Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, accounting for 26% of all female and 28% of
all male cancer deaths in 2013 (1).
In China, the crude mortality rates in 2008 were 47.51 per 100,000
men and 22.69 per 100,000 women (2). Of all lung cancer occurrences, ~85%
are non-small cell lung cancer (NSCLC) (3), which is a lethal malignancy with a
5-year survival rate of only ~15% (4,5).
Standard treatment for patients with NSCLC typically includes
radiotherapy, platinum-based chemotherapy and non-platinum agent
(6,7). However, the prognosis of lung cancer
remains poor, owing mainly to the acquired or inherent drug
resistance of cancer cells.
Drug resistance is a highly common phenomenon in the
clinical chemotherapy of leukemia or other solid tumors, and these
cancer cells may also become cross-resistant to various
chemotherapeutics, leading to multiple drug resistance (MDR).
Previous research found several mechanisms for MDR, such as
overexpression of transporter superfamily members, mutation or
alteration in drug target genes, activation of mitogen-activated
protein kinase (MAPK) cascade and phosphatidylinositol-3-kinase
(PI3K)/Akt signaling pathway (8,9).
Nitric oxide (NO) has been shown to play important
roles in the innate immune response, neovascularization, cancer
metastasis and cell death (10–12).
Recently, long-term exposure to NO was found to render lung cancer
cells resistant to cisplatin, doxorubicin and etoposide in a dose-
and time-dependent manner by increasing the level of caveolin-1
(CAV-1), antiapoptotic B-cell lymphoma-2 (Bcl-2) and activated
protein kinase B (AKT) (13). In
the present study, MDR-related factors glutathione S-transferase-π
(GST-π) and topoisomerase IIα (TOPO IIα) but not P-glycoprotein
(P-gp) were found to be regulated by induced nitric oxide synthase
(iNOS) in A549/CDDP, and this process was directly mediated by the
Wnt signaling pathway. Moreover, we found iNOS was mainly
influenced by canonical Wnt/β-catenin signaling but not
noncanonical Wnt pathways. Furthermore, we detected the expression
of Wnt/β-catenin downstream factors and inhibitors. The results
indicated blocking iNOS could inactivate Wnt/β-catenin signaling,
and this function might be mediated by Dickkopf-1 (DKK-1) and
secreted frizzled-related protein-1 (SFRP-1). Our findings may help
elucidate the relationship between iNOS and Wnt signaling in the
process of drug resistance in NSCLC.
Materials and methods
Cell lines and reagents
The human cisplatin-tolerant NSCLC cell line
A549/CDDP was obtained from the American Type Culture Collection
(ATCC). Cells were cultured at 37°C in 5% CO2 in
Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen), containing
10% FBS (Clontech) and penicillin streptomycin solution (Hyclone).
Human TNF-α, IL-1β and IFN-γ obtained from R&D Systems were
used to induce the production of NO as previously described
(14). iNOS selective inhibitor
S-methylisothiourea sulfate (SMT) was obtained from Beyotime
(China). Recombinant human DKK-1 was from PeproTech, used to
generally block Wnt pathways. XAV939 and SP600125 (both from
Selleck) and Xec (Merck) were chosen to inhibit Wnt/β-catenin,
Wnt/JNK and Wnt/Ca2+ pathways respectively. Protein
levels were normalized to β-actin.
Analysis of mRNA levels by RT-PCR
Total cellular RNA was isolated with TRIzol reagent
(Invitrogen) and reverse transcribed into cDNA using Sprint RT
complete products kit (Clontech). The gene-specific primers for
RT-PCR are listed in Table I.
 | Table IPrimer sequences used in RT-PCR. |
Table I
Primer sequences used in RT-PCR.
