Introduction
BAG-1 is an anti-apoptotic protein that binds to
bcl-2. It is a multi-functional protein that interacts with many
other cellular proteins (1–4). Apoptosis-related factors are
candidates for cancer therapy. Downregulation of the expression of
bcl-2 by ribozymes or antisense oligonucleotides was found to
result in apoptosis (5,6). BAG-1 was reported to be a partner of
bcl-2 collaborating in the suppression of cell death (7). To date, BAG-1 expression has been
implicated in breast, lung, laryngeal and oral cavity cancers
(8–11).
Esophageal carcinoma is a malignant digestive tract
tumor and is associated with a poor prognosis. The pathogenesis and
development of esophageal carcinoma are complicated processes, and
in most cases, esophageal carcinoma exhibits no symptoms until the
cancer is at an advanced stage. The overall survival rate still
remains low, and less than 20% of patients survive for more than
five years (12). To date, the
pathological progression of esophageal cancer has been thoroughly
described, while the molecular mechanisms are less well understood
(13).
Few studies have investigated the role of BAG-1 in
the pathogenesis and development of esophageal carcinoma (14). Therefore, in this study, we
initially studied the expression of BAG-1 in esophageal carcinoma
and adjacent normal tissues. RNA interference (RNAi) provides a new
approach with which to study gene functions (15,16).
Using this method we downregulated BAG-1 expression in esophageal
cancer cells to investigate the function of BAG-1. We constructed
the siRNA vector of BAG-1, transfected it into the Eca109 cell line
to downregulate the expression of BAG-1, and investigated its role
in cell proliferation, invasion and the apoptosis of esophageal
carcinoma.
Materials and methods
The present study was approved and registered by the
Ethics Committee of the First Affiliated Hospital of Liaoning
Medical University in January, 2010. The related screening and
analysis of the resected samples were approved by the Ethics
Committee of Liaoning Medical University, and written consent forms
for the use of these samples were signed and participation in the
study was agreed upon by all subjects.
Sample collection
A total of 92 esophageal carcinoma and 15 adjacent
normal tissue samples (at least 5 cm away from the edge of the
cancer tissue) were collected from the sample preservation center
of our hospital. These esophageal carcinoma (and adjacent normal
tissue) samples were all resected at the Department of Thoracic
Surgery from January 2010 to December 2012. The inclusion criteria
of these sample were: i) patients had not received any prior
radiotherapy and chemotherapy treatment; ii) each patient had
received a medical examination including cranial CT scan, chest CT
scan, abdominal CT scan and ECT, which could define the TNM stage
of the patient clearly; iii) patients had received radical surgery
with sufficient tissue samples prepared in paraffin blocks for
further testing; iv) patients who had at least 2 concurrent primary
tumors were excluded. All samples were fixed in 10% formaldehyde
and paraffin-embedded. The samples were routinely and serially
sectioned with a thickness of 5 μm, and then were
immunohistochemically stained. The esophageal carcinomas were
staged according to the tumor-node-metastasis (TNM) staging system
stipulated by the 7th edition of the American Joint Committee on
Cancer (AJCC) Cancer Staging Manual (2009).
Immunohistochemistry
Sections were deparaffinized and hydrated stepwise
in xylene and graded ethanol, washed with phosphate-buffered saline
(PBS), and recovered through microwave irradiation.
H2O2 solution (3%) was added and cultured for
10 min, and then washed with PBS. Goat blocking serum was supplied
and cultured at room temperature, and the diluted primary antibody
(1:100; rabbit anti-human BAG-1 polyclonal antibody; Santa Cruz)
was applied. Sections, after remaining overnight at 4°C, received
PBS washing, and then the secondary antibody (1:500; goat
anti-rabbit antibody; Santa Cruz) was added and cultured at 37°C
for 20 min. Newly prepared DAB chromogenic reagent was applied and
cultured at 37°C for 5–10 min. Nuclei were then stained with
hematoxylin and eosin (H&E). The staining proportion was
classified as − (≤10%), + (11–50%) and ++ (>50%).
Expression of BAG-1 protein in tissue
samples
Western blotting was used to determine the protein
level in the cancer tissues. Briefly, frozen tissues were
homogenized with lysis buffer, and then centrifuged at 4°C for 30
min (12,000 rpm). The supernatant was collected and the BCA method
was used to determine protein concentration. A 10% polyacrylamide
gel was prepared to load the protein samples, and 5% nonfat dry
milk was added to block the non-specific antigen. The primary
antibody (1:250; rabbit anti-human BAG-1 polyclonal antibody) and
secondary antibody (1:500; goat anti-rabbit anti-body) were
applied. Each sample was also probed with β-actin antibody
(Sigma-Aldrich) as a loading control.
Cell lines
Esophageal carcinoma cell line Eca109 was purchased
from Shanghai Biological Sciences Institute (China). It was
cultured in RPMI-1640 medium, supplemented with 10% FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin and maintained in a 5%
CO2 humidified atmosphere at 37°C.
