Introduction
In the present study, we focused on the β-isoform of
Lifeguard. This transmembrane protein belongs to the
uncharacterised protein family UPF0005. The long version termed
Lifeguard has already been identified as a molecule that inhibits
death mediated by Fas in tumour cells (1–3).
Although the mechanism and action of Lifeguard remain unclear, it
is hypothesised that it either plays a role by interacting with Fas
or at the level of the Fas/FADD complex. Nevertheless, it was
previously shown that Lifeguard interacts with Bax and is localised
in cellular membranes of the endoplasmic reticulum and in the
plasma membrane (3–5). Previously, Lifeguard was found to be
highly expressed in breast carcinoma tissues. The expression of
Lifeguard was found to correlate with high tumour grades in primary
breast tumours and it was dependent on the activity of Akt/LEF-1
signalling (6).
To date, there are no published data evaluating the
role of the Lifeguard β-isoform in carcinogenesis. In the present
study we eavaluated the expression of Lifeguard β-isoform in breast
cancer cell lines in vitro and its expression in human
breast cancer tissue samples by western blotting and
immunofluorescence. We tested the functional relationship between
Fas and Lifeguard β-isoform expression by demonstrating the
correlation between Lifeguard β-isoform expression and resistance
against cell death stimulation by an agonist Fas antibody.
Materials and methods
Cell lines
A normal human mammary cell line (MCF10A), derived
from normal breast epithelium, three human breast carcinoma cell
lines (MCF-7, MDA-MB-231, T47D) and a liposarcoma carcinoma U2OS
cell line were used in this study. All of the cell lines were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). MCF-7, MDA-MB-231 and U2OS cell lines were grown in
Dulbecco’s modified Eagle’s medium (DMEM; PAA, Cölbe, Germany)
supplemented with 10% FCS (Biochrom, Berlin, Germany) and 50 mg/ml
penicillin-streptomycin. T47D cells were grown in RPMI-1640 medium
with 0.2 U/ml bovine insulin (10 mg/ml). The MCF-10A cells were
cultured in defined mammary epithelial growth media (CC-2571; MEGM
Bullet Kit; Lonza, Inc.). All of the cells were maintained at 37°C
in 5% carbon dioxide in a humidified atmosphere. The cells were
subcultured every 2 to 3 days by treatment with 0.25% trypsin/0.53
mM ethylenediaminetetraacetic acid (EDTA) solution. Primary human
breast cancer-derived epithelial cells (HBCECs) were obtained from
explant cultures of human breast cancer biopsies after negative
testing for HIV-1, hepatitis B and C, bacteria, yeast and fungi,
respectively, as previously described (34). Informed written consent was obtained
from each patient for the use of individual biopsy material, and
the study was approved by the Institutional Review Board, Project
#3916 on June 15, 2005. The primary HBCECs were cultured further in
serum-free and phenol red-free mammary epithelial cell growth
medium (MEGM) (Lonza, Basel, Switzerland) in a humidified
atmosphere at 37°C. Half of the cell culture medium was replaced
approximately every fourth day to maintain a conditioned
medium.
cDNA plate array
cDNA plate array analysis is a plate-based
hybridization profiling technique that is used for monitoring the
expression of dozens of genes through reverse transcription of mRNA
into cDNA. For this analysis, total RNA was isolated from cells
transfected with pMK-Lifeguard or with pMK-Lifeguard β-isoform
vector (GeneArt, Regensburg, DE, USA), using the NucleoSpin RNA II
kit (Macherey-Nagel, Düren, Germany). RNA samples (8 μg) were
analyzed by microarray analysis using an Akt pathway-regulated cDNA
plate array (Signosis, Sunnyvale, CA, USA) according to the
manufacturer’s instructions. Each well on the plate contained a
cDNA probe for one of the 24 Akt pathway-regulated genes. After
reverse transcription, in situ hybridisation, blocking and
extensive washing, the wells were incubated with streptavidin-HRP,
and the resulting chemiluminescence was measured within 5 min using
a luminometer (Tecan Schweiz AB, Zurich, Switzerland).
Western blot analysis
For the western blot analysis, the cells were lysed
in RIPA buffer containing 0.3 M NaCl, 1% sodium desoxycholate, 0.1%
sodium dodecyl sulfate (SDS), 1% Triton X-100, 20 mM Tris-HCL (pH
8.0), 1 mM EDTA and 1 mM phenylmethylsulfonyl fluoride (PMSF).