| Gene | Primer |
|---|
| iNOS | F: 5′-ACAAGCTGGCCTCGC
TCTGGAAAGA-3′
R: 5′-TCCATGCAGACAACCTTGGGGTTGAAG-3′ |
| P-gp | F:
5′-ACTTCCACATCTGCTTCGTCAGTG-3′
R: 5′-ATTCAGCCACAGGAGGTAGAGAGC-3′ |
| GST-π | F:
5′-TGGGCATCTGAAGCCTTTTG-3′
R: 5′-GATCTGGTCACCCACGATGAA-3′ |
| TOPO IIα | F:
5′-AAGGTTTGGGCACCAGCAC-3′
R: 5′-CTCGCTTGTCATTCCGTTTG-3′ |
| Wnt-3a | F:
5′-TCCACGCCATTGCCTCAG-3′
R: 5′-GACCACCAGCATGTCTTCACC-3′ |
| Wnt-5a | F:
5′-ACAACCTGGCTGATGTGGC-3′
R: 5′-CGTCTGCACGGTCTTGAACT-3′ |
| Wnt-8a | F:
5′-CCTATCTGACCTACACGACTAGTGT-3′
R: 5′-CGTTCCCAAGCAAACTGG-3′ |
| Wnt-11 | F:
5′-AAGGACTCGGAACTCGTCTATC-3′
R: 5′-GCAGCACCAGTGGTACTTACAG-3′ |
| Wif-1 | F:
5′-ACCTGGATTCTATGGAGTGAACTGT-3′
R: 5′-GTATGAGGCTGGCTTCGTACCT-3′ |
| SFRP-1 | F:
5′-GCTTCCAGTCGGACATCG-3′
R: 5′-AGCATCTCGGGCCAGTAG-3′ |
| DKK-1 | F:
5′-TTCCAACGCTATCAAGAACCT-3′
R: 5′-CCAAGGTGCTATGATCATTACC-3′ |
| β-actin | F:
5′-ATGGATGATGATATCGCCGCGCT-3′
R: 5′-GACTCGATGCCCAGGAAGGA-3′ |
Western blot analysis
A549/CDDP cells were plated in 6-well plates
(3×106 cells/well). Following inflammatory cytokine
mixture stimulation for 4 h, inhibitors of iNOS and Wnt pathways
were added to the medium. After 8 h treatment of these antagonists,
cells were harvested and homogenized with lysis buffer. Total
protein was separated by denaturing 10% SDS-polyacrylamide gel
electrophoresis. Detection was performed with Odyssey system
(Gene). The primary antibodies for iNOS, P-gp, TOPO IIα, GST-π,
Wnt-3a/5a/8a/11, Fzd-8, β-catenin, Axin, APC, phospho-GSK-3β
(Ser9), GSK3β, Wif-1, DKK-1, SFRP-1 and β-actin were all obtained
from Santa Cruz Biotechnology. The animal-matched horseradish
peroxidase-conjugated secondary antibody was purchased from Santa
Cruz Biotechnology.
ELISA
NO is rapidly oxidized to nitrite and nitrate which
are used to quantitate NO production. BioVision’s Nitric Oxide
Colorimetric Assay Kit provided an accurate, convenient measure of
total nitrate/nitrite in a simple two-step process. The amount of
the azo chromophore accurately reflected NO amount in samples.
Statistical analysis
Data are presented as the mean ± SD. Experiments
were carried out in duplicate or triplicate, and were all conducted
a minimum of three times. Data were analyzed by the Student’s
t-test or ANOVA where appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibition of Wnt signaling decreases the
NO-induced drug resistance in A549/CDDP
In inflammation conditions, the iNOS gene is often
activated, resulting in the production of NO. Thus, the
pro-inflammatory cytokines TNF-α, IL-1β and IFN-γ were used to
trigger the expression of iNOS in our experiment (14–17).
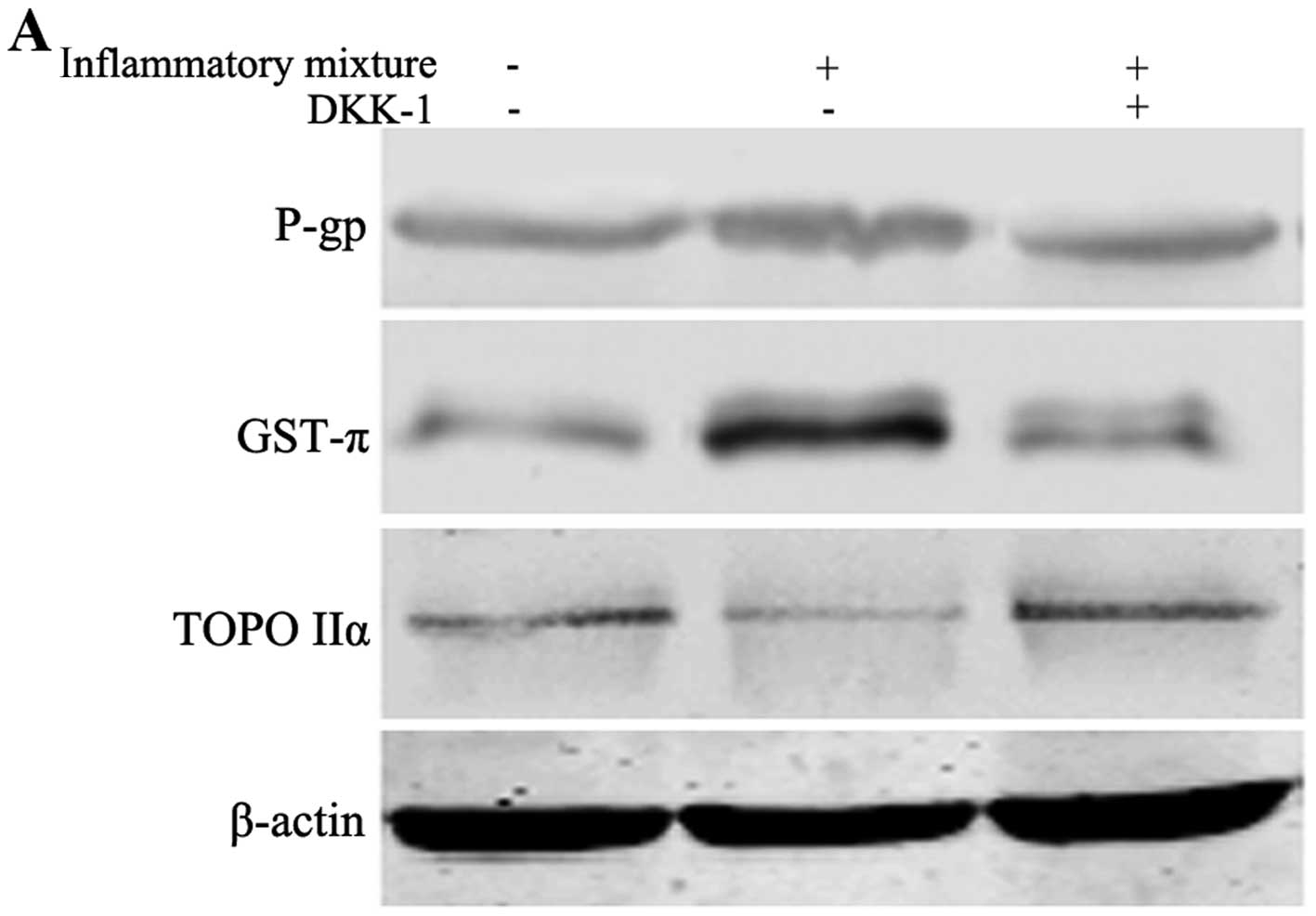
To investigate the Wnt signaling pathway, DKK-1 was added into the
medium to inhibit the Wnt pathway. The expressions of P-gp, TOPO
IIα and GST-π were chosen to reflect the extent of the drug
resistance, and the level of NO in the culture media was evaluated
by ELISA.