Vector construction and transfection
Small interfering RNAs (siRNAs) targeting BAG-1 were
chemically synthesized by Takara Biotechnology Co. Ltd. (Dalian,
China). The three siRNA sequences were: (si-1) forward,
5′-GATCCGTCGAG CAATGAGAGGTATGACCTTCATCGATGAAGGTCATAC
CTCTCATTGCCTTTTTTG-3′ and reverse, 5′-AATTCAAAA
AAGGCAATGAGAGGTATGACCTTCATCGATGAAGGT CATACCTCTCATTGCCTAGCG-3′;
(si-2) forward, 5′-GAT CCGTCGATGGTCGTCACCCACAGCAATATCGATATTG
CTGTGGGTGACGACCACTTTTTTG3′ and reverse, 5′-AAT
TCAAAAAAGTGGTCATCACCCACAGCAATATCGATA TGCTGTGGGTGACGACCACTAGCG-3′.
The negative siRNA sequence was: (si-N) forward, 5′GATCCGCGAGAC
CTCAGTATGTTACCTGTGAAGCCACAGATGGGGTAA CATACTGAGGTTCGCTTTTTTG 3′
(control group). The DNA sequence was fully ligated into the
pRNAT-U6.1/Neo-siRNA vector (Takara Biotechnology, Dalian, China)
at 4°C overnight using the Takara DNA Ligation kit. There were two
targeted recombinant plasmids (BAG-1-siRNA-1, BAG-1-siRNA-2), one
negative control (BAG-1-siRNA-N), and the untreated cells served as
the blank control. Eca109 cells were seeded (2×105
cells/well) in a 6-well plate. After 24 h of incubation, the cells
were transfected with BAG-1-siRNA-1, BAG-1-siRNA-2 or BAG-1-siRNA-N
in serum-free medium using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA). Vectors (5 μg) and 10 μl of Lipofectamine 2000
were mixed and incubated for 15 min at room temperature. The
mixture was then added to the Eca109 cells (no serum and
antibiotics), and after incubation for 6 h, the mixture was
replaced by full medium (including serum but no antibiotics).
MTT assay
Twenty-four hours after transfection, cells from the
4 groups were loaded in a 96-well plate at 2×103
cells/well, cultured with the RPMI-1640 medium with 10% FBS, at
time points of 24, 48 and 72 h. The medium was removed from each
well, and 20 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
5 mg/ml in PBS) was added in the absence of light, and the formazan
crystals were produced over a 4-h incubation period. Then the
supernatant was removed, and 150 μl of DMSO was added into each
well. The dark-blue crystals of MTT were dissolved by shaking the
plates at room temperature for 10 min, and the absorbance was then
measured on a Bio-Rad microplate reader (Bio-Rad, Hercules, CA,
USA) using a test wavelength of 490 nm and a reference wavelength
of 570 nm.
Transwell assay
The invasiveness of Eca109 cells was assayed using
modified Transwell chambers. Polycarbonate filter (pore size, 8 μm)
separating the upper and lower compartments was coated with 50 μg
of reconstituted basement membrane (Matrigel; BD, Bedford, MA,
USA). Thirty-six hours after transfection, the full medium was
replaced with serum-free culture medium. Eight hours later, they
were digested to a suspension at a density of 1×104/ml.
Cells were seeded into the Transwell chamber. The chamber was
placed into a 24-well culture plate with 500 μl of RPMI-1640 medium
containing 15% serum added outside of the chamber, and 200 μl cell
suspension was added to the chamber. After 48 h of incubation at
37°C, cells on the upper surface of the filter that had not invaded
through the Matrigel were removed completely with cotton swabs.
Cells that had invaded remained on the filter. Cells on the
polycarbonate filter were fixed with H&E. The number of
invasive cells was counted under a microscope (magnification,
×200).
Flow cytometry
Thirty-six hours after transfection, the cells were
washed with PBS twice, then digested and centrifuged. Then
apoptosis detection was carried out according to the instructions
of the detection kit (PE Annexin V Apoptosis I). Cells were
resuspended in 1X binding buffer (1×106/ml). A total of
100 μl was drawn and 5 μl of PE Annexin V and 5 μl of 7-AAD were
added. Cells were cultured in a rotary system at room temperature
for 15 min, and 400 μl of 1X binding buffer was added, and analysis
was performed within 1 h.
Western blotting of BAG-1 and bcl-2
Cells were washed three times with ice-cold PBS,
then centrifuged at 4°C for 30 min (12,000 rpm). The supernatant
was collected and the BCA method was used to determine the protein
concentration. A 10% polyacrylamide gel was prepared to load
protein samples, and 5% nonfat dry milk was added to block the
non-specific antigen. The primary antibody (1:250; rabbit
anti-human BAG-1 or bcl-2 polyclonal anti-body) and the secondary
antibody (1:500; goat anti-rabbit antibody) were applied. Each
sample was also probed with β-actin antibody as a loading
control.
Statistical analysis
The images were analyzed by Quantity One software.
All laboratory data are represented as mean ± standard deviation
(SD). The χ2 test and single factor analysis of variance
(ANOVA) were performed with SPSS 17.0 software, and P<0.05 was
considered to indicate a statistically significant difference.
Results
A total of 92 samples were successfully selected,
among which 67 were resected from males and 25 from females. The
average patient age was 59 years (range 35–76). Of the total
samples, 39 cases were well differentiated, 37 cases were
moderately differentiated, and 16 cases were poorly differentiated.
