Introduction
Over the last decade, genome-wide transcriptome
studies have shown that the mammalian genome is abundantly
transcribed and that at least 80% of this transcription is
exclusively associated with long non-coding RNAs (lncRNAs; >200
bp), which map to intronic and intergenic regions (1,2).
Initially, lncRNAs were regarded as ‘transcriptional noise’, since
they lack the capacity to code for proteins (3). To date, a growing body of evidence
indicates that lncRNAs are important players in a wide range of
biological processes, including chromatin remodeling, gene
transcription, mRNA translation and protein function (4–6).
Previous studies have found that the mechanisms
underlying gene regulation also involve non-coding RNAs, including
lncRNAs (7–9). The lncRNA molecular functions in gene
regulation have been classified into four archetypes (5): archetype I, as signals, lncRNA
expression can faithfully reflect the combinatorial actions of
transcription factors or signaling pathways to indicate gene
regulation in space and time; archetype II, as decoys, lncRNAs can
titrate transcription factors and other proteins away from
chromatin or titrate the protein factors into nuclear subdomains;
archetype III, as guides, lncRNAs can recruit chromatin-modifying
enzymes to target genes; and archetype IV, as scaffolds, lncRNAs
can bring together multiple proteins to form ribonucleoprotein
complexes. However, the study of lncRNA is still in its infancy
(10,11), as only a small portion of lncRNAs
has been examined for its biological activities and the molecular
mechanisms of action have been characterized for very few
lncRNAs.
Urothelial carcinoma associated 1 (UCA1), a novel
lncRNA, has been reported to play a potential role in the
progression of bladder cancer and could serve as a biomarker for
diagnosis of bladder cancer (12,13).
Ectopic expression of UCA1 lncRNA in BLS-211, a bladder cancer cell
line, significantly enhanced tumorigenicity and invasive potential
of cells (14). Other studies found
that UCA1 is associated with tumor-linked genes such as WNT6
(15), CYP1A1 (16) and AURKC (17). These findings suggested that UCA1
may regulate some aspects of the tumorigenic processes,
particularly in urothelial carcinoma, but its specific function and
mechanisms remain unknown.
The BRG1 protein is the functional ATPase subunit of
the mammalian SWI/SNF family of ATP-dependent chromatin remodeling
complexes. BRG1 has a widespread role in tumor suppression
(18), cell differentiation
(19) and cellular senescence
(20). Previous studies have
demonstrated that BRG1 generally functions as a tumor suppressor
via various mechanisms, including upregulation of the cell cycle
inhibitor p21, a direct target gene of BRG1 (20,21).
In the present study, we first examined the function
of UCA1 in 5637 bladder cancer cells, which express high levels of
UCA1. We found that UCA1 plays an oncogene-like role in this
bladder cancer cell line, which is consistent with previous
reports. Furthermore, we found UCA1 promotes 5637 cell
proliferation by antagonizing the activities of BRG1, by reducing
its binding to the p21 promoter and inhibiting its chromatin
remodeling activity.
Materials and methods
Cell culture and tissue samples
Human bladder cancer 5637 and EJ cells were cultured
in RPMI-1640, T24 cells were cultured in McCoy’s 5A, and 293T and
HeLa cells were cultured in Dulbecco’s modified Eagle’s medium, all
supplemented with 10% fetal bovine serum. All cells were maintained
in a humidified atmosphere of 5% CO2 at 37°C. All tissue
samples were obtained from a tissue bank at the Institute of
Urology (Peking University, China). These tissue samples were
operative samples of bladder cancers and were diagnosed by
pathology. Every pair of samples included one bladder cancer tissue
sample and one normal bladder tissue sample. The licensing
committee approved the experiments undertaken and both ethical
approval and informed consent were obtained. The ethical approval
document no. is IRB00001052-13057.
Vector constructs
To generate lentivirus vector constructs stably
expressing small interfering RNA (siRNA) targeting BRG1 or UCA1, a
short double strand DNA sequence was cloned into the pll3.7
lentivirus vector. The oligonucleotides were as follows: iBRG1
sense, 5′-TCATGCACCAGATGCAC AAGTTCAAGAGACTTGTGCATCTGGTGCATGTTTTT
TC-3′ and iBRG1 antisense, 5′-TCGAGAAAAAACATGCAC
CAGATGCACAAGTCTCTTGAACTTGTGCATCTGGTG CATGA-3′ (22); iUCA1 sense, 5′-TGGTAATGTATCATCGG
CTTAGTTCAAGAGACTAAGCCGATGATACATTACCTT TTTTC-3′ and iUCA1 antisense,
5′-TCGAGAAAAAAGGTA ATGTATCATCGGCTTAGTCTCTTGAACTAAGCCGATG
ATACATTACCA-3′; and control sense, 5′-TTTCTCCGAACG
TGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAA TTTTTTC-3′ and control
antisense, 5′-TCGAGAAAAAATT CTCCGAACGTGTCACGTTCTCTTGAAACGTGACACG
TTCGGAGAAA-3′. The resulting constructs were designated as
pll3.7-iBRG1, pll3.7-iUCA1 and pll3.7-NC. All constructs were
confirmed by sequencing.
The UCA1 expressing lentivirus vector pZsG-UCA1 was
generated by inserting full-length UCA1 into the pZsG vector, which
contains a puromycin resistant gene. We used the primers
5′-(NotI)-GCGGCCGCTGACATTCTTCTGGACA ATGAGTC-3′ and
5′-(BamHI)-GGATCCGGCATATTAGCT TTAATGTAGGTG-3′ to obtain
full-length UCA1 cDNA from 5637 cells. PCR amplification was
performed using High-Fidelity DNA polymerase (NEB, Ipswich, MA,
USA) with the following conditions: 98°C, 10 sec; 55°C, 30 sec;
72°C, 30 sec, repeat 32 times. We cloned BRG1 into the pcDNA3.1
using EcoRV restriction site. We used the primers
5′-AGCTCCCGTGAAGATGTCCAC-3′ and 5′-TCTGCTGCTACCCGTTACTGCT-3′ to
obtain BRG1 from pBJ5-hBRG1. PCR amplification was performed using
High-Fidelity DNA polymerase with the following conditions: 98°C,
10 sec; 61°C, 30 sec; 72°C, 90 sec; for 32 cycles. The pZsG-UCA1
and pcDNA3.1-BRG1 vectors were confirmed by sequencing.
Lentivirus packaging and infection
Lentivirus was produced by transfecting the
packaging plasmid psPAX2, envelope plasmid pMD2.G and the
experiment or control lentiviral vector (pll3.7 or pZsG with
respective inserts) into 293T cells. Cells were transfected using
VigoFect (Vigorous, Beijing, China) according to the manufacturer’s
protocol. Viruses in the culture medium were harvested at 36 and 60
h after transfection and centrifuged at 4°C at 83,000 × g for 2 h.
Viruses were resuspended with 100 μl phosphate-buffered saline
(PBS) and stored at −80°C. Cells were infected in the presence of 8
μg/ml polybrene. After 6 h, the medium was changed and 24-h media
was changed with the appropriate selection conditions.
MTT and colony formation assays
For
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT)] assay, 5637 cells (103/well) were seeded into a
96-well plate and incubated at 37°C. Growth of 5637 cells was
measured following addition of 20 μl of MTT (5 mg/ml) into the
culture medium. MTT reduction was determined using an automated
microplate reader (Bio-Rad, Hercules, CA, USA). All the assays were
performed in triplicate, and the data are presented as means ±
SD.