Protein (25 μg) was fractionated by 15% SDS-PAGE and transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore Corporation,
Bedford, MA, USA). The membranes were blocked in Odyssey buffer
(Li-COR Biosciences, Lincoln, NE, USA) for 1 h. The protein
expression levels were determined by immunoblotting with the
polyclonal antibodies anti-hLifeguard β-isoform (1:200 dilution;
generated in our laboratory) and monoclonal anti-hNGF-R, TrK-A/B,
Act-p, actin (all used at 1:500; Abcam, Cambridge, UK) at 4°C
overnight. To quantify the protein expression levels, Odyssey
680/800 nm secondary conjugates were used, and the PVDF membranes
were analyzed using the Odyssey infrared imaging system and
software (Li-COR Biosciences).
Immunofluorescence
Breast tissue slides were deparaffinised in xylene
followed by an alcohol gradient. To reduce the non-specific
background staining, the slides were incubated in 0.3% bovine serum
albumin/1X Tris-buffered saline for 30 min and incubated with
hLifeguard-β-iso rabbit primary antibodies at 4°C overnight. The
slides were washed twice for 5 min with phosphate-buffered saline
(PBS) and incubated for 30 min with goat anti-rabbit Alexa Fluor
488 (Invitrogen) secondary antibody. The signals were detected
using the Axiovert 200M fluorescence microscope (Zeiss) equipped
with the appropriate barrier filters.
Caspase-3/7 assay
Activation of caspase-3/7 was determined using the