Following stimulation with the TNF-α/IL-1β/IFN-γ
combination, the expression of GST-π was clearly upregulated, while
that of TOPO IIα decreased. Although P-gp was also reduced after
Wnt pathway blocking, its expression was not significantly altered
(Fig. 1A and B). An increasing
concentration of NO in the culture media was observed, as shown in
Fig. 1C, demonstrating the
activation of iNOS. The results indicated that the resistance of
A549/CDDP to cisplatin was positively increased by high level of
iNOS, and DKK-1 reversed the drug resistance mainly by regulating
GST-π and TOPO IIα.
The level of iNOS is positively
correlated with the canonical but not the noncanonical
Wnt/β-catenin signaling
Although the effect of Wnt signaling in iNOS-induced
drug resistance was confirmed in our experiment, the differences
between canonical and noncanonical Wnt pathways in regulating the
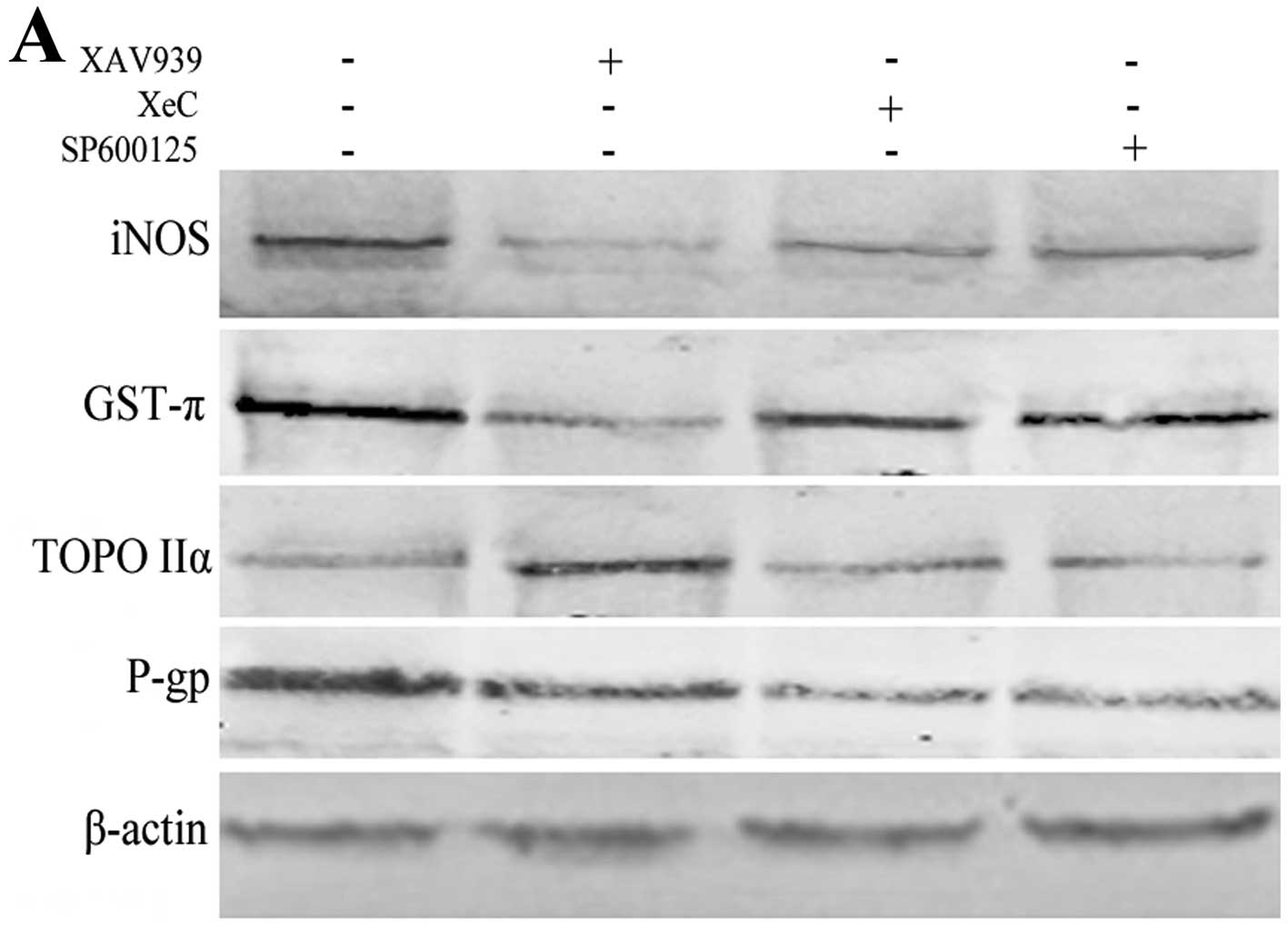
level of iNOS were still unclear. By treatment with Wnt/β-catenin
inhibitor XAV939 (18,19), Wnt/Ca2+ inhibitor XeC
(20,21) and Wnt/JNK inhibitor SP600125
respectively in A549/CDDP, we found a lower iNOS and GST-π, and a
higher TOPO IIα in the Wnt/β-catenin-blocking group. However,
neither Wnt/JNK nor Wnt/Ca2+ pathway were correlated
with iNOS, GST-π and TOPO IIα as shown in Fig. 2A. P-gp was clearly downregulated in
noncanonical Wnt pathways, and that might be related to other
signals influenced by XeC and SP600125.
Furthermore, we detected the effect of iNOS on
canonical and noncanonical Wnt signaling represented secretions
(Wnt-3a/Wnt-8a and Wnt-5a/Wnt-11, respectively) (22–24).
Consistent with our previous results, inhibition of iNOS led to an
obviously decreased expression of Wnt-3a and Wnt-8a which indicated
