Introduction
Gastric cancer is the second most common cause of
cancer-related mortality with little improvement in long-term
survival during the past decades (1). Chemotherapy constitutes an important
treatment regimen for gastric cancer in addition to surgical
resection. However, in clinical application, there are still no
recognized standards on chemotherapy regimens (2). Thus, novel agents that are non-toxic,
efficacious as new recognized standards are urgently required.
All-trans retinoic acid (ATRA) is one of the vitamin
A metabolites. Since FDA approved ATRA for treating acute
promyelocytic leukemia in 1995, ATRA has been widely studied in
cancer since it plays important roles in cell differentiation,
growth and apoptosis (3). However,
the extensive use of ATRA in oncology is hampered by both the
toxicity and the development of ATRA resistance during chemotherapy
(4). Thus, there is a constant need
to find more effective and low-poisonous derivatives of ATRA.
4-Amino-2-trifluoromethyl-phenyl retinate (ATPR) is a novel ATRA
derivative which was designed and synthesized by our team (5). The superior antitumor effects of ATPR
compared to ATRA have been demonstrated on a human breast cancer
cell line in our previous studies (6). In the present study, we compared the
effects of ATPR and ATRA on proliferation, differentiation and
migration of human gastric carcinoma cell line BGC-823 in
vitro. BGC-823 is a poorly-differentiated human gastric
adenocarcinoma cell line with fast-proliferation and high-malignant
characteristics, commonly used in China and also adopted in the UK,
Germany, Italy and Russia (7).
Similar to other solid human cancers, recurrence and
metastasis are the biggest obstacles in the treatment of gastric
cancer. Cell migration is critical for a variety of biological
processes in normal and pathological conditions including cancer
metastasis (8). In cell migration,
a contractile force drives the cell body forward, and the rear part
of the cell is detached from the substrate. Myosin II is believed
to be involved in the generation of the contractile force for cell
migration. The activity of myosin II is mainly controlled by myosin
light chain (MLC II) phosphorylation, which is considered to
promote myosin assembly and increase the actomyosin-based
contractility (9). Furthermore, the
phosphorylation of MLC II is regulated by some enzymes. Myosin
light chain kinase (MLCK) and Rho-associated coiled-coil containing
kinase (ROCK) are two major enzymes that phosphorylate MLC II;
however, myosin phosphatase (MLCP) plays opposite roles in MLC II
phosphorylation (10). MLCP is a
trimeric complex consisting of a regulatory myosin-binding subunit
(MYPT1), a catalytic subunit, and a subunit of unknown function.
MLCP drives the dephosphorylation of MLC II, which becomes inactive
when MYPT1 is phosphorylated (11).
In particular, ROCK can not only directly phosphorylate MLC II, but
it can also increase the phosphorylation level of MLC II indirectly
by phosphorylating MYPT1, thus inhibiting its phosphatase activity
(12). Based on these findings, in
the present study we surveyed whether ATPR can inhibit the
migration of BGC-823 cells in vitro, and observed whether
ATPR affects the position of claudin-18, one of the tight junction
proteins, and identified whether ATPR play its anti-migration roles
by suppressing the expression of MLCK and ROCK.
It is well known that ATRA plays its roles by
activating retinoic acid receptors (RARs) that bind to retinoid X
receptors (RXRs) as a heterodimer, and then this heterodimer binds
to a regulatory DNA element (retinoic acid response elements;
RAREs) and regulates the transcriptional expression of downstream
target genes (13). However,
whether ATPR works by activating RARs and its downstream genes is
not very clear. In the present study, we used the BGC-823 cell
line, which is a RARα and RARβ-positive cell line (14), and identified whether ATPR affects
the expression of RARα and RARβ by targeting MLCK and ROCK as
downstream genes.
Materials and methods
Cell lines and major reagents
The human poorly-differentiated gastric carcinoma
cell line BGC-823 was obtained from the Cell Bank at the Chinese
Academy of Science (Shanghai, China). Cells were cultured in
RPMI-1640 medium (Gibco, USA) supplemented with 10% fetal bovine
serum (Tianhang, China), 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2.
ATPR was synthesized by the School of Pharmacy,
Anhui Medical University, and was dissolved in dimethyl sulfoxide
(DMSO) at the stock concentration of 10 mmol/l and stored at −20°C.
ATRA, MTT and DMSO were purchased from Sigma-Aldrich (USA). Primary
antibodies, including anti-RARα (C-20) (sc-551), anti-RARβ (C-19)
(sc-552), anti-MLCK (L-18) (sc-9452), anti-p-MLC II (Thr18/Ser19)
(sc-12896), anti-MLC II (D-9) (sc-48414), anti-p-MYPT1 (Thr853)
(sc-17432), anti-MYPT1 (H-130) (sc-25618), anti-claudin-18 (P-14)
(sc-17687) and anti-β-actin (C4) (sc-47778) were purchased from
Santa Cruz Biotechnology (USA). All secondary antibodies were
purchased from Millipore (USA).
MTT assay
Cell proliferation after ATPR administration was
estimated by the MTT assay. The BGC-823 cells were plated on
96-well cell culture plates at a density of 4×103
cells/well. After incubation for 24 h, ATPR was added to the wells
at gradual concentrations of 5, 10, 15, 20, 30 and 40 μmol/l, and
then incubated in a humidified incubator at 37°C with 5%
CO2. The cells treated with 40 μmol/l ATRA were taken as
the positive control and the cells treated with 0.1% DMSO were the
solvent control. After a 48-h incubation period, the medium was
removed and 5 mg/ml MTT solution was added to each well. Cells were
incubated for 4 h, followed by the addition of 100 μl DMSO and the
mixture was incubated for 10 min to dissolve formazan crystals. The
absorbance was measured at a wavelength of 570 nm using a
microplate reader (BioTek Model ELx800). Inhibition rate (%) was
calculated according to the following formula: (1-OD570
of drug/OD570 of control) × 100%.
Plate colony formation assay
The colony formation ability of BGC-823 cells in
vitro was measured by plate colony formation assay. BGC-823
cells in the exponential phase of growth were exposed to ATPR for
48 h, and then harvested as single cell suspensions. Approximately
1,000 cells were added to each well of a 6-well cell culture plate.