Apo-One Homogeneous Caspase-3/7 assay (Promega, Madison, WI, USA)
according to the manufacturer’s instructions. Briefly, MCF-10A
breast cells were seeded (1×104/well) in a 96-well plate
and transfected with pMK-Lifeguard and pMK-Lifeguard β-isoform
vector for 24 h. After 24 h, the cells were incubated with 50 ng/ml
agonistic anti-Fas (clone CH11; Abcam) for an additional 24 h.
Following treatment, the cells were mixed with the same volume of
Apo-One Homogeneous Caspase-3/7 reagent and incubated at room
temperature for 2 h. Caspase-3/7 activation was estimated from
sample fluorescence at the excitation wavelength of 492 nm and the
emission wavelength of 521 nm using the fluorescence plate reader
Tecan GENios (Tecan Schweiz AB, Zurich, Switzerland).
Results
Expression of Lifeguard β-isoform in
human breast cancer cells and tissues
To elucidate the role of the Lifeguard β-isoform in
the regulation of apoptosis in breast cancer, we first assessed the
protein expression of the Lifeguard β-isoform in different human
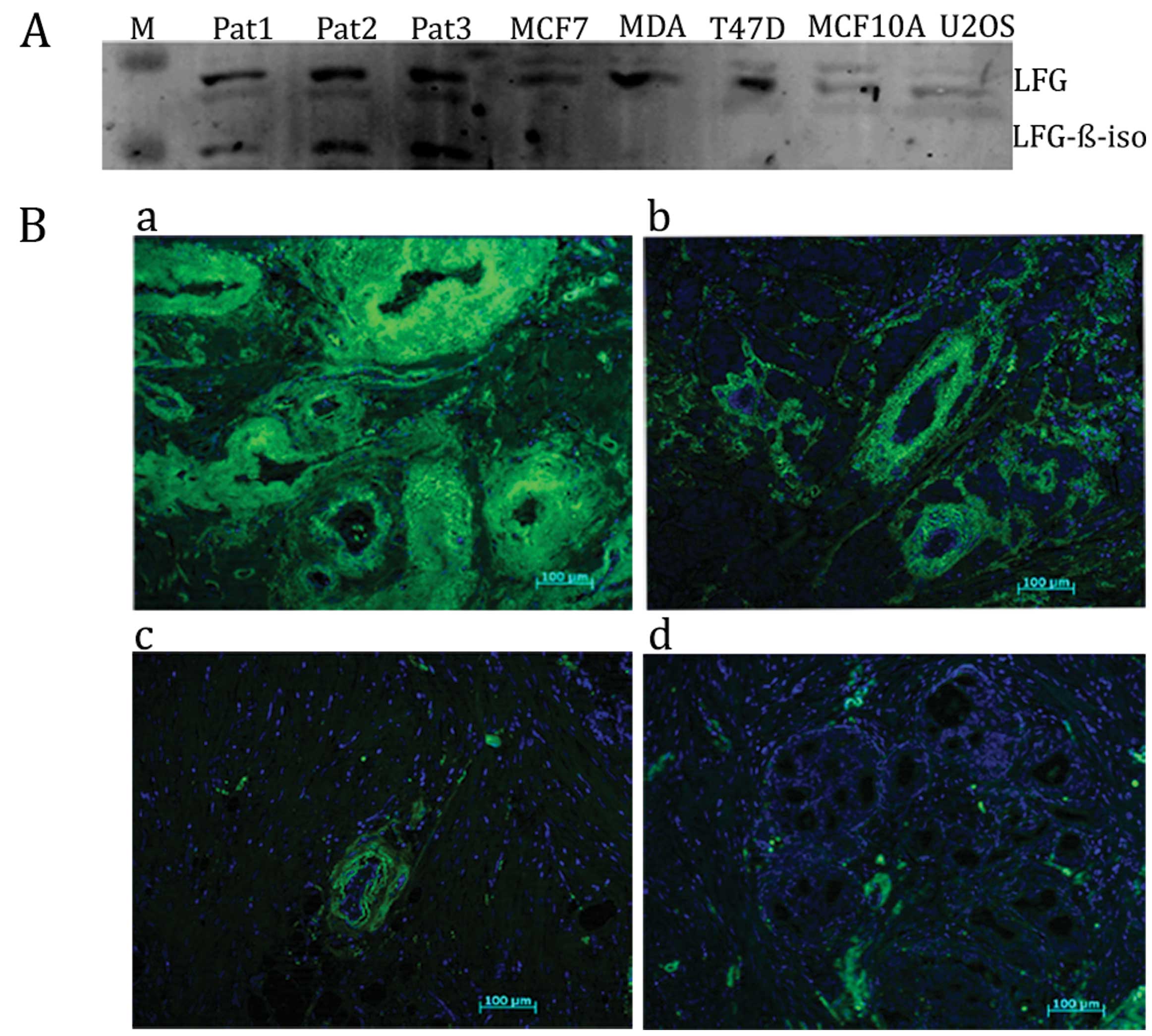
breast cancer cells and tissues. The human Lifeguard β-isoform was
observed only in the patient primary cell cultures from invasive
breast carcinoma with tumour grade III (cells appeared abnormal and
tended to grow and spread more aggressively), but was not detected
in the breast cancer cell lines and the liposarcoma carcinoma U2OS
cell line (Fig. 2A).
