Introduction
Colorectal cancer (CRC) causes an average of 50,000
deaths per year in the US and has emerged as the second leading
cause of cancer-related mortality in the US and worldwide (1,2). While
early diagnosis and surgical intervention, along with combination
chemotherapy, has led to improved outcomes, few effective
strategies have emerged with which to treat colon cancer once
first-line approaches have been exhausted (1,2). The
initiation and progression of CRC development are characterized by
the accumulation of growing numbers of genetic and epigenetic
changes (3,4), while the aberrant activation of the
Wnt pathway, either by inactivation of tumor-suppressor adenomatous
polyposis coli (APC) or oncogenic activation of β-catenin, has been
demonstrated as the essential initial step of tumorigenesis
(3,5). Nonetheless, other alternative pathways
have been implicated in CRC development. One involves the formation
of serrated adenomas that are associated with mutations in BRAF
(6). Another alternative pathway
involves the formation of a hamartoma as a precursor lesion, which
is in this last rare pathway to CRC that mutations in the bone
morphogenetic protein (BMP) pathway were identified (7).
BMPs belong to the transforming growth factor β
(TGFβ) superfamily (8). BMPs bind
to a heterodimeric complex of transmembrane serine threonine kinase
receptors type 1 and 2, triggering the phosphorylation and
activation of the type 1 receptor by the type 2 receptor kinase.
The activated type 1 receptor phosphorylates a receptor-associated
SMAD which subsequently complexes with SMAD4 and translocates to
the nucleus to regulate gene transcription (9). The importance of BMP signaling in
colon cancer development has been highlighted by the identification
of mutations in the BMP pathway in colorectal carcinogenesis
(7). SMAD4 was identified as
being frequently deleted in CRC, although the biological
significance of this genetic change has always been attributed to
loss of TGFβ signaling rather than BMP signaling (10). Mutations in BMP receptor 1A
(BMPR1A) were found in patients with juvenile polyposis
(JP), a rare autosomal dominant hamartomatous polyposis syndrome
with an increased risk for the development of CRC (11). Mutations in SMAD4 and
BMPR1A account for approximately half of all cases of JP
(12–14). Moreover, forced expression of the
BMP antagonist noggin in the mouse intestine results in the
formation of intestinal hamartomatous polyps (15).
However, conflicting results have been reported
concerning the possible roles of BMPs in sporadic colon cancer. For
example, several BMPs were found to be growth suppressive and may
have their promoters methylated in colon cancer, compatible with a
tumor-suppressor role for BMPs in CRC (16–18).
However, the expression of BMP4 and BMP7 was found to increase with
progression through the adenoma-carcinoma sequence and to correlate
with a worse prognosis (19,20). A
more recent report showed that BMP signaling promotes the growth of
primary human colon cancer in vivo (21). Therefore, the biological effects of
BMPs on colon cancer development and progression remain to be fully
elucidated.
In the present study, we investigated the effect of
BMP2 on the proliferation, migration, invasiveness and tumor growth
capabilities of human colon cancer cells. To achieve high levels of
exogenous BMP2 expression, we constructed an adenovirus vector that
overexpresses BMP2 and also generated the piggyBac
transposon-mediated stable BMP2 overexpression cell line using the
commonly used human colon cancer line HCT116. We found that
exogenous BMP2 effectively inhibited HCT116 cell proliferation and
colony formation. BMP2 was shown to suppress colon cancer cell
migration and invasiveness as assessed by cell wound healing assay
and Boyden chamber Transwell assay. Under a low serum condition,
forced expression of BMP2 induced a significantly higher percentage
of apoptosis in HCT116 cells than that in the controls. Using a
xenograft tumor model, we found that forced expression of BMP2 in
HCT116 cells suppressed tumor growth, accompanied by decreased
proliferative activity. Thus, our results strongly suggest that
BMP2 may play an important inhibitory role in controlling the
proliferation and aggressive features of colon cancer cells.
Materials and methods
Cell culture and chemicals
Human colon cancer cell lines HCT116 and HEK-293
were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The cells were maintained in complete DMEM
containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 100
units of penicillin and 100 μg of streptomycin at 37°C in 5%
CO2 as previously reported (22–27).
Unless otherwise indicated, all chemicals were purchased from
Sigma-Aldrich (St. Louis, MO, USA) or Thermo Fisher (Pittsburgh,
PA, USA).
Recombinant adenoviral vectors expressing
BMP2 or GFP
Recombinant adenoviruses were generated using AdEasy
technology (28–32). Briefly, the coding regions of human
BMP2 and green fluorescent protein (GFP) were PCR amplified and
cloned into adenoviral shuttle vectors, which were subsequently
used to generate recombinant adenoviruses in HEK-293 cells as
previously described (29,32). The resultant recombinant
adenoviruses were designated as AdGFP and AdBMP2, respectively. The
amplified adenoviruses were titrated and stored at −80°C.
Establishment of BMP2/FLuc and FLuc
expression stable cell lines
In order to construct BMP2 and/or firefly luciferase
(FLuc) stable expression cell lines, the coding regions of human
BMP2 and/or FLuc were PCR amplified and subcloned into a homemade
piggyBac vector pMPB5, resulting in pMPB-BMP2/FLuc and
pMPB-FLuc, respectively. The PCR amplified sequences were verified
by DNA sequencing. To construct stable cell lines, exponentially
growing HCT116 cells were co-transfected with pMPB-BMP2/FLuc or
pMPB-FLuc and the Super piggyBac transposase expression
vector (System Biosciences, Mountain View, CA, USA) using
Lipofectamine transfection reagents by following the manufacturer’s
instructions (Life Technologies, Grand Island, NY, USA). At 24 h
after transfection, stable clones were selected in the presence of
blasticidin S (10 μg/ml) for 5 days. The resultant stable cell
lines were designated as HCT116-BMP2/FLuc and HCT116-FLuc,
respectively. The stable cell lines were verified by RT-PCR for
BMP2 expression and/or firefly luciferase activity assay.