Forty cases were classified as stage I and stage II, and 52 cases
were stage III (Table I).
 | Table IThe relationship between the positive
expression of BAG-1 protein in esophageal squamous cell carcinoma
and the patient characteristics. |
Table I
The relationship between the positive
expression of BAG-1 protein in esophageal squamous cell carcinoma
and the patient characteristics.
| | BAG-1 protein | | |
|---|
| |
| | |
|---|
| n (92) | − | + | ++ | χ2 | P-value |
|---|
| Gender | | | | | 0.473 | 0.491 |
| Male | 67 | 21 | 13 | 33 | | |
| Female | 25 | 6 | 8 | 11 | | |
| Age (years) | | | | | 0.915 | 0.339 |
| ≤60 | 44 | 15 | 12 | 17 | | |
| >60 | 48 | 12 | 9 | 27 | | |
| Location | | | | | 0.070 | 0.791 |
| Mid-thoracic | 56 | 17 | 14 | 25 | | |
| Lower-thoracic | 36 | 10 | 7 | 19 | | |
| Degree of
differentiation | | | | | 15.713 | <0.001 |
| Well | 39 | 20 | 8 | 11 | | |
| Moderate | 37 | 5 | 10 | 22 | | |
| Poorly | 16 | 2 | 3 | 11 | | |
| TNM stage | | | | | 5.904 | 0.015 |
| I+II | 40 | 17 | 13 | 10 | | |
| III | 52 | 10 | 9 | 33 | | |
BAG-1 expression is low in adjacent
normal tissues while high in esophageal carcinoma tissues
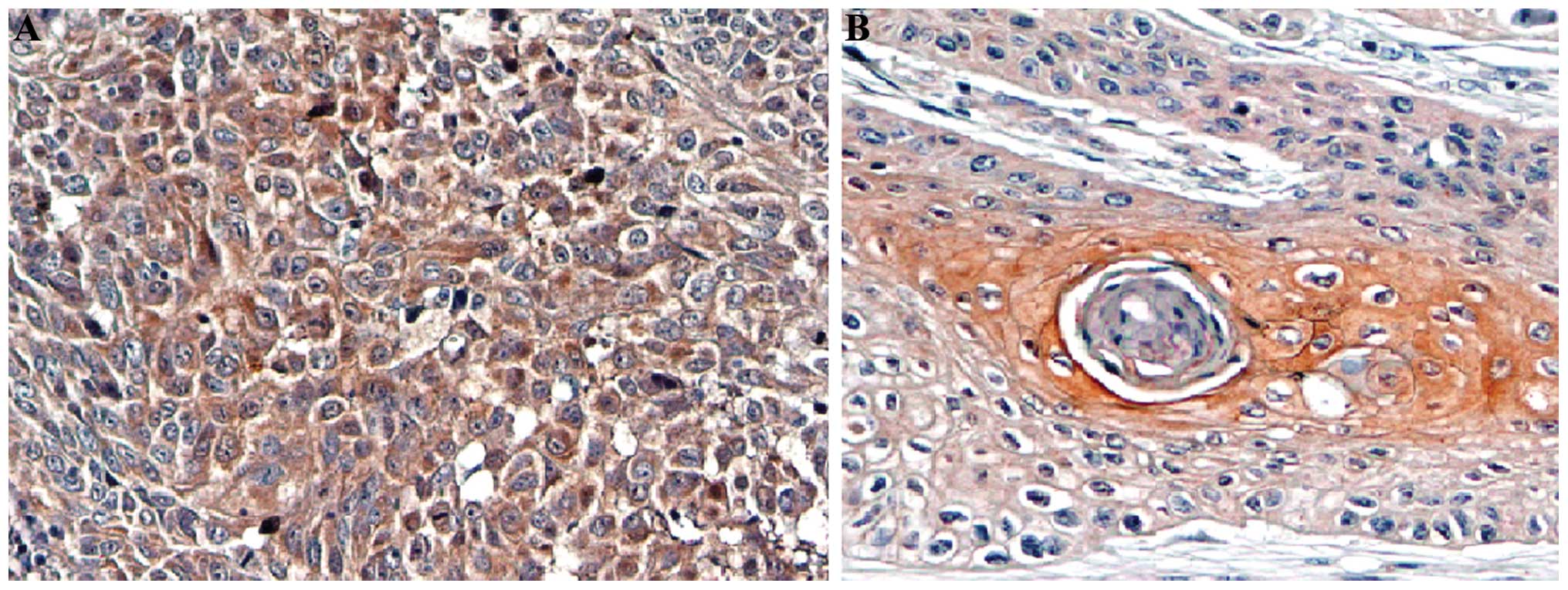
Immunohistochemistry showed that the BAG-1 protein
was mainly expressed in the cytoplasm and the nuclei and exhibited
yellow or brown-color staining. Its expression was significantly
higher in the poorly differentiated cells when compared with the
well differentiated cells (Fig. 1).
