Introduction
Gastroenteric cancer (GE) is one of the most common
cancers in the world with high mortality rates worldwide (1). Although the incidence of GE tumor has
decreased considerably, it remains the second leading cause of
cancer-related mortality. The current treatment strategy includes
surgical removal followed by adjuvant chemotherapy (2,3).
Therefore, reoccurrence and relapse highly depend on the efficiency
of the chemotherapeutic treatment. In this regard, paclitaxel
(PTX), a microtubule destabilizing agent, has shown considerable
potential against gastric-related cancers (4). PTX exerts its effect by facilitating
tubulin polymerization and by stabilization of microtubules in G2-M
phase arrest and mitotic cell death (5). However, its poor aqueous solubility
and low therapeutic index limits its clinical application (6). To counter this problem, Taxol, a
cremophore based alcoholic mixture, was introduced and although it
improved the solubility, it was associated with severe adverse
effects including neurotoxicity and nephrotoxicity (7). Consequently, cremophore free
albumin-bound PTX nanoparticles (NPs; Abraxane) were launched to
overcome the side-effects of Taxol. Although Abraxane was
effective, it showed a poor colloidal stability in vivo
(8). Therefore, the need for
biocompatible, stable, tunable release controlled delivery system
for PTX remains.
N,N,N-trimethyl chitosan (TMC) is a water soluble
cationic polyelectrolyte useful for the oral and intravenous drug
delivery of PTX (9). TMC is a
biocompatible and biodegradable polymer which can be effectively
used to form NPs of 150–200 nm via ionic gelation process with
tripolyphosphate (TPP) as an anionic counterpart (10). Previously, many therapeutic moieties
including antioxidants, enzymes, vaccines, antimicrobials and small
molecules were successfully encapsulated and administered in
vivo (11). The encapsulation
of PTX in TMC NPs could effectively overcome the solubility issues,
provide a controlled release profile and can prolong the half-life
of drug in the in vivo conditions. Moreover, the excellent
mucoadhesive property of TMC would further facilitate the PTX
transport across the GE cancer cells in the body (12).
Thus, the purpose of the present study was to
prepare PTX-encapsulated TMC NPs and to investigate their effect on
the gastroenteric tumors. To achieve this purpose, nanosized TMC
NPs of 200 nm were prepared by cross-linking with TPP counter ion.
Various physicochemical characteristics including particle size,
surface charge, loading efficiency, release kinetics and stability
were investigated. Biological investigations including cellular
uptake and cytotoxicity assays were performed. Most importantly,
antitumor efficacy was carried out in tumor bearing xenograft nude
mice to establish its tumor regression and safety profile.
Materials and methods
Materials
PTX, chitosan (viscosity 20–200 cps; degree of
acetylation 85%) and sodium tripolyphosphate (TPP) were procured
from Sigma-Aldrich (Shanghai, China) and used as delivered.
Dimethyl sulfate was purchased from Vetec (Brazil). All other
chemicals were of analytical grade and used without further
modifications.
Methods
Synthesis of TMC
The N-TMC polymer was synthesized as previously
reported. Briefly, methylation of chitosan was carried out with
dimethyl sulfate at 70°C. Approximately 1 g of chitosan was reacted
with 16 ml of dimethyl sulfate and the rest of the methylation
process was followed as mentioned in the literature (13).
Preparation of PTX-loaded TMC NPs
PTX-TMC NPs were formulated by ionic gelation method
using complexation between oppositely charged macromolecules
(14). Briefly, PTX was dissolved
in a hydro-alcoholic TMC solution and gently vortexed for 30 min.
TPP at a concentration ranging from 10 to 50% w/w was added
dropwise on the TMC solution. The mixture was sonicated for 20 min
followed by dynamic light scattering experiments.
Particle size and ζ-potential
measurements
Zetasizer (Malvern, UK) was used to measure the
hydrodynamic particle size, polydispersity index and ζ-potential
measurements using dynamic light scattering (DLS) techniques. All
measurements were performed at a fixed angle of 90° at 25°C room
temperature. The results were expressed as the size ± SD.
Morphology
The structural morphology was examined by
transmission electron microscopy (TEM). Briefly, liquid sample was
placed in a carbon-coated copper grid and counter stained with
phosphotungstic acid, followed by air drying for 2 h. The surface
topography was further confirmed by the atomic force microscopy
(AFM) where in samples were instilled on the MICA surface and air
dried for 2 h.
PTX loading efficiency
Loading efficiency was calculated from the total
amount of drug added vs. amount of drug entrapped in the NPs.
Briefly, PTX-TMC NP was filtered by Amicon centrifugal filter by
centrifuging at a high speed of 5,000 rpm for 10 min. The filtrate
was analyzed for unentrapped drug by the HPLC method. The mobile
phase (acetonitrile;methanol;water, 28;25;47, pH 3.2) was set at 1
ml/min with an absorbance of 250 nm.
In vitro release study
PTX-TMC NP was centrifuged at 10,000 × g for 5 min
to separate the free PTX from the conjugated one. The supernatant
was removed and PTX-TMC was collected and re-suspended in required
amount of distilled water. The samples were placed in a dialysis
bag which was in turn placed in release media containing conical
tube in a shaker bath (37°C). At a specified time, 1 ml of release
media (phosphate-buffered saline) was removed and replaced with
equal amount of fresh buffer. The amount of drug released was
plotted against time.
In vitro cellular uptake
The cellular uptake of PTX-TMC NP was analyzed by
the HPLC method. Briefly, 1×106 Caco-2 cells were seeded
into 6-well plates and allowed to attach for 18 h. The cells were
treated with free PTX and PTX-TMC NP and incubated for 6 h. The
cells were washed, trypsinized and lysed by high sonication. The
resultant solutions were centrifuged and supernatant was used to
quantify the amount of drug internalized.
Cytotoxicity assay
The gastric cancer cell lines NCI-N87 and SGC-7901
were seeded into 96-well plates at a density of 1×106
cells/well in RPMI media supplemented with 10% FBS and incubated at
37°C for 24 h. Cells were then exposed to various doses of blank,
free PTX and PTX-TMC and further incubated for 24 h. The cells were
washed and treated with 25 μl of MTT (5 mg/ml) and kept in the dark
for 4 h. The formazan crystals were extracted and absorption was
noted at 570 nm using an ELISA (ELX800 Bio-Tek, Winooski, VT, USA)
plate reader.
Apoptosis study
The apoptosis of gastric cells was investigated by
flow cytometry (BD FACS Atira II, BD Company, USA) with a cell
apoptosis kit (Alexa Fluor 488 Annexin V/Dead cell apoptosis kit
with Alexa Fluor 488 Annexin V and PI for flow cytometry;
Invitrogen). NCI-N87 and SGC-7901 cells were seeded in 6-well
plates at a density of 3×105 cells/well with RPMI media
for 24 h. Cells were then exposed to various doses of free PTX and
PTX-TMC and further incubated for 24 h. The cells were extracted
with trypsin and PI and fluorescence labeled Annexin V was added as
per the manufacturer’s instructions. The apoptotic cell ratio was
calculated by the ratio of apoptosis cells and dead cells to total
cells.