For colony formation assay, cells (~103
cells) were plated onto 35-mm petri dishes. Cells were cultured for
10–14 days. Colonies were stained using crystal violet and
counted.
RNA pull-down assay
To generate antisense and sense UCA1 RNA
oligonucleotides, we first inserted UCA1 DNA oligonucleotides with
an EcoRI site at the 5′ end, and a BamHI site at the
3′ end into pcDNA3.1(+) and pcDNA3.1(−) plasmids, respectively,
which harbor a T7 promoter. They were confirmed by sequencing. We
linearized the vectors with EcoRI or BamHI to obtain
pcDNA3.1(+)-UCA1-EcoRI and pcDNA3.1(−)-UCA1-BamHI. We
incubated T7 RNA polymerase biotin RNA labeling mix (Roche, Basel,
Switzerland) and pcDNA3.1(+)-UCA1-EcoRI (or
pcDNA3.1(−)-UCA1-BamHI) DNA template in vitro
according to the manufacturer’s instructions and then purified
these RNAs by phenol chloroform extraction. RNA pull-down assays
were performed with HeLa cell lysate as previously described
(23).
Identification of BRG1 by mass
spectrometry
Proteins precipitated by RNA pull-down assays were
subjected to NuPAGE 4–12% Bis-Tris gel electrophoresis and examined
by silver stain using the Pierce Silver Stain kit (24612; Thermo
Fisher Scientific, Rockford, IL, USA) according to the
manufacturer’s instructions. Specific bands only in the sense UCA
lane (Fig. 2A) were excised and
analyzed by mass spectrometry (GeneSci Biotech Company, Beijing,
China).
RNA-binding protein immunoprecipitation
assay
RNA-binding protein immunoprecipitation assay was
performed with the Magna RIP RNA-Binding Protein
Immunoprecipitation kit (17–700; Merck KGaA, Darmstadt, Germany, )
according to the manufacturer’s instructions. UCA1 (primer
sequences as above) was detected from the pulled down RNA by
real-time PCR with the primers 5′-GCCCAAG GAACATCTCACCAATTT-3′ and
5′-TTGAGGGGTCAG ACTTTTGACAAGG-3′ using the ABI PRISM 7500 sequence
detection system (Applied Biosystems, Rockford, IL, USA) according
to the manufacturer’s instructions. The PCR conditions were: 95°C,
30 sec; 60°C, 30 sec, repeat 40 times.
RNA extraction and PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
protocol. First-strand cDNA was synthesized using SuperScript™ III
first-strand kits (Invitrogen) for RT-PCR. The p21 mRNA was
analyzed by PCR on cDNA with primers 5′-GAAGACCATGTGGACCTGTCA-3′
and 5′-GGCTTCCTCTTGGAGAAGATCA-3′. GAPDH was used as an
internal control, 5′-ACGGATTTGGTCGTATTGGG-3′ and
5′-TGATTTTGGAGGGATCTCGC-3′. The UCA1 RNA was analyzed by PCR
with the primers, 5′-GCCCAAGGAAC ATCTCACCAATTT-3′ and
5′-TTGAGGGGTCAGACTTTT GACAAGG-3′. The BRG1 RNA was analyzed
by PCR with the primers: 5′-AGTGCTGCTGTTCTGCCAAAT-3′ and
5′-GGCTCGTTGAAGGTTTTCAG-3′.
Western blot analysis
Cells were lysed in RIPA buffer (Applygen, Beijing,
China) and total cell lysates were separated by SDS-PAGE,
transferred to PVDF membranes (Merck Millipore, Darmstadt,
Germany), immunoblotted with antibodies, and visualized using a
ChemiDoc XRS+ Imaging System (Bio-Rad) or film. Antibodies used for
immunoblotting were anti-β-actin antibody (PM053; MBL, Japan)
(1:5,000), anti-human p21 (3733-1; Abcam Epitomics, Cambridge, UK)
(1:2,000), anti-H3K9me3 (49–1008; Novex, Carlsbad, CA, USA)
(1:1,000), anti-H3K4m3 (ab8580) (1:2,000) and anti-human BRG1
antibodies (ab4081) (both from Abcam) (1:2,000). Signals were
detected using secondary antibody anti-rabbit IgG-HRP (7077; Cell
Signaling, Beverly, MA, USA) (1:5,000).
Chromatin immunoprecipitation (ChIP)
assays
ChIP assays were performed with the ChIP kit
(Pierce, Cambridge, UK) according to the manufacturer’s
instructions. Briefly, 5637 cells were transfected with pll3.7-NC
or pll3.7-iUCA1 viruses and selected with G418 for 5 days. The
post-confluent cells were then washed in PBS and fixed with 1%
formaldehyde for 10 min at 37°C. Cells were harvested, washed twice
and homogenized by bead beating. Chromatin DNA was sheared using
ultrasound to a size of 0.5–1 kb. ChIP was performed overnight at
4°C using the BRG1 antibody (ab4081; Abcam) or an isotype control
IgG. After a 1 h incubation in the presence of salmon sperm
DNA/protein A agarose beads, the immunoprecipitated DNA/protein
complexes were then washed and eluted from the beads with 1% SDS
and 0.1 M NaHCO3 solution. Protein/DNA cross-links were
reversed by adding 5 M NaCl and protein K at 65°C for 4 h. DNA was
purified and amplified by PCR with primers for detecting human p21
promoter sequences: forward primer, 5′-GGAAATGTGTCCAGCGCACCAAC-3′
and reverse primer, 5′-CAGCGCGGCCCTGATATACAACC-3′.
ATP hydrolysis assays
The measurements of the ATPase activity of BRG1 in
the presence of nucleosome particles (using Nucleosome Assembly kit
E5350S; NEB, Ipswich, MA, USA) was carried out as previously
described (24). Briefly, 100 ng of
reconstituted nucleosomes were mixed with 1 μl of BRG1 and 1 μl Ci
of [γ-32P] ATP in a final volume of 10 μl (10 mM HEPES,
pH 7.8, 50 mM KCl, 5 mM DTT, 0.5 mM PMSF, 200 g/ml BSA, 5%
glycerol, 3.5 mM MgCl2). Aliquots of 1 μl were obtained
at the time points indicated, and the reaction was stopped with 10
μl of gel loading buffer containing 90% formamide, 0.2% SDS, 10 mM
EDTA and dyes. ATP hydrolysis was analyzed on 15% denaturing
polyacrylamide gels. Gels were dried and exposed with phosphoimager
screens, and quantified using the ImageQuant software.
Micrococcal nuclease (MNase) assays
Cells were permeabilized with 0.01%
L-a-lysophosphatidylcholine in 150 mM sucrose, 80 mM KCl, 35 mM
HEPES pH 7.4, 5 mM K2HPO4, 5 mM
MgCl2 and 0.5 mM CaCl2 for 90 sec, followed
by digestion for 60 sec with 2 U/ml micrococcal nuclease (NEB) in
20 mM sucrose, 50 mM Tris-HCl pH 7.5, 50 mM NaCl and 2 mM
CaCl2 at room temperature for various durations.
Digestion of the DNA was arrested by adding 50 mM EDTA. DNA was
then purified by Tris-buffered phenol/chloroform/isoamyl alcohol
extraction. DNA was precipitated using 0.3 M NaOAc (pH 6.5) and two
volumes of ethanol on dry ice for 30 min, and then resuspended in
Tris-EDTA (pH 8.0). DNA concentration was evaluated with a
spectrophotometer. DNA separation was performed by agarose (0.8%)
gel electrophoresis.