The plate was incubated for 8–10 days in a humidified atmosphere at
37°C until the colonies were clearly visible to the naked eye. We
removed the medium from each well, gently washed each well by
phosphate-buffered saline (PBS) two times, fixed the cells by 4%
paraform and stained with 1% crystal violet. The colonies
containing >50 cells were counted in each well for five random
visual fields. The mean colony count for each of the treatments was
calculated.
Flow cytometry
Detection of cell cycle was performed by PI stained
flow cytometry. BGC-823 cells in the exponential phase of growth
were exposed to ATPR for 48 h, then harvested as single cell
suspensions, washed with cold PBS two times and then tested by
Coulter DNA-Prep Reagents kit (Beckman Coulter, USA) according to
the manufacturer’s manual. Cell cycle distribution was analyzed by
FACSCalibur flow cytometry (Becton-Dickinson).
Colorimetric detection of the activities
of alkaline phosphatase (ALP) and lactate dehydrogenase (LDH)
ALP and LDH activities of BGC-823 cells treated with
ATPR for 48 h were assayed. The cells were collected, washed with
PBS, and then lysed with 0.2% Triton-X 100 for 30 min on ice. The
cell lysate was centrifuged at 14,000 rpm at 4°C for 15 min. The
ALP and LDH activities in the supernatant were measured by the
colorimetric method using Olympus AU400. The total protein
concentration in the supernatant was determined by BCA assay. The
enzyme activity was expressed as activity/total protein (U/g).
Wound healing assay
The migration ability of BGC-823 cells was measured
by the wound healing assay. BGC-823 cells were cultured in a
24-well cell culture cluster to form a monolayer that was >90%
confluent. Then, the cell monolayer was scraped with a sterile
pipette tip to generate the wound. Thereafter, the cells were
washed with RPMI-1640 medium and then re-cultured in fresh medium
with ATPR or ATRA for 48 h. Images were captured at 0, 24 and 48 h
on the same position of the wound with an inverted phase contrast
microscope (Leica DMI3000 B). The width of the wound was measured
with Quantity One software.
Immunofluorescence assay
The position of claudin-18 was observed by
immunofluorescence assay. BGC-823 cells were seeded at a density of
1×104 cells/ml into a 6-well cell culture cluster with
sterile coverslips and cultured. When cells grew in form of
monolayer, cells were treated with 25 μmol/l ATPR or ATRA for 72 h,
and cells treated with 0.1% DMSO were used as a control group.
After treatment, the cells were washed with PBS 3 times and fixed
with 4% paraformaldehyde for 20 min at room temperature, then
washed and blocked with blocking buffer (PBS/5% non-fat dry milk)
for 2 h at room temperature. The coverslips with cells were
incubated with goat anti-human claudin-18 (1:50) primary antibody
overnight at 4°C. Subsequently, the coverslips were washed and
incubated with donkey anti-goat IgG-FITC (1:100) for 2 h at room
temperature away from light, then washed and incubated with DAPI
for 5 min, and then washed and mounted with aqueous-based anti-fade
mounting medium, finally fixed with colorless nail polish on
microscope slides. Images of stained cells were captured by
fluorescence microscope (Leica DMI4000 B).
Real-time quantitative PCR (qRT-PCR)
qRT-PCR was performed to quantify the mRNA levels of
MLCK, ROCK1 and ROCK2. Total cellular RNA was isolated from
cultured cells by TRIzol solution (Invitrogen, USA), and 1 μg of
total RNA was reverse-transcribed by PrimeScript™ RT reagent kit
with gDNA Eraser (Takara, China) according to the manufacturer’s
protocol. The complementary DNAs were amplified by UltraSYBR
Mixture (CWBiotech, China) by using a Real- Time PCR System
(Thermo, USA) with the following primer sets: MLCK,
5′-ggactttcagccttgtgattc-3′ (forward) and 5′-cgca
aaacttccttctactgtc-3′ (reverse); ROCK1, 5′-ctctaccactttcctgc caa-3′
(forward) and 5′-gtggcacttaacatggcatc-3′ (reverse); ROCK2,
5′-accaatgctttactgcgaac-3′ (forward) and 5′-tctccagcaggcagttttta-3′
(reverse); 18S rRNA, 5′-cagccacccgagattgag ca-3′ (forward) and
5′-tagtagcgacgggcggtgtg-3′ (reverse). All primers were synthesized
by Sangon Biotech (China). 18S rRNA served as a reference gene. The
PCR program was: template denaturation at 95°C for 10 min; 40
cycles of template denaturation at 95°C for 10 sec, primer
annealing at 56°C for 30 sec, product extension at 72°C for 32 sec;
a final PCR product extension at 60°C for 30 sec. Results are shown
by the mean normalized values of cDNA levels among each group with
the reference gene.
Western blot analysis
To determine the changes of protein levels, the
cellular total protein of BGC-823 was extracted after different
treatment. At specific time points, cells were washed with PBS 3
times and protein extracts were prepared with RIPA buffer and
quantified by BCA protein assay (Beyotime, China). Thirty
micrograms of protein were resolved in SDS sample buffer,
fractionated in SDS-PAGE gels and transferred to a PVDF membrane.
The membranes were blocked for 1 h at room temperature with TBST
containing 5% non-fat dry milk and then incubated with the primary
antibodies for RARα (1:500), RARβ (1:500), MLCK (1:500), p-MLC II
(1:250), MLC II (1:500), p-MYPT1 (1:500), MYPT1 (1:700) and β-actin
(1:1,000) overnight at 4°C. The reactive bands were visualized with
enhanced chemiluminescence (Beyotime).
Statistical analysis
All experiments were repeated a minimum of 3 times.
Data presented in images are from one representative experiment.
Statistical significance was determined with one-way ANOVA with
SPSS 17.0 software. A value of P<0.05 was considered to indicate
a statistically significant difference.
