Introduction
Breast cancer, a type of cancer originating from
breast tissue, is a common cancer and the second leading cause of
cancer-related mortality among women (1,2).
Although there has been a sustained decline in the mortality rates
in recent decades due to development of increasingly effective
adjuvant medical treatments, relapse after the first 5 years is
frequent and is a major contributor to breast cancer mortality
(3–5). The major reason for relapse is
chemoresistance acquired during the chemotherapeutic process
(6–8). Therefore, overcoming acquired
chemoresistance is critically important to reducing the rate of
relapse and improving prognosis.
Doxorubicin, also known as adriamycin, is an
anthracycline antibiotic that functions by intercalating DNA
(9). It is commonly used in the
treatment of a wide range of cancers, including breast cancer,
hematological malignancies, soft tissue sarcomas and many types of
carcinoma (10–12). However, a long duration of
doxorubicin treatment often causes cancer cell resistance to
treatment and has the serious adverse effect of life-threatening
heart damage (13,14). Elucidating the molecular mechanisms
that underlie chemoresistance and overcoming established
chemoresistance to doxorubicin are clinically relevant challenges
in breast cancer treatment.
Genistein, first isolated from the dyer’s broom in
1899, is a phytoestrogen and belongs to the category of
isoflavones. Various studies have found that genistein has
inhibitory effects on many types of cancers, including breast
cancer (15,16). More importantly, several studies
have shown that genistein can enhance chemotherapeutic efficacy and
overcome chemoresistance in breast cancer (17–19).
However, the molecular mechanisms underlying genistein-mediated
reversal of chemoresistance are still largely unknown.
Materials and methods
Materials and equipment
The human breast cancer cell line MCF-7 and
resistant derivative cell line MCF-7/Adr were gifted by the
Department of Surgery, Wuhan Union Hospital, Hubei, China.
Sensitive human breast cancer MCF-7/s cells were obtained from ATCC
(USA). The MCF-7/adr cells (Michigan Cancer Foundation-7/adriamycin
resistant) were kindly provided by Dr. Huang Tao (University of
Science and Technology of Xiehe Hospital at Huazhong Surgery
Laboratory Center, Wuhan, Hubei). The cell line is derived from the
parental drug-sensitive MCF-7 cells by stepwise selection with Dox.
To maintain the drug-resistant phenotype, the cells were cultured
in the presence of 1 μg/ml Dox in RPMI-1640 culture medium
supplemented with 10% heat-inactivated fetal bovine serum
(Invitrogen), 10 mM glutamine, and antibiotics (100 U/ml penicillin
and 100 μg/ml streptomycin). Cells were maintained at 37ºC in 5%
CO2/95% air (20–22).
Genistein and rhodamine (Rh123) were obtained from
Sigma-Aldrich (USA). Doxorubicin hydrochloride was purchased from
Shanghai Biological Engineering Co., Ltd. (China). Oligo(dt) was
synthesized by Shanghai Biological Engineering Co. Taq PCR
MasterMix was purchased from Tiangen (China). Primers were
synthesized by Shanghai Biological Engineering Co. Anti-human
permeability glycoprotein (P-gp) monoclonal antibody was purchased
from Abcam (USA). Anti-c-erbB2 [human epidermal growth factor
receptor 2 (Her2/Neu)] polyclonal antibody was purchased from Dako
Corp. (USA). Anti-mouse IgG-horseradish peroxidase (HRP) and
anti-rabbit IgG-HRP were purchased from Beijing Ding National
Biotechnology Co. (China). A Coulter DNA PREP™ reagents kit was
purchased from Beckman-Coulter (USA). An Annexin V-FITC apoptosis
detection kit was purchased from Shanghai Yan-Bin Chemical
Technology (China). The F-7000 fluorescence spectrometer was
purchased from Hitachi (Japan), and the Hitachi UV
spectrophotometer was from Eppendorf (Germany).
Cells and culture
Cells were cultured in RPMI-1640 culture medium with
10% fetal calf serum at 37ºC with 5% CO2. Cells were
trypsinized with 0.25% trypsin and passed every 2 or 3 days.
MCF-7/Adr cells were maintained in medium supplemented with
doxorubicin (1.0 μg/ml) for two weeks. Cells were diluted from
5×104 to 5×105/ml for use in the
experiments.