To test the relevance of Lifeguard β-isoform protein
expression in patient primary breast carcinoma cells derived from
human breast cancer specimens, we examined the expression of
Lifeguard β-isoform protein in carcinoma breast tissue sections.
Representative image pairs, detected by microscopy from tissue
samples with different tumour grades with varying levels of the
Lifeguard β-isoform protein expression compared with the control
staining, are shown in Fig. 2B.
Overexpression of the Lifeguard β-isoform
inhibits apoptosis and induces the expression of genes of the Akt2
pathway
To investigate the ability of Lifeguard β-isoform
expression to suppress Fas-induced apoptosis, the human breast cell
line MCF10A without endogenous Lifeguard β-isoform expression was
selected. Two vectors were designed, pMK-Lifeguard and
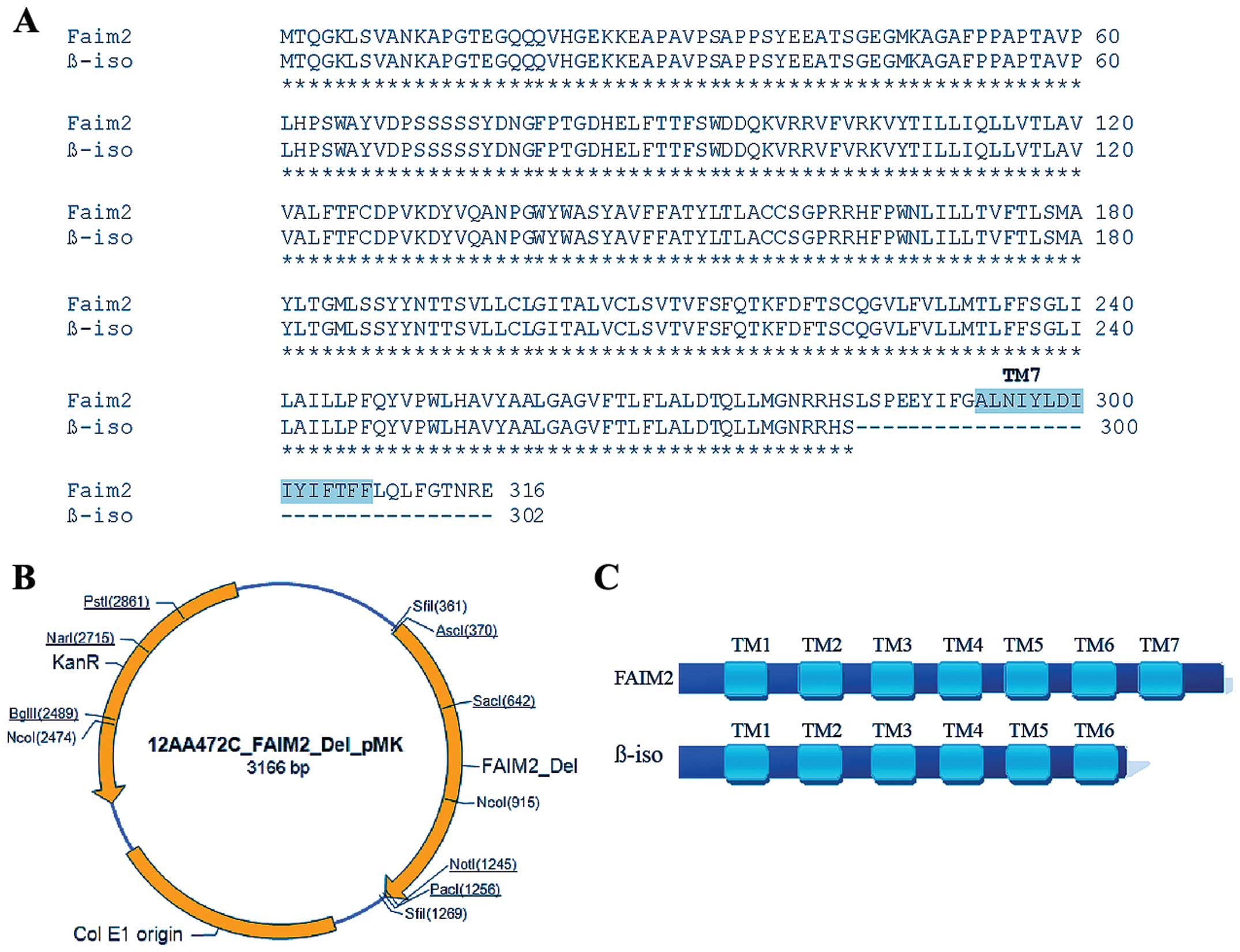
pMK-Lifeguard β-isoform (Fig. 1A and
B) and were tested for their activity (data not shown). The
MCF10A cells were transfected with pMK-Lifeguard β-isoform for 24 h
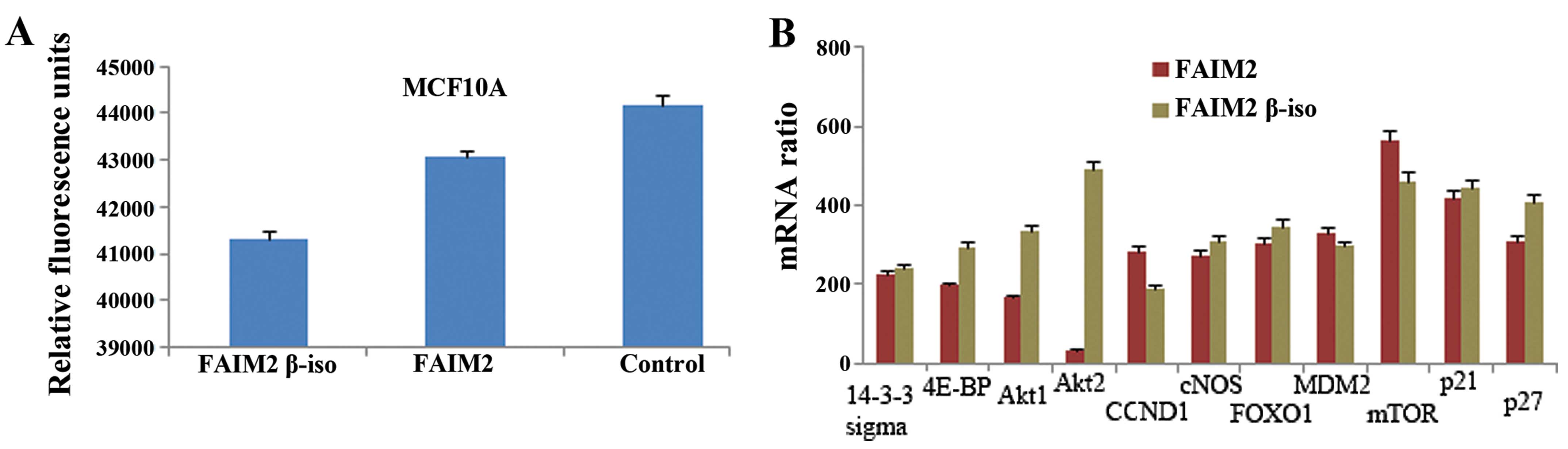
and treated with 50 ng/ml of agonistic anti-Fas. Following 24 h of
incubation, significantly increased levels of caspase 3/7 were
detected in the non-transfected cells when compared to the levels
in the Lifeguard and Lifeguard β-isoform transfectants (Fig. 3A). The inactivation of apoptosis
revealed the Lifeguard β-isoform to be a potential regulator of
apoptosis in tumour cells.