Results
ATPR inhibits the proliferation of
BGC-823 cells
To test the proliferation inhibitory effect of ATPR
on BGC-823 cells, we performed an MTT assay and a plate colony
formation assay. As shown in Table
I, the proliferation ability of BGC-823 cells was significantly
decreased in the treatment group compared to the solvent group. For
the 40 μmol/l ATPR-treated cells, the OD 570 nm value was
0.289±0.015 while the value of the ATRA group was 0.429±0.007 at
the same concentration (P<0.01). The results also showed that
the proliferation of BGC-823 cells was inhibited by ATPR in a
concentration-dependent manner. According to the above data, the
IC50 value of ATPR was 27.86 μmol/l. Finally, 25 μmol/l
was chosen as the treatment concentration for most of the following
assays.
 | Table IThe dose-effect of ATPR on BGC-823
cell proliferation. |
Table I
The dose-effect of ATPR on BGC-823
cell proliferation.
| Group | Concentration
(μmol/l) | OD570
(mean ± SD) | Inhibition rate
(%) |
|---|
| Cell | | 0.758±0.020 | |
| DMSO-treated | | 0.745±0.010 | |
| ATRA-treated | 40 | 0.429±0.007 | 42.4±0.93 |
| ATPR-treated | 5 | 0.741±0.007 | 0.64±0.08 |
| 10 | 0.732±0.016 | 1.78±0.02 |
| 15 | 0.540±0.075 | 27.61±1.00 |
| 20 | 0.466±0.083 | 37.5±1.12 |
| 30 | 0.297±0.031 | 60.15±4.1 |
| 40 | 0.289±0.015 | 61.19±2.0a |
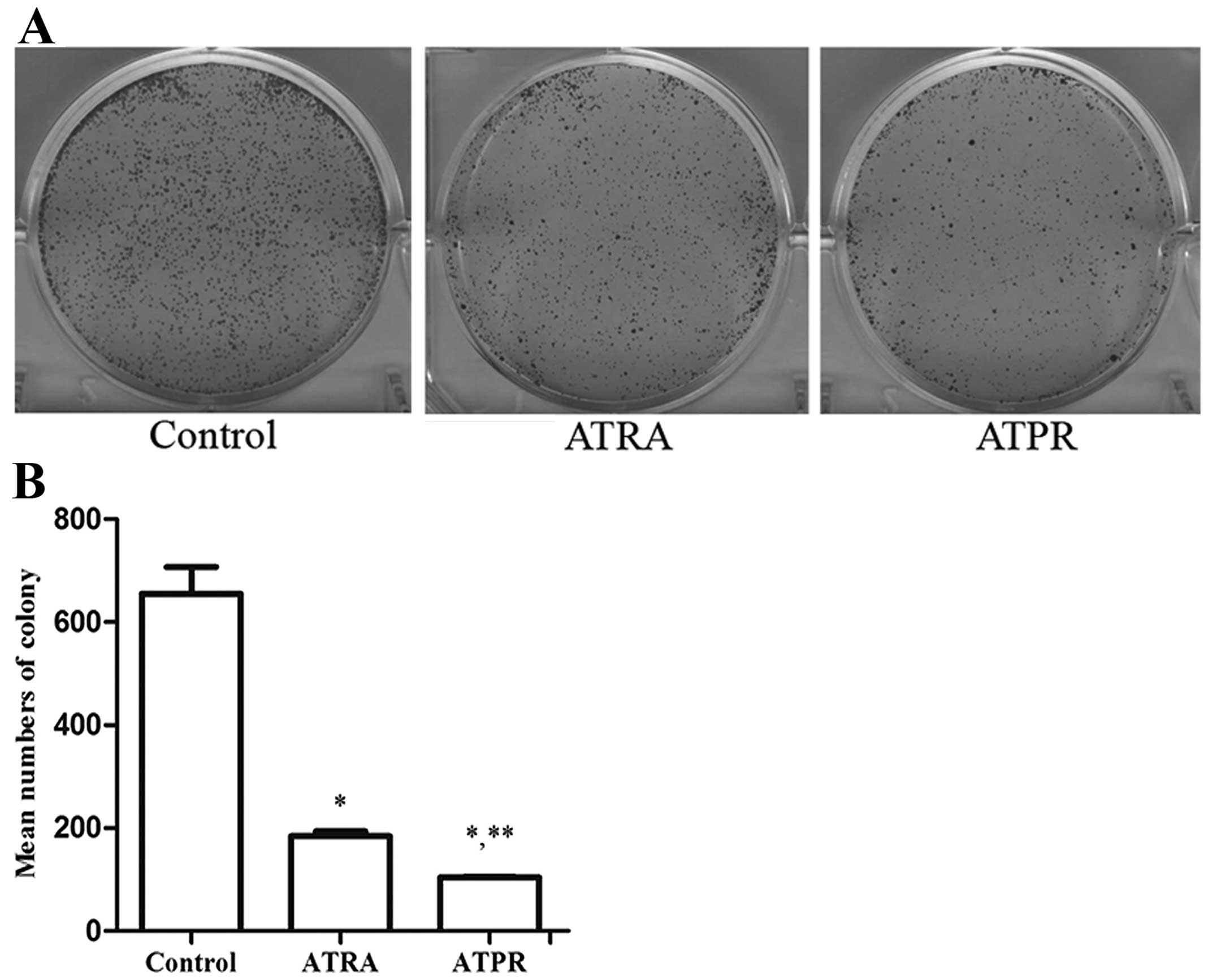
On the other hand, the colony formation ability of
BGC-823 cells in vitro was determined by plate colony
formation assay. BGC-823 cells treated with 25 μmol/l ATPR or 25
μmol/l ATRA were taken as the treatment groups and cells treated
with 0.1% DMSO as the control group. After 8–10 days, we captured
the images and statistically accounted the mean numbers of
colonies. Fig. 1 shows that the
colony formation ability of BGC-823 cells in the treatment groups
was markedly reduced compared to the control group (P<0.01).
Furthermore the mean numbers of colonies in the ATPR-treated group
were lower than in the ATRA-treated group (P<0.01).
ATPR induces the differentiation of
BGC-823 cells
To investigate the effect of ATPR on differentiation
of BGC-823 cells, we observed the cell morphological changes, the
cell cycle distribution and the activities of ALP and LDH. These
results suggested that BGC-823 cells not only undergo
differentiation following ATPR treatment, but also that ATPR is
more efficient than ATRA in inducing the differentiation of BGC-823
cells.
