Introduction
Neuregulin-1 (NRG1) was originally identified by its
ability to stimulate the phosphorylation of HER2 receptor tyrosine
kinase (1). A large number of
different isoforms are produced through the alternative splicing of
the NRG1 gene, including heregulins (HRGs), glial growth
factors (GGFs) and sensory and motor neuron-derived factor (SMDF).
Furthermore, the isoforms are tissue-specifically expressed, and
through interaction with HER receptors, NRG1 isoforms induce the
growth and differentiation of epithelial, neuronal, glial and other
types of cells (2).
NRG1 is known as a ligand for the HER3 receptor,
which has no intrinsic tyrosine kinase activity. HER3 receptors
activated by NRG1 binding form heterodimers with other HER family
receptors and mediate downstream signaling pathways. HER2–HER3 have
been shown to be a potent signaling pair in preclinical models of
HER2-positive breast cancer. Signals from dimerized, activated HER
receptors can impact downstream signaling cascades, such as the
mitogen-activated protein kinase (MAPK) pathway, the Janus tyrosine
kinase (JAK)/signal transducer and activator of transcription
(STAT) pathway and the phosphatidylinositol 3′-kinase (PI3K)-Akt
pathway, leading to multiple effects, including growth,
proliferation, decreased apoptosis, cellular migration and
angiogenesis (3–8)
Research using breast cancer cell lines has shown
that NRG1, the ligand of HER3, induces epithelial-mesenchymal
transition (EMT) in breast cancer cells, induces expression of
proteins that are involved in invasion and metastasis (e.g., matrix
metalloproteinase) and drives tyrosine kinase inhibitor-resistant
growth and invasion of breast cancer cells (9–11).
In EMT, epithelial cells lose cell-cell contact,
transform to take on a spindle shape and express mesenchymal cell
proteins, which are favorable for cellular migration and invasion
(12–14). Studies suggesting that EMT
accompanies cancer stem cell-like characteristics have recently
come into the spotlight. Cancer stem cells (CSCs), a subgroup of
cancer cells, display features of stem cells such as the abilities
of self-renewal and pluripotent differentiation into other types of
mature cells (15). CSCs and their
relationships with tumor recurrence, metastasis and chemoresistance
have lately drawn much attention (16–19).
In human cancer, a relationship between the malignant potential of
cancer and the proportion of CSCs present has been reported
(15,20,21).
Meanwhile, various growth factors such as transforming growth
factor β (TGF-β) and epidermal growth factor (EGF) have been found
to induce the signaling pathways related to EMT and CSC-like
characteristics (22–26). These results may indicate that
expression of CSC characteristics is a transient, common and
dynamic process according to the tumor microenvironment during
cancer progression (27).
The aim of this study was to determine whether NRG1
induces CSC characteristics in breast cancer cells and whether the
induced CSC characteristics are a transient and dynamic process.
Using breast cancer cell lines, MCF-7, SKBr-3 and MDA-MB 468,
changes related to CSC characteristics were observed following NRG1
treatment.
Materials and methods
Cell culture
Breast cancer cell lines SKBr-3, MCF-7, T47D,
Hs578T, MDA-MB 231, MDA-MB 453 and MDA-MB 468 were obtained from
the American Type Culture Collection (ATCC; Rockville, MD, USA).
Cells were grown in RPMI-1640 medium (Gibco, Grand Island, NY, USA)
containing 100 units/ml penicillin, 100 mg/ml streptomycin
(Gibco-BRL, USA) and 10% fetal bovine serum (FBS; Gibco-BRL) at
37°C under a humidified 95-5% (v/v) mixture of air and
CO2. Cells were harvested by trypsinization, and
2×106 cells were seeded in a 100-mm culture dish.
For experiments, cells were incubated in serum-free
medium for 16 h. After 16 h of serum starvation, cells in the test
group were treated with human heregulin (HRG)-β1 (25 ng/ml) in
serum-free medium for a specified period of time. HRG-β1 is a
well-known isoform of NRG1. Cells in the control group were
incubated in serum-free medium for the same time but without any
treatment. Recombinant human HRG-β1 (purity >97%) was purchased
from R&D Systems (Minneapolis, MN, USA) and aliquoted in small
amounts in PBS and stored at −70°C.
Flow cytometry
Cells were mechanically harvested from 100-mm
culture dishes and resuspended in fluorescence-activated cell
sorting buffer [2% bovine serum albumin (BSA) in PBS]. Cells were
incubated with anti-CD44-FITC (20 μl/106 cells),
anti-CD24-PE (20 μl/106 cells), or isotype-matched IgG
for 20 min at room temperature. Anti-CD44-FITC, anti-CD24 PE and
isotype-matched IgG were obtained from Immunotech, a Beckman
Coulter Co. (Marseille, France). After fixation, all samples were
analyzed using a Cytomics FC500 (Beckman Coulter) within 2 h.
Western blot analysis
Cells were harvested mechanically and lysed with
RIPA buffer containing 20 mM Tris-HCl (pH 7.5), 2 mM EDTA, 150 mM
NaCl, 1 mM sodium vanadate, 10 mM NaF, 2.5 mM sodium pyrophosphate,
1% sodiumdeoxycholate, 0.1% SDS, 1% NP-40, 1 mM PMSF and a protease
inhibitor cocktail (Roche, Germany). Cell lysates were cleared by
centrifugation at 14,000 rpm for 20 min at 4°C, and the
supernatants were used as total cellular protein. The protein
concentration of each sample was determined using a BCA protein
assay kit (Pierce, Rockford, IL, USA). Proteins were
electrophoresed on a sodium dodecyl sulfate (SDS)-polyacrylamide
gel and transferred onto polyvinylidene fluoride membranes
(Bio-Rad, USA) in a transfer buffer. The blocked membranes were
then incubated with the indicated antibodies, and immunoreactive
bands were developed by an enhanced chemiluminescence detection kit
(Amersham Pharmacia Biotech) using secondary antibodies coupled
with horseradish peroxidase.