Taking into consideration the anti-apoptotic
activity of Lifeguard β-isoform, we next aimed to ascertain the
genes that are expressed at higher levels when the β-isoform is
present by analysis using the human Akt pathway regulated cDNA
plate array kit. Before RNA isolation, the MCF10A cells were
transfected with pMK-Lifeguard or pMK-Lifeguard β-isoform,
respectively, for 24 h. The results showed that the Lifeguard
β-isoform-transfected cells exhibited upregulated expression of
4E-BP and p27 expression as well as the expression of Akt1 and Akt2
compared to the Lifeguard-transfected cells (Fig. 3B).
Nerve growth factor-induced Lifeguard
β-isoform expression
In order to demonstrate a direct effect of the nerve
growth factor (NGF) on Lifeguard β-isoform expression, we treated
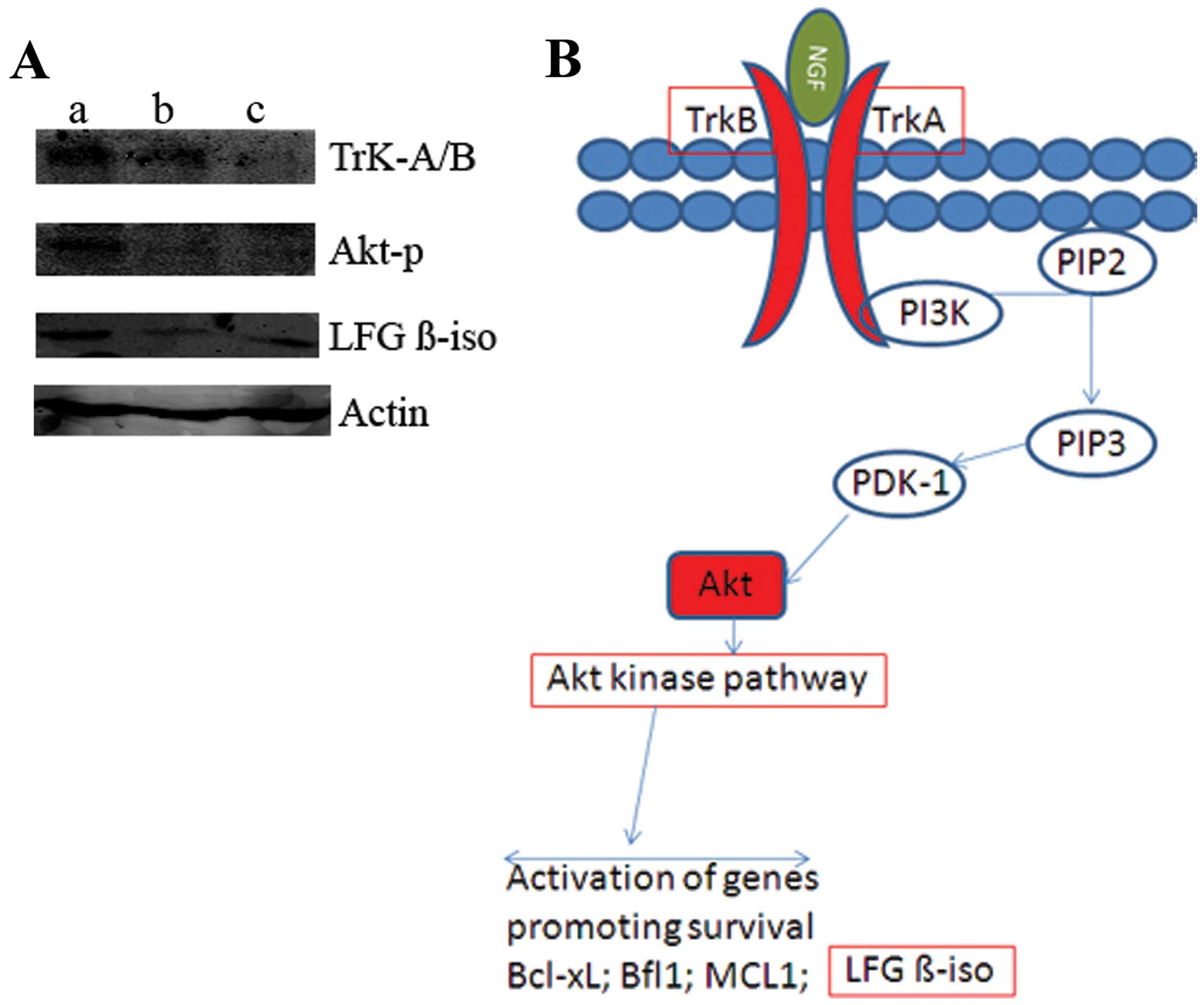
the MCF10A breast cell line with 0.5 or 1.5 μg/ml NGF for 24 h.