The expression of BAG-1 in the esophageal carcinoma tissues was not
correlated with patient age, gender and tumor location (Table I). Statistical difference was noted
in BAG-1 protein levels among the esophageal carcinoma tissues with
different degrees of differentiation (χ2=15.733,
P<0.001), and the degree of differentiation was negatively
correlated with the expression of BAG-1 (Fig. 1). A statistical difference was found
in the expression of BAG-1 among the TNM stages in the esophageal
carcinoma cases (χ2=5.904; P=0.015), and the expression
was positively correlated with TNM stage. There were 70.7% (65/92)
of cases with positive expression of BAG-1 protein in the
esophageal carcinoma tissues while the rate of positive expression
was significantly low in the adjacent normal tissues (20%; 3/15;
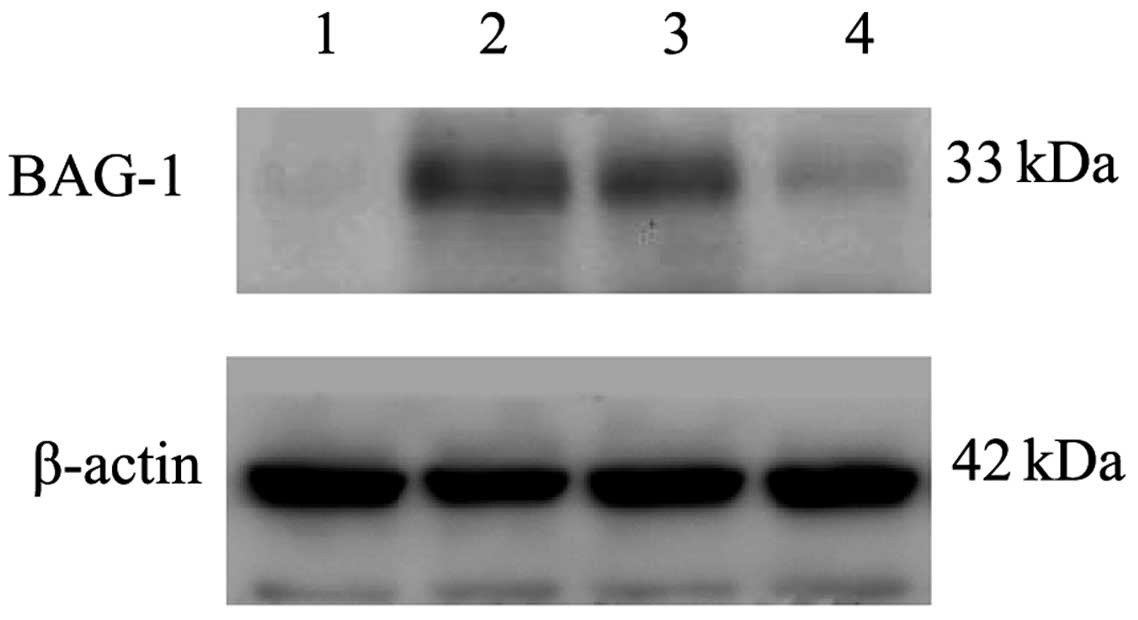
P<0.001; Table II). The western
blotting results (Fig. 2) showed
that BAG-1 was expressed weakly in the adjacent normal tissues of
the esophageal carcinoma, while highly expressed in the esophageal
carcinoma tissues.
 | Table IIRelationship between the positive
expression of BAG-1 protein in esophageal squamous tumor tissues
and adjacent normal esophageal tissues. |
Table II
Relationship between the positive
expression of BAG-1 protein in esophageal squamous tumor tissues
and adjacent normal esophageal tissues.
| | BAG-1 | | |
|---|
| |
| | |
|---|
| n | − | + | ++ | χ2 | P-value |
|---|
| Tissue |
| Tumor | 92 | 27 | 21 | 44 | 14.285 | <0.001 |
| Adjacent
normal | 15 | 12 | 2 | 1 | | |
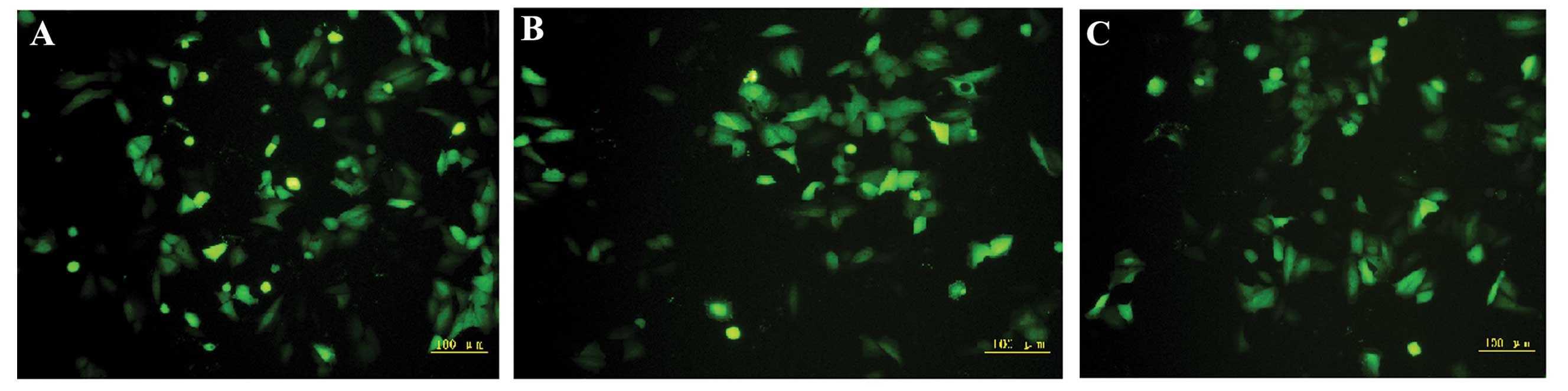
Fluorescence microscopy
After the Eca109 cells were transfected with
BAG-1-siRNA-1, BAG-1-siRNA-2 or BAG-1-siRNA-N, green fluorescence
was observed in the cytoplasm. As shown in Fig. 3, under fluorescence microscopy, the
transfection efficiency of the three groups (BAG-1-siRNA-1,
BAG-1-siRNA-2 and BAG-1-siRNA-N) was satisfactory with all
exceeding 70%. We observed that some of the cells treated with
BAG-1-siRNA were less confluent or became smaller and orbicular
compared with the control. Consistently, there were fewer cells in
both the BAG-1-siRNA-1 and BAG-1-siRNA-2 transfected cells as
compared with the BAG-1-siRNA-N transfected and control cells
cultured for 72 h after transfection. The BAG-1-siRNA treatment
decreased the number of Eca109 cells implying that BAG-1
participates in cell cycle progression and cell survival.