Cell cycle analysis
NCI-N87 and SGC-7901 cells were seeded in 6-well
plates at a density of 3×105 cells/well with RPMI media
for 24 h. Cells were exposed to various doses of free PTX and
PTX-TMC and further incubated for 24 h. The cells were washed and
incubated with propidium iodide (PI; 20 μg/ml) for 30 min. The DNA
content was measured for 10,000 events for each sample by flow
cytometry (BD FACS Atira II, BD Company) and the data was plotted
using Cell Quest software.
In vivo antitumor efficacy
All animal experiments were carried out according to
the guidelines set by the Animal Ethics Committee (AEC), Cancer
Hospital of Jiangxi Province, China. The animals were provided good
care throughout the study period under 12 h day/night cycle. Female
SCID nude mice (7 weeks) were used to grow tumor subcutaneously.
Gastric cancer NCI-N87 cells (5×106) were subcutaneously
injected into the left flank of each mouse. The tumor growth was
monitored until it reached an average tumor size of 100–150
mm3. The mice were randomly divided into 4 groups with 8
mice in each group. Each group was administered blank vehicle, free
PTX and PTX-TMC (5 mg/kg PTX equivalent) 2 times a week for 14 days
with one group being untreated control. The tumor size was measured
twice weekly via caliper and tumor volume (V) was calculated by
using the formula: V = 1/2[L × (W)2], L= length and W=
width.
Results and Discussion
Preparation of TMC-TPP NPs
The ionic gelation method is considered one of the
most suitable methods for the preparation of NPs. In this method,
charged macromolecules electrostatically interact with the
oppositely charged species resulting in the formation of stable
carrier. Such physical cross-linking process eliminates the
undesirable side-effects of chemical cross-linkers (15). In this study, various weight ratios
of TPP were complexed with positively charged TMC moiety to observe
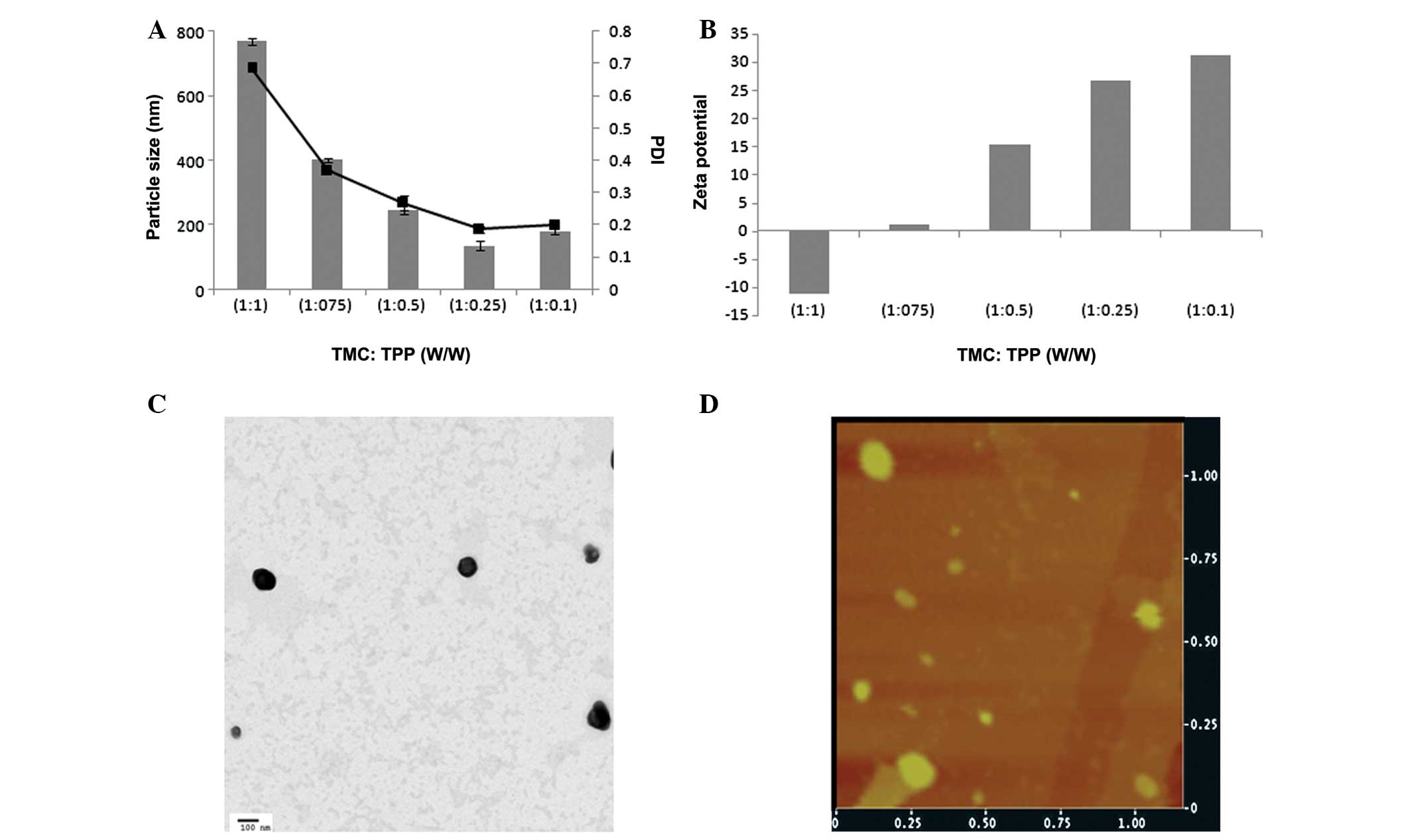
a nanosized particle with uniform size distribution. Fig. 1A shows the influence of TPP ratio on
the final size of blank NP. The particle size was big when equal
proportion of both complexing moieties was added, while the
particle size decreased gradually when the TPP concentration
decreased. In particular, at 1:0.25 (TMC-TPP) ratios it undergoes
ionic gelation and precipitation to yield a nanosize particle with
uniform size distribution (PDI ~0.15).
Generally, specific ratio of TMC and TPP plays an
important role in the NP structure which is largely governed by the
neutralization process. The TPP containing
P3O105− ions neutralizes the
chitosan -NH3+ amino groups (16). In the case of TMC, this process
becomes slightly complex due to the presence of multiple functional
groups including quaternary site
(-N(CH3)3+), monomethylated
(-N(CH3)2H+), dimethylated
(-N(CH3)H2+) and even pure
protonated amine site (-NH3+) (17).
Similarly, ζ-potential is an important indicator of
NP formation. At a higher concentration of TPP, surface charge was
slightly negative due to the high cross-linking density of
anionically charged TPP (Fig. 1B).