Statistical analysis
Results are expressed as means ± SD. The statistical
significance of differences in experimental data was analyzed using
the two-sample Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
UCA1 promotes proliferation of 5637
cells
To investigate the function of UCA1 lncRNA in
urinary bladder cancer, we used the 5637 urinary bladder cell line
that highly expresses UCA1 (Fig.
2D). Based on previous studies indicating that UCA1 exhibits
tumorigenic activities, we first examined whether UCA1 promotes
proliferation of 5637 cells. Cells were infected with lentiviruses
harboring UCA1-shRNA or control-shRNA under G418 selection for
>1 week, and knockdown of UCA1 in UCA1-shRNA-transfected cells
was confirmed by real-time PCR (Fig.
1A). We next performed colony formation and MTT assays in the
two cell lines. Colonies of UCA1-shRNA-transfected cells were fewer
and smaller than those of the control group (Fig. 1C). Additionally, MTT assay results
showed that UCA1-shRNA-infected cells grew slower than the control
cells (Fig. 1E). To confirm these
results, we generated a 5637 cell line ectopically expressing UCA1.
Cells were infected with lentiviruses harboring UCA1 or controls
under puromycin selection for 1 week, and UCA1 overexpression was
confirmed by real-time PCR (Fig.
1B). Ectopic expression of UCA1 in 5637 cells promoted colony
formation (Fig. 1D) and cell
proliferation (Fig. 1F). These
results indicate that UCA1 promotes 5637 cell proliferation,
supporting its role as an oncogene-like factor.
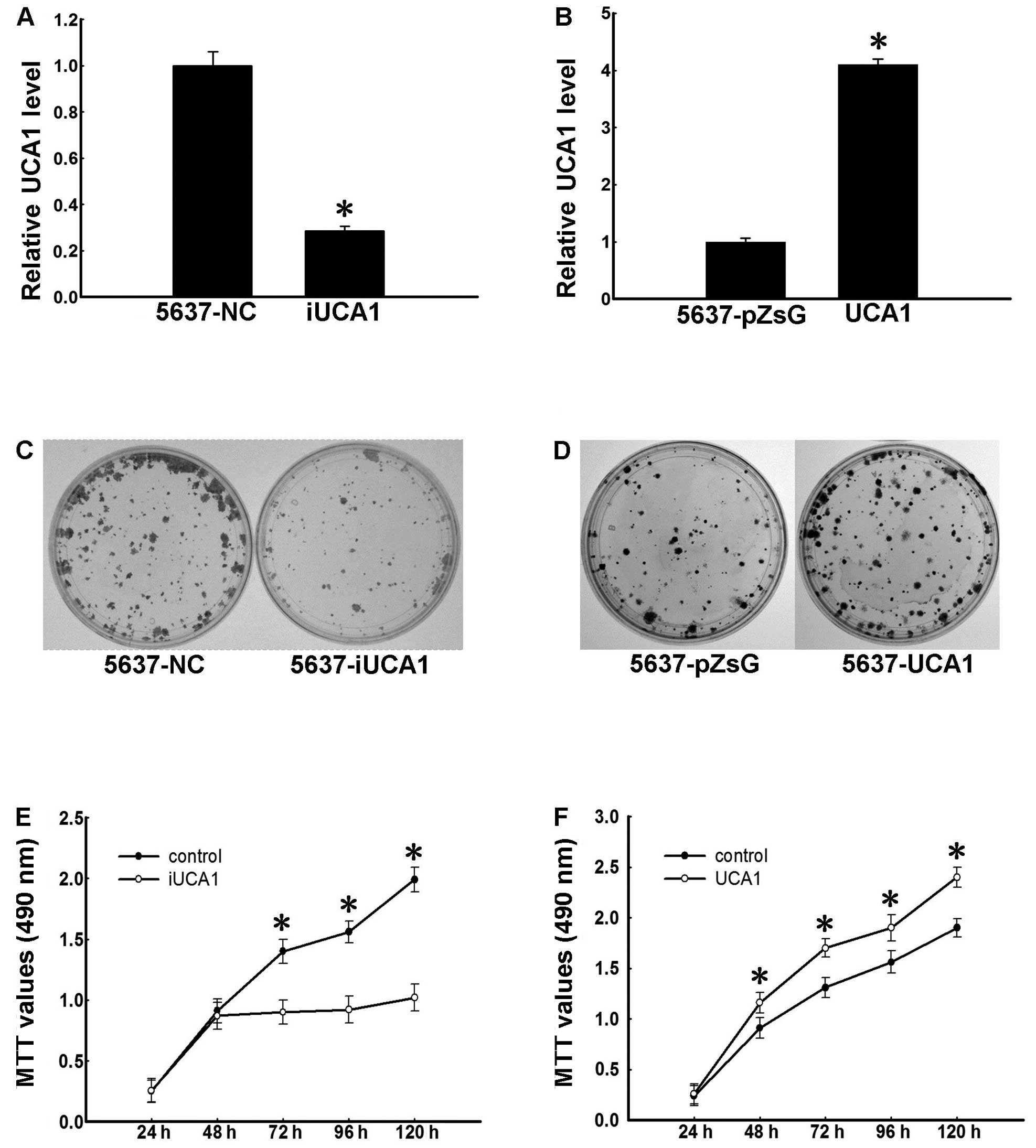 | Figure 1UCA1 promotes proliferation of 5637
cells. (A) Establishment of UCA1 knockdown 5637 cells. The 5637
cells were infected with pll3.7-NC (control) and pll3.7-iUCA1
viruses and selected with G418 for a week to generate 5637-NC and
5637-iUCA1 cells, respectively. Total RNA was isolated and the
level of UCA1 was determined using real-time PCR. Data were
normalized to GAPDH and expressed as the means ± SD of three
independent experiments. *P<0.05 (t-test). (B)
Establishment of UCA1 overexpressing 5637 cells. The 5637 cells
were infected with pZsG or pZsG-UCA1 viruses and selected with
puromycin for 7 days to generate 5637-pZsG and 5637-UCA1 cells,
respectively. Total RNA was isolated and the level of UCA1 was
determined using real-time PCR. Data are expressed as the means ±
SD of three independent experiments. *P<0.05
(t-test). (C) Colony formation of 5637-NC and 5637-iUCA1 cells. The
cells were cultured for 14 days. Colonies were stained using
crystal violet. (D) Colony formation of 5637-pZsG and 5637-UCA1
cells. The cells were cultured for 14 days. Colonies were stained
using crystal violet. (E) Growth curve of 5637-iUCA1 cells.
Cellular proliferation was measured using MTT assays at 24, 48, 72,
96 and 120 h. The differences in data of 72, 96 and 120 h were
significant, *P<0.05 (t-test). (F) Growth curves of
5637-UCA1 cells. Cellular proliferation was measured using MTT
assays at 24, 48, 72, 96 and 120 h. The differences in data of 48,
72, 96 and 120 h were significant, *P<0.05 (t-test).