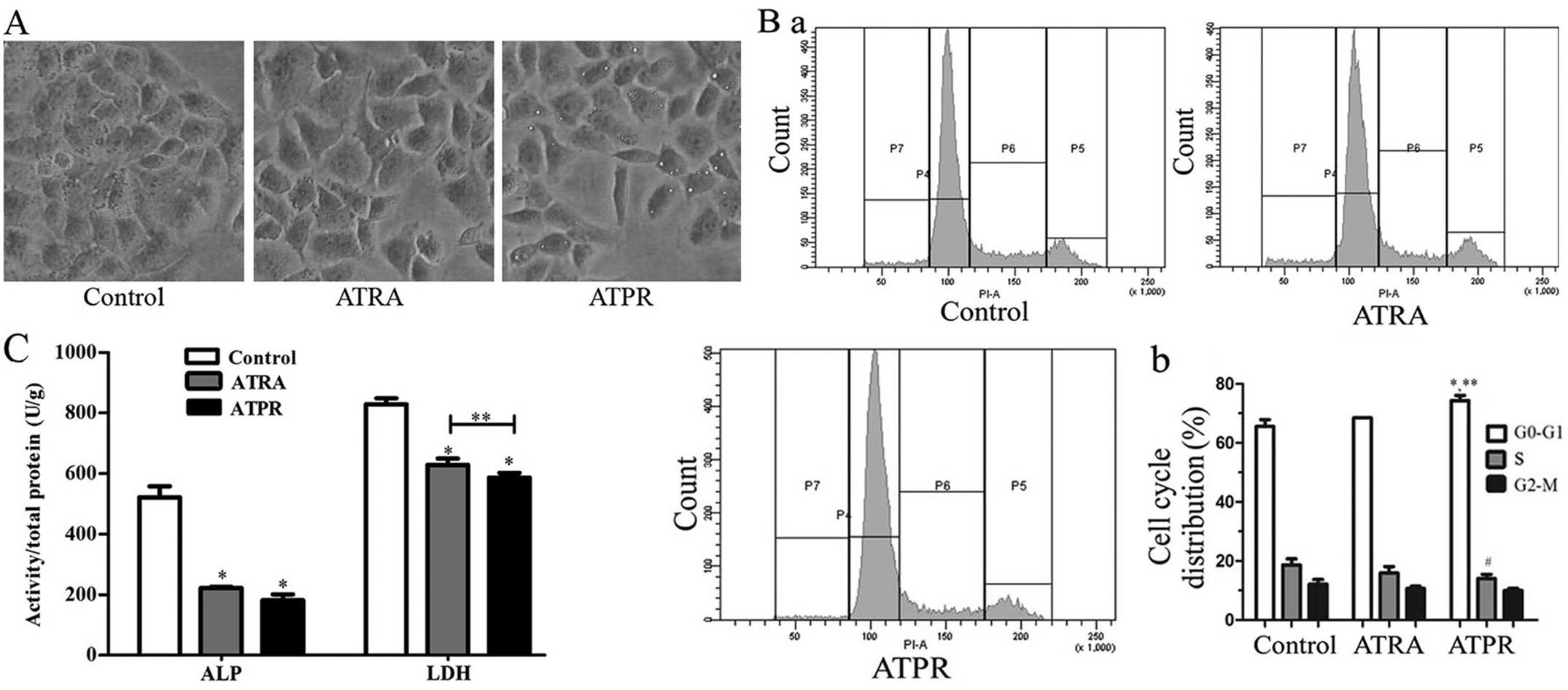
After the BGC-823 cells were treated with 25 μmol/l
ATPR for 48 h, we observed the cell morphological changes with an
inverted phase contrast microscope. ATPR-treated cells showed
morphological changes with characteristics of differentiating
cells, such as elongation or stretching of the cells as shown in
Fig. 2A. ATPR induced more obvious
changes in cell morphology than ATRA-treated cells at the same
concentration. However, the control cells appeared hexagonal.
A cell cycle analysis of BGC-823 cells after
treatment with 25 μmol/l ATPR for 48 h was performed by a flow
cytometry. As shown in Fig. 2B, an
accumulation of cells in G0/G1 phase with a
reduction in the number of cells in S phase was observed. For the
0.1% DMSO-treated cells (the control group), 65.55±2.35 and
18.6±2.1% cells were detected in G0/G1 and S
phases, respectively. For the cells treated with 25 μmol/l ATPR,
74.35±1.75 and 14.05±1.35% were detected in
G0/G1 and S phases, respectively. The
differences of G0/G1 phase (P<0.01) and S
phase (P<0.05) between the control and ATPR-treated cells were
statistically significant. However, there was no statistically
significant difference between the control and the ATRA-treated
cells. In addition, ATPR induced greater accumulation in
G0/G1 phase than ATRA (P<0.01).
At the same time, we also detected the activities of
ALP and LDH after ATPR treatment. The results (Fig. 2C) showed that the activities of ALP
and LDH in ATPR-treated cells were lower than those in control
cells. The activities of ALP and LDH in control cells were
522.17±35.69 and 828.75±19.67 U/g, respectively. In the cells
treated with ATPR, the activities of ALP and LDH were 181.95±19.21
and 587.25±14.55 U/g, respectively. In the cells treated with ATRA,
the activities of ALP and LDH were 221.90±4.40 and 628.72±21.10
U/g, respectively. The difference in the activity of the two
enzymes between the control and treated cells was statistically
significant (P<0.01). Moreover, ATPR suppressed the activity of
LDH more than ATRA (P<0.05).