The ζ-potential, however, reversed to strong positive charge when
the concentration of negatively charged species decreased. At this
stage, cationic surface charge of NPs attributed to the large
presence of cationic head groups of chitosan (18). According to the literature, physical
stability of NPs is good when the ζ-potential value is >20 mV.
Therefore, TMC:TPP (1:0.25) ratio which produced nanosized particle
with strong positive charge was selected to incorporate PTX.
Preparation of TMC-PTX NPs
Table I presents the
effect of PTX incorporation on the physicochemical property of
TMC-TPP NPs. The particle size of TMC-PTX NP increased more than
~25 nm for the addition of PTX indicating a successful entrapment
within the NP. This finding is in agreement with previously
published reports which showed that based on the molecular weight
and charge of small molecules, size varied between 50–200 nm
(17,19). In the present study, PTX possessed a
small molecular weight and a neutral charge which does not
interfere in the electrostatic interaction process between TMC and
TPP. It was also shown that PTX was efficiently incorporated into
the NP matrix with 95% of entrapment efficiency. Similarly, loading
capacity was also found to be more than ~25% of total NP mass.
 | Table IPhysicochemical characterization of
TMC-PTX nanoparticles (NPS). |
Table I
Physicochemical characterization of
TMC-PTX nanoparticles (NPS).
| Size (nm) | PDI | ζ-potential (mV) | Entrapment efficiency
(%) | Loading efficiency
(%) |
|---|
| Blank TMC NP | 135.4±1.75 | 0.168±0.018 | 26.65±0.98 | - | - |
| TMC-PTX NP | 158.8±1.4 | 0.211±0.002 | 25.82±1.51 | 95.8±2.3 | 27.8±1.98 |
Morphology and topography
TEM was used to confirm the morphology and structure
of TMC-PTX NP. The imaging showed spherical shaped particles with
perfect boundary with individual objects. The particles were
uniformly distributed in the copper grid. A dense black core might
be due to the high electron density of phosphate group of TPP
complex (Fig. 1C). The TEM size
(~100 nm) was consistent with the hydrodynamic particle size
measured by Zetasizer. The shape was further confirmed by AFM
analysis (Fig. 1D). The NPs were
uniformly spread and flatted on the cover slip with clear round and
circular shape. The particle angles were not as sharp as observed
under TEM as the analysis was performed in contact mode that may
distort the actual structure of particles.
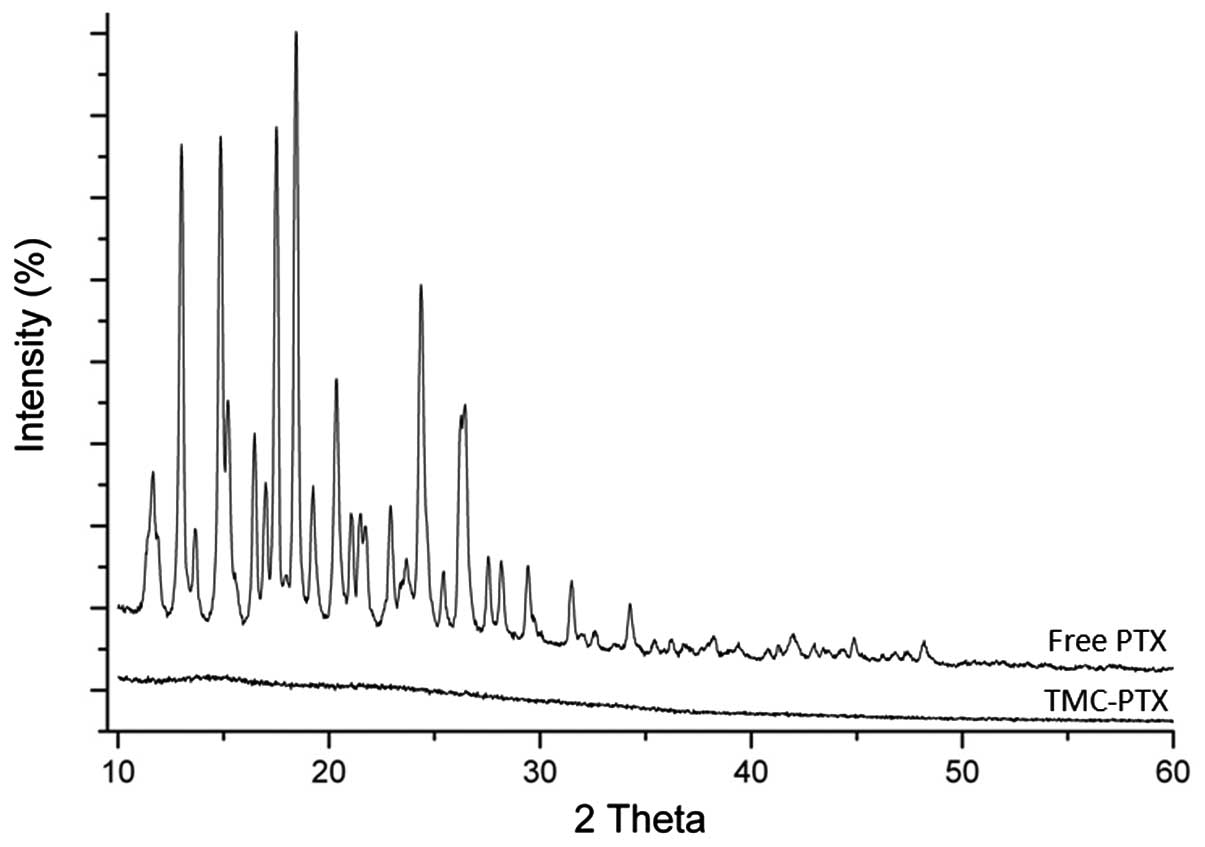
XRD analysis
XRD analysis was performed to confirm the nature of
drug incorporation. XRD patterns of free PTX and TMC-PTX are
presented in Fig. 2. Free PTX
showed numerous sharp characteristic peaks of 2Θ between 10–30°
indicating its prominent crystalline nature. TMC-PTX pattern,
however, did not show any such peaks suggesting the presence of
drug in the amorphous form in the NPs.
