and signal
transduction pathway molecules remain to be determined (3,8).
In addition, we tested retinoblastoma
protein-interacting zinc finger gene 1 (RIZ1) expression after
SMYD3 downregulation. Oncogene activation and tumor-suppressor gene
inactivation are important mechanisms of tumorigenesis and
progression. RIZ1 belongs to the nucleoprotein methyltransferase
superfamily (9), which was first
reported by Buyse et al, who identified a retinal cell
tumor-suppressor protein by functional screening (9). RIZl methylates histones through its PR
structure domain on H3K9, leading to the inhibition of gene
transcription. Unlike RIZ1, RIZ2 starts transcription from exon 6,
thereby lacking the first five exons, which encode the
tumor-suppressor PR domain. Imbalances between RIZ1 and RIZ2 are an
underlying cause of tumorigenesis. RIZl is widely expressed in
multiple tissues and cells; however, its expression is low or
absent in tumor tissue, both in vivo and in vitro,
which correlates with its tumor inhibitory function (10,11).
Further research confirmed that the expression of RIZl is
significantly related to tumorigenesis and progression in malignant
tumors. Our laboratory has reported many research results for
tumor-suppressor gene RIZ1 in ESCC, further confirming that RIZ1 is
an important inhibitory factor in ESCC (12–15).
In the present study, we showed that the mRNA and protein
expression of RIZ1 both increased in the SMYD-shRNA group compared
with the negative control and blank groups. There were no
significant differences between the negative control and blank
group or between the internal reference β-actin in all three
groups. Thus, suppression of SMYD3 expression could increase RIZ1
expression in ESCC cells. This suggests the existence of a
signaling pathway between SMYD3 and RIZ1, where alterations in the
expression of the SMYD3 gene could affect tumor cell biology.
As a histone methylation transferase, the substrate
of SMYD3 is mainly histone. Although the SET domain of SMYD3 is the
structural domain responsible for the histone methyltransferase
function, SMYD3 also possess a zf-MYND zinc finger structural
domain. The downregulation of the expression of RIZ1 may involve
the zf-MYND zinc finger structure of SMYD3. Zinc finger structure
domains are important transcription elements that can combine with
the specific binding elements (SBEs) of the target DNA promoter.
The presence of the zf-MYND in SMYD3 suggests that SMYD3 could not
only regulate gene expression via histone methylation by the SET
domain, but also directly through the zinc finger domain. Related
research has identified the SBEs for zf-MYND as 5′-CCCTCC-3′ and
5′-GGAGGG-3′ (5). Notably, SBE
5′-GGAGGG-3′ is present in the RIZ1 promoter sequence (16). Thus, SMYD3 could directly regulate
RIZ1 expression by the binding of its zf-MYND zinc finger to the
RIZ1 SBE sequence. Of course, it is possible that SMYD3 indirectly
regulates the RIZ1 gene by binding to an SBE in another DNA
sequence. For example, SMYD3 binding to the SBE of DNA
methyltransferase (DNMT) could affect the RIZ1 promoter methylation
levels, which would regulate the expression of RIZ1. The exact
mechanism of the regulation of RIZ1 expression by SMYD3 and the
effect of the proposed SMYD3-RIZ1 signaling pathway in ESCC remain
undetermined. We hypothesize that the mechanism is closely related
to epigenetics. We will perform further studies in this area.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81201945), the Science
Foundation of Tianjin Medical University (no. 2011KY08), the
Doctoral Program of Higher Education Research Fund (no.
20121202110009), and the Natural Science Foundation of Tianjin (no.
10JCYBJC11300).
References
|
1
|
Dong SW, Ma L, Xu N, Yan HQ, Liu HY, Li YW
and Zhang P: Research on the reactivation of Syk expression caused
by the inhibition of DNA promoter methylation in the lung cancer.
Neoplasma. 58:89–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamamoto R, Fumkawa Y, Morita M, et al:
SMYD3 encodes a histone methyltransferase involved in the
proliferation of cancer cells. Nat Cell Biol. 6:731–740. 2004.
View Article : Google Scholar
|
|
3
|
Sims RJ III and Reinberg D: From chromatin
to cancer: a new histone lysine methyltransferase enters the mix.
Nat Cell Biol. 6:685–687. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sliva FP, Hamamoto R, Kunizaki M, et al:
Enhanced methyltransferase activity of SMYD3 by the cleavage of its
N-terminal region in human cancer cells. Oncogene. 27:2686–2692.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen LB, Xu JY, Yang Z and Wang GB:
Silencing SMYD3 in hepatoma demethylates RIZI promoter induces
apoptosis and inhibits cell proliferation and migration. World J
Gastroenterol. 13:5718–5724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kunizaki M, Hamamoto R, Silva FP,
Yamaguchi K, Nagayasu T, Shibuya M, Nakamura Y and Furukawa Y: The
lysine 831 of vascular endothelial growth factor receptor 1 is a
novel target of methylation by SMYD3. Cancer Res. 67:10759–10765.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu C, Fang X, Ge Z, et al: The
telomerase reverse transcriptase (hTERT) gene is a direct
target of the histone methyltransferase SMYD3. Cancer Res.
67:2626–2631. 2007. View Article : Google Scholar
|
|
8
|
Luo XG, Xi T, Guo S, et al: Effects of
SMYD3 overexpression on transformation, serum dependence, and
apoptosis sensitivity in NIH3T3 cells. IUBMB Life. 61:679–684.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buyse IM, Shao G and Huang S: The
retinoblastoma protein binds to RIZ, a zinc-finger protein that
shares an epitope with the adenovirus E1A protein. Proc Natl Acad
Sci USA. 92:4467–4471. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steele-Perkins G, Fang W, Yang XH, et al:
Tumor formation and inactivation of RIZ1, an Rb-binding member of a
nuclear protein-methyltransferase superfamily. Genes Dev.
15:2250–2262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robert MF, Morin S, Beaulieu N, Gauthier
F, Chute IC, Barsalou A and MacLeod AR: DNMT1 is required to
maintain CpG methylation and aberrant gene silencing in human
cancer cells. Nat Genet. 33:61–65. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong SW, Cui YT, Zhong RR, Liang DC, Liu
YM, Wang YG, He Z, Wang S, Liang SJ and Zhang P: Decreased
expression of retinoblastoma protein-interacting zinc-finger gene 1
in human esophageal squamous cell cancer by DNA methylation. Clin
Lab. 58:41–51. 2012.PubMed/NCBI
|
|
13
|
Dong SW, Zhang P, Liu YM, et al: 2012
Study on RIZ1 gene promoter methylation status in human
esophageal squamous cell carcinoma. World J Gastroenterol.
18:576–582. 2012.
|
|
14
|
Dong SW, Li D, Xu C, Sun P, Wang YG and
Zhang P: Alteration in gene expression profile and oncogenicity of
esophageal squamous cell carcinoma by RIZ1 upregulation.
World J Gastroenterol. 19:6170–6177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong S, Zhang P, Liang S, Wang S, Sun P
and Wang Y: The role of the retinoblastoma protein-interacting zinc
finger gene 1 tumor suppressor gene in human esophageal squamous
cell carcinoma cells. Oncol Lett. 6:1656–1662. 2013.PubMed/NCBI
|
|
16
|
Sasaki O, Meguro K, Tohmiya Y, et al:
Altered expression or retinoblastoma protein-interacting zinc
finger, RIZ, in human leukemia. Br J Haematol. 119:940–948. 2002.
View Article : Google Scholar : PubMed/NCBI
|