ATPR suppresses the migration of BGC-823
cells
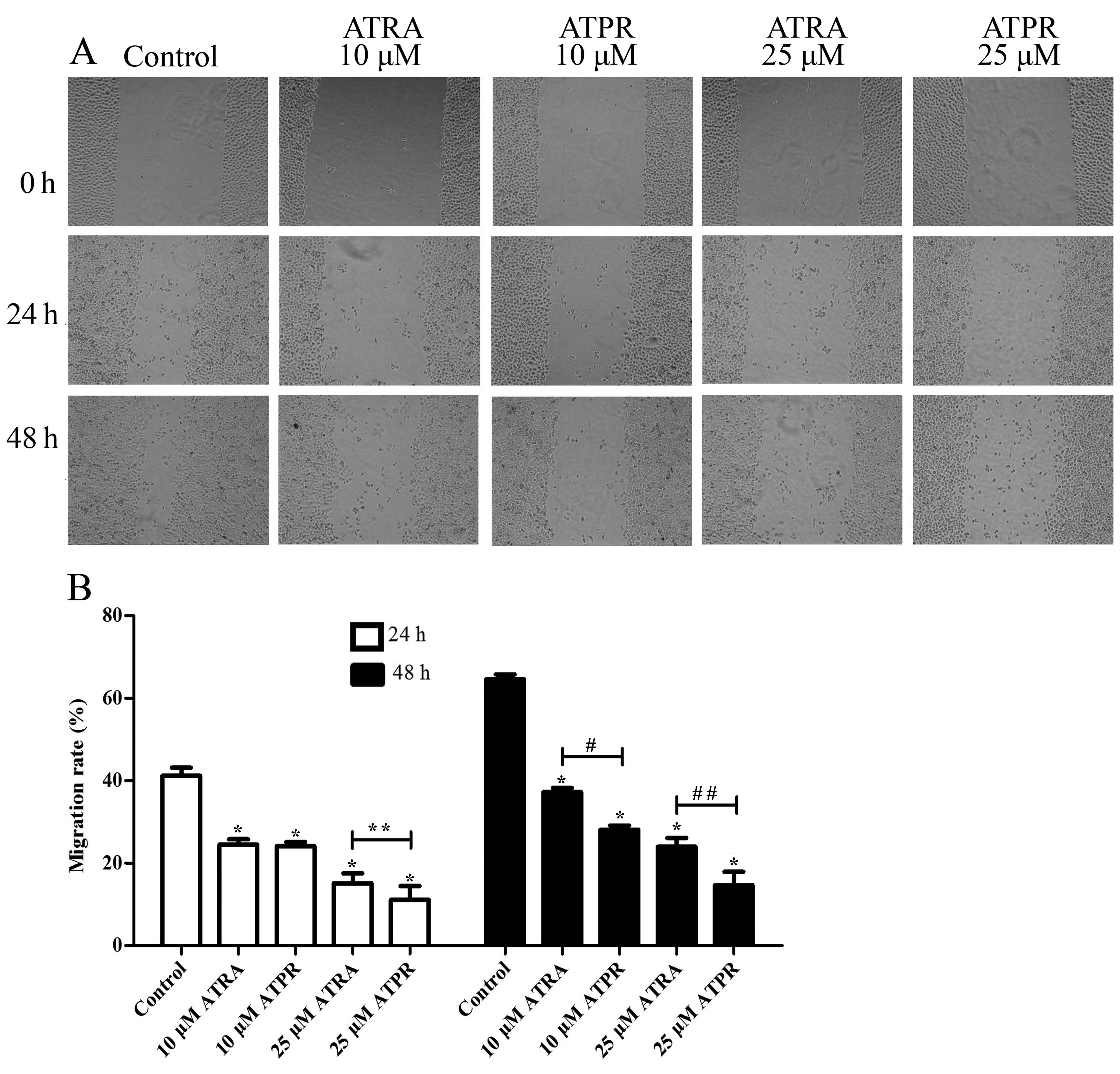
To investigate the effect of ATPR on migration in
BGC-823 cells, wound healing assay was performed. As shown in
Fig. 3, wound healing assay
illustrated that the migration rate of BGC-823 cells was inhibited
by ATPR, and the effect reached statistical significance compared
with the control group (P<0.01). Furthermore, ATPR had a
significant effect on the migration of cells compared to ATRA at
the same concentration. After culturing for 24 h, the migration
rate of the 25 μmol/l ATPR group was 11.08±3.35% while that of the
ATRA group was 15.12±2.40% (P<0.05). After culturing for 48 h,
the migration rate of the 10 μmol/l ATPR group was 28.08±0.99%
while that of the ATRA group was 37.16±1.09% (P<0.01).
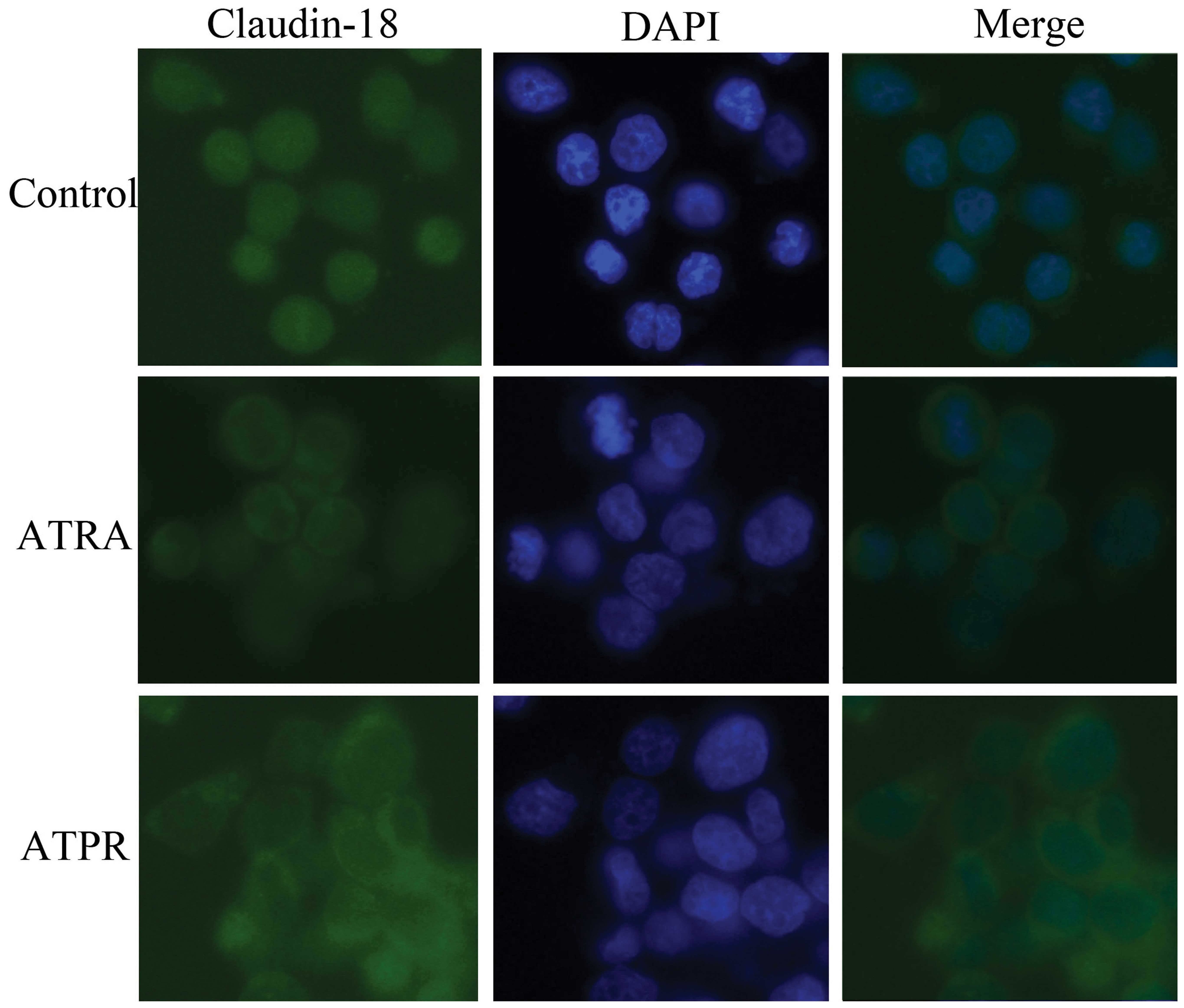
ATPR changes the localization of
claudin-18 in BGC-823 cells
As shown in Fig. 4,
immunofluorescence assay displayed that, in the control group,
claudin-18 labeled with green fluorescent protein positioned at the
cytoplasm well-distributed, while in the treatment groups,
claudin-18 mainly concentrated on the cell surface. Moreover, in
the ATPR group, claudin-18 accumulation was clearly increased
compared to the ATRA group.