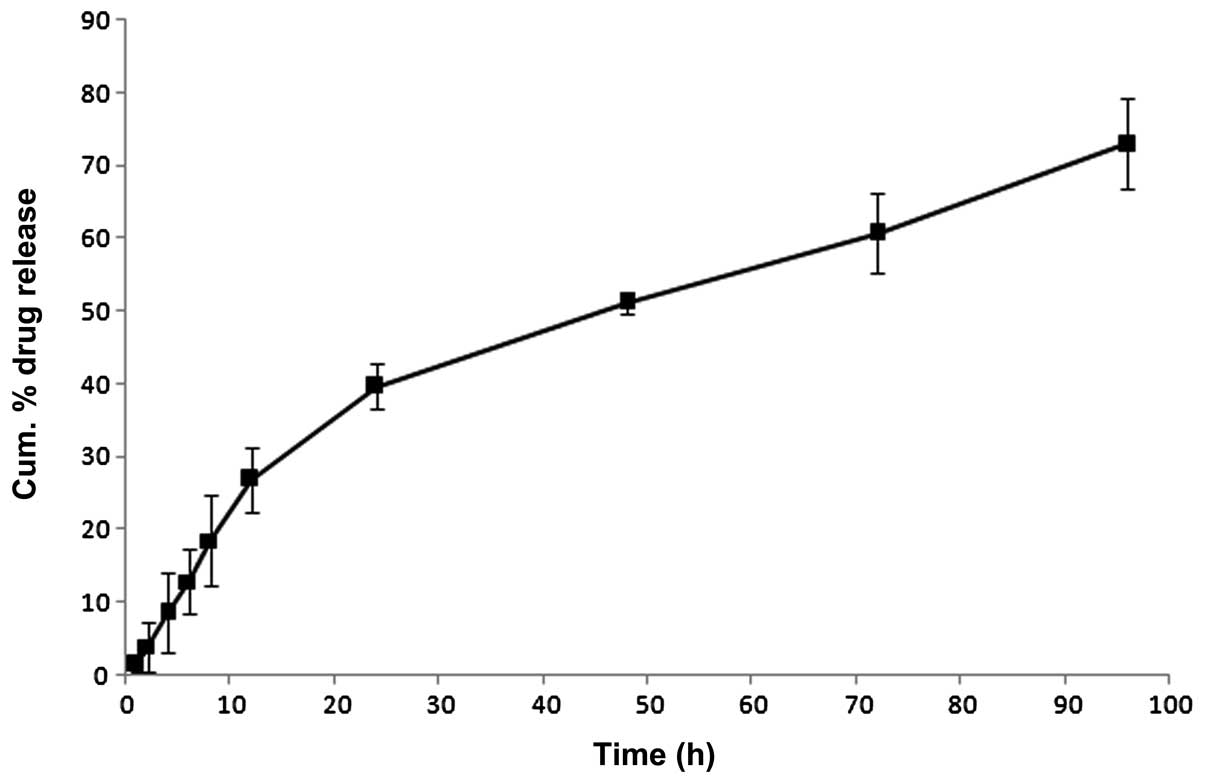
In vitro release study
The in vitro release profiles of TMC-PTX NP
are presented in Fig. 3. The NPs
exhibited a biphasic release profile with >25% of drug released
within 12 h of study period, followed by a relatively sustained
release pattern up to 96 h (~75% PTX). The initial fast release
attributed to the dissolution and diffusion of PTX either located
on the surface or poorly entrapped in the polymer matrix. The
sustained release however enabled the slow diffusion of drug from
the hydrophobic core matrix. Specifically, hydrophobic interaction
between PTX and methylated chitosan could be anticipated. PTX
molecule has a hydrophobic region and a partially hydrophilic
region with hydroxyl and secondary amine groups which can form
hydrogen bonds with chitosan molecules. Such slow release has
significant importance in the systemic application wherein the drug
will be available for therapeutic action in a steady manner.
Furthermore, release profile best fitted the Higuchi model
(r2=0.9865) suggesting a diffusion controlled release
mechanism. The Korsmeyer-Peppas model was applied to gain further
insight into the mechanisms of release. An n value of 0.65
indicates the presence of anomalous transport which means a
combination of drug diffusion and polymer matrix relaxation.
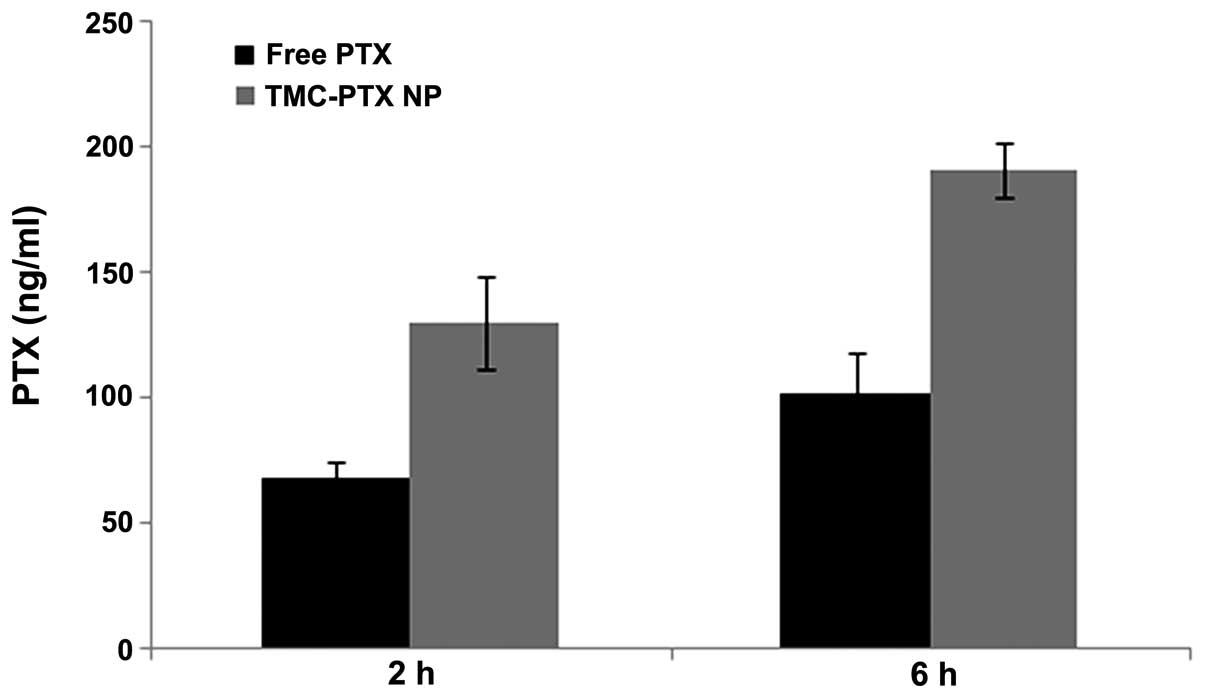
Cellular uptake
Intracellular drug uptake studies were performed
using HPLC. Since gastroenteric cancer lies in the gastrointestinal
tract, cellular uptake of free PTX and TMC-PTX was studied in
Caco-2 cells. The drug internalization in Caco-2 cells from both
groups are presented in Fig. 4.
After 2 h of incubation, a considerable amount of PTX was
accumulated in cells with significantly (P<0.005) higher
internalization of PTX from NPs over free drug. As expected, the
same trend was observed at a longer incubation time (6 h) with
nearly 2-fold higher uptake from both groups. Higher drug
accumulation from TMC-PTX might be attributed to the positive
charge of NPs that facilitated the drug internalization. Generally,
anionic heparin covers the outer cell surface that may be
responsible for the binding affinity/favorable interaction with
cationic species (20).