canonical Wnt signaling, but noncanonical Wnt-5a and Wnt-11 were
not significantly influenced by iNOS, as shown in Fig. 2B.
Inhibition of iNOS is positively
associated with the Wnt/β-catenin signaling pathway and its
downstream factors
The signaling transduction of canonical
Wnt/β-catenin pathway has been well described. Following binding of
Wnt to its receptor frizzled (FZD) and lipoprotein receptor-related
protein 5/6 (LRP5/6), dishevelled proteins (DSH) become activated,
leading to the inactivation of the Axin/adenomatous polyposis
coli (APC)/glycogen synthase kinase (GSK)3β complex which
mediated β-catenin degradation, and resulting in the accumulation
of β-catenin. Then, the β-catenin proteins translocated to the
nucleus and interacted with transcription factors of the T cell
factor (TCF) and lymphoid-enhancing factor (LEF) families,
promoting the transcription of many oncogenic factors, such as
c-Myc, cyclin D1 and VEGF (25–29).
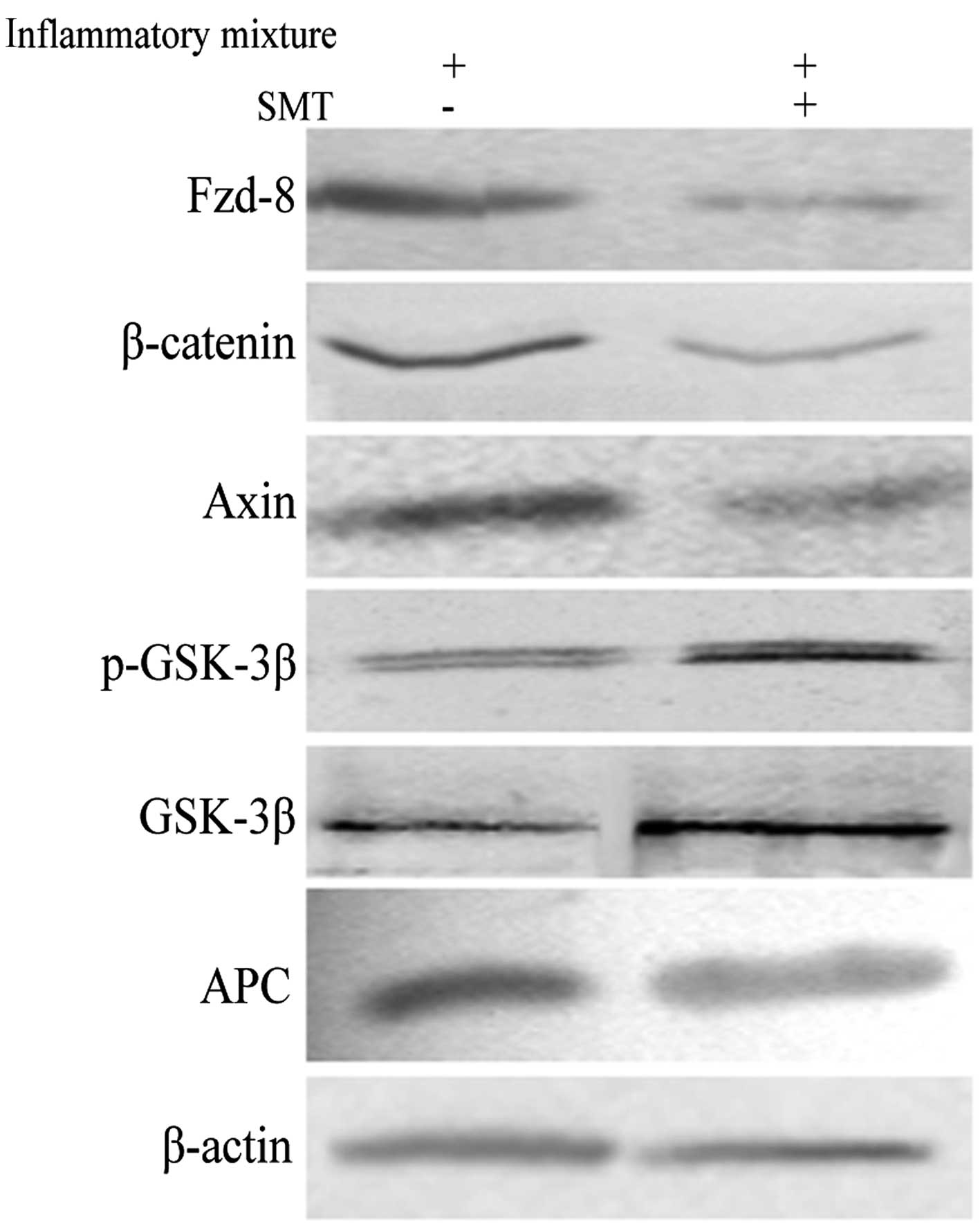
By preventing the expression of iNOS by its highly selective
inhibitor SMT in A549/CDDP, we observed a decreasing level of
Fzd-8, β-catenin and Axin, and an increased p-GSK-3β and
GSK-3β-expression. However, the change in APC showed no statistical
significance compared with that in no-SMT control as shown in
Fig. 3.
The level of DKK-1 and SFRP-1 inversely
regulate the iNOS and Wnt/β-catenin signaling
As a core modulator, Wnt/β-catenin transduction
pathway was regulated by a precise mechanism, containing positive
and negative feedback. The general opposite control has been
considered to be mediated by Wnt antagonists such as endogenic
DKK-1, SFRP-1 and Wif-1. The main inhibitory mechanism is the
interference of the combination between Wnt and its receptors