ATPR reduces the protein expression of
RARα and RARβ in BGC-823 cells
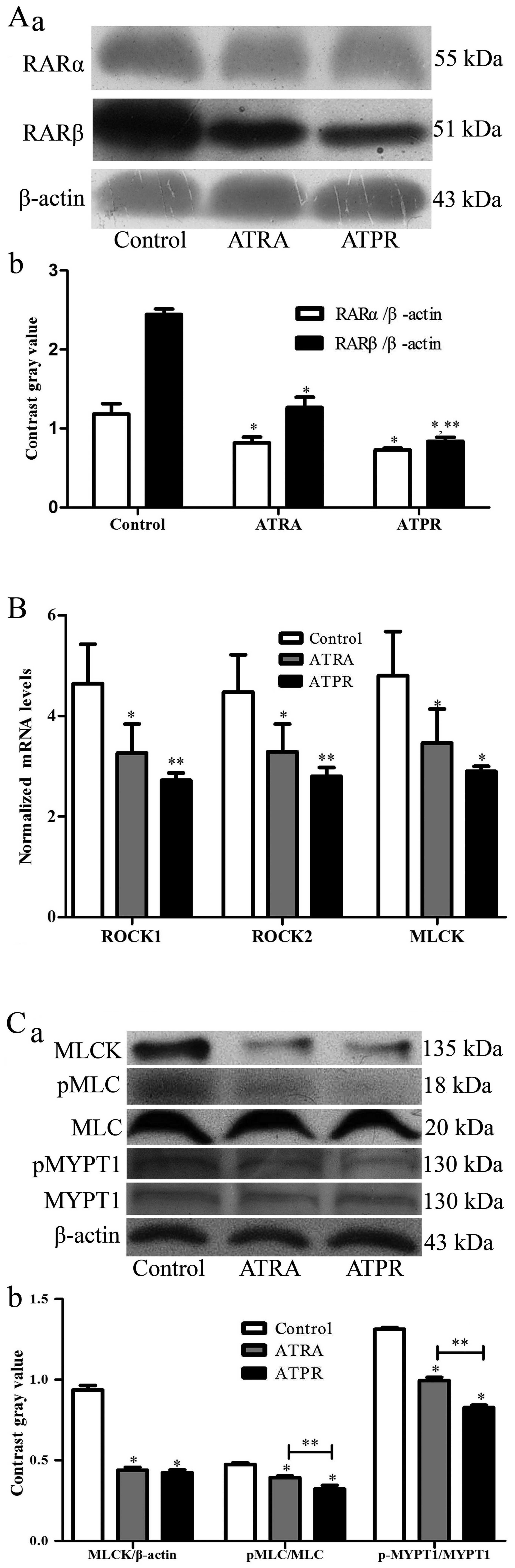
Fig. 5A shows that
the protein expression levels of RARα and RARβ after treatment with
drugs were all decreased compared with the DMSO control group
(P<0.05). However, the protein expression levels in the ATPR
group decreased more than in the ATRA group, particularly in the
protein expression of RARβ (P<0.01).
ATPR suppresses MLCK and ROCK at
transcriptional and translational levels in BGC-823 cells
In order to investigate whether MLCK and ROCK play a
potential role in the antimigration effects of ATPR, qRT-PCR and
western blotting were applied to analyze the mRNA and protein
changes of the MLCK and ROCK after treatment with ATPR. Two days
after treatment, we harvested cells for RNA isolation. As shown in
Fig. 5B, the mRNA levels of MLCK
(P<0.05), ROCK1 (P<0.01) and ROCK2 (P<0.01) were
significantly downregulated in the ATPR group compared to the
control group. Although the mRNA levels in the ATPR group decreased
more than in the ATRA group, there was no statistical significance
between the two groups.
Western blot analysis, shown in Fig. 5C, revealed that in the treatment
groups, the protein expression of MLCK, the phosphorylation levels
of MLC II and MYPT1 were decreased compared with the control group
(P<0.05). In addition, ATPR showed greater effects than ATRA on
the phosphorylation status of MLC II and MYPT1 (P<0.05).