Furthermore, it has been reported that cellular uptake is
size-dependent and smaller sized NPs were preferably uptaken over
larger or bulk sized NPs (21).
Collectively, it can be said that positive charge and nanosize of
particles contributed to the enhanced cellular uptake of
TMC-PTX.
Cytotoxicity assay
Advanced gastroenteric tumors are life threatening
diseases that present formidable challenges. Many double and triple
drug combination regimens have been shown to evoke poor clinical
response and are often associated with severe side-effects.
Therefore, the present study focused on the NP-based PTX delivery
to overcome the drawbacks associated with single drug or multiple
drug combinations. Cytotoxicity assays were performed on gastric
cancer cell lines (NCI-N87 and SGC-7901) to confirm the superior
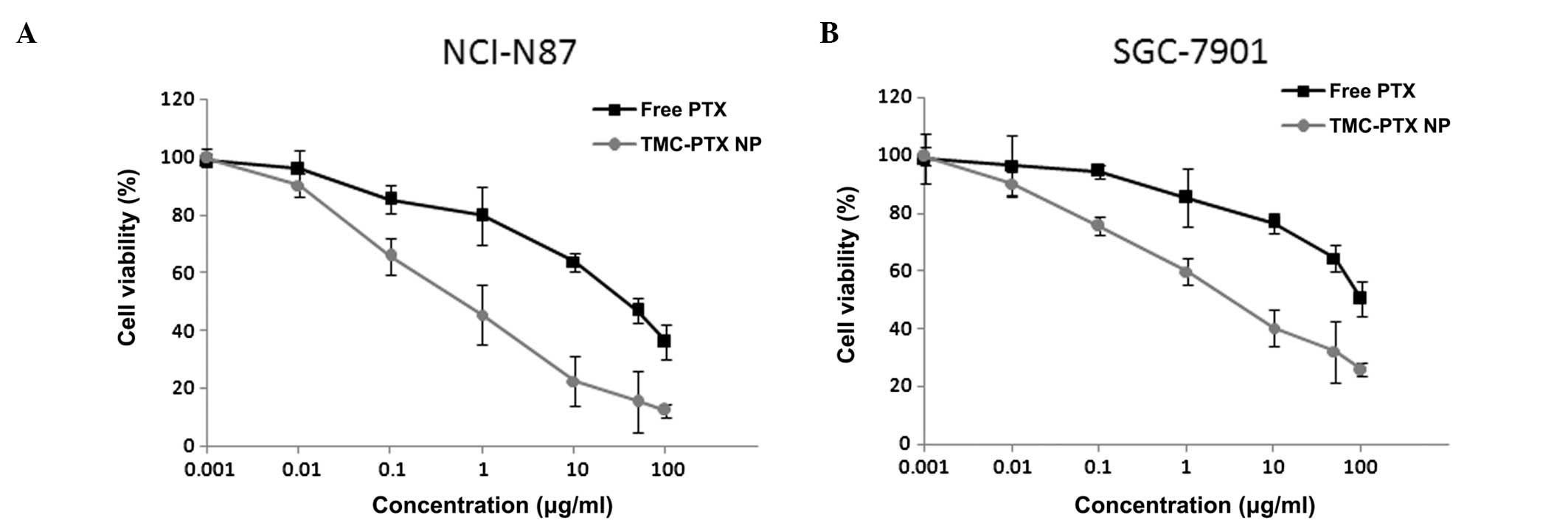
effect of TMC-PTX. Free PTX as well as TMC-PTX inhibited gastric
cancer cell proliferation in a dose-dependent manner (Fig. 5A and B). Specifically, TMC-PTX
exhibited the maximum anti-proliferative effects by comparison to
free PTX, consistent with the cellular uptake observations which
showed higher intracellular accumulation for the NPs. NCI-N87 was
relatively more sensitive to PTX than SGC-7901 which was slightly
less responsive to the drug. The IC50 value of free PTX
and TMC-PTX in NCI-N87 cell was 0.6 and 12.5 μg, respectively. In
the case of SGC-7901 cells, IC50 values were
significantly higher at 1.26 and 28.8 μg, respectively for both
groups. The difference in cytotoxic effect on cell lines may be
attributed to the difference in genetic origin and indigenous
biological behavior. It can be expected that at longer incubation
periods, many cell populations enter the G2 and M phases at which
it is highly active. Drug-free blank polymeric NPs, however, did
not exhibit obvious cytotoxicity in either cell line in all tested
concentrations indicating high biocompatibility.
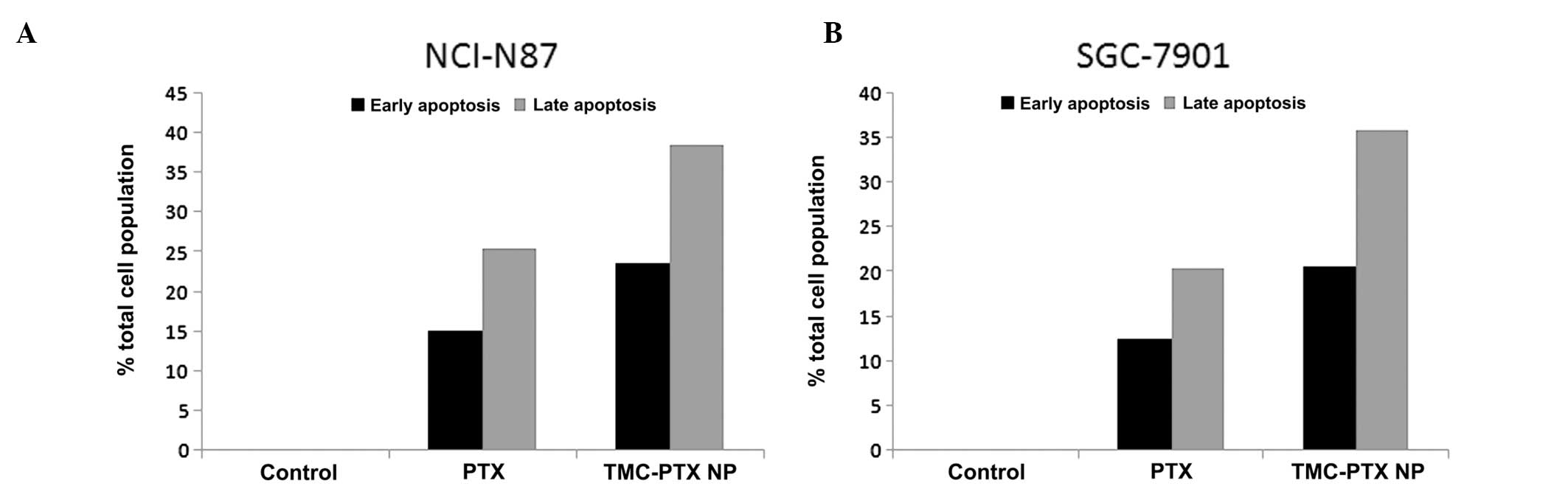
Apoptosis study
PTX binds to the β-subunit of the α/β-tubulin
dimerin in the microtubule that induces the polymerization of
tubulin which in turn inhibits the growth of rapidly dividing cells
resulting in apoptosis (22). To
confirm the cytotoxic potential of free PTX and TMC-PTX, cells were
treated with Annexin V and PI and analyzed using flow cytometry.