Antibodies for CD44 (8E2), HER1/epidermal growth
factor receptor and HER2/ErbB2 were obtained from Cell Signaling
Technology (Beverly, MA, USA). Antibodies for HER3/ErbB3 and
HER4/ErbB4 were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The antibody for CD49f (integrin α6) was purchased
from Millipore (Temecula, CA, USA). The CD29 (integrin β1) antibody
was from BD Biosciences (San Diego, CA, USA), while monoclonal
anti-β-actin was obtained from Sigma (St. Louis, MO, USA). The
anti-mouse immunoglobulin horseradish peroxidase-linked F(ab)2
fragment was from Perkin-Elmer (Boston, MA, USA).
Immunofluorescence
Approximately 2×104 cells were seeded on
a 2-well Lab-Tek™ II chamber slide™ (Thermo Fisher Scientific Inc.,
USA). After 16 h of serum starvation, the cells in the test group
were treated with HRG-β1 (25 ng/ml) (R&D Systems) in serum-free
medium for 24 h. Cells in the control group were incubated in
serum-free medium for the same amount of time without any
treatment. Then, cells were washed three times with PBS and fixed
with 4% paraformaldehyde for 10 min. Following three washes with
PBS, cells were permeabilized for 20 min with 0.1% Triton X-100.
After being again washed in PBS, the slides were blocked with 3%
BSA for 1 h at room temperature, and the cells were incubated with
mouse monoclonal anti-CD44 primary antibodies (Cell Signaling
Technology) overnight at 4°C. After three washes, the cells were
incubated with Alexa fluor® 488 anti-mouse IgG secondary
antibodies (Invitrogen, New Zealand). Following further washing,
cells were mounted with mounting medium containing DAPI (Vector
Laboratories, Burlingame, CA, USA) and observed by a Zeiss LSM 700
confocal laser scanning microscope (Carl Zeiss, Thornwood, NY,
USA).
Small interfering RNA (siRNA)
transfection
For transfection, SKBr-3 and MCF-7 cells were seeded
in 6-cm plates and transfection was performed under conditions
described by the manufacturer (Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA). After replacing media with reduced serum Opti-MEM,
the cells were transfected with control siRNA or ErbB3 siRNA, using
the siRNA transfection reagent system (Santa Cruz). Six hours after
the incubation, the media were replaced with the standard culture
media described above. After an additional 24-h incubation, the
transfected cells were treated with HRG-β1 (25 ng/ml) and then used
in the following tests.
Statistical analysis
The data are expressed as the mean value ± SE,
unless indicated otherwise. Statistical analysis was performed
using a two-tailed Student’s t-test. Significance was defined as
P<0.05.
Results
Selection of breast cancer cell lines for
experiments
Since NRG1 is a ligand for the HER3 receptor, the
breast cancer cell lines with considerable HER3 receptor expression
were selected for the experiments. It was also necessary to
determine the HER family receptor expression profiles of each
cancer cell line since the activated HER3 receptor forms
heterodimers with other members of the HER receptor family and
activates downstream signaling pathways. Using western blot
analysis, the expression profiles of HER family receptors were
analyzed semi-quantitatively in various cancer cell lines.
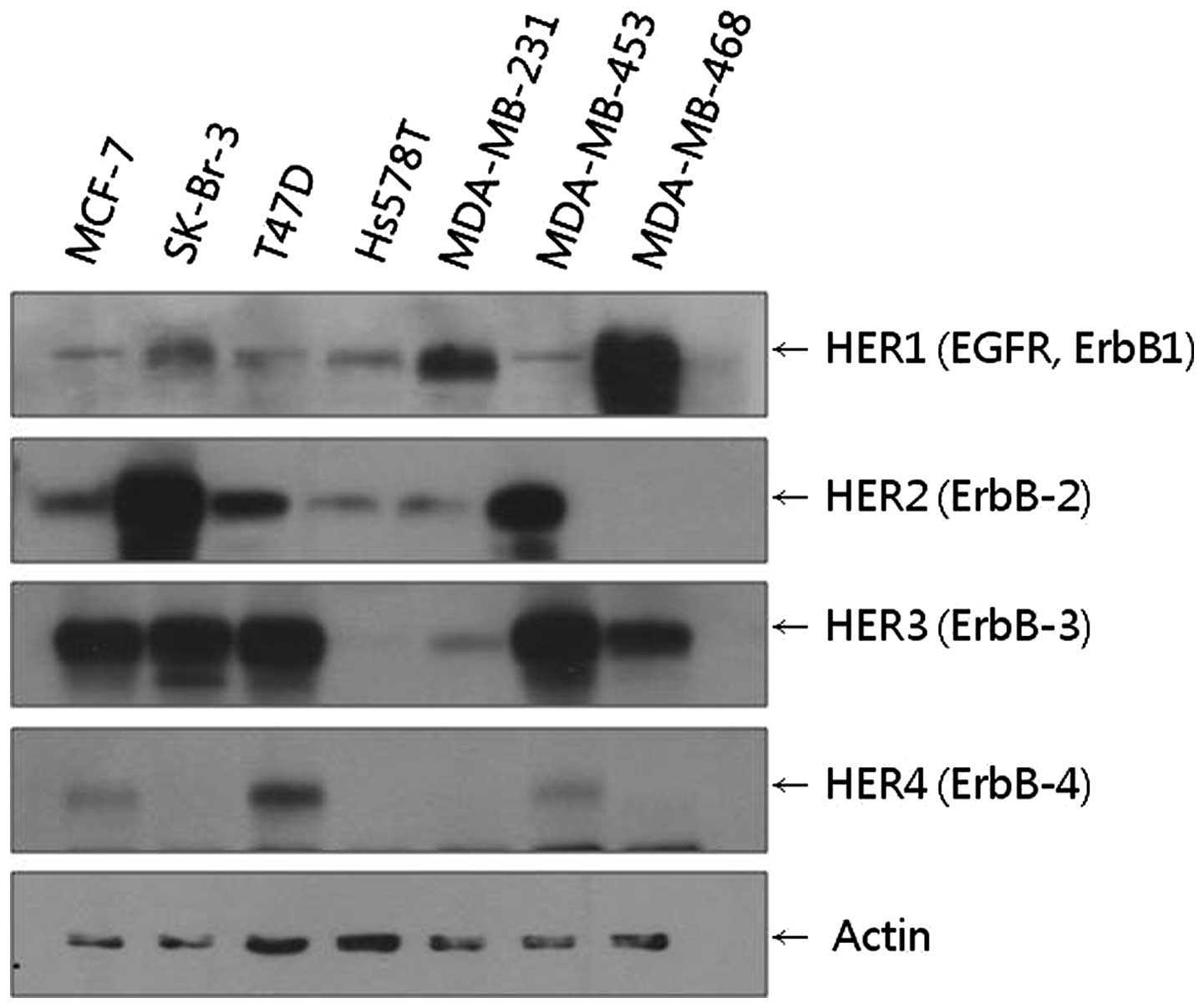
We found that MCF-7, SKBr-3, T47D, MDA-MB 453 and
MDA-MB 468 cell lines all showed high levels of HER3 expression.