UCA1, urothelial carcinoma associated 1. |
BRG1 is a target of UCA1
We next investigated the mechanism by which lncRNA
UCA1 promotes cell proliferation by searching for possible protein
factors that bind UCA1 using RNA pull-down assays. Biotin-labeled
sense UCA1 RNA and antisense UCA1 RNA was transcribed in
vitro and incubated with HeLa cell lysate. Avidin-labeled beads
were added to precipitate biotin-labeled UCA1 RNA and any
associated proteins. Samples were subjected to NuPAGE 4–12%
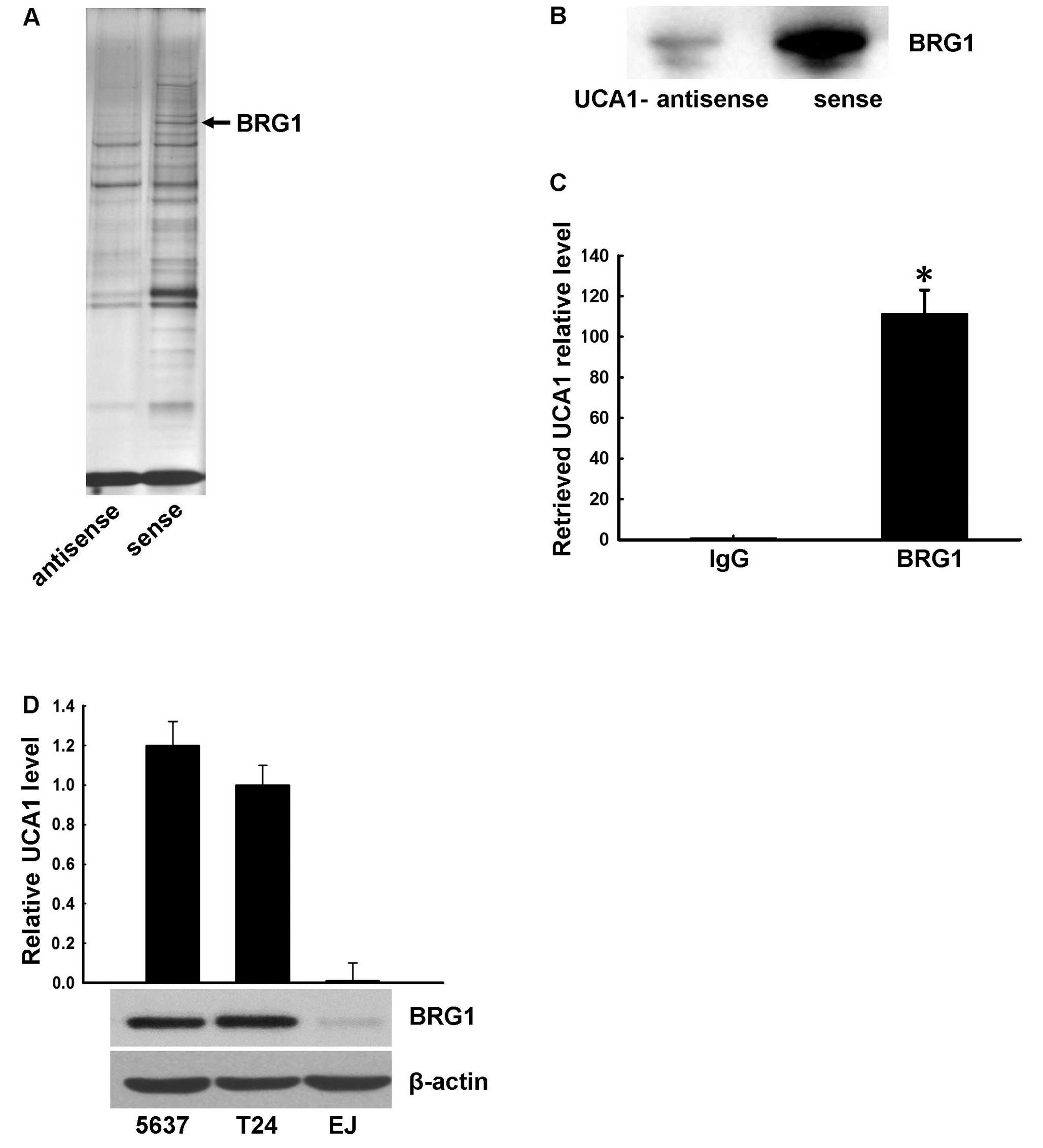
Bis-Tris gel electrophoresis and examined by silver stain (Fig. 2A). Mass spectrometry of the band
indicated by the arrow in the sense UCA1 lane in Fig. 2A revealed the band was likely BRG1,
a chromatin remodeling factor. To verify UCA1-BRG binding, we next
performed RNA pull-down and western blotting experiments in HeLa
cells. As shown in Fig. 2B, western
blotting confirmed UCA1 binding to BRG1 in vitro.
Next, we investigated UCA1 and BRG1 binding in
vivo by RNA-binding protein immunoprecipitation (RIP)
experiments. BRG1 antibody was used to immunoprecipitate BRG1
protein and associated RNAs in 5637 cells, and the presence of UCA1
RNA was determined by quantitative real-time PCR. Mouse IgG was
used as control. The relative UCA1 RNA level in the BRG1 antibody
immunoprecipitated samples was 111-fold higher compared with those
in the IgG sample (Fig. 2C).
Collectively, these data confirm that BRG1 binds UCA1 in
vitro and in vivo. Notably, analysis of UCA1 and BRG1
expression in three bladder cancer cell lines (5637, T24 and EJ
cells) revealed a relationship between BRG1 and UCA1 expression
(Fig. 2D), suggesting that these
two molecules may be functionally related.
UCA1 is a suppressor of BRG1
To explore the significance of the association of
UCA1 with BRG1, we first investigated BRG1 function in bladder
cancer. To this end, BRG1 was depleted using a validated shRNA in
5637 cells. Western blot analysis confirmed that BRG1-shRNA could
effectively suppress BRG1 expression (Fig. 3E). Consistent with previous reports,
p21, a target gene of BRG1, displayed reduced expression at both
the mRNA and protein levels in cells with BRG1 knockdown compared
with controls. Furthermore, our results showed that BRG1 knockdown
resulted in increased colony formation and cell growth (Fig. 3A and C). In contrast, overexpression
of BRG1 in 5637 cells resulted in upregulated p21 expression
(Fig. 3F) and suppressed colony
formation and cell growth (Fig. 3B and
D). These data suggest that BRG1 has anti-oncogenic functions
in bladder cancer cells.