Preparation of genistein
The purity of genistein was greater than 98%.
Twenty-five milligrams of genistein was dissolved in 1 ml
dimethylsulfoxide (DMSO) and stored at 4°C. Before use, genistein
was diluted in culture medium to the required concentration, with
the final percentage of DMSO <0.2%. Twenty-five milligrams of
doxorubicin was dissolved in 10 ml saline and stored at 20°C. For
experiments, doxorubicin was serially diluted to final
concentrations of 0.1, 1, 10 and 100 μM.
MTT assay
MCF-7/Adr cells in the logarithmic growth phase were
seeded in 96-well culture plates. To each well, we added 30 μmol/l
genistein and 0.07, 0.7, 7 or 70 μM doxorubicin, in a final volume
of 200 μl (n=8 for each concentration). Wells with medium only (no
cells) were used to measure the background, and seeded wells given
untreated medium served as the controls. Forty-eight hours after
treatment, MTT was added to measure proliferation. Q-value was
calculated by the formula of Guinness (17–19).
IC50 and the reversal fold were also calculated. The
reversal fold = IC50 (chemotherapy
drugs)/IC50 (genistein).
Fluorospectrophotometry
Quantification of doxorubicin concentrations was
carried out using a standard curve. The standard curve was
established using standard solutions of different doxorubicin
concentrations (0, 0.01, 0.05, 0.25, 1.25, 6.25 and 31.25 μM). Cell
culture medium was added to yield final genistein concentrations of
7, 15, 30, 60 and 120 μmol/l, and 0.7, 7 and 70 μM doxorubicin.
After experimental culture, the supernatants were collected and
centrifuged, and fluorescence intensity was measured. The
concentration of doxorubicin in the supernatant was calculated
according to the standard curve.
Analyses of cell cycle and apoptosis by
flow cytometry
MCF-7/Adr cells in the logarithmic growth phase were
seeded in 24-well plates. The cells were divided into following
groups: control group (culture medium), doxorubicin groups (10 or
100 μM), genistein groups (30 or 60 μmol/l), low-dose combination
group (1 μM doxorubicin + 30 μmol/l genistein), and high-dose
combination group (10 μM doxorubicin + 60 μmol/l genistein). The
final concentration of DMSO was 0.1%. Then cells were harvested and
washed twice with cold PBS. Cells were suspended in cold 70%
ethanol and fixed overnight at -20°C. Fixed cells were centrifuged,
resuspended in resuspension buffer, and 100 μl propidium iodide
(PI; 50 μg/ml) was added and incubated for 30 min at 4°C for
staining. A 200-μl sample was transferred to an injection tube,
along with 100 μl Coulter DNA-PREP reagents kit. After 2 min of
mixing, 1 ml Coulter DNA-PREP reagents kit stain was added and the
solution was mixed. PI single staining was used to assess cell
cycle via flow cytometry. PI and Annexin V-FITC double staining was
used to measure apoptosis. To measure Rh123, a fluorescent
substrate of P-gp, a cell suspension was added to 2 mg/l Rh123 or
Rh123 + different concentrations of genistein and incubated at 37°C
for 45 min. Cells were washed twice with cold PBS, and Rh123
concentrations were measured by flow cytometry.
RT-PCR
MCF-7/Adr cells in the logarithmic growth phase were
seeded in 6-well plates and divided into the following groups:
control, doxorubicin (1 or 10 μM), genistein (15, 30 or 60 μmol/l).
After 48 h of treatment, cells were harvested and total-RNA was
extracted. The A260:A280 ratio was ≥1.8. RT-PCR was performed
according to the two-step RT-PCR amplification kit instructions.
The primers were as follows: mdr-1 forward,
5′-CCCATCATTGCAATAGCAGG-3′ and reverse, 5′-GTTCAAACTTCTGCTCCTCA-3′;
Her2/neu forward, 5′-GCCCTCATCCACCATAACACC-3′ and reverse,
5′-CATTCCTCCACGCACTCCTG-3′; β-actin forward,
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse, 5′-GGG
CACGAAGGCTCATCATT-3′. The PCR product of mdr-1 is 157 bp; the PCR
product of Her2/neu is 220 bp; and the PCR product of control
β-actin is 200 bp. PCR conditions were as follows: 94°C
denaturation for 30 sec, 57°C refolding for 30 sec, and 72°C
extension for 30 sec, for a total of 30 cycles.