Decrease in proliferation and invasion
after transfection
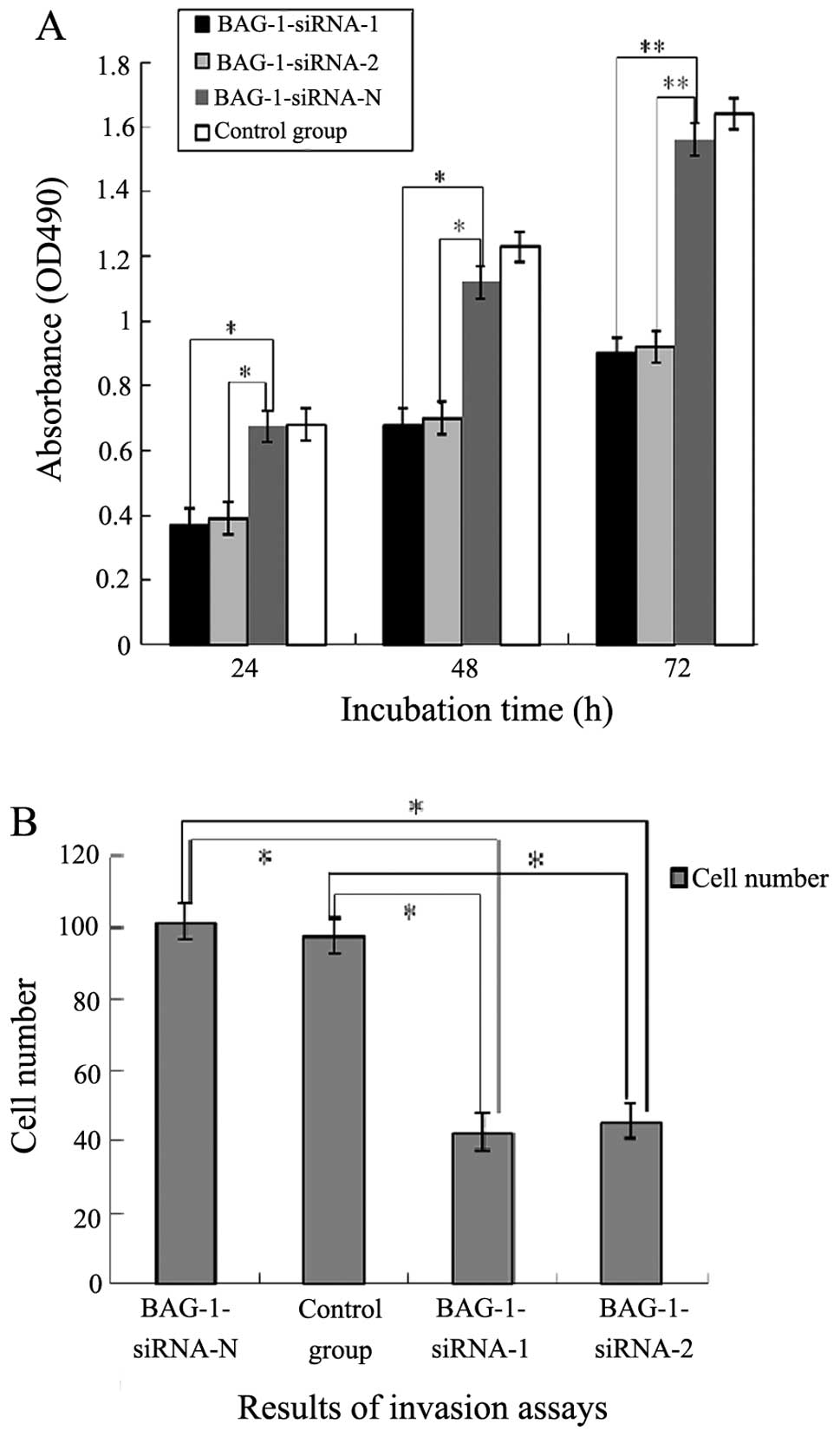
MTT assay showed that the proliferation of cells in
the BAG-1-siRNA-N and blank control groups increased almost 3-fold
from 24 to 72 h after transfection (Fig. 4A), while that in the BAG-1-siRNA-1
and BAG-1-siRNA-2 groups was relatively slow, and doubled at 72 h.
Compared with the negative control, proliferation was significantly
retarded (P<0.01).