Colony formation assay
Exponentially growing HCT116 cells were seeded in
6-well plates at a low density (300 cells/well) and infected with
AdGFP or AdBMP2 (MOI=20) for 2 weeks to form colonies. The medium
was replaced every 3–4 days. The uninfected cells were also
included as a control. The colonies were stained with crystal
violet. Each assay condition was conducted in triplicate and
repeated in at least three batches of independent experiments. The
average colony number for each group was calculated and expressed
as the colony formation rate (colony number/seeded cell number) ×
100%.
Cell proliferation (MTT) assay
In order to assess cell proliferation and viability,
the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide] assay was performed as previously described (33–39).
Briefly, subconfluent HCT116 cells were infected with AdBMP2 or
AdGFP (MOI=20) for 16 h and seeded in 96-well plates (1,000
cells/well). The plated cells were incubated in DMEM supplied with
1% FBS. At the indicated time points, the cells were incubated with
10 μl of the CellTiter 96® Non-Radioactive Cell
Proliferation Assay (MTT) reagent (Promega, Madison, WI, USA) at
37°C for 4 h, followed by addition of 100 μl DMSO to dissolve the
formazan products for 10 min at room temperature with gentle
agitation. The absorbance was measured at 492 nm using a microtiter
plate reader. Each assay condition was carried out in five
replicates. The overall experiments were repeated at least in three
batches of independent experiments.
Cell migration/wound healing assay
Subconfluent HCT116 cells were infected with AdGFP
or AdBMP2 for 16 h and reseeded in 6-well plates at ~90%
confluency. Upon cell attachment, scratches were made with pipette
micro-tips. Floating cells were removed and the attached cells were
maintained in DMEM supplemented with 1% FBS. The width of the
scratched cell gaps were monitored and recorded at different time
points. The scratch assay was carried out in triplicate and at
least three scratch sites were monitored and recorded in each well.
Percentage of the wound area closure was measured using ImageJ
software.
Boyden chamber invasion/migration
assays
The Matrigel cell invasion assay was performed as
previously described (33,34,40).
Briefly, subconfluent HCT116 cells were infected with AdGFP or
AdBMP2 for 24 h. Polycarbonate membranes with 8-μm pores were
coated with Matrigel (BD Biosciences). The membranes were
rehydrated, and 5×105 of the transduced cells were
placed onto each upper chamber of the Transwell unit. Medium with
10% FBS was used as a chemoattractant in the bottom chamber. The
cells were allowed to invade at 37°C in 5% CO2 for 24 h.
Cells were fixed in 10% formalin and washed with PBS. The cells
were stained with hematoxylin and rinsed with water. Cells on the
unmigrated side were gently wiped off with a wet cotton tip
applicator, and the membrane was rinsed with water. The membranes
containing the migrated cells were dried and mounted onto slides
with Permount. The number of migrated cells per high power field
(HPF) was determined by averaging 20 randomly counted HPFs. The
assays were performed in triplicate and repeated in at least three
batches of independent experiments.
Apoptosis and flow cytometric
analysis
Subconfluent HCT116-FLuc and HCT116-BMP2/FLuc cells
were cultured in DMEM containing 1% FBS for 72 h. Both floating and
attached cells were collected, stained with Annexin V-FITC and
propidium iodide (PI) using the Annexin V-FITC apoptosis detection
kit (BD Pharmingen™, BD Biosciences). The stained cells were
subjected to FACS analysis using the BD™ LSR II flow cytometer and
FlowJo software. Each assay was performed in triplicate and
repeated at least three times.
Xenograft tumor growth and xenogen whole
body bioluminescence imaging
All animal experiments reported in this study were
carried out in strict accordance with the recommendations
established in the Guide for the Care and Use of Laboratory Animals
of the National Institutes of Health. The protocol was approved by
the Institutional Animal Care and Use Committee (IACUC). For
subcutaneous xenograft tumor formation, 4–6 week old male athymic
nude (nu/nu) mice were purchased from Harlan Sprague Dawley
(Indianapolis, IN, USA). Exponentially growing HCT116-BMP2/FLuc and
HCT116-FLuc cells were harvested and resuspended in PBS. Cells
(2×106 in 100 μl of PBS) were injected subcutaneously
into the flanks of athymic mice (n=6/group). All animals were
sacrificed 4 weeks after injection.
For weekly whole body bioluminescence imaging, the
animals were anesthetized with isoflurane attached to a nose-cone
mask within the Xenogen IVIS 200 imaging system. Mice were injected
with D-Luciferin sodium salt (Gold BioTechnology, St. Louis, MO,
USA) at 100 mg/kg in 0.1 ml sterile PBS. The pseudo-images were
obtained by superimposing the emitted light over the grayscale
images of the animal. The average signals in
photons/sec/cm2/steradian were calculated. Quantitative
analysis was carried out using the Xenogen’s Living Image software
as previously described (22,35,38,41,42).
Histologic evaluation and
immunohistochemical staining
The retrieved tissues were fixed in 10% buffered
formalin and embedded in paraffin. The 5-μm sections were subjected
to H&E staining. Immunohistochemistry was carried out as
previously described (22,23,30,31,40,43–46).
For immunohistochemical staining, sections were deparaffinized,
rehydrated, subjected to antigen retrieval and probed with a PNCA
antibody (Santa Cruz Biotechnology), followed by incubation with
biotin-secondary antibodies and streptavidin-HRP. PCNA protein was
visualized by 3,3′-diaminobenzidine staining. Control IgG and
minus-primary antibody staining were used as negative controls.
Statistical analysis
All quantitative data were calculated and are
expressed as means ± standard deviation. The differences between
groups were analyzed using one-way ANOVA followed by the
Student-Newman-Keuls test using GraphPad Prism software. A
P<0.05 was considered to indicate a statistically significant
result.