(14,30–33).
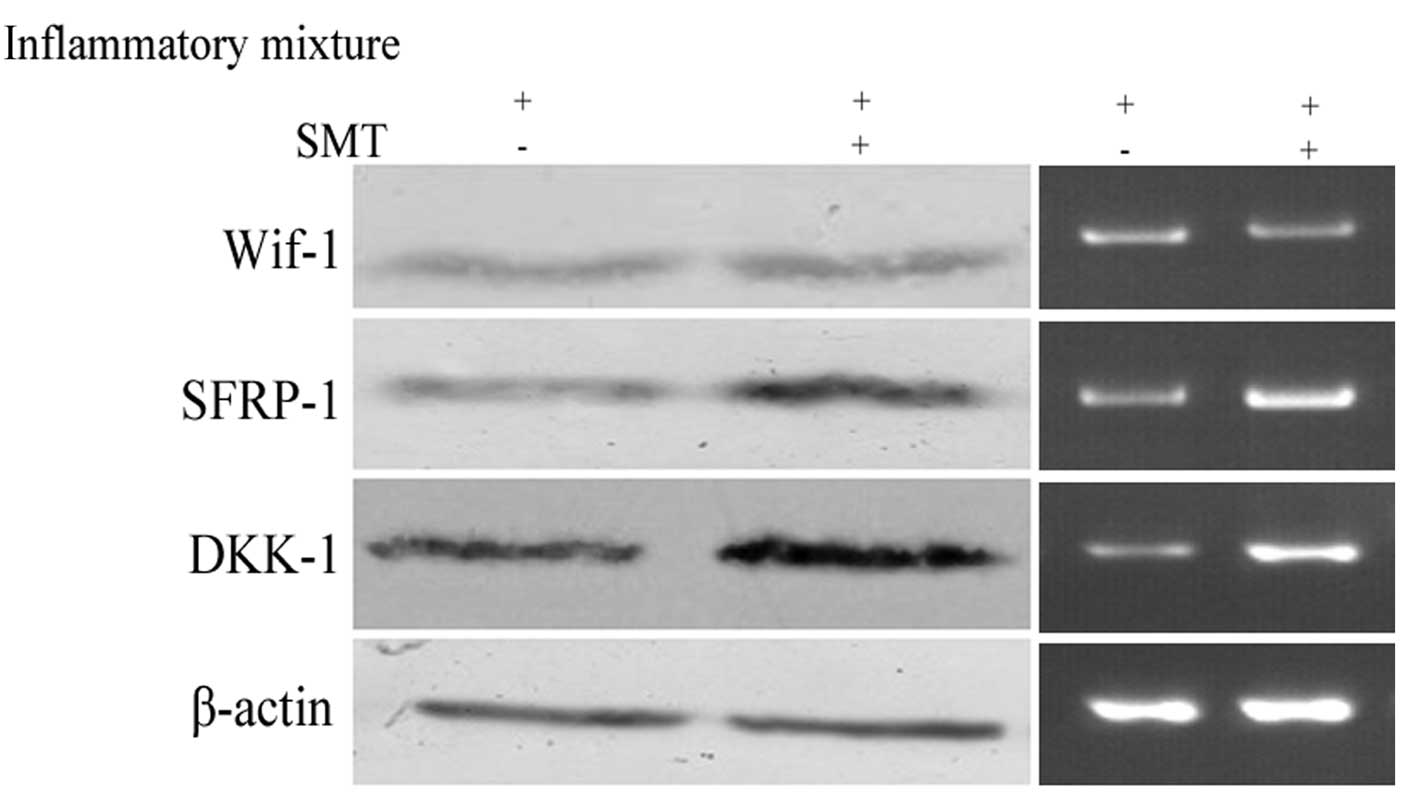
Fig. 4 shows the influence of iNOS
on Wif-1, DKK-1 and SFRP-1. In accordance with a previous study,
the results indicated DKK-1 was increased after iNOS blocking
(14). In addition, the expression
of SFRP-1 also showed a negative correlation with iNOS level, but
Wif-1 appeared to be less associated with this factor. Thus, we
concluded that iNOS could increase drug resistance in NSCLC by
inhibiting DKK-1 and SFRP-1.
Discussion
Extensive studies have been performed to elucidate
the mechanism underlying multiple drug resistance (MDR) in
non-small cell lung cancer (NSCLC) in the past ten years. One of
the important components of the tumor microenvironment, nitric
oxide (NO), has been found to be markedly increased in
drug-resistant NSCLC. As a reactive nitrogen species, NO is
catalytically synthesized by iNOS, promoting tumor formation,
metastasis and differentiation through P53, NF-κB, EGFR and other
transduction pathways, including Wnt signaling, which is also
considered a core pathway highly activated in drug-resistant lung
cancer cells. Previous studies have shown the human iNOS gene is a
transcriptional target of Wnt signaling, while iNOS-overexpression
increased the levels of downstream effectors of the Wnt pathway
such as c-Myc and cyclin D1 (14,34–36).
In this study, we focused on the relationship
between iNOS and Wnt signaling in cisplatin-resistant lung cells
A549/CDDP. By inhibiting the Wnt pathway by DKK-1, the iNOS-induced
drug resistance was confirmed to be reversed. Furthermore, we found
Wnt signaling could influence TOPO IIα and GST-π, but affected P-gp
less directly. As is known, P-gp-related resistance mainly acts
against natural and lipophilic anti-cancer drugs (37,38),