Analysis of cellular protein lysates from the MCF10A cells by
western blotting demonstrated that NGF dose-dependently upregulated
the expression of Lifeguard β-isoform protein. Furthermore, we
found significant increases in the expression of TrK-A/B and Akt-p
protein after treatment with NGF (Fig.
4A). These observations identify Lifeguard β-isoform as a
target of the NGF pathway (Fig.
4B), a regulation which could play a role in breast tumour
progression.
Discussion
Dysregulation of apoptosis plays an important role
in the pathogenesis of human cancers (7). Lifeguard, a member of a unique gene
family with high structural similarity (8), was isolated and identified as a
molecule that inhibits death mediated by Fas in tumour cells. Given
the high structural similarity and phylogenetic relationships among
the Lifeguard proteins, Hu et al reported that it is highly
likely that all Lifeguard family members are in some way apoptosis
modulating (8). Somia et al
showed that Lifeguard binds directly to the Fas receptor but not to
Fas adaptor proteins (1). The
anti-apoptotic role of Lifeguard has been tested in LN-18
astrocytoma, cervical carcinoma HeLa and Jurkat T cell lines
(10), yet the exact mechanism of
action of Lifeguard remains unclear. It is well documented that
dominant-negative Akt/PKB inhibits Lifeguard activity, whereas
overexpression of constitutively active Akt/PKB increases Lifeguard
activity (9,10). In comparison to Lifeguard, the
β-isoform has one transmembrane domain less; specifically the last
(seventh) transmembrane is missing. Due to this, we hypothesised
that the Lifeguard β-isoform exhibits a different function
(Fig. 1A and C).
In the present study, we examined the expression of
Lifeguard β-isoform protein in normal breast and carcinoma cell
lines and carcinoma tissues. We provide convincing evidence that
expression of Lifeguard β-isoform protein was increased in breast
carcinoma versus normal cells and tissues. Moreover, we found
convincing evidence that the expression of the Lifeguard β-isoform
protein was increased in breast carcinoma relative to
differentiated tissues (Fig. 2B).
In contrast to BI-1, for which high expression rates have been
demonstrated in several tumour tissues and cancer cell lines
(11–14) and Lifeguard (3) this is the first time that high
Lifeguard β-isoform expression rates can be phenotypically linked
to human cancer (4). Resistance to
apoptosis and alterations in Fas signalling were initially observed
in breast carcinoma cell lines (15). Several further studies on breast
cancer patients indicated that the Fas/FasL status may have a
significant impact on patient survival (16–19).
These results, together with the evidence obtained during
experiments on other solid malignancies (20–26),
suggest that the tumour levels of Fas/FasL possibly influence the
prognosis of oncology patients. In the present study, we found that
Lifeguard β-isoform protein expression reduced the sensitivity
against stimulation with an agonistic Fas antibody (Fig. 3A) even more than the long version of
Lifeguard, which has been previously identified as a molecule that
inhibits death mediated by Fas in tumour cells (8).
In regards to breast cancer, studies have focused on
the opposing functions of Akt1 and Akt2 on cell migration and
invasion. In this study, we found that the Akt2 isoform was
activated to a greater degree by the Lifeguard β-isoform than by
Lifeguard (Fig. 3B). Several
downstream Akt targets have been shown to contribute to the
differential functions of these two isoforms in motility, including
palladin, nuclear factor of activated T cells, tuberous sclerosis
complex 2 and β1 integrins (27–30).
Concerning breast cancer, it has been shown that NGF
promotes both tumour cell survival and proliferation (31–33).
In the present study, we first demonstrated that addition of NGF
increased Lifeguard β-isoform activity in MCF10A breast cells
(Fig. 4A). Furthermore, we
determined that TrK-A/B and p-Akt protein expression levels were
increased following exposure to NGF. These data suggest that
over-expression of the Lifeguard β-isoform in breast cells has
oncogenic potential.