Results and Discussion
Exogenous BMP2 inhibits the proliferative
activity of human colon cancer cells
As the effects of BMP2 on colon cancer cells remain
to be fully understood, we used an adenoviral vector overexpressing
BMP2 and investigated its effects on the cell proliferation of
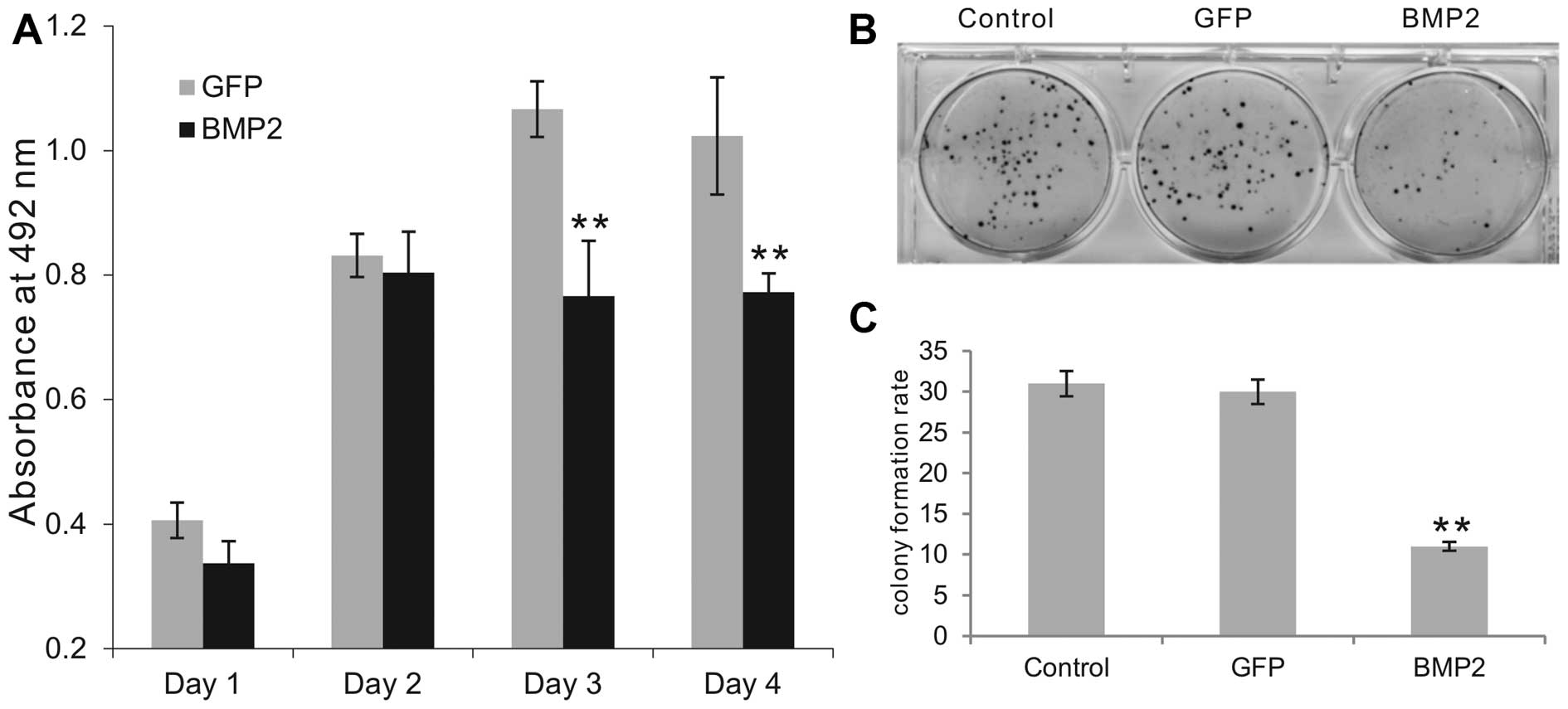
colon cancer HCT116 cells. Using MTT assay, we found that
AdBMP2-infected HCT116 cells exhibited lower proliferative activity
at all tested time points when compared with that of the
AdGFP-transduced cells, although only the differences on days 3 and
4 exhibited statistical significance (P<0.001) (Fig. 1A). When the AdBMP2, AdGFP or
uninfected HCT116 cells were seeded at a very low density and
allowed to form colonies, the BMP2-expressing HCT116 group formed
significantly fewer colonies (Fig.
1B). Quantitatively, the BMP2-expressing HCT116 group formed
approximately one third of the number of colonies when compared
with the number in the GFP or uninfected control groups (Fig. 1C). These results suggest that BMP2
inhibits the proliferative activity of human colon cancer
cells.