Among these cell lines, the SKBr-3 cell line showed the highest
level of HER2 expression, while the MDA-MB 468 cell line showed the
highest level of HER1 expression (Fig.
1). Based on these results, the SKBr-3 cell line with HER2–HER3
receptors and the MDA-MB 468 cell line with HER1–HER3 receptors
were selected for the subsequent experiments. Using the
experimental results of the SKBr-3 and MDA-MB 468 cell lines, the
downstream signaling effects induced by HER2–HER3
heterodimerization and those induced by HER1–HER3
heterodimerization could be compared. In addition to SKBr-3, T47D
and MDA-MB 453 cells also expressed considerable levels of HER2 and
HER3. However, these cell lines were not included in the following
experiments as their HER2 expression levels were lower than those
of SKBr-3.
The MCF-7 cell line, which showed considerable HER3
expression in the western blot analysis, was included in the
subsequent experiments since it is a well-known hormone-responsive
cell line (28). Although the
relevance of a breast cancer cell line as a model for actual breast
cancer is a controversial issue (29), the hormone-responsive cell line
MCF-7 was considered as a model for luminal-type breast cancer in
this study, while SKBr-3 with strong HER2 expression was considered
as a model for HER2-type breast cancer and MDA-MB 468 with strong
HER1 expression was considered as a model for basal-like type
breast cancer.
NRG1 induces increases in CD44-positive
CSC fractions
To test the hypothesis that NRG1 induces CSC-like
properties in breast cancer cells, the expression of CSC markers
was examined. How best to calculate CSCs in a complex tumor cell
population is a subject of active research and controversy. In
breast cancer, CSCs are usually identified by a
CD44high/CD24low phenotype (30). Using flow cytometry, the fraction of
CSCs in each cell line was measured. The differences in CSC
fraction between the control group cells and the NRG1 treatment
group cells were analyzed.
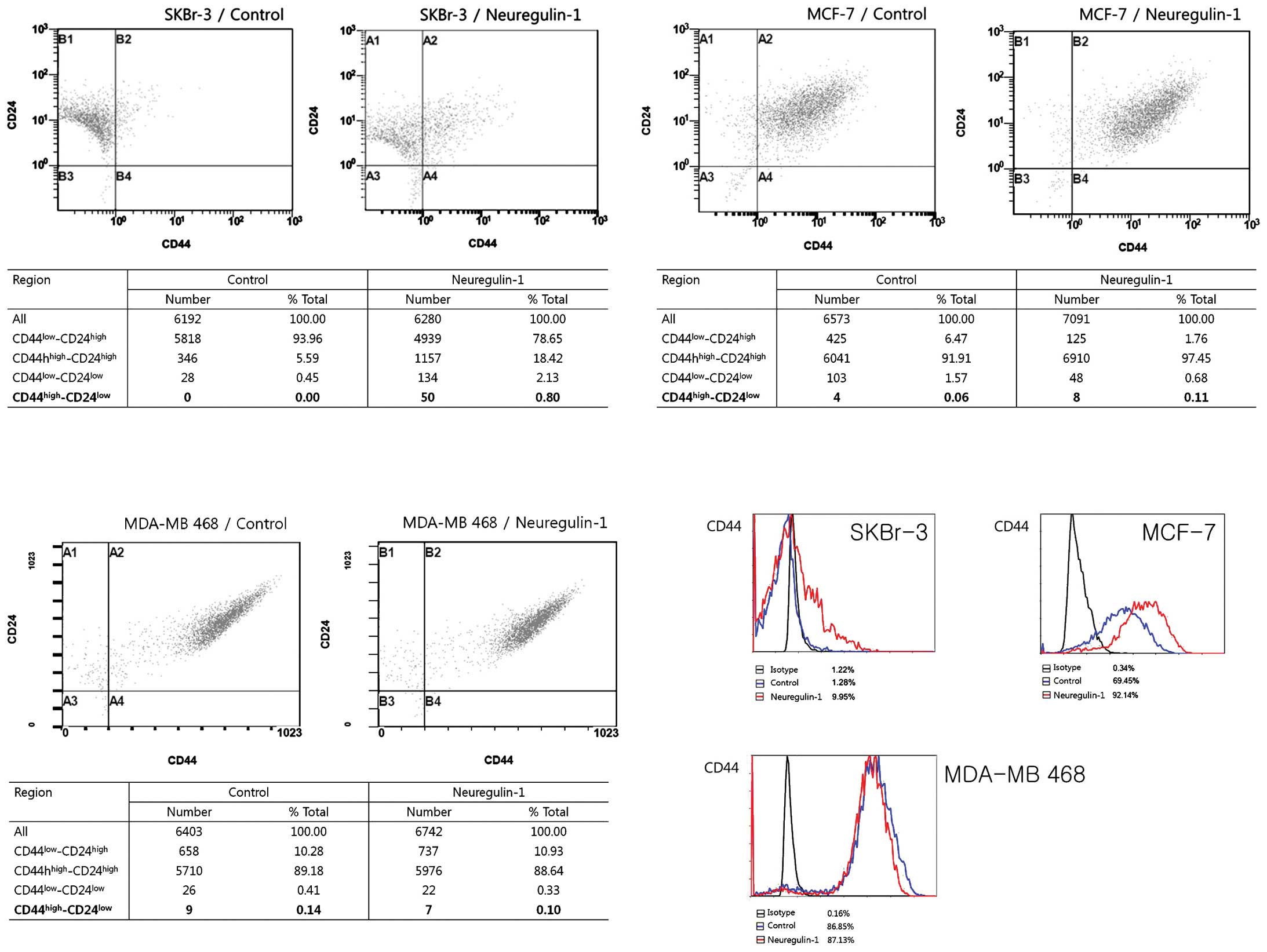
Most SKBr-3 cells in the control group showed the
CD44low/CD24high phenotype (93.96%) and no
cells showed the CD44high/CD24low phenotype.