Western blot analysis
Cells were washed with cold PBS and lysed with
protein lysis buffer for 30 min. Cell lysates were centrifuged at
12,000 × g at 4°C for 15 min. After centrifugation, supernatants
were considered as total protein extract. The total amount of
protein for loading was adjusted using the measured protein
concentration. Six microliters of 5X sample buffer was added to
each sample for a final volume of 30 μl. Samples were boiled at
100°C for 5 min (protein denaturation) and subjected to
electrophoresis at 4°C. Proteins in the gel were transferred to a
polyvinylidene fluoride membrane and blotted with anti-human P-gp
antibody (diluted 1:20 in blocking solution) and the anti-c-erbB2
antibody (1:500) overnight at 4°C. HRP-conjugated secondary
antibodies were added for 2 h.
Statistical methods
SPSS 17.0 software was used for variance and
regression analyses and pairwise comparisons Q-test. Data are
presented as means ± standard deviation (SD). Comparison between
groups was performed via t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
MCF-7/Adr cells are resistant to
doxorubicin-induced cell death
It is known that MCF-7/Adr cells are resistant to
doxorubicin once the cell line is established. To confirm that
MCF-7/Adr cells do have this biological feature, we compared the
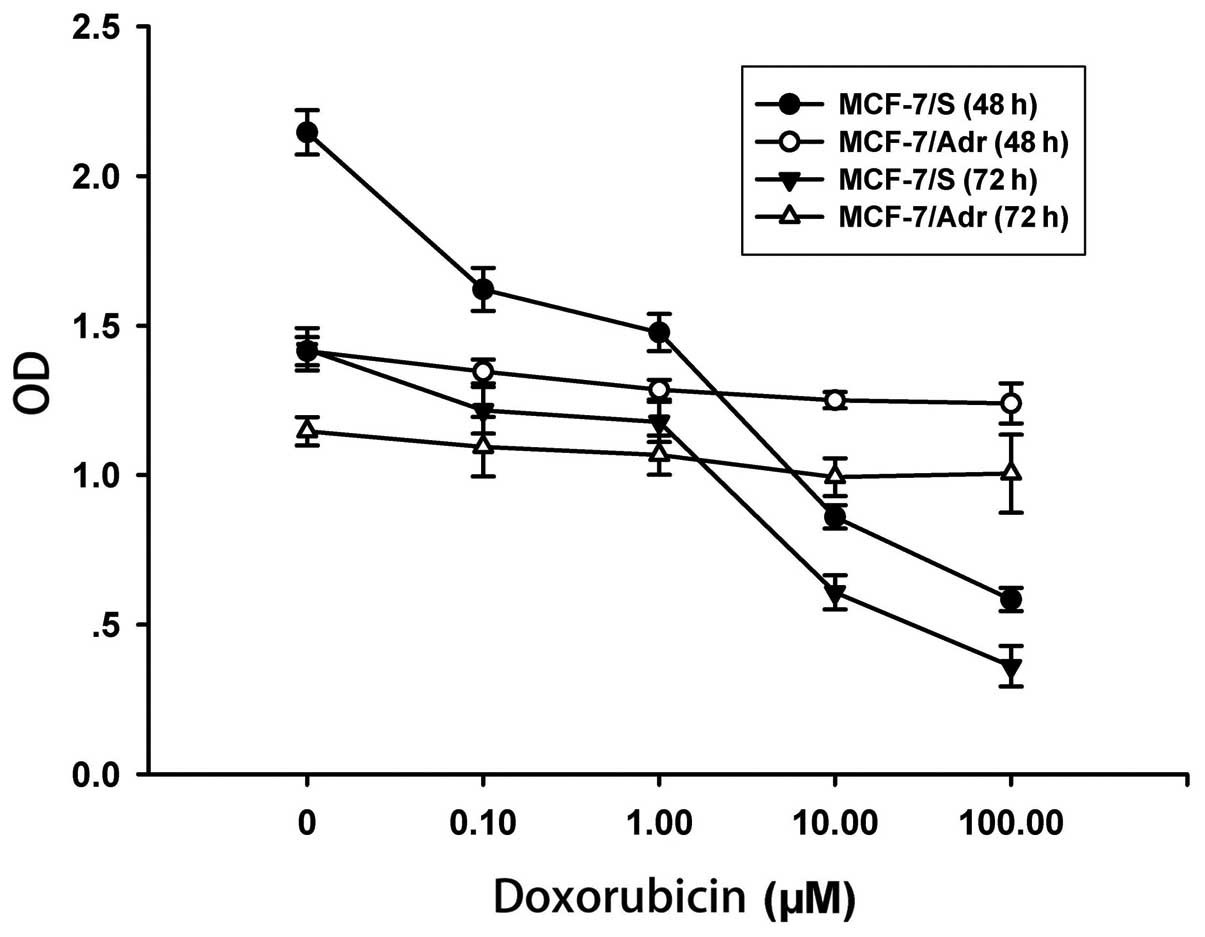
sensitivity of MCF-7/S and MCF-7/Adr cells. As shown in Fig. 1, doxorubicin inhibited the cell
proliferation of MCF-7/S cells in a dose- and time-dependent
manner; however, doxorubicin had no inhibitory effect on MCF-7/Adr
cells. In MCF-7/Adr cells, the IC50 for a 48-h treatment
was 117.15 μM (4.75±0.04 to 556.21±51.35 μM), whereas the
IC50 for a 72-h treatment was 54.42 μM (8.95±0.08 to
486.90±45.33 μM). Taken together, our results confirmed that the
MCF-7/Adr cells were resistant to doxorubicin.
Genistein enhances the cytotoxic effect
of doxorubicin in MCF-7/Adr cells
Several studies have shown that genistein can
enhance the cytotoxic effects of doxorubicin and reduce the
chemoresistance of tumor cells (17–19).
To test this observation, we treated MCF-7/Adr cells with genistein