BMP2 inhibits the cell migration
capability and invasiveness of human colon cancer cells
We further examined whether BMP2 affects the
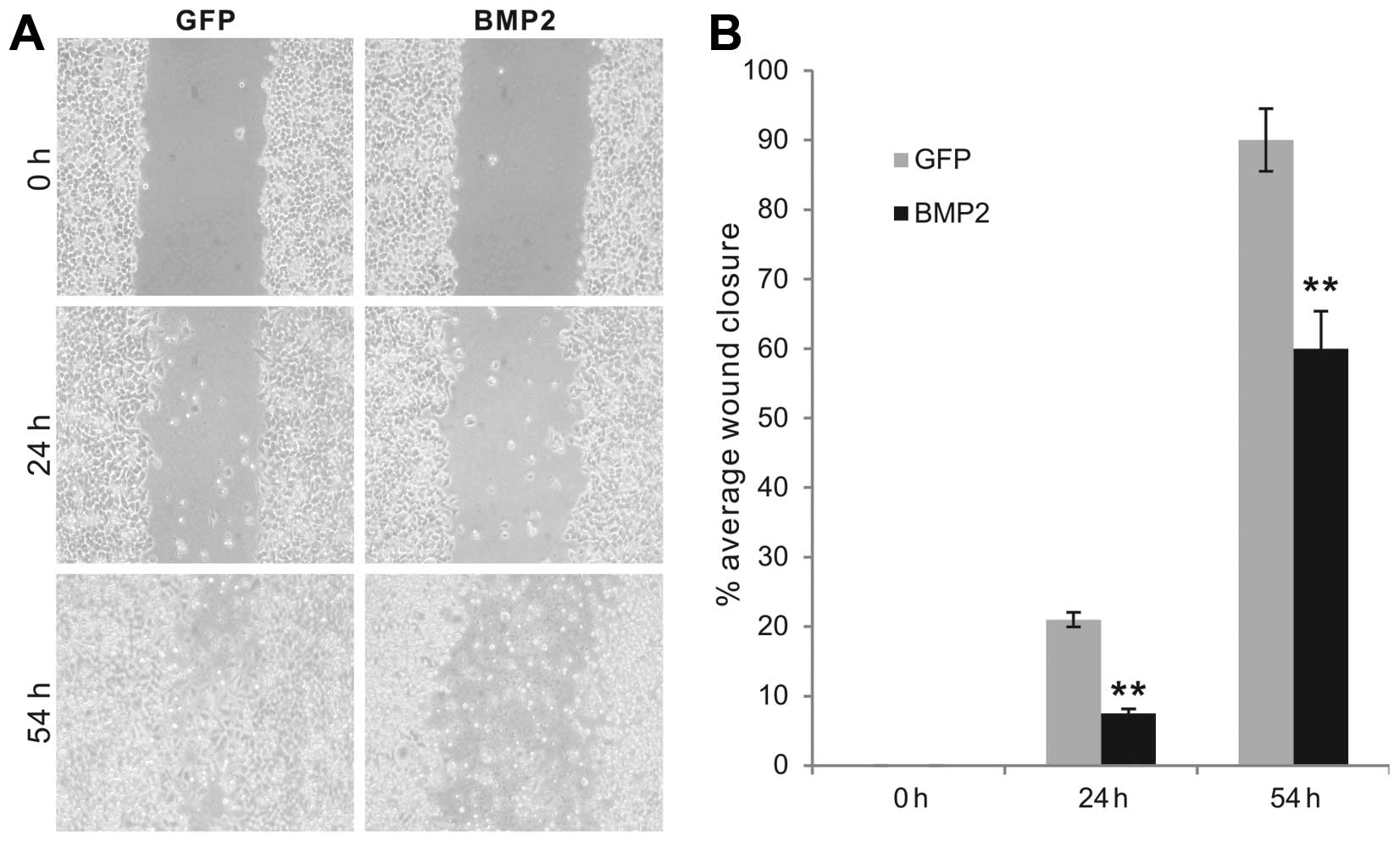
migration capability and invasiveness of colon cancer cells. We
performed the commonly used cell wound healing assay to assess the
effect on cell migration. The AdBMP2-transduced HCT116 cells were
shown to close the scratched gaps on monolayer culture at a much
slower pace than that of the GFP control groups (Fig. 2A). The percentage of wound closure
was significantly higher in the GFP-transduced control cells at all
tested time points (P<0.001) (Fig.
2B). Thus, these results suggest that exogenous BMP2 expression
may significantly inhibit the migratory capability of colon cancer
cells.
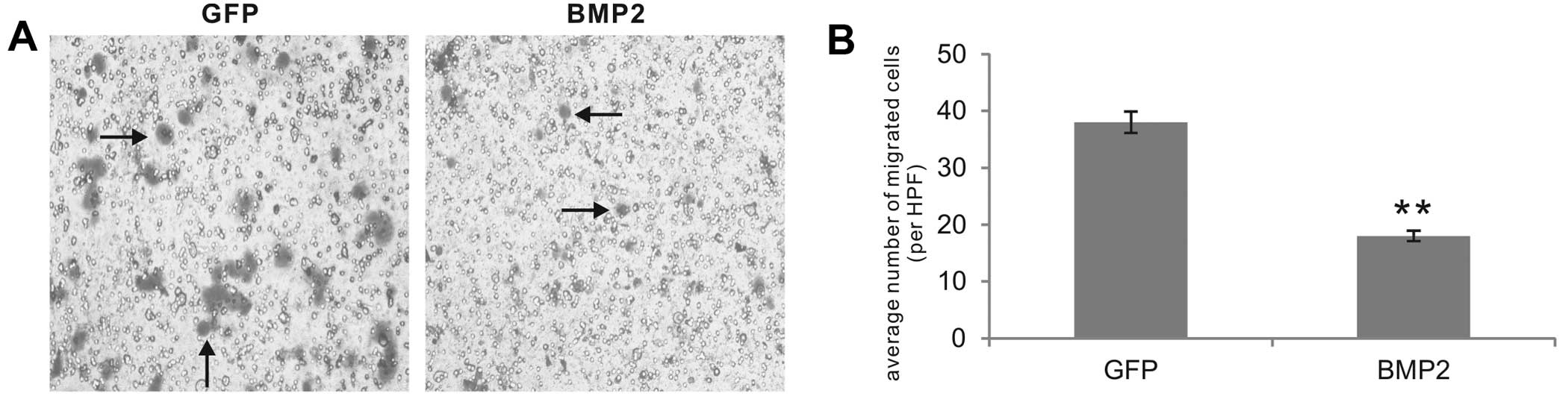
Using the Boyden Transwell extracellular matrix
invasion assay, we analyzed the effect of BMP2 on the invasiveness
of colon cancer cells. Consistent with our previous reports,
GFP-treated HCT116 control cells were fairly aggressive and invaded
the Matrigel-coated Transwell membrane with high efficiency, which
was inhibited by exogenous BMP2 (Fig.
3A). Quantitatively, the BMP2-transduced HCT116 cells exhibited
approximately <50% of the number of invaded cells in the GFP
control group (P<0.001) (Fig.