After 24 h of incubation with NRG1, the cellular fraction with the
CD44low/CD24high phenotype was decreased
(78.65%) and a very small fraction [50 cells (0.8%)] expressing the
CD44high/CD24low phenotype newly appeared.
The results of flow cytometry showed an increased proportion of
CD44-positive cells after NRG1 treatment, from 1.28% in the control
group to 9.95% in the NRG1 treatment group. The proportion of cells
with CD24 expression was not significantly changed (Fig. 2).
In contrast, most of the MCF-7 and MDA-MB 468 cells
in the control group exhibited the
CD44high/CD24high phenotype, with 91.91% of
MCF-7 cells and 89.18% of MDA-MB 468 cells having this phenotype.
Approximately 70% of MCF-7 cells in the control group expressed
CD44. NRG1 treatment induced an increase in the proportion of
CD44-positive cells, up to 92%. The proportion of cells with the
CD44high/CD24low phenotype also increased,
from 0.06% in the control group to 0.11% in the NRG1 treatment
group (Fig. 2).
Regarding MDA-MB 468 cells, NRG1 had no effect. The
cellular fractions depending on the degree of CD44/CD24 expression
were not significantly changed after NRG1 treatment. Unexpectedly,
the proportion of cells with the
CD44high/CD24low phenotype was slightly
decreased, from 0.14% in the control group to 0.10% in the NRG1
treatment group. NRG1 treatment did not make any significant
changes in the proportion of CD44-positive cells (Fig. 2).
The results of the flow cytometry showed that NRG1
induced increases in the proportion of CD44-positive cells in the
SKBr-3 and MCF-7 cell lines but not in the MDA-MB 468 cells. NRG1
treatment made no significant change in the proportion of
CD24-positive cells in any of the three cell lines. Regarding the
cells with the CD44high/CD24low phenotype,
NRG1 treatment induced new appearances of those cells in the SKBr-3
cell line and a slight increase of those cells in the MCF-7 cell
line. While the SKBr-3 cell line had no cells with the
CD44high/CD24low phenotype in the control
group, some of the MCF-7 and MDA-MB 468 control cells had the
CD44high/CD24low phenotype. Although the
proportions of these cells were very small, it is noteworthy that
these cell lines had some cells with the CSC phenotype before any
treatment. Considering the high proportion of CD44-positive cells
in the control group, MCF-7 and MDA-MB 468 cells are considered to
contain a substantial population of CSC before treatment with
NRG1.
NRG1 induces the expression of CSC
markers
Using western blot analysis, the expression levels
of CSC markers were analyzed semi-quantitatively. In addition to
CD44, integrin α6/CD49f and integrin β1/CD29 were used as CSC
markers. These integrins are generally known for their high levels
of expression in normal mammary gland stem cells (MGSCs) and may
also be important in breast CSCs (31–34).
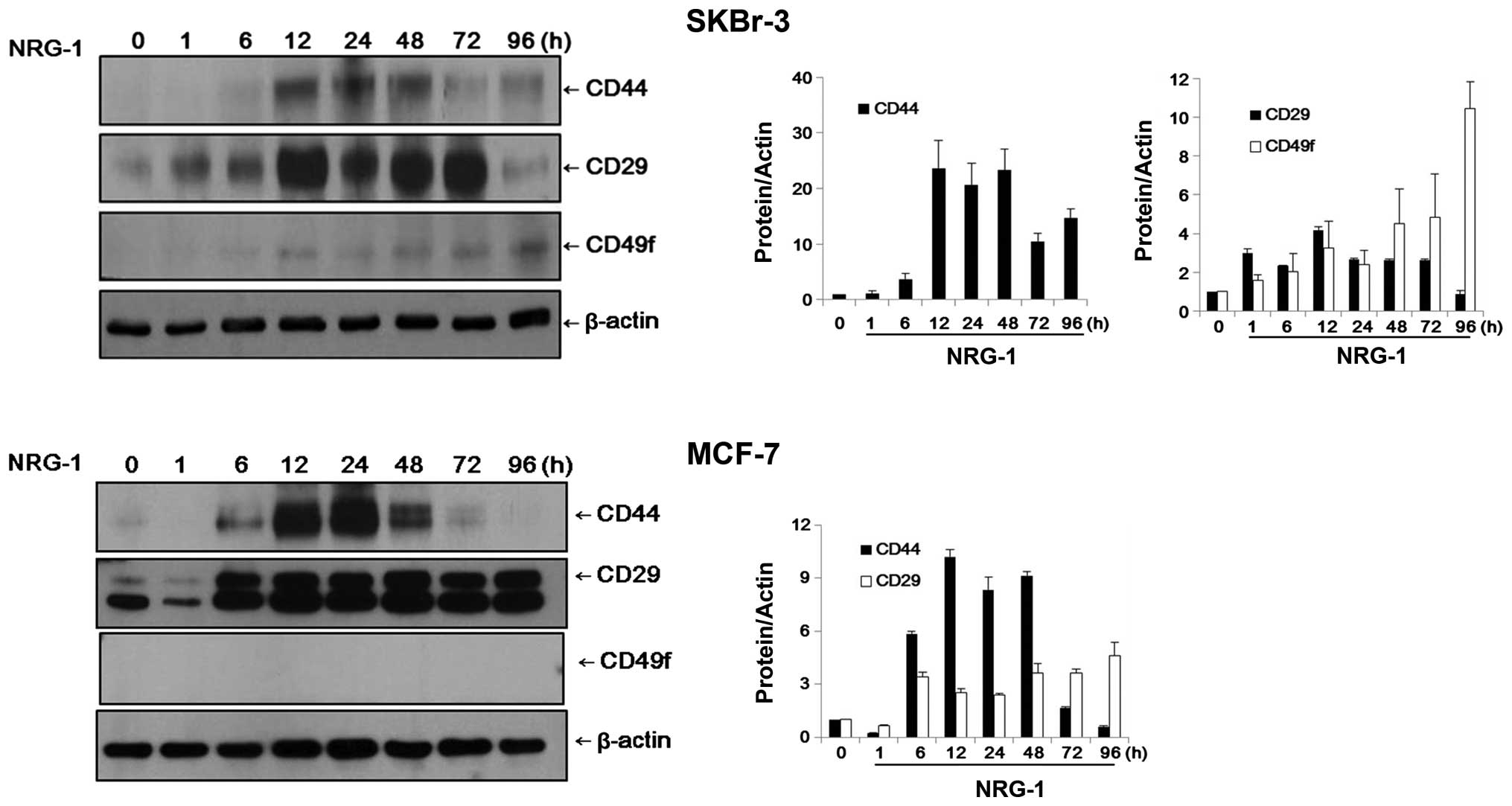
In SKBr-3 cells, the control group cells did not
express CD44 or CD49f and expression of CD29 was negligible.