To determine the possible role of BAG-1-siRNA in the
invasive ability of esophageal cancer cells, we used a Transwell
invasion assay. Eca109 cells and Eca109 cells transfected with
BAG-1-siRNA-1, BAG-1-siRNA-2 and BAG-1-siRNA-N were placed for 48 h
on Matrigel-coated filters, which were stained with H&E and
inspected under a microscope. The cell numbers of invasive Eca109
cells transfected with BAG-1-siRNA-1 and BAG-1-siRNA-2 were
significantly decreased when compared with the invasive cell
numbers in the siRNA-N and control groups (P<0.05). There was no
significant difference between the siRNA-N group and the control
group (P>0.05; Fig. 4B). These
data demonstrated that knockdown of BAG-1 by transient transfection
of BAG-1-siRNA decreased the proliferation and inhibited the
invasion of esophageal carcinoma cells in vitro.
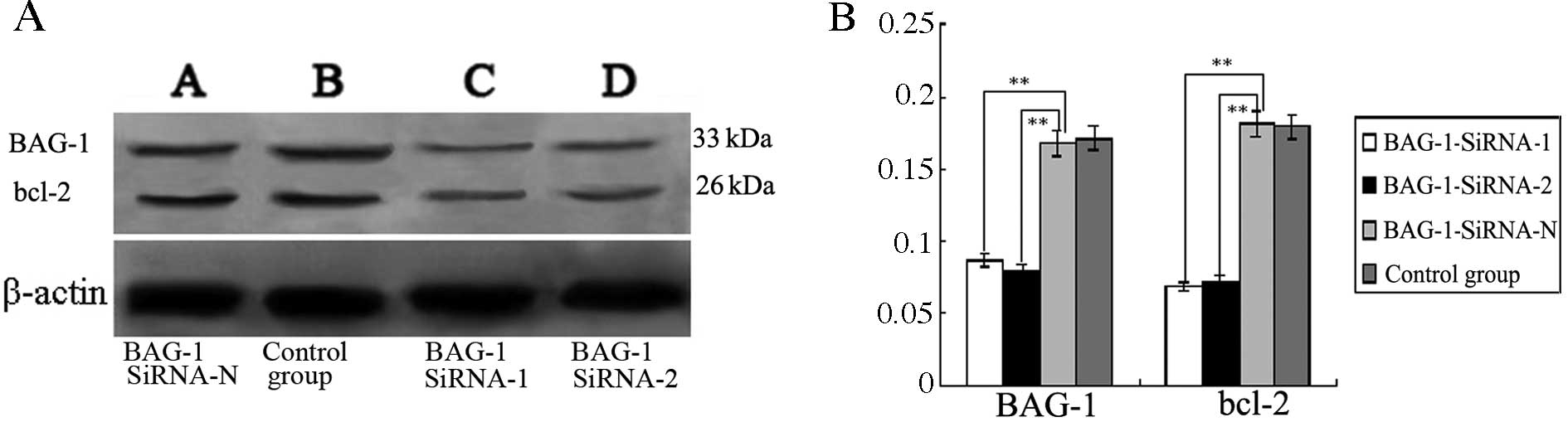
Expression of BAG-1 and bcl-2 in the
transfected Eca109 cells
Compared with the BAG-1-siRNA-N group and the blank
control group, western blotting results showed that the expression
of BAG-1 protein was significantly decreased in the BAG-1-siRNA-1
and BAG-1-siRNA-2 groups compared with expression in the blank
control (P<0.05; Fig. 5), while
BAG-1 protein expression in the BAG-1-siRNA-N group almost remained
unchanged. Concomitantly, the expression of bcl-2 was significantly
reduced in the BAG-1-siRNA-1 and BAG-1-siRNA-2 groups compared with
bcl-2 expression in the blank control (P<0.05; Fig. 5). Expression in the BAG-1-siRNA-N
group was the same as that in the blank control.
Apoptosis rate is increased after
transfection
Flow cytometric analysis showed that the apoptosis
rate was significantly increased (P<0.01) in the BAG-1-siRNA-1
and BAG-1-siRNA-2 transfected cells compared with the negative
control; there was no significant difference in the apoptotic rate
between cells transfected with BAG-1-siRNA-N and the blank control
(P>0.05) (Table III).
 | Table IIIAnalysis of the rate of apoptosis for
each cell group. |
Table III
Analysis of the rate of apoptosis for
each cell group.
| Group | Apoptosis (%) |
|---|
| BAG-1-siRNA-1 | 15.87±1.12a |
| BAG-1-siRNA-2 | 16.26±1.06a |
| BAG-1-siRNA-N | 4.64±0.72 |
| Blank control | 5.14±0.48 |
Discussion
Apoptosis is a cell death process that involves many
genes, and the occurrence and development of tumors are the result
of abnormal expression of oncogenes, tumor-suppressor genes and
apoptosis-related gene changes in cell proliferation and apoptosis.
Apoptosis-related factors are candidates for cancer therapy.
Downregulation of the expression of bcl-2 by ribozymes or antisense
oligonucleotides results in apoptosis. BAG-1 was reported to be a
partner of bcl-2 collaborating in the suppression of cell death.
Overexpression of BAG-1 caused an acceleration in cell motility in
human gastric and cervical cancer cells (17,18).