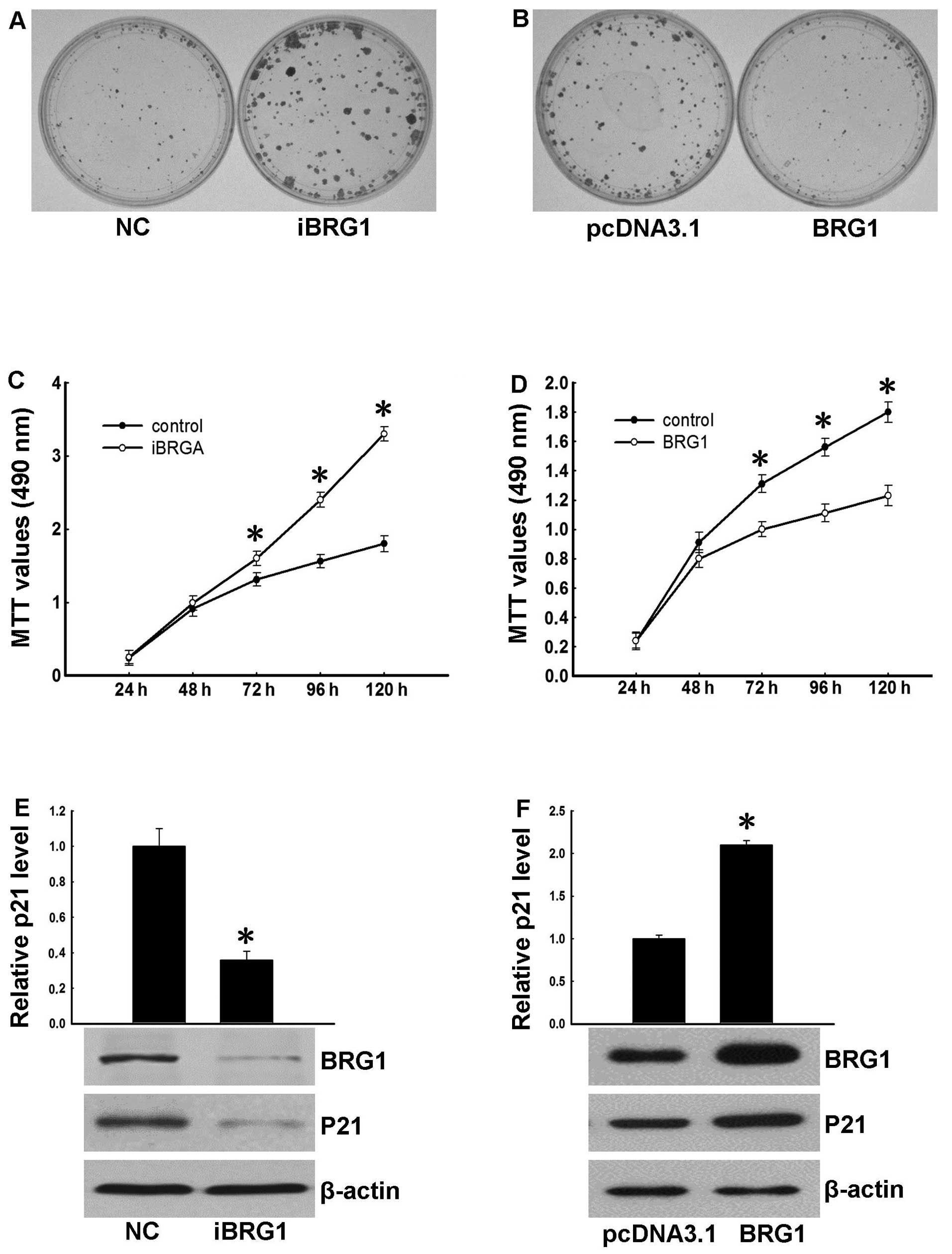 | Figure 3BRG1 plays a tumor suppressor role.
(A) Colony formation of 5637-NC and 5637-iBRG1 cells. The cells
were cultured for 14 days. Colonies were stained using crystal
violet. (B) Colony formation of 5637-pcDNA3.1 and 5637-BRG1 cells.
The cells were cultured for 14 days. Colonies were stained using
crystal violet. (C) Growth curves of 5637 cells after transfection
with BRG1 RNAi. Cellular proliferation was measured using MTT assay
at 24, 48, 72, 96 and 120 h. The differences in data of 72, 96 and
120 h were significant, *P<0.05 (t-test). (D) Growth
curves of 5637 cells overexpressing BRG1. Cellular proliferation
was measured using MTT assay at 24, 48, 72, 96 and 120 h. The
differences in data of 72, 96 and 120 h were significant,
*P<0.05 (t-test). (E) The expression level of p21 in
5637-NC and 5637-iBRG1 cells was determined by real-time PCR. Data
were normalized to GAPDH and are expressed as the means ± SD
of three independent experiments, *P<0.05 (t-test).
Western blot analysis of BRG1 and P21 in 5637-NC and 5637-iBRG1
cells. β-actin served as the internal control. (F) The expression
level of p21 in 5637-pcDNA3.1 and 5637-BRG1 cells was determined by
real-time PCR. Data were normalized to GAPDH and are
expressed as the means ± SD of three independent experiments,
*P<0.05 (t-test). Western blot analysis of BRG1 and
p21 in 5637-pcDNA3.1 and 5637-BRG1 cells. β-actin served as the
internal control. |
Since UCA1 interacts with BRG1 in 5637 cells, we
hypothesized that UCA1 exerts proliferation-promoting functions by
antagonizing BRG1. To confirm this, we performed several
experiments. First, EJ cells, which lack BRG1 and UCA1 expression,
were transfected with plasmids expressing BRG1, BRG1 and UCA1.
Three days after drug selection, BRG1 and UCA1 expressions were
confirmed by western blotting and real-time PCR (Fig. 4C, left). Colony formation and MTT
assays showed that BRG1 overexpression suppressed colony formation
and cell growth (Fig. 4A and B,
left); however this effect was abrogated by overexpressing UCA1,
suggesting that UCA1 antagonizes the growth suppressive function of
BRG1.
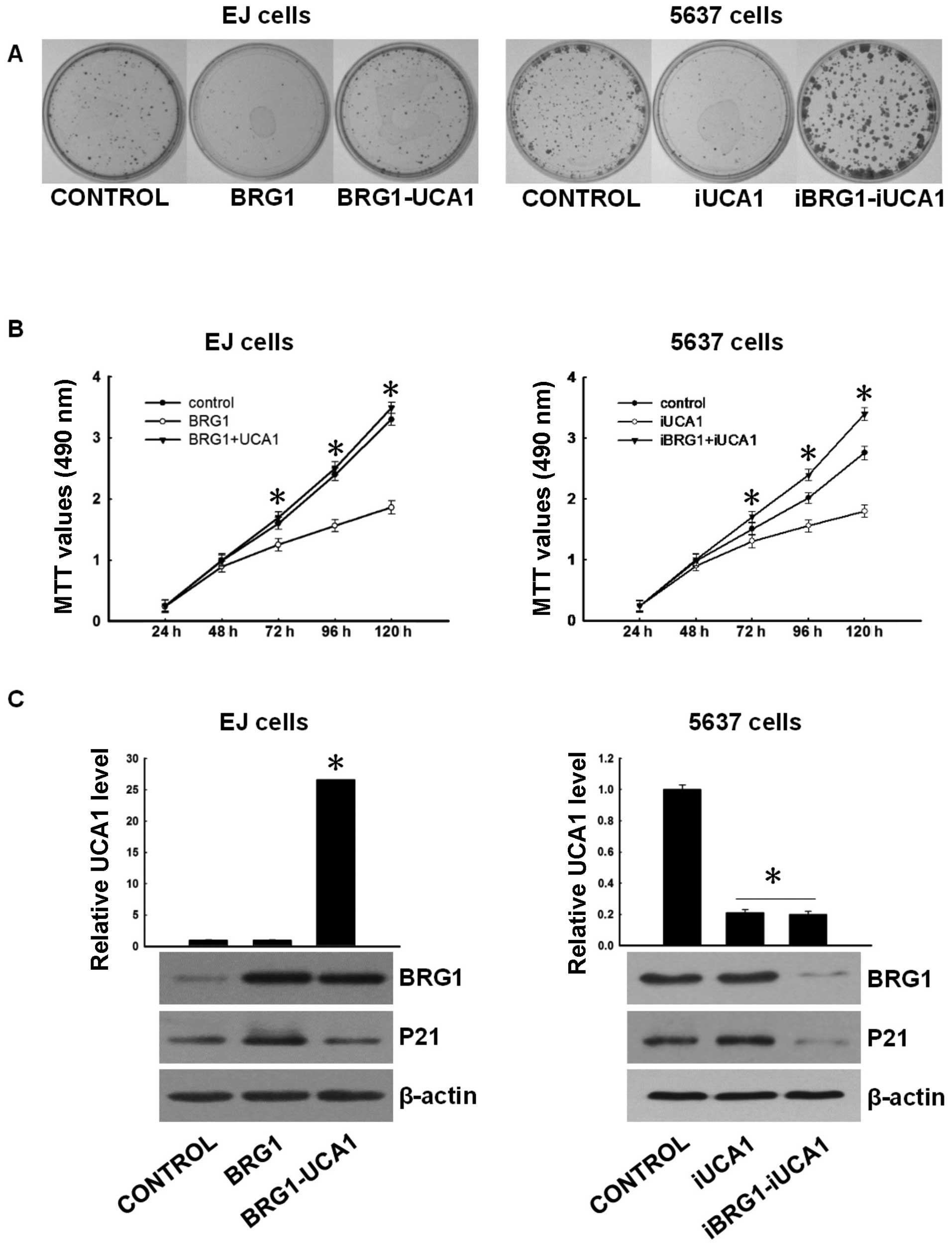 | Figure 4UCA1 antagonizes the tumor
suppressing function of BRG1. (A) (Left) Colony formation of EJ
control, EJ-BRG1 (overexpressed BRG1) and EJ-BRG1-UCA1
(overexpressed BRG1 and UCA1) cells. The cells were cultured with
G418 for 10 days. Colonies were stained using crystal violet.