However, after 12 h of incubation with NRG1, expression of CD44 and
CD49f became evident in the SKBr-3 cells. The expression levels of
CD44 and CD29 appeared to reach their peaks after 12–24 h of
incubation. While the expression levels of CD44 and CD29 were
decreased over time, the expression level of CD49f increased
continuously until 96 h of NRG1 treatment (Fig. 3).
MCF-7 cells exhibited patterns of expression similar
to those of SKBr-3 cells. Expression of CD29 was observed only
faintly in the control cells and CD44 was almost completely absent.
After 6 h of NRG1 treatment, expression of CD44 and CD29 became
evident. Expression levels of CD44 and CD29 appeared to reach their
peaks after 12–24 h of incubation. While the expression level of
CD44 was decreased after 48 h, the elevated expression level of
CD29 was sustained until 96 h of treatment. Expression of CD49f was
not observed in the MCF-7 cells, either in the control group or the
NRG1 treatment group (Fig. 3).
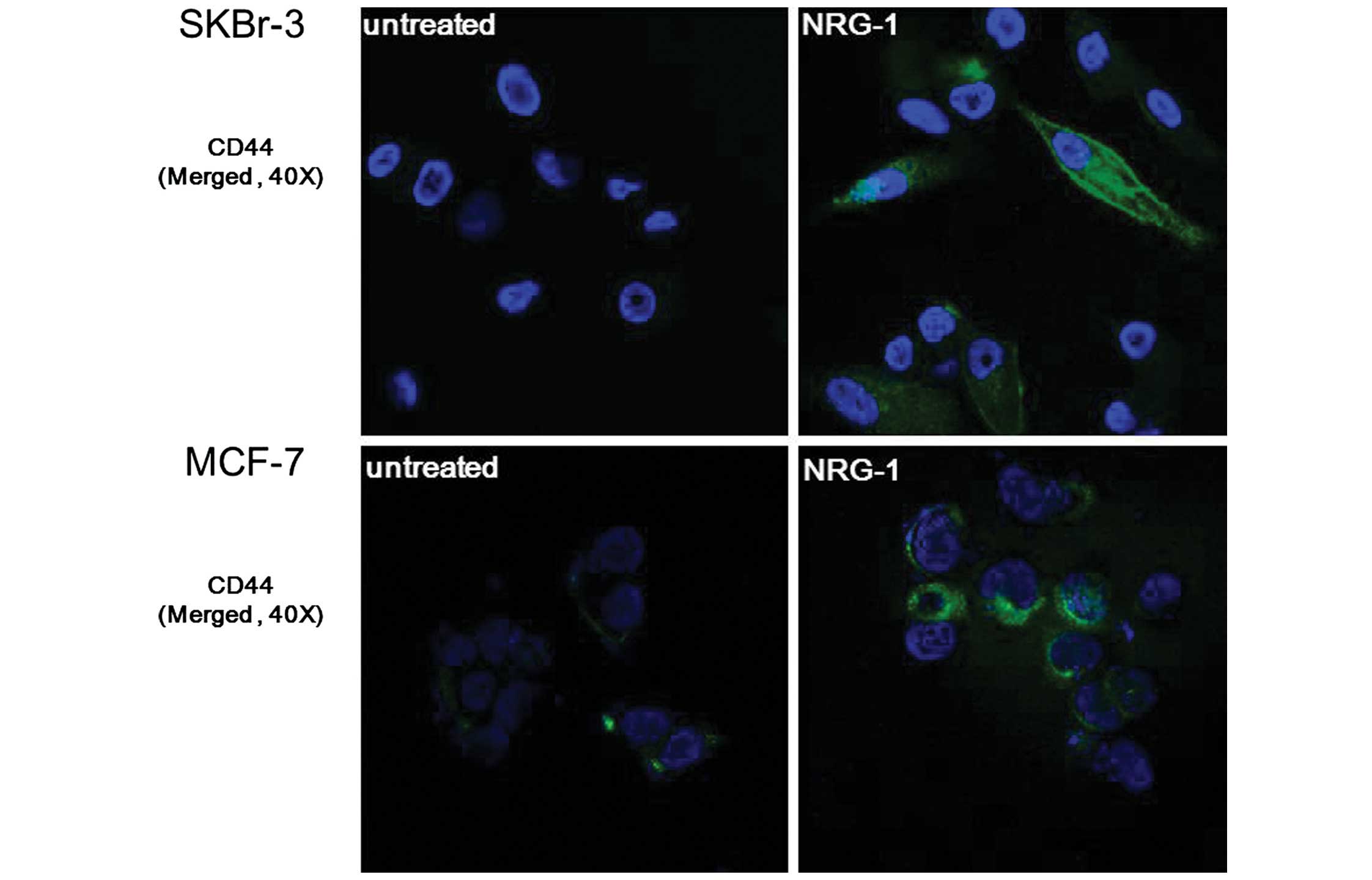
NRG1-induced expression of CSC markers was verified
by immunofluorescence staining. After incubating cells for 24 h
with NRG1, expression of CD44 was observed in the SKBr-3 and MCF-7
cells. Furthermore, SKBr-3 cells showed an outstanding
transformation into spindle-shaped cells following NRG1 treatment,
suggesting that they underwent epithelial-mesenchymal transition
(Fig. 4).