3B), suggesting that BMP2 exerts an inhibitory effect on the
invasiveness of colon cancer cells.
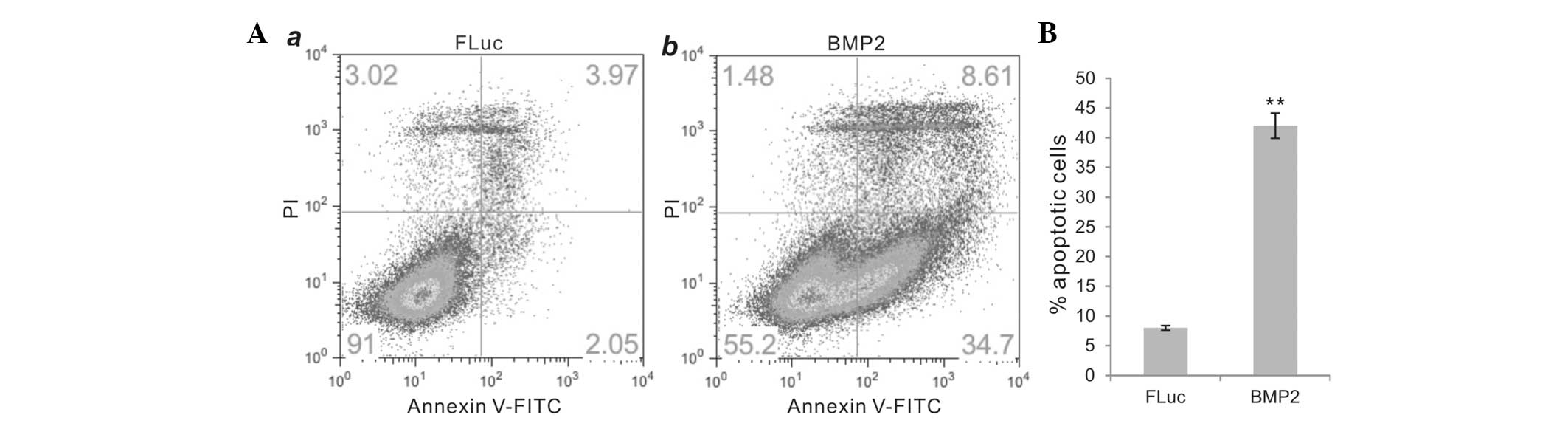
BMP2 effectively induces apoptosis in
human colon cancer cells
We next investigated whether BMP2 induces apoptosis
in colon cancer cells. We established a stable cell line
HCT116-BMP2/FLuc that co-expressed human BMP2 and firefly
luciferase (FLuc), while a control stable cell line HCT116-FLuc
that only expresses FLuc was established in the same fashion. The
exogenous expression of BMP2 was verified by RT-PCR analysis while
the FLuc activity was determined using luciferase assay kits. We
observed that HCT116-BMP2/FLuc cells grew normally in complete DMEM
(with 10% FBS), compared with the parental HCT116 or HCT116-FLuc
cells (data not shown). However, when the BMP2-expressing HCT116
cells were grown under no (0%) or low (1%) FBS condition, a
significant increase in apoptosis was detected using the Annexin V
labeling assay (Fig. 4A, panel a
vs. b). When cultured in 1% FBS/DMEM for 72 h, the BMP2-expressing
HCT116 cells underwent significant apoptosis (~42%), compared to
~8% in the control group (P<0.001) (Fig. 4B). Thus, these results suggest that
BMP2 inhibits colon cancer cell proliferation at least in part
through induction of apoptosis.
BMP2 effectively inhibits the growth of
xenograft tumors derived from human colon cancer
Although the above in vitro data strongly
suggest that BMP2 exhibits an inhibitory effect on colon cancer
cell proliferation, we aimed to verify whether the inhibitory
effect could be extended to in vivo tumor models. We
previously demonstrated that HCT116 cells can reproducibly form
subcutaneous tumors in athymic nude mice (35,36).
We used the FLuc-tagged stable lines, HCT116-FLuc and
HCT116-BMP2/FLuc. Subconfluent HCT116-FLuc and HCT116-BMP2/FLuc
(BMP2) cells were subcutaneously injected into athymic nude mice,
and the tumor growth was monitored at weeks 2 and 4 using whole
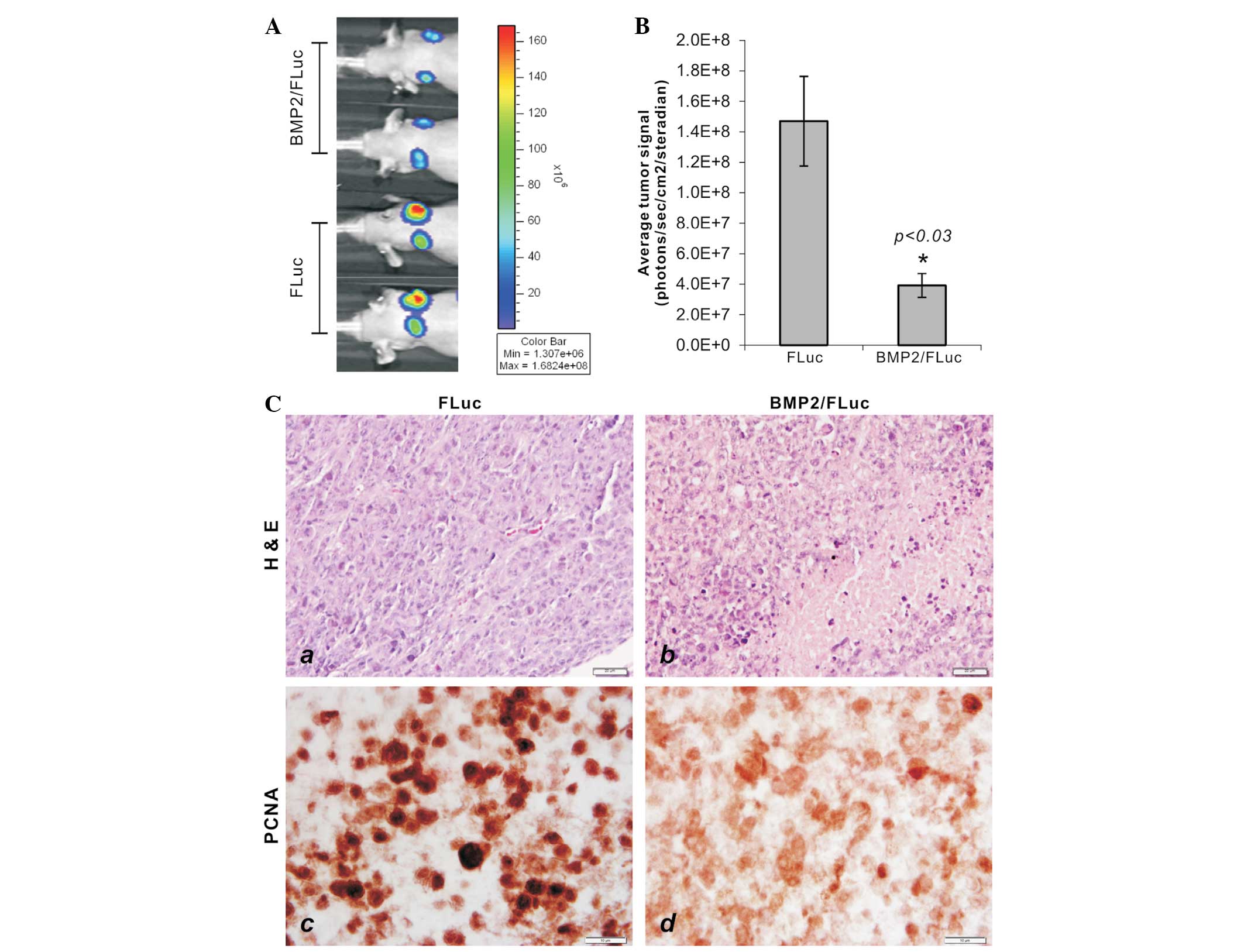
body xenogen bioluminescence. We found that the BMP2-expressing
HCT116 group formed significantly smaller tumor masses at each time
point (Fig. 5A). Quantitative
analysis revealed that the tumor growth in the BMP2-expressing
HCT116 tumors was ~26% when compared with the control group
(P<0.03) (Fig. 5B). When the
retrieved tumor samples were fixed, embedded and sectioned for
H&E staining, the samples from the BMP2/FLuc group exhibited
significant necrosis and low cell proliferation, compared with
these parameters in the FLuc control group (Fig. 5C, panel a vs. b). The embedded
samples were also sectioned and subjected to immunohistochemical
staining with a PCNA antibody. We found that the tumor samples
formed by HCT116 cells expressing BMP2 exhibited a significantly
diminished staining of PCNA expression in the tumor cells (Fig. 5C, panel c vs. d), suggesting that