(30 μmol/l) along with increasing doses of doxorubicin. We found
that genistein had a synergistic effect with doxorubicin at all
tested doses (Q>1.15; Table I).
The inhibitory effect of doxorubicin and genistein was
significantly greater than that of the control and doxorubicin
alone (P<0.01; Table I).
 | Table IGenistein enhances the cytotoxic
effect of doxorubicin in MCF-7/Adr cells (mean ± SD, n=8). |
Table I
Genistein enhances the cytotoxic
effect of doxorubicin in MCF-7/Adr cells (mean ± SD, n=8).
| Gen dose (30
μmol/l) |
|---|
|
|
|---|
| Dox dose (μM) | OD | IR (%) | Q-value |
|---|
| Control | 0.676±0.035 | - | - |
| 0.07 | 0.534±0.062a | 20.91 | 1.42 |
| 0.7 | 0.470±0.063a | 30.40 | 1.67 |
| 7.0 | 0.472±0.051a | 30.13 | 1.83 |
| 70 | 0.457±0.080a | 47.17 | 2.09 |
|
IC50 | 73.89 | |
| Fold | 7.53 | |
Genistein increases the intracellular
accumulation of doxorubicin
Our results revealed that genistein enhanced the
cytotoxic effect of doxorubicin. To better understand the molecular
mechanism of this observation, we investigated whether genistein
increases the intracellular accumulation of doxorubicin in
MCF-7/Adr cells. The intracellular accumulation of doxorubicin was
estimated by the formula y = 152.55 + 99.496x, R2=0.954.
As presented in Table II,
genistein significantly increased the intracellular accumulation of
doxorubicin (P<0.01). In the MCF-7/Adr cells, increased
concentration of doxorubicin alone did not significantly upregulate
the intracellular accumulation of doxorubicin, and the maximal
intracellular loading of doxorubicin was extremely limited in the
doxorubicin alone group (Table
II). However, genistein treatment significantly increased the
intracellular accumulation of doxorubicin in a dose-dependent
manner. More importantly, the maximal loading of doxorubicin was
greatly increased with genistein treatment (Table II).
 | Table IIEffect of genistein on the
intracellular accumulation of doxorubicin in MCF-7/Adr cells
(n=8). |
Table II
Effect of genistein on the
intracellular accumulation of doxorubicin in MCF-7/Adr cells
(n=8).