Due to the results of the flow cytometry, MDA-MB 468
cells were not analyzed further.
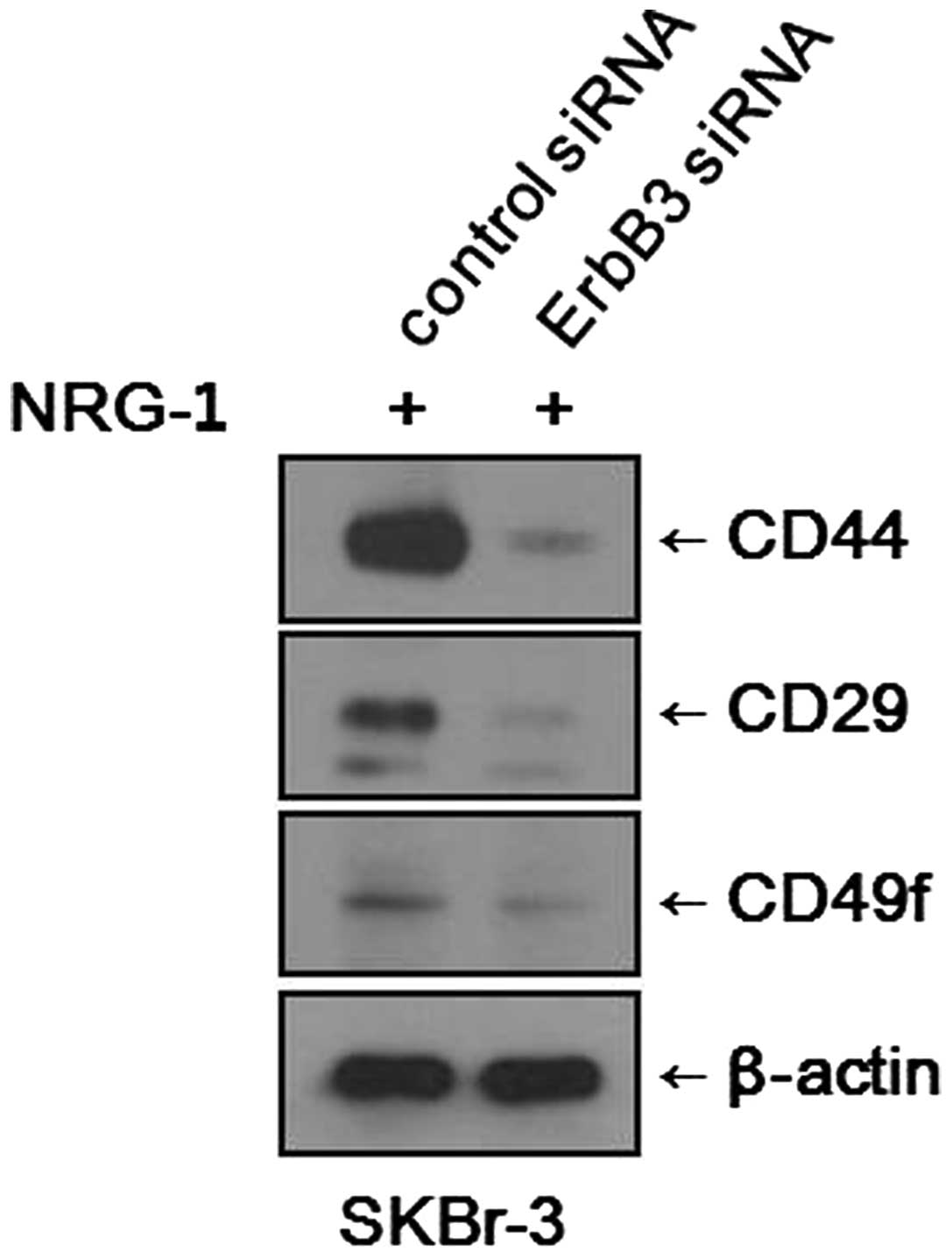
Inhibition of the HER3 receptor
suppresses expression of CSC markers
We found that NRG1 treatment induced increases in
the CSC fraction and expression levels of CSC markers in various
breast cancer cell lines. To determine whether the effects of NRG1
treatment are mediated through the HER3 receptor, we blocked the
expression of the HER3 receptor using siRNA and observed any
changes in CSC marker expression. After NRG1 treatment, SKBr-3
cells treated with control siRNA showed increased expression levels
of CSC markers, as expected. Meanwhile, SKBr-3 cells treated with
the HER3 receptor (ErbB3) siRNA showed only minimal expression of
CSC markers (Fig. 5). It was
evident that the inhibition of HER3 receptor counteracted the
effects of NRG1 treatment. However, the experiment performed with
MCF-7 cells did not show any significant changes. After NRG1
treatment, both the MCF-7 cells treated with control siRNA and
those treated with HER3 receptor (ErbB3) siRNA showed similar
levels of CSC marker expression.
Discussion
The present study demonstrated that NRG1 induces the
expression of CSC-like characteristics in a number of breast cancer
cell lines. Manifestation of CSC-like characteristics in the cells
lines was examined by observing the expression of CSC markers CD44,
CD29 and CD49f.
There were differences among the breast cancer cell
lines in regards to the levels of expression of CSC markers. MDA-MB
468 cells had the highest CSC fraction and CSC marker expression
before NRG1 treatment, and this high basal level of expression made
it impossible to use the MDA-MB 468 cells to illustrate any
increase in CSC properties due to NRG1 treatment. MDA-MB 468 cells
display high levels of HER1 expression and are known to have low or
minor expression of hormonal receptors and HER2. Because of these
features, MDA-MB 468 cells are often used as a model for basal-like
type of breast cancer. The result of this study suggests that the
basal-like type of breast cancer cells may have a high level of CSC
characteristics regardless of HER family receptor activation. Some
studies using a tissue microarray of human breast cancer reported
that breast cancers with basal-like phenotypes are associated with
EMT (35,36). Considering that EMT accompanies
CSC-like characteristics, the results of the present study support
those of the human breast cancer tissue microarray studies.