thus it may not play a key role in this non-lipophilic drug-induced
cell line, leading to a slight change of P-gp levels.
To further differentiate among three Wnt signaling
pathways in the regulation of iNOS, we chose XAV939, XeC and
SP600125 to inhibit Wnt/β-catenin, Wnt/Ca2+ and Wnt/JNK
pathways respectively. The results clearly demonstrated higher TOPO
IIα and lower iNOS/GST-π levels in the XAV939 treatment group
compared with that in the other two inhibitor groups. The
expression of P-gp was only slightly altered in the XAV939 group,
but it was downregulated in the XeC and SP600125 groups. That is
possibly because SP600125 and XeC could disturb other core signal
transductions related to P-gp-expression, except the inhibition of
JNK1/2 and Ca2+.
To confirm the effect of iNOS on canonical and
noncanonical Wnt signaling, we also investigated the corresponding
secretions, Wnt-3a/Wnt-8a and Wnt-5a/Wnt-11, respectively.
Consistent with what we observed, inhibition of iNOS led to an
obviously decreased Wnt-3a and Wnt-8a level, which indicated
canonical Wnt signaling, but noncanonical Wnt-5a and Wnt-11 levels
were less altered. The results indicated the iNOS-induced drug
resistance was mainly mediated by canonical Wnt/β-catenin
signaling, but not by the other two noncanonical pathways.
After establishing the relationship between iNOS and
Wnt/β-catenin signaling in A549/CDDP, we detected the effect of
iNOS on downstream factors of this pathway, containing membrane
co-receptor Fzd, and β-catenin/APC/GSK-3β/Axin compound. In humans,
there are 10 Fzd genes which may be divided into five subgroups:
Fzd-1/2/7, Fzd-3/6, Fzd-5/8, Fzd-9/10 and Fzd-4 (39). Among them, Fzd-8 was confirmed to
form a complex with Wnt3α in vitro (40,41).