the BMP2-expressing colon cancer cells have a decreased
proliferative activity. Collectively, these in vivo results
further confirm that BMP2 exhibits strong inhibitory effects on
colon cancer cells, possibly through inhibiting their proliferation
and migration and inducing apoptosis.
BMP signaling may play an important role
in modulating colorectal tumorigenesis
The findings from our in vitro and in
vivo studies demonstrated that exogenous BMP2 inhibits cell
proliferation, migration and invasion, induces apoptosis and
suppresses in vivo xenograft tumor growth of human colon
cancer cells. The importance of BMP signaling in colorectal
tumorigenesis has been highlighted by the identification of
frequent mutations of SMAD4 in CRC (10) and mutations in BMP receptor 1A
(BMPR1A) in patients with juvenile polyposis (JP) (12–14),
which is associated with an increased risk for the development of
CRC (11). Moreover, forced
expression of the BMP antagonist noggin in mouse intestine was
found to result in the formation of intestinal hamartomatous polyps
(15).
Consistent with our findings are previous reports in
which BMP2, BMP3 or BMP7 were shown to have growth-suppressive
activities in colon cancer cells (16–18),
although the expression of BMP4 and BMP7 was found to correlate
with a worse prognosis (19,20).
Notably, a more recent report showed that BMP signaling promotes
the growth of primary human colon cancer in vivo and the
investigators proposed that blockade of BMP signaling may have
beneficial effects against at least a subset of advanced colon
cancers (21). Nonetheless, studies
have revealed that genetic variations in the BMP signaling pathway
may be associated with the etiology, survival and/or prognosis of
colon and rectal cancer (18,47–49).
Mechanistically, an early study suggested that BMP2
may act as a tumor suppressor promoting apoptosis in mature colonic
epithelial cells (16), although it
was suggested that BMP may also utilize SMAD4-independent pathways
for growth suppression in colon cancers (18). Notably, it was reported that
statins, acting as DNMT inhibitors can demethylate the BMP2
promoter, activate BMP signaling, induce differentiation of colon
cancer stem cells and reduce their ‘stemness’ (50). Moreover, BMP-induced growth
suppression may be mediated in part by p21WAF1, which is
inhibited by RAS/ERK, as in colon cancer cells where BMP-SMAD
signaling and growth suppression are facilitated by
p21WAF1 but diminished by oncogenic K-RAS (51). It has been reported that suppression
of the PI3 kinase/Akt pathway may be correlated with the
development of BMP2 resistance and invasion in BMP2-induced
epithelial-to-mesenchymal transformation (EMT) in colon cancer
(52). It has been reported that
the anti-mitogenic effect of proteasome inhibitors on colon cancer
cells may require BMP signaling (53).