To date, few reports exist concerning the role of the BAG-1 gene in
esophageal carcinoma (19). In the
present study, we observed that BAG-1 was highly expressed in
esophageal cancer tissues, when compared with that in adjacent
normal tissues. Importantly, we observed that the expression of
BAG-1 was closely related to cancer differentiation and TNM stage,
which suggests that BAG-1 may contribute to invasion and metastasis
of esophageal cancer.
The discovery of small interfering RNAs (siRNAs) may
well be one of the transforming events in biology in the past
decade. This technology has become a powerful tool in the studies
of gene function, carcinoma and viral disease therapy (20,21).
Liu et al silenced the BAG-1 gene in lung cancer cell lines
A549 and L9981, resulting in changes in expression of
apoptosis-related genes and sensitization of A549 and L9981 cells
to cisplatin-induced apoptosis (22). Enlightened by this research, we
constructed a BAG-1 siRNA vector and transfected it into the Eca109
cell line to downregulate the expression of the BAG-1 gene. Our
results showed that transfection of BAG-1-siRNA-1 or BAG-1-siRNA-2
into Eca109 cells, significantly inhibited cell proliferation and
invasion. In addition, BAG-1-siRNA induced the apoptosis of Eca109
cells in vitro, which confirmed that BAG-1 plays a major
role in controlling cell proliferation, invasion and apoptosis. Our
results indicate that targeting BAG-1 could be a strategy for the
development of esophageal tumor therapy.
In our study, more than 70% of the plasmids in the
BAG-1-siRNA-1, BAG-1-siRNA-2 and BAG-1-siRNA-N groups were
transfected successfully, while the proliferation, migration and
apoptosis rate in the BAG-1-siRNA-N group showed almost no
difference with the control group. These results indicate that the
safety and efficacy of the interfering vector system was reliable
at the cellular level, and BAG-1 is an ideal target for esophageal
carcinoma.
BAG-1 is an anti-apoptotic protein that binds to
bcl-2. We confirmed that with a decrease in BAG-1, the bcl-2 level
decreased in the esophageal cancer tissues. When the amount of
bcl-2 decreased, the cell apoptosis rate significantly increased,
and the cell proliferation and differentiation capabilities
decreased. This may be one of the mechanisms of BAG-1 influence on
esophageal cancer proliferation, invasion and apoptosis.
At present, a wide array of genes which could
possibly affect the pathogenesis and development of esophageal
carcinoma have been investigated, and targeted RNAi of CXCR4,
MMP-2, XIAP, MTA1 and ABCE1 have been reported for esophageal
carcinoma in vitro or in vivo with downregulation
ranging from 20–80% (23–26). Yet, few studies have researched the
role of the BAG-1 gene in esophageal carcinoma. Our study
demonstrated that BAG-1 could be a future target for the treatment
of esophageal carcinoma. In summary, our study showed that BAG-1
was highly expressed in esophageal cancer tissues, compared with
that in adjacent normal tissues. The expression of BAG-1 was
closely related to cancer cell differentiation and TNM stage. The
transfection vector system we constructed significantly
downregulated the BAG-1 level in esophageal cancer cells and it
also inhibited cell proliferation, invasion, and induced cell
apoptosis. Our findings may provide novel insights for the
development of gene therapy technology with which to treat patients
with esophageal cancer.
Acknowledgements
This research was supported by the National Natural
Science Foundation of China (30973520) and the Science and
Technology Department of Liaoning Province (series nos. 201202143
and 2013022038).
References
|
1
|
Yang L, McBurney D, Tang SC, Carlson SG
and Horton WE Jr: A novel role for Bcl-2 associated-athanogene-1
(Bag-1) in regulation of the endoplasmic reticulum stress response
in mammalian chondrocytes. J Cell Biochem. 102:786–800. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takayama S, Krajewski S, Krajewska M,
Kitada S, Zapata JM, Kochel K, Knee D, Scudiero D, Tudor G, Miller
GJ, Miyashita T, Yamada M and Reed JC: Expression and location of
Hsp70/Hsc-binding anti-apoptotic protein BAG-1 and its variants in
normal tissues and tumor cell lines. Cancer Res. 58:3116–3131.
1998.PubMed/NCBI
|
|
3
|
Knapp RT, Steiner A, Schmidt U, Hafner K,
Holsboer F and Rein T: BAG-1 diversely affects steroid receptor
activity. Biochem J. 441:297–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elliott E, Tsvetkov P and Ginzburg I:
BAG-1 associates with Hsc70. Tau complex and regulates the
proteasomal degradation of Tau protein. J Biol Chem.
282:37276–37284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kunze D, Kraemer K, Erdmann K, Froehner M,
Wirth MP and Fuessel S: Simultaneous siRNA-mediated knockdown of
anti-apoptotic BCL-2, Bcl-xL, XIAP and survivin in bladder cancer
cells. Int J Oncol. 41:1271–1277. 2012.PubMed/NCBI
|
|
6
|
Xu HD, Wu D, Gu JH, Ge JB, Wu JC, Han R,
Liang ZQ and Qin ZH: The pro-survival role of autophagy depends on
Bcl-2 under nutrition stress conditions. PLoS One. 8:e632322013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roth W, Grimmel C, Rieger L, Strik H,
Takayama S, Krajewski S, Meyermann R, Dichgans J, Reed JC and
Weller M: Bag-1 and Bcl-2 gene transfer in malignant glioma:
modulation of cell cycle regulation and apoptosis. Brain Pathol.