In conclusion, in the present study we have shown
that high levels of Lifeguard isoform expression are associated
with the grade of the breast tumour. More notably, we found that
Lifeguard β-isoform plays a greater role than Lifeguard in the
development of breast cancer. Thus, the Lifeguard β-isoform is a
potential target for therapeutic benefit in cancer.
References
|
1
|
Somia NV, Schmitt MJ, Vetter DE, Van
Antwerp D, Heinemann SF and Verma IM: LFG: an anti-apoptotic gene
that provides protection from Fas-mediated cell death. Proc Natl
Acad Sci USA. 96:12667–12672. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernandez M, Segura MF, Sole C, Colino A,
Comella JX and Cena V: Lifeguard/neuronal membrane protein 35
regulates Fas ligand-mediated apoptosis in neurons via microdomain
recruitment. J Neurochem. 103:190–203. 2007.PubMed/NCBI
|
|
3
|
Bucan V, Reimers K, Choi CY, Eddy MT and
Vogt PM: The anti-apoptotic protein lifeguard is expressed in
breast cancer cells and tissues. Cell Mol Biol Lett. 15:296–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reimers K, Choi CY, Bucan V and Vogt PM:
The Bax inhibitor-1 (BI-1) family in apoptosis and tumourigenesis.
Curr Mol Med. 8:148–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reimers K, Choi CY, Bucan V and Vogt PM:
The growth-hormone inducible transmembrane protein (Ghitm) belongs
to the Bax inhibitory protein-like family. Int J Biol Sci.
3:471–476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bucan V, Choi CY, Lazaridis A, Vogt PM and
Reimers K: Silencing of anti-apoptotic transmembrane protein
lifeguard sensitizes solid tumour cell lines MCF-7 and SW872 to
peri-fosine-induced cell death activation. Oncol Lett. 2:419–422.
2011.PubMed/NCBI
|
|
7
|
Nguyen A, Rosner A, Milovanovic T, et al:
Wnt pathway component LEF1 mediates tumour cell invasion and is
expressed in human and murine breast cancers lacking ErbB2
(her-2/neu) overexpression. Int J Oncol. 27:949–956.
2005.PubMed/NCBI
|
|
8
|
Hu L, Smith TF and Goldberger G: LFG: a
candidate apoptosis regulatory gene family. Apoptosis.
14:1255–1265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beier CP, Wischhusen J, Gleichmann M, et
al: FasL (CD95L/APO-1L) resistance of neurons mediated by
phosphatidylinositol 3-kinase-Akt/protein kinase B-dependent
expression of lifeguard/neuronal membrane protein 35. J Neurosci.
25:6765–6774. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Satoh A, Bryant SV and Gardiner DM:
Regulation of dermal fibroblast dedifferentiation and
redifferentiation during wound healing and limb regeneration in the
Axolotl. Dev Growth Differ. 50:743–754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grzmil M, Thelen P, Hemmerlein B, Schweyer
S, Voigt S, Mury D and Burfeind P: Bax inhibitor-1 is overexpressed
in prostate cancer and its specific down-regulation by RNA
interference leads to cell death in human prostate carcinoma cells.
Am J Pathol. 163:543–552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grzmil M, Kaulfuss S, Thelen P, Hemmerlein
B, Schweyer S, Obenauer S, Kang TW and Burfeind P: Expression and
functional analysis of Bax inhibitor-1 in human breast cancer
cells. J Pathol. 208:340–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanaka R, Ishiyama T, Uchihara T, Inadome
Y, Iijima T, Morishita Y, Kano J, Goya T and Noguchi M: Expression
of the Bax inhibitor-1 gene in pulmonary adenocarcinoma. Cancer.
106:648–653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Villalva C, Trempat P, Greenland C, Thomas
C, Girard JP, Moebius F, Delsol G and Brousset P: Isolation of
differentially expressed genes in NPM-ALK-positive anaplastic large
cell lymphoma. Br J Haematol. 188:791–798. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Keane MM, Ettenberg SA, Lowrey GA, Russell
EK and Lipkowitz S: Fas expression and function in normal and
malignant breast cell lines. Cancer Res. 56:4791–4798.
1996.PubMed/NCBI
|
|
16
|
Sjöström J, Blomqvist C, von Boguslawski
K, et al: The predictive value of bcl-2, bax, bcl-xL, bag-1, fas,
and fasL for chemotherapy response in advanced breast cancer. Clin
Cancer Res. 8:811–816. 2002.PubMed/NCBI
|
|
17
|
Botti C, Buglioni S, Benevolo M, et al:
Altered expression of FAS system is related to adverse clinical
outcome in stage I–II breast cancer patients treated with adjuvant
anthracycline-based chemotherapy. Clin Cancer Res. 10:1360–1365.