In summary, we investigated the effect of BMP2 on
the proliferation, migration, invasiveness and tumor growth
capabilities of human colon cancer cells. We found that exogenous
BMP2 effectively inhibited HCT116 cell proliferation and colony
formation. BMP2 also suppressed colon cancer cell migration and
invasiveness. Forced expression of BMP2 induced significant
apoptosis in HCT116 cells. Using an xenograft tumor model, we found
that forced expression of BMP2 in HCT116 cells suppressed tumor
growth, accompanied by decreased proliferative activity.
Collectively, our results strongly suggest that BMP2 plays an
inhibitory role in controlling the proliferation and aggressive
features associated with colon cancer cells.
Acknowledgements
The present study was supported in part by research
grants from the National Institutes of Health (CA106569, AT004418,
AR50142 and AR054381 to T-C.H, R.C.H. and H.H.L.) and the 973
Program of Ministry of Science and Technology (MOST) of China
(#2011CB707900 to T-C.H.). This study was also supported in part by
The University of Chicago Core Facility Subsidy grant from the
National Center for Advancing Translational Sciences (NCATS) of the
National Institutes of Health through grant no. UL1 TR000430.
References
|
1
|
Bertrand FE, Angus CW, Partis WJ and
Sigounas G: Developmental pathways in colon cancer: crosstalk
between WNT, BMP, Hedgehog and Notch. Cell Cycle. 11:4344–4351.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
3
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kinzler KW, Nilbert MC, Su LK, et al:
Identification of FAP locus genes from chromosome 5q21. Science.
253:661–665. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan TL, Zhao W, Leung SY and Yuen ST:
BRAF and KRAS mutations in colorectal hyperplastic
polyps and serrated adenomas. Cancer Res. 63:4878–4881.
2003.PubMed/NCBI
|
|
7
|
Hardwick JC, Kodach LL, Offerhaus GJ and
van den Brink GR: Bone morphogenetic protein signalling in
colorectal cancer. Nat Rev Cancer. 8:806–812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Massague J: TGF-beta signal transduction.
Annu Rev Biochem. 67:753–791. 1998. View Article : Google Scholar
|
|
9
|
Massague J: How cells read TGF-beta
signals. Nat Rev Mol Cell Biol. 1:169–178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiagalingam S, Lengauer C, Leach FS, et
al: Evaluation of candidate tumour suppressor genes on chromosome
18 in colorectal cancers. Nat Genet. 13:343–346. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Howe JR, Bair JL, Sayed MG, et al:
Germline mutations of the gene encoding bone morphogenetic protein
receptor 1A in juvenile polyposis. Nat Genet. 28:184–187. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Howe JR, Roth S, Ringold JC, et al:
Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science.
280:1086–1088. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Howe JR, Sayed MG, Ahmed AF, et al: The
prevalence of MADH4 and BMPR1A mutations in juvenile
polyposis and absence of BMPR2, BMPR1B, and ACVR1
mutations. J Med Genet. 41:484–491. 2004.PubMed/NCBI
|
|
14
|
Sayed MG, Ahmed AF, Ringold JR, et al:
Germline SMAD4 or BMPR1A mutations and phenotype of juvenile
polyposis. Ann Surg Oncol. 9:901–906. 2002.PubMed/NCBI
|
|
15
|
Haramis AP, Begthel H, van den Born M, et
al: De novo crypt formation and juvenile polyposis on BMP
inhibition in mouse intestine. Science. 303:1684–1686. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hardwick JC, Van Den Brink GR, Bleuming
SA, et al: Bone morphogenetic protein 2 is expressed by, and acts
upon, mature epithelial cells in the colon. Gastroenterology.
126:111–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Loh K, Chia JA, Greco S, et al: Bone
morphogenic protein 3 inactivation is an early and frequent event
in colorectal cancer development. Genes Chromosomes Cancer.
47:449–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beck SE, Jung BH, Fiorino A, et al: Bone
morphogenetic protein signaling and growth suppression in colon
cancer. Am J Physiol Gastrointest Liver Physiol. 291:G135–G145.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng H, Makizumi R, Ravikumar TS, Dong H,
Yang W and Yang WL: Bone morphogenetic protein-4 is overexpressed
in colonic adenocarcinomas and promotes migration and invasion of
HCT116 cells. Exp Cell Res. 313:1033–1044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Motoyama K, Tanaka F, Kosaka Y, et al:
Clinical significance of BMP7 in human colorectal cancer. Ann Surg
Oncol. 15:1530–1537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lorente-Trigos A, Varnat F, Melotti A and
Ruiz i Altaba A: BMP signaling promotes the growth of primary human
colon carcinomas in vivo. J Mol Cell Biol. 2:318–332. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo X, Chen J, Song WX, et al: Osteogenic
BMPs promote tumor growth of human osteosarcomas that harbor
differentiation defects. Lab Invest. 88:1264–1277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang N, Song WX, Luo J, et al:
BMP-9-induced osteogenic differentiation of mesenchymal progenitors
requires functional canonical Wnt/beta-catenin signaling. J Cell
Mol Med. 13:2448–2464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao Y, Huang E, Zhang H, et al: Crosstalk
between Wnt/β-catenin and estrogen receptor signaling
synergistically promotes osteogenic differentiation of mesenchymal
progenitor cells. PloS One. 8:e824362013.
|
|
25
|
Kong Y, Zhang H, Chen X, et al:
Destabilization of heterologous proteins mediated by the GSK3β
phosphorylation domain of the β-catenin protein. Cell Physiol
Biochem. 32:1187–1199. 2013.PubMed/NCBI
|
|
26
|
Zhang W, Zhang H, Wang N, et al:
Modulation of β-catenin signaling by the inhibitors of MAP kinase,
tyrosine kinase, and PI3-kinase pathways. Int J Med Sci.
10:1888–1898. 2013.
|
|
27
|
Chen X, Luther G, Zhang W, et al: The E–F
hand calcium-binding protein S100A4 regulates the proliferation,
survival and differentiation potential of human osteosarcoma Cells.