10:223–234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nadler Y, Camp RL, Giltnane JM, Moeder C,
Rimm DL, Kluger HM and Kluger Y: Expression patterns and prognostic
value of Bag-1 and Bcl-2 in breast cancer. Breast Cancer Res.
10:R352008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YD, Ha MW, Cheng J, Zhang WL, Cong X,
Tong CY and Sun J: The role of expression and polymorphism of the
BAG-1 gene in response to platinum-based chemotherapeutics in
NSCLC. Oncol Rep. 27:979–986. 2012.PubMed/NCBI
|
|
10
|
Yamauchi H, Adachi M, Sakata K, Hareyama
M, Satoh M, Himi T, Takayama S, Reed JC and Imai K: Nuclear BAG-1
localization and the risk of recurrence after radiation therapy in
laryngeal carcinomas. Cancer Lett. 165:103–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coutinho-Camillo CM, Lourenço SV,
Nishimoto IN, Kowalski LP and Soares FA: Expression of Bcl-2 family
proteins and association with clinicopathological characteristics
of oral squamous cell carcinoma. Histopathology. 57:304–316. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
13
|
Zhu L, Yan W, Rodriguez-Canales J,
Rosenberg AM, Hu N, Goldstein AM, Taylor PR, Erickson HS,
Emmert-Buck MR and Tangrea MA: MicroRNA analysis of microdissected
normal squamous esophageal epithelium and tumor cells. Am J Cancer
Res. 1:574–584. 2011.PubMed/NCBI
|
|
14
|
Noguchi T, Takeno S, Shibata T, Fumoto S,
Uchida Y, Yokoyama S, Gabbert HE and Müller W: Nuclear BAG-1
expression is a biomarker of poor prognosis in esophageal squamous
cell carcinoma. Dis Esophagus. 16:107–111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang B, Zhou H, Wang X and Liu Z:
Silencing SATB1 with siRNA inhibits the proliferation and invasion
of small cell lung cancer cells. Cancer Cell Int. 13:82013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang B, Gao Y, Tian D and Zheng M: A
small interfering ABCE1-targeting RNA inhibits the proliferation
and invasiveness of small cell lung cancer. Int J Mol Med.
25:687–693. 2010.PubMed/NCBI
|
|
17
|
Zheng HC, Xu XY, Xing YN, Wei ZL,
Takahashi H, Masuda S and Takano Y: Nuclear or cytoplasmic
localization of Bag-1 distinctly correlates with pathologic
behavior and outcome of gastric carcinomas. Hum Pathol. 41:724–736.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hassumi-Fukasawa MK, Miranda-Camargo FA,
Zanetti BR, Galano DF, Ribeiro-Silva A and Soares EG: Expression of
BAG-1 and PARP-1 in precursor lesions and invasive cervical cancer
associated with human papillomavirus (HPV). Pathol Oncol Res.
18:929–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noguchi T, Takeno S, Shibata T, Fumoto S,
Uchida Y, Yokoyama S, Gabbert HE and Müller W: Nuclear BAG-1
expression is a biomarker of poor prognosis in esophageal squamous
cell carcinoma. Dis Esophagus. 16:107–111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia L, Guan W, Wang D, Zhang YS, Zeng LL,
Li ZP, Wang G and Yang ZZ: Killing effect of Ad5/F35-APE1 siRNA
recombinant adenovirus in combination with hematoporphrphyrin
derivative-mediated photodynamic therapy on human nonsmall cell
lung cancer. Biomed Res Int. 2013:9579132013.
|
|
21
|
Yi X, Zhao G, Zhang H, Guan D, Meng R,
Zhang Y, Yang Q, Jia H, Dou K, Liu C, Que F and Yin JQ: MITF-siRNA
formulation is a safe and effective therapy for human melasma. Mol
Ther. 19:362–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu H, Liang Y, Li Y, Li YW, Wang J, Wu H,
Wang Y, Tang SC, Chen J and Zhou Q: Gene silencing of BAG-1
modulates apoptotic genes and sensitizes lung cancer cell lines to
cisplatin-induced apoptosis. Cancer Biol Ther. 9:832–840. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang T, Mi Y, Pian L, Gao P, Xu H, Zheng Y
and Xuan X: RNAi targeting CXCR4 inhibits proliferation and
invasion of esophageal carcinoma cells. Diagn Pathol. 8:1042013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen YG, Xu YJ, Shi ZL, Han HL, Sun DQ and
Zhang X: Effects of RNAi-mediated matrix metalloproteinase-2 gene
silencing on the invasiveness and adhesion of esophageal carcinoma
cells, KYSE150. Dig Dis Sci. 57:32–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang S, Ding F, Luo A, Chen A, Yu Z, Ren
S, Liu Z and Zhang L: XIAP is highly expressed in esophageal cancer
and its downregulation by RNAi sensitizes esophageal carcinoma cell
lines to chemotherapeutics. Cancer Biol Ther. 6:973–980. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang B, Gong X, Zhou H, Xiong F and Wang
S: Depleting ABCE1 expression induces apoptosis and inhibits the
ability of proliferation and migration of human esophageal
carcinoma cells. Int J Clin Exp Pathol. 7:584–592. 2014.PubMed/NCBI
|