On the other hand, MCF-7 and SKBr-3 cells showed
increased CSC fractions and expression levels of CSC markers after
NRG1 treatment. This finding implies the existence of a mechanism
by which the activated HER receptors contribute to the acquisition
of CSC-like characteristics. It is known that signals from
activated, dimerized HER receptors can impact downstream signaling
cascades such as the MAPK pathway, the JAK/STAT pathway and the
PI3K/Akt pathway, leading to multiple effects (4,6,37,38).
Acquisition of CSC-like characteristics could be the result of one
of these multiple effects. Some studies have shown that NRG1
induces EMT in breast cancer cells and induces expression of
proteins related to invasion and metastasis by cancer cells (e.g.,
matrix metalloproteinase) (9–11).
Therefore, activation of HER receptors by NRG1 induces EMT and
CSC-like characteristics in tumor cells and ultimately the
acquisition of a phenotype that is favorable for the survival of
the tumor cell. NRG1 is a tissue-specifically expressed growth
factor that normally affects variable types of cells. It may have a
role in the settlement of circulating tumor cells and the formation
of metastatic tumor masses, particularly in some specific sites,
such as the brain and liver, in which normal parenchymal cells
express NRG1.
Regarding the expression of CSC markers, the results
of our western blot analysis showed another significant finding. In
SKBr-3 and MCF-7 cells, the expression levels of CSC markers
reached a peak after 12–24 h of incubation with NRG1. After that
time, the expression levels of most CSC markers, particularly CD44,
showed a decreasing trend with the passage of time. This result
suggests that the expression of CSC characteristics is a transient
and reversible phenomenon. This finding supports the hypothesis
that the expression of CSC characteristics is a transient and
dynamic process that can advance or retreat according to the tumor
microenvironment during cancer progression (27).
It is possible that NRG1-induced CSC-like
characteristics may play a role in a mechanism of chemoresistance.
Studies have shown that activation of HER receptors and their
downstream signaling cascades may be related to the resistance of
cancer cells to chemotherapy and hormonal therapy (39). Particularly, HER2–HER3 dimerization
appears to play a role as a scavenger of HER2-targeted therapy and
contributes to the restoration of the malignant phenotype of breast
cancers cells (40,41). Unpublished data from our laboratory
revealed that the expression level of NRG1 is elevated in
tamoxifen-resistant MCF-7 cells. It is assumed that chemoresistant
tumor cells secrete NRG1 and activate autocrine induction of HER
receptor-related downstream signals. As a consequence, the tumor
cells may then be induced to express proteins related to the EMT
and acquisition of CSC-like properties to ultimately attain a more
favorable phenotype for survival.
Although this study is a novel one, it has some
limitations. The acquisition of CSC characteristics was evaluated
using expression of cancer stem cell markers only. It was not
confirmed that the cells which expressed CSC markers had
self-renewal activity or pluripotent differentiation potential. In
addition, the highly tumorigenic nature of these cells needs to be
demonstrated through mammosphere assays or xenografts in NOD/SCID
mice.
In summary, the present study revealed that NRG1
treatment induces CSC characteristics in breast cancer cell lines.
In MCF-7 and SKBr-3 cells, increases in the CSC fraction and
expression levels of CSC markers were observed after NRG1
treatment. However, MDA-MB 468 cells showed high intrinsic
expression of CSC markers and a high cellular fraction of CSCs. In
the MDA-MB 468 cells, NRG1 treatment induced no significant change
in CSC characteristics.
These results imply the existence of a mechanism by
which HER receptors activated by NRG1 contribute to the acquisition
of CSC-like characteristics in certain types of breast cancer. CSCs
are known to be related to tumor recurrence and metastasis,
chemoresistance and poor prognosis. This mechanism could be a
strategy through which tumor cells that have been activated by NRG1
gain a favorable phenotype for survival. Understanding the
mechanism of action of NRG1 and HER receptors could be a clue to
overcoming the chemoresistance observed in certain types of
cancers. Furthermore, NRG1, HER receptors and the downstream
signaling molecules could serve as new targets for cancer
therapeutics.
References
|
1
|
Holmes WE, Sliwkowski MX, Akita RW, et al:
Identification of heregulin, a specific activator of p185erbB2.
Science. 256:1205–1210. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Britsch S: The neuregulin-I/ErbB signaling
system in development and disease. Adv Anat Embryol Cell Biol.
190:1–65. 2007.PubMed/NCBI
|
|
3
|
Atlas E, Cardillo M, Mehmi I,
Zahedkargaran H, Tang C and Lupu R: Heregulin is sufficient for the
promotion of tumorigenicity and metastasis of breast cancer cells
in vivo. Mol Cancer Res. 1:165–175. 2003.PubMed/NCBI
|
|
4
|
Vijapurkar U, Kim MS and Koland JG: Roles
of mitogen-activated protein kinase and phosphoinositide 3′-kinase
in ErbB2/ErbB3 coreceptor-mediated heregulin signaling. Exp Cell
Res. 284:291–302. 2003.
|
|
5
|
Hsieh AC and Moasser MM: Targeting HER
proteins in cancer therapy and the role of the non-target HER3. Br
J Cancer. 97:453–457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: receptor heterodimerization in
development and cancer. EMBO J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lupu R, Cardillo M, Cho C, et al: The
significance of heregulin in breast cancer tumor progression and
drug resistance. Breast Cancer Res Treat. 38:57–66. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hijazi MM, Thompson EW, Tang C, et al:
Heregulin regulates the actin cytoskeleton and promotes invasive
properties in breast cancer cell lines. Int J Oncol. 17:629–641.
2000.PubMed/NCBI
|
|
9
|
Cheng L, Zha Z, Lang B, Liu J and Yao X:
Heregulin-beta1 promotes metastasis of breast cancer cell line
SKBr-3 through upregulation of Snail and induction of
epithelial-mesenchymal transition. Cancer Lett. 280:50–60. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan G, Qian L, Song L, et al:
Heregulin-beta promotes matrix metalloproteinase-7 expression via
HER2-mediated AP-1 activation in MCF-7 cells. Mol Cell Biochem.