Annexin V (35–36 kDa), a Ca2+-dependent
phospholipid-binding protein shows high affinity for phospholipid
phosphatidylserine lining the outer plasma membrane in cell
apoptosis (23). PI is a standard
molecular probe used to distinguish viable cells from nonviable
cells. Generally, viable cells having intact membranes do not
interact with PI, while membranes of dead cells are permeable to PI
(24). Therefore, Annexin V-FITC/PI
staining can distinguish early apoptosis from late apoptosis in
cell populations. As expected, a considerable proportion of cells
was in early and late apoptosis stages suggesting the potent action
of free drug and drug-loaded NP (Fig.
6A and B). Specifically, TMC-PTX NP showed 25 and 40% cell
death in early and late apoptosis which was significantly higher
than the free drug (15 and 28%, respectively) in NCI-N87 cells. The
trend was similar in SGC-7901 cells; however, percentage of cell
populations in both stages was relatively lower compared with
NCI-N87 cells. The apoptosis result was consistent with the higher
cytotoxicity potential of nanoparticulate formulations in these
cell lines (25). These
observations indicate that PTX was intact in the TMC NP and
released in a controlled manner to induce apoptosis.
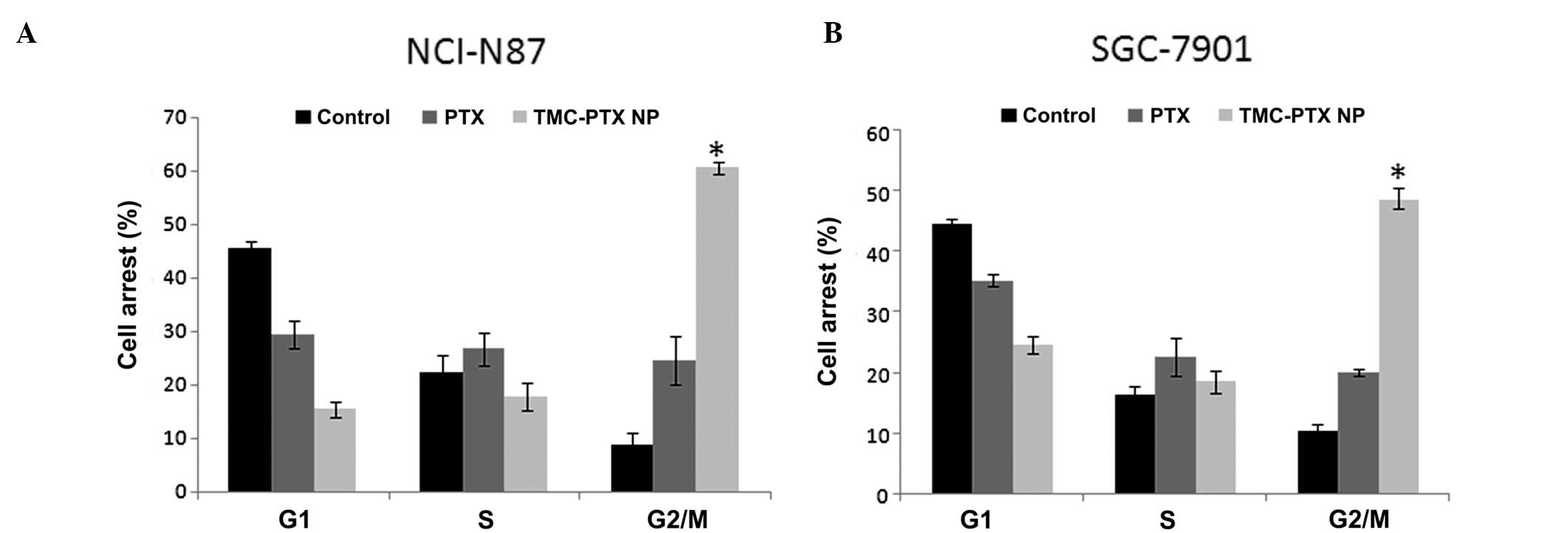
Cell cycle analysis
The toxicity of PTX and TMC-PTX was further
confirmed by cell cycle analysis. It is known that PTX induced cell
cycle arrest by impairing mitosis and PTX-induced G2/M arrest is
associated with the breakage of double-strain DNA and considerable
chromosome damage (26). In this
perspective, it follows that increased G2/M phase arrest is
associated with the inhibition of cell division and cell growth
arrest (27). Results of the
present study clearly showed the presence of cell populations in
different phases upon treatment with respective formulations. As
can be seen, nearly 45% of cells were present in the G1 phase while
only <10% of cells were in the G2/M phase in the
control/untreated cells in NCI-N87 cells. Upon PTX treatment,
however, G1 phase population decreased to 28% with nearly 25% of
cells having entered the G2/M where PTX exhibited the maximum
action (Fig. 7A and B). Notably,
TMC-PTX exhibited a marked 60% of cells in G2/M phase which is
almost 2-fold higher than compared to the free PTX group and 6-fold
higher than the control. A considerable proportion of cells was
present in the subG0 (11%) phase of the cell cycle, while 50% of
cells were present in the G2/M phase in the case of SGC-7901 cells.
These observations clearly reveal the fact that PTX exhibits
prominent G2/M phase cell accumulation. These results are
consistent with the above cytotoxicity study and apoptotic
assay.