Discussion
In the present study, we investigated the antitumor
effects of ATPR, a novel ATRA derivative, in human gastric cancer
cell line BGC-823, and explored the initial mechanism involved in
the anti-migration effect. Our results demonstrated that ATPR has a
promising inhibitory effect compared to ATRA on BGC-823 in
vitro. MTT assay and plate colony formation assay demonstrated
that ATPR inhibits the proliferation ability of BGC-823 cells. Cell
cycle analysis, detection of the activities of alkaline phosphatase
(ALP) and lactate dehydrogenase (LDH) combined cell morphological
changes indicated that ATPR clearly induces the differentiation in
BGC-823 cells. Wound healing assay showed that ATPR suppresses the
migration activity of BGC-823 cells in vitro, and
immunofluorescence assay revealed that claudin-18 translocates from
the cytoplasm to the cell surface after ATPR treatment. To explore
the preliminary mechanism, we performed real-time quantitative
RT-PCR and western blot analyses, and then found that
ATPR decreases the expression of MLCK and ROCK in
BGC-823 cells at transcriptional and translational levels. ATPR is
one of the ATRA derivatives designed and synthesized by our team
(5). It is well-known that ATRA is
a classical differentiation agent. Nevertheless, in the present
study, ATPR exhibited superior differentiation-inducing activity
compared to ATRA on human gastric cancer cell line BGC-823. Several
studies have shown that ATRA-induced differentiation is tightly
coupled to growth arrest in the G0/G1 phase,
which usually represents the cell differentiation (7,15). The
present study also demonstrated the coincidental result that
stimulation under ATRA or ATPR arrested the cell cycle of BGC-823
cells in G0/G1 phase. In addition, the
accumulation effect of ATPR was stronger than that of ATRA. ALP and
LDH are two enzymes known for their involvement in the
differentiation of gastric cells. The activities of these two
enzymes are both upregulated in gastric cancer; however, both
enzymes are decreased in the process of normal gastric cell
differentiation, indicating that the enzymes function as
dedifferentiation factors in the stomach (16,17).
In the present study, we found that the activities of ALP and LDH
in BGC-823 cells were markedly decreased by ATPR. The activities of
the two enzymes were also reduced by ATRA, but the effect was
weaker than by ATPR.
It is well established that inhibition of cancer
cell growth by ATRA is mediated by retinoic acid receptors (RARs).
Activated RARs regulate the expression of multiple target genes,
including genes involved in differentiation, cell cycle control and
migration, and thus often inhibit cell growth (13,18).
The structure of ATPR is similar to ATRA. However, little is known
about whether ATPR plays its role by binding RARs. In this study,
we detected the expression of RARα and RARβ after exposure to ATPR,
and the results of western blot analyses showed that the expression
levels of RARα and RARβ were decreased, indicating that ATPR can
influence the expression of RARs; however, further studies are
required to confirm whether ATPR can bind to RARs.
Phosphorylation of MLC II is important for the
activity of myosin which is responsible for actomyosin
contractility and hence cell migration. Phosphorylation of MLC II
is regulated mainly by two distinct signal transduction cascades.
One pathway requires phosphorylation of MLC by MLCK, while the
second pathway involves ROCK activation. It has been found that
ML-7, a selective inhibitor of MLCK, could significantly inhibit
the migration of breast cancer cell lines (19), and MLCK shows DNA hypermethylation
in gastric cancer (20). The mRNA
expression level of MLCK increases in non-small cell lung cancer
and is associated with recurrence and metastasis (21). On the other hand, there is
considerable evidence to suggest that ROCK becomes hyperactivity in
many cancers, including gastric cancer, and contributes to more
aggressive tumor properties such as metastasis and invasion
(22–24). Two isoforms of ROCK have been
described that are encoded by two separate genes, ROCK1 and ROCK2
(25). In the present study,
real-time quantitative RT-PCR showed that the ATPR can reduce the
mRNA levels of MLCK, ROCK1 and ROCK2. This means ATPR can affect
MLCK and ROCK at the transcriptional level. Moreover, ATPR has an
impact on MLCK and ROCK at the translational level for the data as
western blot analyses revealed that the MLCK, p-MLC II and p-MYPT1
were downregulated by ATPR. The phosphorylation of MLC II and MYPT1
has been widely used as surrogate markers of ROCK activity. Since
ROCK can phosphorylate MLC II directly and inhibit the
dephosphorylation of MLC II by phosphorylating the myosin-binding
subunit (MYPT1) of MLCP, it can phosphorylate MLC II
indirectly.
To a certain extent, we verified that ATPR inhibits
the phosphorylation of MLC II in a MLCK- and ROCK-dependent manner,
and then suppresses the cell migration. Although both MLCK and ROCK
can phosphorylate MLC, Totsukawa et al hypothesized that
MLCK would result in a high turnover rate of MLC phosphorylation
while ROCK may achieve a slower phosphorylation of MLC (10). Although in this study we did not
observe a clear distinction in ATPR on the role of suppressing
these two kinases, the effect of ATPR on inhibiting the MLC
phosphorylation may be different at different time points due to
the active MLCP increased by inhibiting ROCK.
On the other hand, cytoskeletal contraction induced
by phosphorylation of MLC can regulate tight junction barrier
function and result in disruption of tight junction structure
(26). Claudins are the major
elements of tight junction complexes, such as occludins, JAMs and
ZO-1 (27). Claudin-18 is mainly
expressed in stomach and lung epithelial cells, and may be a good
marker for gastric cancer (28,29).
Oshima et al showed that downregulation of claudin-18 is
related to the proliferative potential at the invasive front of
gastric cancer (30). Our data
showed that after ATPR stimuli, claudin-18 proteins positioned from
cytoplasm to cell surface, which indicated that the translocation
of claudin-18 to enhance the tight junction may be contributed to
the anti-migration effect of ATPR.
According to the above results, ATPR can suppress
the migration by influencing claudin-18, MLCK and ROCK,
consequently inhibiting the proliferation and inducing the
differentiation in BGC-823 cells. Our findings provide an
experimental basis for the use of ATPR as chemotherapy drugs
against gastric caner cells, thereby facilitating the development
of new anticancer agents.
Acknowledgements
This study was supported by a National Nature
Science Research Grant (no. 81272399).