Thus, we tested the expression of Fzd-8, β-catenin, APC and Axin,
and the phosphorylation of GSK-3β was assessed as well. By blocking
iNOS by SMT, we observed a decreasing level of Fzd-8, β-catenin and
Axin, a higher p-GSK-3β and GSK-3β expression, but a slight change
of APC. Thus the effect of iNOS on the Wnt/β-catenin pathway was
mainly mediated by Fzd-8 and p-GSK-3β. It is of note that Axin, as
a negative modulator in the canonical Wnt pathway, was
downregulated after iNOS inhibition, and we speculated it might be
because Axin has multi functions influenced by iNOS in tumor
proliferation or other processes.
To explain the mechanism of iNOS-induced positive
regulation on Wnt/β-catenin signaling, we further investigated
three widely accepted antagonists of this pathway, Wif-1, DKK-1 and
SFRP-1. Human Wif-1 protein contains a Wnt inhibitory factor (Wif)
domain, can bind to seven Wnts (3a, 4, 5a, 7a, 8, 9a and 11)
(42,43), directly competing with Wnt for
binding to its membrane receptors. DKK-1 works by inhibiting Wnt
co-receptors LRP5/6 through binding cell surface Kremen-1 or
Kremen-2 and thus promoting the internalization of LRP5/6. As a
type of secreted frizzled-related protein, SFRP can suppress the
transduction of Wnt pathway signaling by competitively binding with
Fzd receptor. Consistent with other reports that DKK1 expression is
inversely correlated with iNOS and β-catenin translocation, we also
observed the negative correlation between DKK-1 and iNOS.
Furthermore, SFRP-1 was indicated to be inversely regulated by iNOS
as well, but Wif-1 seemed to be less associated with this factor.
In the present study, we presumed that there exists a balance
between Fzd and its relative secreted protein SFRP, and blocking
iNOS might promote the balance switches from Fzd to SFRP, inducing
Wnt/β-catenin pathway inactivation, and finally increasing the
sensitivity of A549/CDDP cells to cisplatin.
The relationship between iNOS and Wnt signaling has
attracted considerable attention for its multiple functions in
tumor; hence, clarifying the detailed mechanism of its regulation
may help to better understand the mechanism of drug resistance, and
may aid in the development of new targets for reversing drug
resistance in NSCLC.
Acknowledgements
This study was supported by The Fourth Youth
Foundation of the First Hospital of Jilin University
(JDYY42013008).
References
|
1
|
Keith RL and Miller YE: Lung cancer
chemoprevention: current status and future prospects. Nat Rev Clin
Oncol. 10:334–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Li H, Hou S, Hu B, Liu J and Wang
J: The noncoding RNA expression profile and the effect of lncRNA
AK126698 on cisplatin resistance in non-small-cell lung cancer
cell. PLoS One. 8:e653092013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grutters JPC, Kessels AGH,
Pijls-Johannesma M, de Ruysscher D, Joore MA and Lambin P:
Comparison of the effectiveness of radiotherapy with photons,
protons and carbon-ions for non-small cell lung cancer: a
meta-analysis. Radiother Oncol. 95:32–40. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Herbst R, Heymach J and Lippman S:
Molecular origins of cancer. N Engl J Med. 359:1367–1380. 2008.
|
|
6
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med. 346:92–98. 2002.
View Article : Google Scholar
|
|
7
|
Scagliotti GV, Parikh P, von Pawel J, et
al: Phase III study comparing cisplatin plus gemcitabine with
cisplatin plus pemetrexed in chemotherapy-naive patients with
advanced-stage non-small-cell lung cancer. J Clin Oncol.
26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mackay HJ and Twelves CJ: Protein kinase
C: a target for anticancer drugs? Endocr Relat Cancer. 10:389–396.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hiss D: Optimizing molecular-targeted
therapies in ovarian cancer: the renewed surge of interest in
ovarian cancer biomarkers and cell signaling pathways. J Oncol.
2012:7379812012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jenkins DC, Charles IG, Thomsen LL, et al:
Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA.
92:4392–4396. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu WM, Liu LZ, Loizidou M, Ahmed M and
Charles IG: The role of nitric oxide in cancer. Cell Res.
12:311–320. 2002. View Article : Google Scholar
|
|
12
|
Chen GG, Lee TW, Xu H, Yip JH, Li M, Mok
TS and Yim AP: Increased inducible nitric oxide synthase in lung
carcinoma of smokers. Cancer. 112:372–381. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wongvaranon P, Pongrakhananon V, Chunhacha
P and Chanvorachote P: Acquired resistance to chemotherapy in lung
cancer cells mediated by prolonged nitric oxide exposure.
Anticancer Res. 33:5433–5444. 2013.PubMed/NCBI
|
|
14
|
Du Q, Zhang X, Liu Q, Zhang X, Bartels CE
and Geller DA: Nitric oxide production upregulates Wnt/β-catenin
signaling by inhibiting Dickkopf-1. Cancer Res. 73:6526–6537.