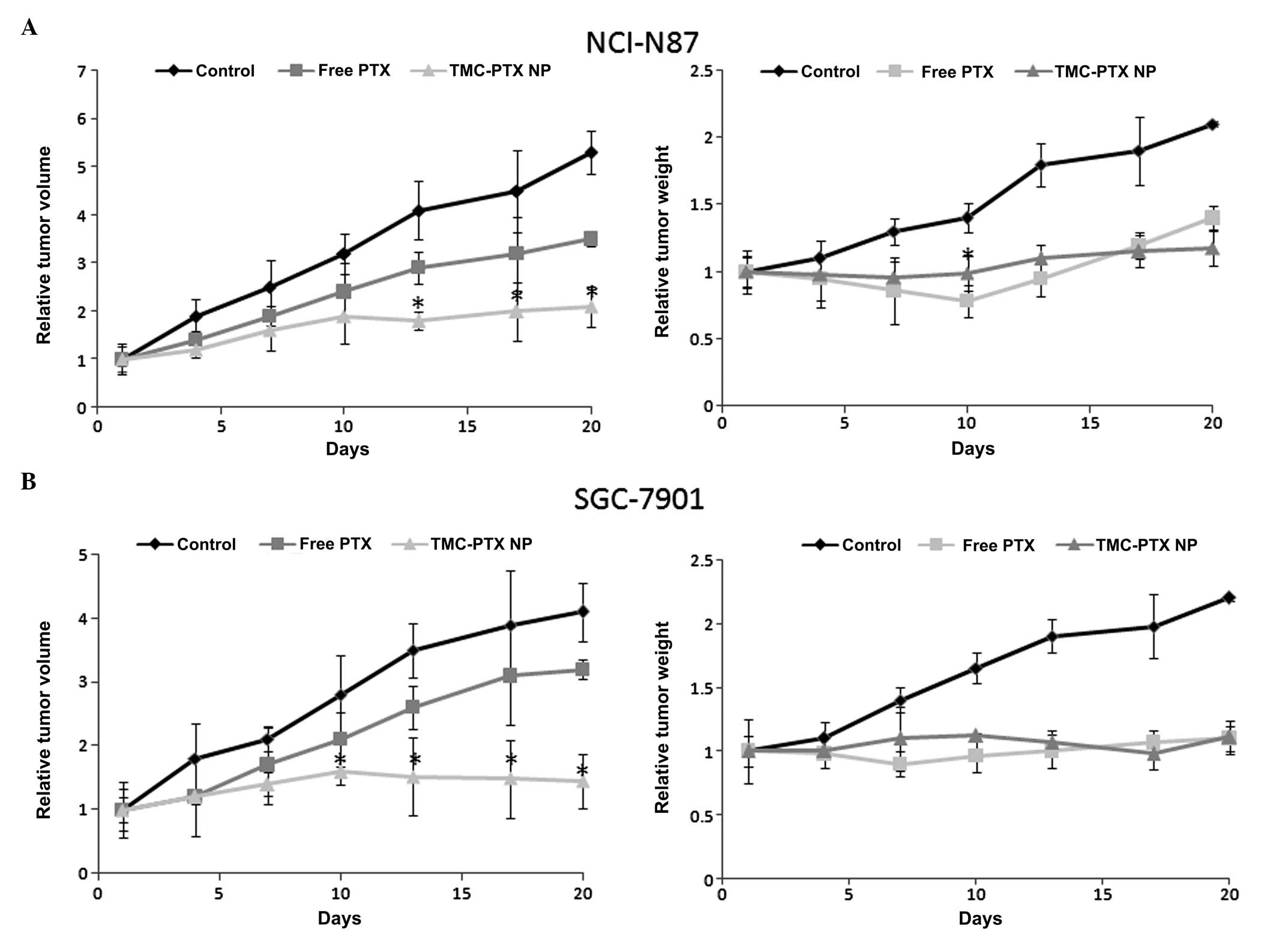
Antitumor efficacy
The antitumor efficacy study was investigated on
NCI-N87 and SGC-7901 gastric cancer cells bearing xenograft tumor
models. As can be seen, the tumor in the untreated group grew
rapidly and attained maximum size at the study period. In contrast,
free PTX and TMC-PTX significantly inhibited the tumor growth in
both xenograft mice models. The tumor volume was the same for the
two groups until day 7, after which time the tumor growth was
accelerated in the PTX group and the tumor size was significantly
(P<0.005) different from each other at the end of day 20. In a
key finding, in the case of TMC-PTX, the tumor volume gradually
increased until day 10 after which complete tumor regression was
observed and tumor size did not increase further in either tumor
model (shrunk) (Fig. 8A and B). It
has previously been reported that repeated administrations of
PTX-NPs could markedly increase the drug in tumor targets,
resulting in maximum therapeutic efficacy (28). The free PTX group, however, showed
constant increment in the tumor volume throughout the study period
although it was less than that of control. Specifically, TMC-PTX
showed 70 and 64% tumor regression in NCI-N87 and SGC-7901
xenograft mice, compared to only 30 and 25% growth inhibition,
respectively, in the case of free PTX. The result was consistent
with our in vitro cell proliferations and apoptosis
observations which were prominent for TMC-PTX.
Tumor weight is an indicator of drug-related
systemic toxicity and therefore tumor weights were recorded
simultaneously with the tumor volume measurements. From the
results, it can be interpreted that free PTX was toxic and mice
shed at least 20% of their weight in the NCI-N87 model indicating a
severe toxicity at the present dosage form (5 mg/kg). In contrast,
TMC-PTX was completely safe and at 5 mg/kg twice a week was well
tolerated and the same body weight was maintained without any overt
signs of toxicity throughout the study period. In the untreated
group, body weight constantly increased, which may have been due to
the growth of tumor. When the tumor mass was excised, the mean
tumor weight from TMC-PTX group was significantly smaller compared
to other groups.
The enhanced antitumor efficacy with appreciable
safety profile could be explained on several bases; first, it can
be anticipated that NP incorporation of PTX (TMC-PTX) could have
greatly improved the blood circulation time resulting in preferable
accumulation in tumor tissues via EPR effect (29). Secondly, sustained release pattern
of TMC-PTX enabled the slow release of drug which could enter the
tumor cells gradually. Thirdly, nanosized particle of ~150 nm could
escape from the reticuloendothelial system (RES) and can easily
penetrate the tumor fenestration (30). Therefore, our results clearly reveal
the superior antitumor efficacy of TMC-PTX in experimental gastric
cancers and appears to be a potent single drug chemotherapeutic
agent in clinical settings. These observations support the clinical
evaluation of polymer-loaded PTX as a significant microtubule
inhibiting agent.
In conclusion, TMC polymer was synthesized and
TMC-PTX NP was successfully prepared. The nanosized particles (~150
nm) were formed with uniform size distribution and spherical
dimensions. The TMC NP exhibited a sustained release profile for
PTX with 75% of drug release by 96 h at physiological pH. Augmented
internalization of NP across Caco-2 cell monolayers was confirmed
by the HPLC method. The PTX-bound NP showed potent cytotoxic effect
in both gastric cancer cells, NCI-N87 and SGC-7901, while the
former were more sensitive to PTX than the latter. The cytotoxic
effect was further confirmed by apoptosis assay, which clearly
revealed the majority of cell populations in early and late
apoptosis chamber. Consistently, TMC-PTX showed prominent 60 and
50% G2/M phase arrest in NCI-N87 and SGC-7901 cells. Most
importantly, it showed 70 and 60% tumor regression in these cell
lines with no obvious signs of any systemic toxicity. The augmented
chemotherapeutic effects were due to the preferential accumulation
of NPs in the gastric tumor cells via EPR effects. Collectively,
the present study highlighted the therapeutic efficiency of TMC-PTX
in the treatment of experimental gastric cancer with the
possibility of clinical application.
Acknowledgements
The authors thank Dr Xian for proofreading the
manuscript. The authors also thank the hospital staff members for
suggestions and advice during the course of the study.