References
|
1
|
Vogiatzi P, Vindigni C, Roviello F,
Renieri A and Giordano A: Deciphering the underlying genetic and
epigenetic events leading to gastric carcinogenesis. J Cell
Physiol. 211:287–295. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mackenzie M, Spithoff K and Jonker D:
Systemic therapy for advanced gastric cancer: a clinical practice
guideline. Curr Oncol. 18:e202–e209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Connolly RM, Nguyen NK and Sukumar S:
Molecular pathways: current role and future directions of the
retinoic acid pathway in cancer prevention and treatment. Clin
Cancer Res. 19:1651–1659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garattini E, Gianni M and Terao M:
Retinoids as differentiating agents in oncology: a network of
interactions with intracellular pathways as the basis for rational
therapeutic combinations. Curr Pharm Des. 13:1375–1400. 2007.
View Article : Google Scholar
|
|
5
|
Gui SY, Chen FH, Zhou Q and Wang Y:
Effects of novel all-trans retinoic acid retinamide derivatives on
the proliferation and apoptosis of human lung adenocarcinoma cell
line A549 cells. Yakugaku Zasshi. 131:1465–1472. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang B, Yan Y, Zhou J, Zhou Q, Gui S and
Wang Y: A novel all-trans retinoid acid derivatives inhibits the
migration of breast cancer cell lines MDA-MB-231 via myosin light
chain kinase involving p38-MAPK pathway. Biomed Pharmacother.
67:357–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang G, Wang G, Wang S, Li Q, Ouyang G
and Peng X: Applying proteomic methodologies to analyze the effect
of hexamethylene bisacetamide (HMBA) on proliferation and
differentiation of human gastric carcinoma BGC-823 cells. Int J
Biochem Cell Biol. 36:1613–1623. 2004. View Article : Google Scholar
|
|
8
|
Olson MF and Sahai E: The actin
cytoskeleton in cancer cell motility. Clin Exp Metastasis.
26:273–287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Somlyo AP and Somlyo AV: Signal
transduction by G-proteins, rho-kinase and protein phosphatase to
smooth muscle and nonmuscle myosin II. J Physiol. 522:177–185.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Totsukawa G, Wu Y, Sasaki Y, et al:
Distinct roles of MLCK and ROCK in the regulation of membrane
protrusions and focal adhesion dynamics during cell migration of
fibroblasts. J Cell Biol. 164:427–439. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ito M, Nakano T, Erdodi F and Hartshorne
DJ: Myosin phosphatase: structure, regulation and function. Mol
Cell Biochem. 259:197–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wong CC, Wong CM, Ko FC, et al: Deleted in
liver cancer 1 (DLC1) negatively regulates Rho/ROCK/MLC pathway in
hepatocellular carcinoma. PLoS One. 3:e27792008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soprano DR, Qin P and Soprano KJ: Retinoic
acid receptors and cancers. Annu Rev Nutr. 24:201–221. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Q, Chen ZM and Su WJ: Anticancer effect
of retinoic acid via AP-1 activity repression is mediated by
retinoic acid receptor α and β in gastric cancer cells. Int J
Biochem Cell Biol. 34:1102–1114. 2002.PubMed/NCBI
|
|
15
|
Fang Y, Zhou X, Lin M, et al: The
ubiquitin-proteasome pathway plays essential roles in ATRA-induced
leukemia cells G0/G1 phase arrest and
transition into granulocytic differentiation. Cancer Biol Ther.
10:1157–1167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nowak G, Griffin JM and Schnellmann RG:
Hypoxia and proliferation are primarily responsible for induction
of lactate dehydrogenase activity in cultured cells. J Toxicol
Environ Health. 49:439–452. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fishman WH: Recent developments in
alkaline phosphatase research. Clin Chem. 38:24841992.PubMed/NCBI
|
|
18
|
Lu J, Zhang F, Yuan Y, Ding C, Zhang L and
Li Q: All-trans retinoic acid upregulates the expression of
p53 via Axin and inhibits the proliferation of glioma cells. Oncol
Rep. 29:2269–2274. 2013.
|
|
19
|
Zhou X, Liu Y, You J, Zhang H, Zhang X and
Ye L: Myosin light-chain kinase contributes to the proliferation
and migration of breast cancer cells through cross-talk with
activated ERK1/2. Cancer Lett. 270:312–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Su L, Li J, et al: Hypermethylated
FAM5C and MYLK in serum as diagnosis and pre-warning
markers for gastric cancer. Dis Markers. 32:195–202. 2012.
|
|
21
|
Minamiya Y, Nakagawa T, Saito H, et al:
Increased expression of myosin light chain kinase mRNA is related
to metastasis in non-small cell lung cancer. Tumour Biol.
26:153–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lochhead PA, Wickman G, Mezna M and Olson
MF: Activating ROCK1 somatic mutations in human cancer. Oncogene.
29:2591–2598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu N, Bi F, Pan Y, et al: Reversal of the
malignant phenotype of gastric cancer cells by inhibition of RhoA
expression and activity. Clin Cancer Res. 10:6239–6247. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuoka T, Yashiro M, Kato Y, Shinto O,
Kashiwagi S and Hirakawa K: RhoA/ROCK signaling mediates plasticity
of scirrhous gastric carcinoma motility. Clin Exp Metastasis.
28:627–636. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakagawa O, Fujisawa K, Ishizaki T, Saito
Y, Nakao K and Narumiya S: ROCK-I and ROCK-II, two isoforms of
Rho-associated coiled-coil forming protein serine/threonine kinase
in mice. FEBS Lett. 392:189–193. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vicente-Manzanares M, Ma X, Adelstein RS
and Horwitz AR: Non-muscle myosin II takes centre stage in cell
adhesion and migration. Nat Rev Mol Cell Biol. 10:778–790. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Runkle EA and Mu D: Tight junction
proteins: from barrier to tumorigenesis. Cancer Lett. 337:41–48.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sanada Y, Oue N, Mitani Y, Yoshida K,
Nakayama H and Yasui W: Down-regulation of the claudin-18 gene,
identified through serial analysis of gene expression data
analysis, in gastric cancer with an intestinal phenotype. J Pathol.
208:633–642. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iravani O, Tay BW, Chua PJ, Yip GW and Bay
BH: Claudins and gastric carcinogenesis. Exp Biol Med. 238:344–349.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oshima T, Shan J, Okugawa T, et al:
Down-regulation of claudin-18 is associated with the proliferative
and invasive potential of gastric cancer at the invasive front.
PLoS One. 8:e747572013. View Article : Google Scholar : PubMed/NCBI
|