(Right) Colony formation of 5637 control, 5637-iUCA1 (UCA1 knocked
down) and 5637-iUCA1-iBRG1 (UCA1 and BRG1 both knocked down) cells.
The cells were cultured with G418 and puromycin for 10 days.
Colonies were stained using crystal violet. (B) (Left) Growth
curves of EJ control, EJ-BRG1 and EJ-BRG1-UCA1 cells. Cellular
proliferation was measured using MTT assays at 24, 48, 72, 96 and
120 h. The differences between EJ-BRG1 and EJ-BRG1-UCA1 were
significant. *P<0.05 (t-test). (Right) Growth curves
of 5637 control, 5637-iUCA1 and 5637-iUCA1-iBRG1 cells. Cellular
proliferation was measured using MTT assays at 24, 48, 72, 96 and
120 h. The differences between 5637-iUCA1 and 5637-iUCA1-iBRG1,
5637-iUCA1 and control were significant *P<0.05
(t-test). (C) (Left) The expression levels of UCA1 in EJ
cells control EJ-BRG1 and EJ-BRG1-UCA1 were determined by real-time
PCR. Data were normalized to GAPDH and are expressed as the
means ± SD of three independent experiments, the UCA1 level
in EJ-BRG1-UCA1 was significantly high, *P<0.05
(t-test). The expression levels of BRG1 and p21 were determined by
western blotting with β-actin as the internal control. (Right) The
expression levels of UCA1 in 5637 cells control 5637-iUCA1
and 5637-iUCA1-iBRG1 were determined by real-time PCR. Data were
normalized to GAPDH and are expressed as the means ± SD of
three independent experiments. The effect of RNAi was significant
*P<0.05 (t-test). The expression levels of BRG1 and
p21 were determined by western blotting with β-actin as the
internal control. UCA1, urothelial carcinoma associated 1. |
To further analyze the functional association of
BRG1 with UCA1, 5637 cells were transfected with UCA1-shRNA,
UCA1-shRNA and BRG1-shRNA. Seven days after G418 selection, colony
formation and MTT assay were performed. Results showed that
UCA1-shRNA led to decreased colony formation and proliferation
(Fig. 4A and B, right). However,
BRG1-shRNA may weaken the suppressive role of UCA1-shRNA.
Collectively, these data indicate that UCA1 acts as a suppressor of
BRG1.
UCA1 blocks recruitment of BRG1 to
chromatin
BRG1 is a catalytic subunit of the ATP-dependent
nucleosome remodeling complex SWI/SNF and has helicase/ATPase
activity. The SWI/SNF complex has no intrinsic ability to bind DNA
and is commonly recruited to target promoters by other factors,
such as transcription factors. We considered two possible pathways
for UCA1 suppression of BRG1: i) UCA1 binds BRG1 and inhibits the
ATPase activity of BRG1; ii) UCA1 binds BRG1 and blocks recruitment
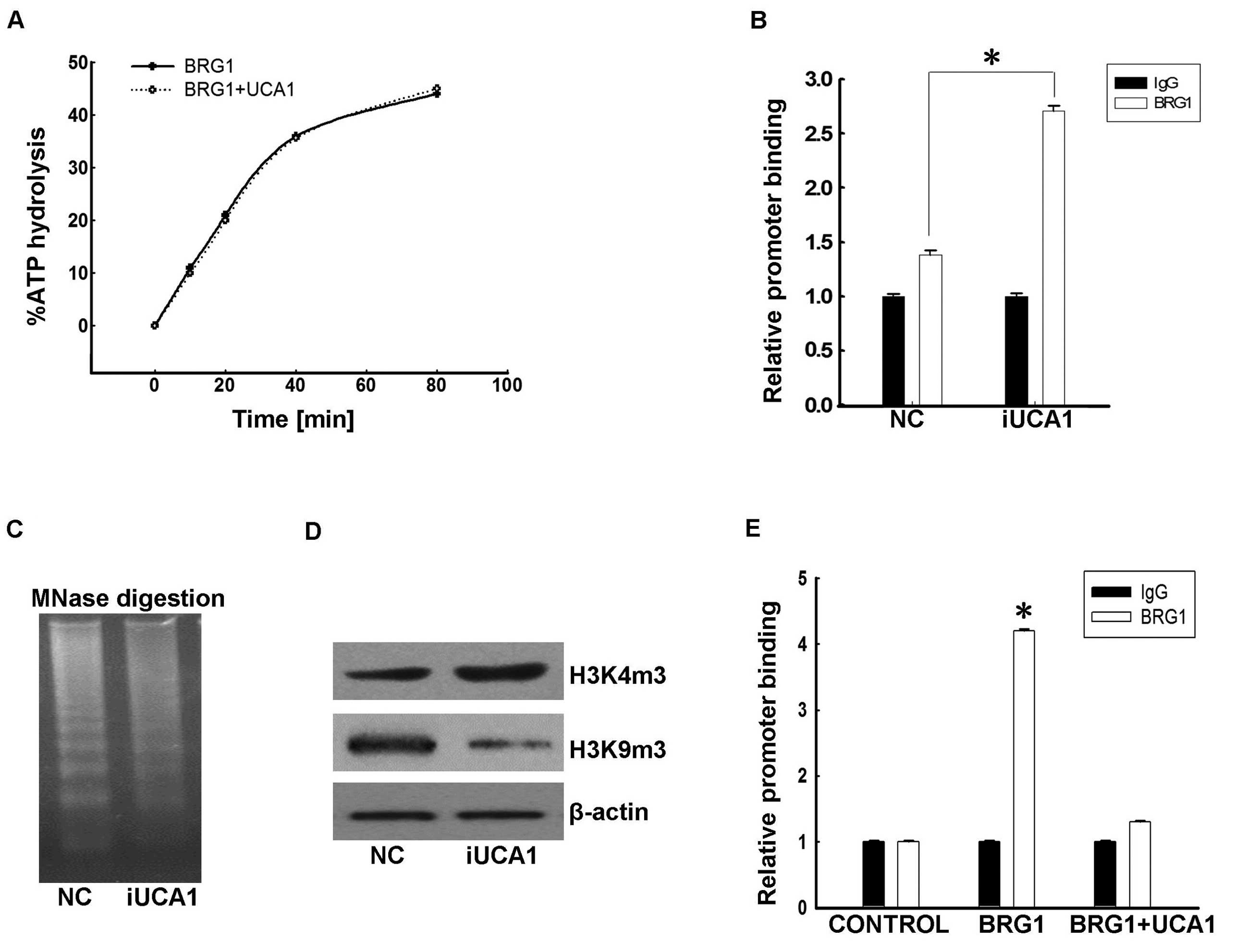
of BRG1 to target promoters. The in vitro ATPase activity
assay results (Fig. 5A) showed that
the presence of UCA1 did not result in changes of BRG1 ATPase
activity, thus we suspected that UCA1 blocks recruitment of BRG1 to
target promoters.