Cell Physiol Biochem. 32:1083–1096. 2013.
|
|
28
|
Cheng H, Jiang W, Phillips FM, et al:
Osteogenic activity of the fourteen types of human bone
morphogenetic proteins (BMPs. J Bone Joint Surg Am. 85-A:1544–1552.
2003.PubMed/NCBI
|
|
29
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang Q, Song WX, Luo Q, et al: A
comprehensive analysis of the dual roles of BMPs in regulating
adipogenic and osteogenic differentiation of mesenchymal progenitor
cells. Stem Cells Dev. 18:545–559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang Q, Sun MH, Cheng H, et al:
Characterization of the distinct orthotopic bone-forming activity
of 14 BMPs using recombinant adenovirus-mediated gene delivery.
Gene Ther. 11:1312–1320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo J, Deng ZL, Luo X, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luu HH, Kang Q, Park JK, et al: An
orthotopic model of human osteosarcoma growth and spontaneous
pulmonary metastasis. Clin Exp Metastasis. 22:319–329. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luu HH, Zhou L, Haydon RC, et al:
Increased expression of S100A6 is associated with decreased
metastasis and inhibition of cell migration and anchorage
independent growth in human osteosarcoma. Cancer Lett. 229:135–148.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He BC, Gao JL, Luo X, et al: Ginsenoside
Rg3 inhibits colorectal tumor growth through the down-regulation of
Wnt/β-catenin signaling. Int J Oncol. 38:437–445. 2011.PubMed/NCBI
|
|
36
|
He BC, Gao JL, Zhang BQ, et al:
Tetrandrine inhibits Wnt/β-catenin signaling and suppresses tumor
growth of human colorectal cancer. Mol Pharmacol. 79:211–219.
2011.
|
|
37
|
Su Y, Luo X, He BC, et al: Establishment
and characterization of a new highly metastatic human osteosarcoma
cell line. Clin Exp Metastasis. 26:599–610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Su Y, Wagner ER, Luo Q, et al:
Insulin-like growth factor binding protein 5 suppresses tumor
growth and metastasis of human osteosarcoma. Oncogene.
30:3907–3917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang J, Zhang H, Zhang W, et al: Bone
morphogenetic protein-9 (BMP9) effectively induces
osteo/odontoblastic differentiation of the reversibly immortalized
stem cells of dental apical papilla. Stem Cells Dev. Mar
21–2014.(Epub ahead of print).
|
|
40
|
Luo Q, Kang Q, Si W, et al: Connective
tissue growth factor (CTGF) is regulated by Wnt and bone
morphogenetic proteins signaling in osteoblast differentiation of
mesenchymal stem cells. J Biol Chem. 279:55958–55968. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luther GA, Lamplot J, Chen X, et al:
IGFBP5 domains exert distinct inhibitory effects on the
tumorigenicity and metastasis of human osteosarcoma. Cancer Lett.
336:222–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He BC, Chen L, Zuo GW, et al: Synergistic
antitumor effect of the activated PPARgamma and retinoid receptors
on human osteosarcoma. Clin Cancer Res. 16:2235–2245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sharff KA, Song WX, Luo X, et al: Hey1
basic helix-loop-helix protein plays an important role in mediating
BMP9-induced osteogenic differentiation of mesenchymal progenitor
cells. J Biol Chem. 284:649–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen L, Jiang W, Huang J, et al:
Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced
osteogenic differentiation and bone formation. J Bone Miner Res.
25:2447–2459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang W, Deng ZL, Chen L, et al: Retinoic
acids potentiate BMP9-induced osteogenic differentiation of
mesenchymal progenitor cells. PloS One. 5:e119172010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luo J, Tang M, Huang J, et al: TGFbeta/BMP
type I receptors ALK1 and ALK2 are essential for BMP9-induced
osteogenic signaling in mesenchymal stem cells. J Biol Chem.
285:29588–29598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Slattery ML, Lundgreen A, Herrick JS,
Wolff RK and Caan BJ: Genetic variation in the transforming growth
factor-β signaling pathway and survival after diagnosis with colon
and rectal cancer. Cancer. 117:4175–4183. 2011.
|
|
48
|
Slattery ML, Lundgreen A, Herrick JS, et
al: Genetic variation in bone morphogenetic protein and colon and
rectal cancer. Int J Cancer. 130:653–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xiang L, Wang S, Jin X, Duan W, Ding X and
Zheng C: Expression of BMP2, TLR3, TLR4 and COX2 in colorectal
polyps, adenoma and adenocarcinoma. Mol Med Rep. 6:973–976.
2012.PubMed/NCBI
|
|
50
|
Kodach LL, Jacobs RJ, Voorneveld PW, et
al: Statins augment the chemosensitivity of colorectal cancer cells
inducing epigenetic reprogramming and reducing colorectal cancer
cell ‘stemness’ via the bone morphogenetic protein pathway. Gut.
60:1544–1553. 2011.PubMed/NCBI
|
|
51
|
Beck SE, Jung BH, Del Rosario E, Gomez J
and Carethers JM: BMP-induced growth suppression in colon cancer
cells is mediated by p21WAF1stabilization and modulated
by RAS/ERK. Cell Signal. 19:1465–1472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kang MH, Kang HN, Kim JL, Kim JS, Oh SC
and Yoo YA: Inhibition of PI3 kinase/Akt pathway is required for
BMP2-induced EMT and invasion. Oncol Rep. 22:525–534.
2009.PubMed/NCBI
|
|
53
|
Wu WK, Sung JJ, Wu YC, Li ZJ, Yu L and Cho
CH: Bone morphogenetic protein signalling is required for the
anti-mitogenic effect of the proteasome inhibitor MG-132 on colon
cancer cells. Br J Pharmacol. 154:632–638. 2008. View Article : Google Scholar : PubMed/NCBI
|