2004.PubMed/NCBI
|
|
18
|
Mottolese M, Buglioni S, Bracalenti C, et
al: Prognostic relevance of altered Fas (CD95)-system in human
breast cancer. Int J Cancer. 89:127–132. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reimer T, Herrnring C, Koczan D, Richter
D, Gerber B, Kabelitz D, Friese K and Thiesen HJ: FasL: Fas ratio -
a prognostic factor in breast carcinomas. Cancer Res. 60:822–828.
2000.PubMed/NCBI
|
|
20
|
Qin LX and Tang ZY: The prognostic
molecular markers in hepatocellular carcinoma. World J
Gastroenterol. 8:385–392. 2002.PubMed/NCBI
|
|
21
|
Yamana K, Bilim V, Hara N, Kasahara T,
Itoi T, Maruyama R, Nishiyama T, Takahashi K and Tomita Y:
Prognostic impact of FAS/CD95/APO-1 in urothelial cancers:
decreased expression of Fas is associated with disease progression.
Br J Cancer. 93:544–551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Onodera H, Mori A, Nagayama S, Fujimoto A,
Tachibana T, Yonenaga Y and Tsuruyama T: Fas/CD95 signaling rather
than angiogenesis or proliferative activity is a useful prognostic
factor in patients with resected liver metastases from colorectal
cancer. Int J Colorectal Dis. 20:477–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sträter J, Hinz U, Hasel C, Bhanot U,
Mechtersheimer G, Lehnert T and Möller P: Impaired CD95 expression
predisposes for recurrence in curatively resected colon carcinoma:
clinical evidence for immunoselection and CD95L mediated control of
minimal residual disease. Gut. 54:661–665. 2005.PubMed/NCBI
|
|
24
|
Lee WC, Yu MC and Chen MF: Prognostic
impact of Fas ligand on hepatocellular carcinoma after hepatectomy.
World J Surg. 28:792–796. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murakami M, Sasaki T, Miyata H, Yamasaki
S, Kuwahara K and Chayama K: Fas and Fas ligand: expression and
soluble circulating levels in bile duct carcinoma. Oncol Rep.
11:1183–1186. 2004.PubMed/NCBI
|
|
26
|
Kanauchi H, Wada N, Ginzinger DG, Yu M,
Wong MG, Clark OH and Duh QY: Diagnostic and prognostic value of
fas and telomeric-repeat binding factor-1 genes in adrenal tumours.
J Clin Endocrinol Metab. 88:3690–3693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chin YR and Toker A: The actin-bundling
protein palladin is an Akt1-specific substrate that regulates
breast cancer cell migration. Mol Cell. 38:333–344. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoeli-Lerner M, Yiu GK, Rabinovitz I,
Erhardt P, Jauliac S and Toker A: Akt blocks breast cancer cell
motility and invasion through the transcription factor NFAT. Mol
Cell. 20:539–550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu H, Radisky DC, Nelson CM, Zhang H,
Fata JE, Roth RA and Bissell MJ: Mechanism of Akt1 inhibition of
breast cancer cell invasion reveals a protumourigenic role for
TSC2. Proc Natl Acad Sci USA. 103:4134–4139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arboleda MJ, Lyons JF, Kabbinavar FF, Bray
MR, Snow BE, Ayala R, et al: Overexpression of AKT2/protein kinase
Bbeta leads to up-regulation of beta1 integrins, increased
invasion, and metastasis of human breast and ovarian cancer cells.
Cancer Res. 63:196–206. 2003.PubMed/NCBI
|
|
31
|
Tagliabue E, Castiglioni F, Ghirelli C, et
al: Nerve growth factor cooperates with p185(HER2) in activating
growth of human breast carcinoma cells. J Biol Chem. 275:5388–5394.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiarenza A, Lazarovici P, Lempereur L,
Cantarella G, Bianchi A and Bernardini R: Tamoxifen inhibits nerve
growth factor-induced proliferation of the human breast cancerous
cell line MCF-7. Cancer Res. 61:3002–3008. 2001.PubMed/NCBI
|
|
33
|
Descamps S, Toillon RA, Adriaenssens E, et
al: Nerve growth factor stimulates proliferation and survival of
human breast cancer cells through two distinct signaling pathways.
J Biol Chem. 276:17864–17870. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hass R and Bertram C: Characterization of
human breast cancer epithelial cells (HBCEC) derived from long term
cultured biopsies. J Exp Clin Cancer Res. 28:1272009. View Article : Google Scholar : PubMed/NCBI
|