Indeed, ChIP analysis showed that depletion of UCA1
with shRNA enhanced BRG1 occupancy on the p21 promoter in 5637
cells (Fig. 5B). Furthermore, the
genomic DNA in UCA1-shRNA cells became more sensitive to MNase
digestion than that in cells with control-shRNA (Fig. 5C). Additionally, the level of
H3K4m3, which is an active chromatin marker, were also increased in
UCA1-shRNA cells, while H3K9m3, a repressive one, were decreased
(Fig. 5D). Collectively, these data
suggest that UCA1 suppresses the recruitment of BRG1 to its target
promoters. To further confirm the above hypothesis, we ectopically
expressed BRG1 and/or UCA1 in EJ cells as described above. ChIP
results showed that BRG1 was enriched on the p21 promoter region in
EJ cells overexpressing BRG1 alone (Fig. 5E). However, co-expression of UCA1
led to decreased occupancy of the p21 promoter by BRG1, and these
suppressive effects of UCA1 showed dose-dependent
characteristics.
Expression of UCA1 is positively
correlated with BRG1 in bladder cancer tissue specimens
The above observation that UCA1 promotion of
proliferation is achieved at least partly by antagonizing BRG1
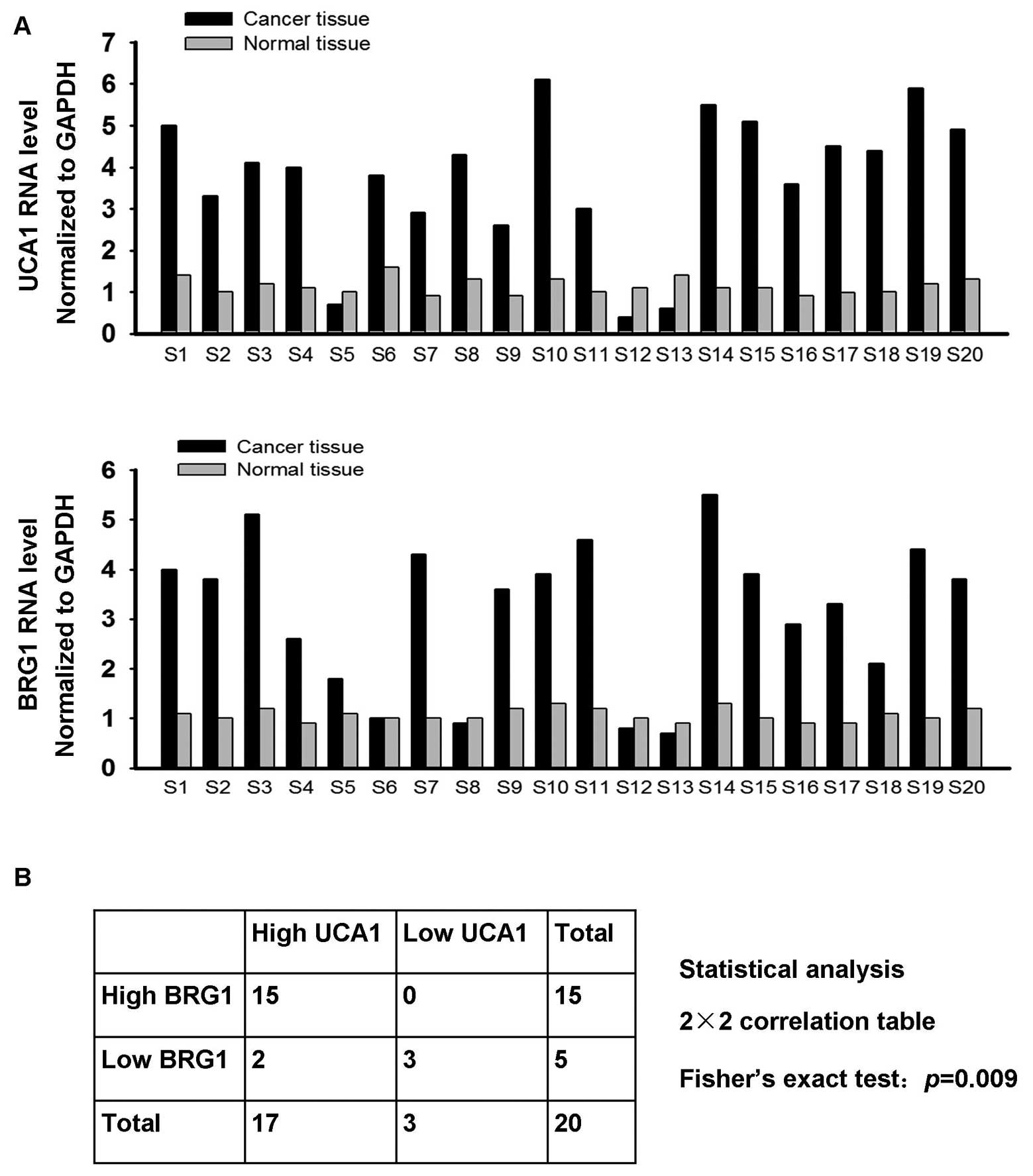
indicates that BRG1 is likely upregulated in bladder cancer. For
this reason, we investigated BRG1 and UCA1 expression in tissue
specimens of 20 bladder cancer patients with real-time PCR. As
shown in Fig. 6A, 17 out of 20
bladder cancer tissue samples showed elevated UCA1 mRNA levels,
whereas elevated BRG1 mRNA levels were detected in 15 cancer tissue
samples. We used Fisher’s exact test to analyze the correlation
between UCA1 and BRG1 (Fig. 6B).
The results indicated a positive correlation between BRG1
expression and UCA1 expression and were significant, P=0.009. These
data suggest that UCA1 antagonizes the suppressive effect of BRG1
on bladder cancer cells in vivo.
Discussion
The long non-coding RNA UCA1 was first identified
and termed by Wang et al in bladder cancer tissues. The
authors found that UCA1 could serve as a potential biomarker for
bladder cancer since it is specifically highly expressed in bladder
cancer (12). Previous studies
reported that UCA1 seems to function as a tumor-promoting factor in
bladder cancers (14,25). However, the general function and
underlying mechanisms of UCA1 in bladder cancer cells have remained
elusive and require further investigation. In the present study,
BRG1 was identified as target protein of UCA1. Our data showed that
BRG1 exhibits anti-oncogenic features in bladder cancer cells. UCA1
bound BRG1 and antagonized its suppressive effects in bladder
cancer cells. UCA1 blocked recruitment of BRG1 to its target
promoter, and thus led to reduced expression of p21 and accelerated
cell growth.