References
|
1
|
Oh SC: Update of adjuvant chemotherapy for
resected gastric cancer. J Gastric Cancer. 12:3–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwarz RE and Smith DD: Clinical impact
of lymphadenectomy extent in resectable gastric cancer of advanced
stage. Ann Surg Oncol. 14:317–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
4
|
Rowinsky EK, Cazenave LA and Donehower RC:
Taxol: a novel investigational antimicrotubule agent. J Natl Cancer
Inst. 82:1247–1259. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mancuso A, Oudard S and Sternberg CN:
Effective chemotherapy for hormone-refractory prostate cancer
(HRPC): present status and perspectives with taxane-based
treatments. Crit Rev Oncol Hematol. 61:176–185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiss RB, Donehower RC, Wiernik PH, Ohnuma
T, Gralla RJ, Trump DL, et al: Hypersensitivity reactions from
taxol. J Clin Oncol. 8:1263–1268. 1990.PubMed/NCBI
|
|
7
|
Akhlaghi SP, Saremi S, Ostad SN, Dinarvand
R and Atyabi F: Discriminated effects of thiolated chitosan-coated
pMMA paclitaxel-loaded nanoparticles on different normal and cancer
cell lines. Nanomedicine. 6:689–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang W, Shi Y, Chen Y, Yu S, Hao J, Luo
J, Sha X and Fang X: Enhanced antitumor efficacy by
paclitaxel-loaded pluronic P123/F127 mixed micelles against
non-small cell lung cancer based on passive tumor targeting and
modulation of drug resistance. Eur J Pharm Biopharm. 75:341–353.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Britto D and Assis OBG: A novel method
for obtaining a quaternary salt of chitosan. Carbohydr Polym.
69:305–310. 2007.
|
|
10
|
Slütter B and Jiskoot W: Dual role of CpG
as immune modulator and physical crosslinker in ovalbumin loaded
N-trimethyl chitosan (TMC) nanoparticles for nasal vaccination. J
Control Release. 148:117–121. 2010.PubMed/NCBI
|
|
11
|
Subbiah R, Ramalingam P, Ramasundaram S,
et al: N,N,N-trimethyl chitosan nanoparticles for controlled
intranasal delivery of HBV surface antigen. Carbohydr Polym.
89:1289–1297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hamman JH, Stander M and Kotze AF: Effect
of the degree of quaternisation of N-trimethyl chitosan chloride on
absorption enhancement: in vivo evaluation in rat nasal epithelia.
Int J Pharm. 232:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Britto D, Forato LA and Assis OBG:
Determination of the average degree of quaternization of
N,N,N-trimethylchitosan by solid state C-13 NMR. Carbohydr Polym.
74:86–91. 2008.
|
|
14
|
de Moura MR, Aouada FA, Avena-Bustillos
RJ, McHugh TH, Krochta JM and Mattoso LHC: Improved barrier and
mechanical properties of novel hydroxypropyl methylcellulose edible
films with chitosan/tripolyphosphate nanoparticles. J Food Eng.
92:448–453. 2009.
|
|
15
|
de Britto D, de Moura MR, Aouada FA,
Mattoso LHC and Assis OBG: N,N,N-trimethyl chitosan nanoparticles
as a vitamin carrier system. Food Hydrocoll. 27:487–493. 2012.
|
|
16
|
Bhumkar DR and Pokharkar VB: Studies on
effect of pH on cross-linking of chitosan with sodium
tripolyphosphate: a technical note. AAPS PharmSciTech. 7:E502006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen F, Zhang ZR and Huang Y: Evaluation
and modification of N-trimethyl chitosan chloride nanoparticles as
protein carriers. Int J Pharm. 336:166–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gan Q and Wang T: Chitosan nanoparticle as
protein delivery carrier - systematic examination of fabrication
conditions for efficient loading and release. Colloids Surf B
Biointerfaces. 59:24–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi L, Xu Z, Jiang X, Hu C and Zou X:
Preparation and antibacterial activity of chitosan nanoparticles.
Carbohydr Res. 339:2693–2700. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davda J and Labhasetwar V:
Characterization of nanoparticle uptake by endothelial cells. Int J
Pharm. 233:51–59. 2002. View Article : Google Scholar
|
|
21
|
Yu B, Zhang Y, Zheng W, Fan C and Chen T:
Positive surface charge enhances selective cellular uptake and
anticancer efficacy of selenium nanoparticles. Inorg Chem.
51:8956–8963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ganesh T, Yang C, Norris A, et al:
Evaluation of the tubulin-bound paclitaxel conformation: synthesis,
biology, and SAR studies of C-4 to C-3′ bridged paclitaxel
analogues. J Med Chem. 50:713–725. 2007.PubMed/NCBI
|
|
23
|
Sharma AK, Zhang L, Li S, Kelly DL,
Alakhov VY, Batrakova EV and Kabanov AV: Prevention of MDR
development in leukemia cells by micelle-forming polymeric
surfactant. J Control Release. 131:220–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo DD, Moon HS, Arote R, Seo JH, Quan JS,
Choi YJ and Cho CS: Enhanced anticancer effect of conjugated
linoleic acid by conjugation with Pluronic F127 on MCF-7 breast
cancer cells. Cancer Lett. 254:244–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo DD, Xu CX, Quan JS, Song CK, Jin H,
Kim DD, Choi YJ, Cho MH and Cho CS: Synergistic anti-tumor activity
of paclitaxel-incorporated conjugated linoleic acid-coupled
poloxamer thermosensitive hydrogel in vitro and in vivo.
Biomaterials. 30:4777–4785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahmed F, Pakunlu RI, Brannan A, Bates F,
Minko T and Discher DE: Biodegradable polymersomes loaded with both
paclitaxel and doxorubicin permeate and shrink tumors, inducing
apoptosis in proportion to accumulated drug. J Control Release.
116:150–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ng SSW, Tsao MS, Chow S and Hedley DW:
Inhibition of phosphatidylinositide 3-kinase enhances
gemcitabine-induced apoptosis in human pancreatic cancer cells.
Cancer Res. 60:5451–5455. 2000.PubMed/NCBI
|
|
28
|
Kim K, Kim JH, Park H, Kim YS, Park K, Nam
H, et al: Tumor-homing multifunctional nanoparticles for cancer
theragnosis: simultaneous diagnosis, drug delivery, and therapeutic
monitoring. J Control Release. 146:219–227. 2010. View Article : Google Scholar
|
|
29
|
Zhang L, He Y, Ma G, Song C and Sun H:
Paclitaxel-loaded polymeric micelles based on
poly(ɛ-caprolactone)-poly(ethylene glycol)-poly(ɛ-caprolactone)
triblock copolymers: in vitro and in vivo evaluation. Nanomedicine.
8:925–934. 2012.
|
|
30
|
Xiao K, Luo J, Fowler WL, Li Y, Lee JS,
Xing L, Cheng RH, Wang L and Lam KS: A self-assembling nanoparticle
for paclitaxel delivery in ovarian cancer. Biomaterials.
30:6006–6016. 2009. View Article : Google Scholar : PubMed/NCBI
|