2013.PubMed/NCBI
|
|
15
|
Vane JR, Mitchell JA, Appleton I, et al:
Inducible isoforms of cyclooxygenase and nitric-oxide synthase in
inflammation. Proc Natl Acad Sci USA. 91:2046–2050. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nathan C and Xie QW: Nitric oxide
synthases: roles, tolls, and controls. Cell. 78:915–918. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gonzalez D, Rojas A, Herrera MB and Conlan
RS: iNOS activation regulates β-catenin association with its
partners in endothelial cells. PLoS One. 7:e529642012.
|
|
18
|
Sumi T, Oki S, Kitajima K and Meno C:
Epiblast ground state is controlled by canonical Wnt/β-catenin
signaling in the post-implantation mouse embryo and epiblast stem
cells. PLoS One. 8:e633782013.PubMed/NCBI
|
|
19
|
Tian XH, Hou WJ, Fang Y, et al: XAV939, a
tankyrase 1 inhibitor, promotes cell apoptosis in neuroblastoma
cell lines by inhibitingWnt/β-catenin signaling pathway. J Exp Clin
Cancer Res. 32:1002013.
|
|
20
|
Gafni J, Munsch JA, Lam TH, et al:
Xestospongins: potent membrane permeable blockers of the inositol
1,4,5-trisphosphate receptor. Neuron. 19:723–733. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Westfall TA, Brimeyer R, Twedt J, et al:
Wnt-5/pipetail functions in vertebrate axis formation as a negative
regulator of Wnt/beta-catenin activity. J Cell Biol. 162:889–898.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cadigan KM and Nusse R: Wnt signaling: a
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moon RT, Brown JD and Torres M: WNTs
modulate cell fate and behavior during vertebrate development.
Trends Genet. 13:157–162. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakajima M, Fukuchi M, Miyazaki T, Masuda
N, Kato H and Kuwano H: Reduced expression of Axin correlates with
tumour progression of oesophageal squamous cell carcinoma. Br J
Cancer. 88:1734–1739. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bryja V, Andersson ER, Schambony A, et al:
The extracellular domain of Lrp5/6 inhibits noncanonical Wnt
signaling in vivo. Mol Biol Cell. 20:924–936. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andersson ER, Bryjova L, Biris K,
Yamaguchi TP, Arenas E and Bryja V: Genetic interaction between
Lrp6 and Wnt5a during mouse development. Dev Dyn. 239:237–245.
2010.PubMed/NCBI
|
|
28
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mikels AJ and Nusse R: Purified Wnt5a
protein activates or inhibits beta-catenin-TCF signaling depending
on receptor context. PLoS Biol. 4:570–582. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Surana R, Sikka S, Cai W, Dharmarajan AM,
Kumar AP, et al: Secreted frizzled related proteins: implications
in cancers. Biochim Biophys Acta. 1845:53–65. 2014.PubMed/NCBI
|
|
31
|
Zhang J, Zhou B, Liu Y, et al: Wnt
inhibitory factor-1 functions as a tumor suppressor through
modulating Wnt/β-catenin signaling in neuroblastoma. Cancer Lett.
348:12–19. 2014.PubMed/NCBI
|
|
32
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bafico A, Liu G, Yaniv A, Gazit A and
Aaronson SA: Novel mechanism of Wnt signalling inhibition mediated
by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol.
3:683–686. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Du Q, Park KS, Guo Z, et al: Regulation of
human nitric oxide synthase 2 expression by Wnt beta-catenin
signaling. Cancer Res. 66:7024–7031. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Du Q, Zhang X, Cardinal J, et al:
Wnt/beta-catenin signaling regulates cytokine-induced human
inducible nitric oxide synthase expression by inhibiting nuclear
factor-kappaB activation in cancer cells. Cancer Res. 69:3764–3771.
2009. View Article : Google Scholar
|
|
36
|
Du Q and Geller DA: Cross-regulation
between Wnt and NF-κB signaling pathways. For Immunopathol Dis
Therap. 1:155–181. 2010.
|
|
37
|
Pakos EE and Ioannidis JP: The association
of P-glycoprotein with response to chemotherapy and clinical
outcome in patients with osteosarcoma. A meta-analysis. Cancer.
8:581–589. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Geng G, Wang L, Chen X, Cao R and Li P:
The association between chemosensitivity and Pgp, GST-π and Topo II
expression in gastric cancer. Diagn Pathol. 8:1982013.PubMed/NCBI
|
|
39
|
MacDonald BT and He X: Frizzled and LRP5/6
receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect
Biol. 4:a0078802012.
|
|
40
|
Bourhis E, Tam C, Franke Y, et al:
Reconstitution of a Frizzled8-Wnt3a-LRP6 signaling complex reveals
multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem.
285:9172–9179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tamai K, Semenov M, Kato Y, et al:
LDL-receptor-related proteins in Wnt signal transduction. Nature.
407:530–535. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsieh JC, Kodjabachian L, Rebbert ML, et
al: A new secreted protein that binds to Wnt proteins and inhibits
their activities. Nature. 398:431–436. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Surmann-Schmitt C, Widmann N, Dietz U, et
al: Wif-1 is expressed at cartilage-mesenchyme interfaces and
impedes Wnt3a-mediated inhibition of chondrogenesis. J Cell Sci.
122:3627–3637. 2009. View Article : Google Scholar : PubMed/NCBI
|