Three possibilities exist for the mechanisms
underlying UCA1 blockage of BRG1 recruitment to promoter regions:
i) in addition to BRG1, UCA1 binds another protein that blocks BRG1
access to promoter regions; ii) BRG1 is recruited to target
promoter through specific transcription factors (26,27),
thus UCA1 may hinder BRG1 interaction with specific transcription
factors; and iii) UCA1 titrates BRG1 away from chromatin. Our
studies cannot distinguish between these mechanisms. Titration
would be a simple mechanism to prevent BRG1 access to promoter
sequences even without impairing BRG1 ATPase activity. Furthermore,
we cannot rule out the possibility of multiple and coexisting
mechanisms of BRG1 control.
The present study revealed a notable phenomenon,
namely, that although BRG1 has an antiproliferative role, its
expression is elevated in bladder cancer tissue samples. Analysis
of cDNA sequences showed that BRG1 mutation did not take place in
these tissue samples (data not shown), thus, the possibility that
mutation leads to inactivation of BRG1 function is ruled out. The
elevated expression of BRG1 is likely associated with cellular
senescence, which is an important tumor suppression mechanism. Upon
exposure to external insults such as γ-irradiation or
constitutively active oncogenes, normal cells become senescent. If
senescence is inhibited, however, cells become prone to
carcinogenesis. Previous studies have shown that overexpression of
BRG1 may lead to cellular senescence (28). Additionally, BRG1 is required for
formation of senescence-associated heterochromatin foci (SAHF)
(20). We speculate that external
insults lead to increased expression of BRG1, thus inducing cell
senescence, and that UCA1 suppresses BRG1-mediated senescence, thus
promoting cells carcinogenesis. P21 is also associated with cell
senescence (29,30) and its increased level by BRG1 is
consistent with levels in senescent cells. Our studies cannot
confirm that p21 plays a key role in UCA1 inhibition of BRG1, since
BRG1 has widespread functions via widespread pathways. However, p21
can be a clear indicator of BRG1 activity in 5637 cells.
Acknowledgements
This study was funded by the National Science
Foundation of China, no. 81170318. The authors thank Dr Keji Zhao
and Dr Alan H. Deutch (SBC, NHLBI, National Institutes of Health)
for providing us with pBJ5-hBRG1 plasmids.
References
|
1
|
ENCODE Project Consortium. Birney E,
Stamatoyannopoulos JA, et al: Identification and analysis of
functional elements in 1% of the human genome by the ENCODE pilot
project. Nature. 447:799–816. 2007.
|
|
2
|
Khachane AN and Harrison PM: Mining
mammalian transcript data for functional long non-coding RNAs. PLoS
One. 5:e103162010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsang WP, Wong TW, Cheung AH, Co CN and
Kwok TT: Induction of drug resistance and transformation in human
cancer cells by the noncoding RNA CUDR. RNA. 13:890–898.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar
|
|
6
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Costa FF: Non-coding RNAs, epigenetics and
complexity. Gene. 410:9–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faghihi MA and Wahlestedt C: Regulatory
roles of natural antisense transcripts. Nat Rev Mol Cell Biol.
10:637–643. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang XS, Zhang Z, Wang HC, et al: Rapid
identification of UCA1 as a very sensitive and specific unique
marker for human bladder carcinoma. Clin Cancer Res. 12:4851–4858.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie XJ, Li X, Wang F and Chen W: Cellular
localization and tissue expression pattern of UCA1, a non-coding
RNA. Nan Fang Yi Ke Da Xue Xue Bao. 30:57–60. 2010.(In
Chinese).
|
|
14
|
Wang F, Li X, Xie X, Zhao L and Chen W:
UCA1, a non-protein-coding RNA up-regulated in bladder
carcinoma and embryo, influencing cell growth and promoting
invasion. FEBS Lett. 582:1919–1927. 2008. View Article : Google Scholar
|
|
15
|
Peifer M and Polakis P: Wnt signaling in
oncogenesis and embryogenesis - a look outside the nucleus.
Science. 287:1606–1609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimada T and Fujii-Kuriyama Y: Metabolic
activation of polycyclic aromatic hydrocarbons to carcinogens by
cytochromes P450 1A1 and 1B1. Cancer Sci. 95:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katayama H, Brinkley WR and Sen S: The
Aurora kinases: role in cell transformation and tumorigenesis.
Cancer Metastasis Rev. 22:451–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilson BG and Roberts CW: SWI/SNF
nucleosome remodellers and cancer. Nat Rev Cancer. 11:481–492.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kidder BL, Palmer S and Knott JG:
SWI/SNF-Brg1 regulates self-renewal and occupies core
pluripotency-related genes in embryonic stem cells. Stem Cells.
27:317–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tu Z, Zhuang X, Yao YG and Zhang R: BRG1
is required for formation of senescence-associated heterochromatin
foci induced by oncogenic RAS or BRCA1 loss. Mol Cell Biol.
33:1819–1829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang H, Cui K and Zhao K: BRG1 controls
the activity of the retinoblastoma protein via regulation of
p21CIP1/WAF1/SDI. Mol Cell Biol. 24:1188–1199. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Y, Zhang J and Chen X: The activity of
p53 is differentially regulated by Brm- and Brg1-containing SWI/SNF
chromatin remodeling complexes. J Biol Chem. 282:37429–37435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai MC, Manor O, Wan Y, et al: Long
noncoding RNA as modular scaffold of histone modification
complexes. Science. 329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bochar DA, Savard J, Wang W, et al: A
family of chromatin remodeling factors related to Williams syndrome
transcription factor. Proc Natl Acad Sci USA. 97:1038–1043. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang C, Li X, Wang Y, Zhao L and Chen W:
Long non-coding RNA UCA1 regulated cell cycle distribution via CREB
through PI3-K dependent pathway in bladder carcinoma cells. Gene.
496:8–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barker N, Hurlstone A, Musisi H, Miles A,
Bienz M and Clevers H: The chromatin remodelling factor Brg-1
interacts with β-catenin to promote target gene activation. EMBO J.
20:4935–4943. 2001.
|
|
27
|
DiRenzo J, Shang YF, Phelan M, et al:
BRG-1 is recruited to estrogen-responsive promoters and cooperates
with factors involved in histone acetylation. Mol Cell Biol.
20:7541–7549. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alessio N, Squillaro T, Cipollaro M,
Bagella L, Giordano A and Galderisi U: The BRG1 ATPase of chromatin
remodeling complexes is involved in modulation of mesenchymal stem
cell senescence through RB-P53 pathways. Oncogene. 29:5452–5463.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aravinthan A, Mells G, Allison M, et al:
Gene polymorphisms of cellular senescence marker p21 and disease
progression in non-alcohol-related fatty liver disease. Cell Cycle.
13:1489–1494. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai J, Chen J, Ma M, et al: Inhibiting
enhancer of zeste homolog 2 promotes cellular senescence in gastric
cancer cells SGC-7901 by activation of p21 and p16. DNA Cell Biol.
33:337–344. 2014. View Article : Google Scholar : PubMed/NCBI
|