318:73–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho SJ, Chae MJ, Shin BK, Kim HK and Kim
A: Akt- and MAPK-mediated activation and secretion of MMP-9 into
stroma in breast cancer cells upon heregulin treatment. Mol Med
Rep. 1:83–88. 2008.PubMed/NCBI
|
|
12
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial- mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kokkinos MI, Wafai R, Wong MK, Newgreen
DF, Thompson EW and Waltham M: Vimentin and epithelial-mesenchymal
transition in human breast cancer - observations in vitro and in
vivo. Cells Tissues Organs. 185:191–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial- mesenchymal transition in cancer: parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ebben JD, Treisman DM, Zorniak M, Kutty
RG, Clark PA and Kuo JS: The cancer stem cell paradigm: a new
understanding of tumor development and treatment. Expert Opin Ther
Targets. 14:621–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blick T, Hugo H, Widodo E, et al:
Epithelial mesenchymal transition traits in human breast cancer
cell lines parallel the CD44(hi/)CD24(lo/-) stem cell phenotype in
human breast cancer. J Mammary Gland Biol Neoplasia. 15:235–252.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Creighton CJ, Chang JC and Rosen JM:
Epithelial-mesenchymal transition (EMT) in tumor-initiating cells
and its clinical implications in breast cancer. J Mammary Gland
Biol Neoplasia. 15:253–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
May CD, Sphyris N, Evans KW, Werden SJ,
Guo W and Mani SA: Epithelial-mesenchymal transition and cancer
stem cells: a dangerously dynamic duo in breast cancer progression.
Breast Cancer Res. 13:2022011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Almqvist PM, Mah R, Lendahl U, Jacobsson B
and Hendson G: Immunohistochemical detection of nestin in pediatric
brain tumors. J Histochem Cytochem. 50:147–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dahlstrand J, Collins VP and Lendahl U:
Expression of the class VI intermediate filament nestin in human
central nervous system tumors. Cancer Res. 52:5334–5341.
1992.PubMed/NCBI
|
|
22
|
Miyazono K: Transforming growth
factor-beta signaling in epithelial-mesenchymal transition and
progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci.
85:314–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taylor MA, Parvani JG and Schiemann WP:
The pathophysiology of epithelial-mesenchymal transition induced by
transforming growth factor-beta in normal and malignant mammary
epithelial cells. J Mammary Gland Biol Neoplasia. 15:169–190. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Wu J, Zhang Y, et al: Transforming
growth factor β-induced epithelial-mesenchymal transition increases
cancer stem-like cells in the PANC-1 cell line. Oncol Lett.
3:229–233. 2012.
|
|
25
|
Hardy KM, Booth BW, Hendrix MJ, Salomon DS
and Strizzi L: ErbB/EGF signaling and EMT in mammary development
and breast cancer. J Mammary Gland Biol Neoplasia. 15:191–199.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim J, Jung J, Lee SJ, Lee JS and Park MJ:
Cancer stem-like cells persist in established cell lines through
autocrine activation of EGFR signaling. Oncol Lett. 3:607–612.
2012.PubMed/NCBI
|
|
27
|
Li Y and Laterra J: Cancer stem cells:
distinct entities or dynamically regulated phenotypes? Cancer Res.
72:576–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Levenson AS and Jordan VC: MCF-7: the
first hormone-responsive breast cancer cell line. Cancer Res.
57:3071–3078. 1997.PubMed/NCBI
|
|
29
|
Lacroix M and Leclercq G: Relevance of
breast cancer cell lines as models for breast tumours: an update.
Breast Cancer Res Treat. 83:249–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cariati M, Naderi A, Brown JP, et al:
Alpha-6 integrin is necessary for the tumourigenicity of a stem
cell-like subpopulation within the MCF-7 breast cancer cell line.
Int J Cancer. 122:298–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shackleton M, Vaillant F, Simpson KJ, et
al: Generation of a functional mammary gland from a single stem
cell. Nature. 439:84–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taddei I, Deugnier MA, Faraldo MM, et al:
Beta1 integrin deletion from the basal compartment of the mammary
epithelium affects stem cells. Nat Cell Biol. 10:716–722. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
White DE, Kurpios NA, Zuo D, et al:
Targeted disruption of beta1-integrin in a transgenic mouse model
of human breast cancer reveals an essential role in mammary tumor
induction. Cancer Cell. 6:159–170. 2004. View Article : Google Scholar
|
|
35
|
Sarrio D, Rodriguez-Pinilla SM, Hardisson
D, Cano A, Moreno-Bueno G and Palacios J: Epithelial-mesenchymal
transition in breast cancer relates to the basal-like phenotype.
Cancer Res. 68:989–997. 2008. View Article : Google Scholar
|
|
36
|
Jeong H, Ryu YJ, An J, Lee Y and Kim A:
Epithelial-mesenchymal transition in breast cancer correlates with
high histological grade and triple-negative phenotype.
Histopathology. 60:E87–E95. 2012. View Article : Google Scholar
|
|
37
|
Kim S, Choi JH, Lim HI, et al: EGF-induced
MMP-9 expression is mediated by the JAK3/ERK pathway, but not by
the JAK3/STAT-3 pathway in a SKBr-3 breast cancer cell line. Cell
Signal. 21:892–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park S, Jung HH, Park YH, Ahn JS and Im
YH: ERK/MAPK pathways play critical roles in EGFR ligands-induced
MMP1 expression. Biochem Biophys Res Commun. 407:680–686. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Frogne T, Benjaminsen RV, Sonne-Hansen K,
et al: Activation of ErbB3, EGFR and Erk is essential for growth of
human breast cancer cell lines with acquired resistance to
fulvestrant. Breast Cancer Res Treat. 114:263–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Garrett JT, Olivares MG, Rinehart C, et
al: Transcriptional and posttranslational up-regulation of HER3
(ErbB3) compensates for inhibition of the HER2 tyrosine kinase.
Proc Natl Acad Sci USA. 108:5021–5026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sergina NV, Rausch M, Wang D, et al:
Escape from HER-family tyrosine kinase inhibitor therapy by the
kinase-inactive HER3. Nature. 445:437–441. 2007. View Article : Google Scholar : PubMed/NCBI
|