| Dox (μM) |
|---|
|
|
|---|
| Gen (μmol/l) | 0.7 | 7 | 70 |
|---|
| 0 | 0.16 | 0.20a | 0.22a |
| 7 | 0.45 | 0.71 | 0.99 |
| 15 | 0.79 | 0.95 | 1.21 |
| 30 | 0.85b | 2.32c | 2.66c |
| 60 | 1.73c | 3.59c | 4.55c |
| 120 | 2.04c | 4.05c | 4.92c |
Genistein has no effect on the
intracellular accumulation or excretion of Rh123
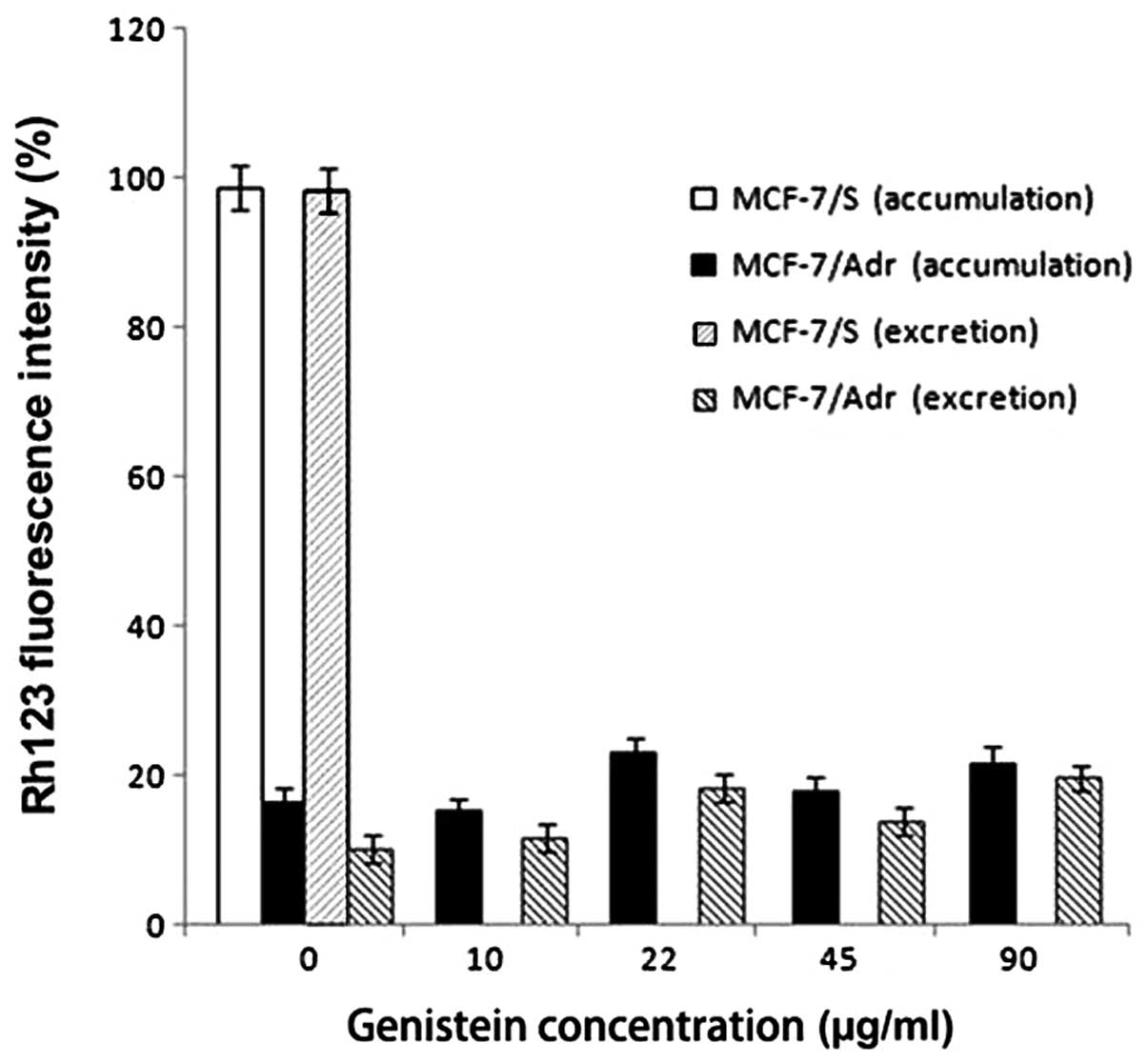
To further understand the mechanism underlying
doxorubicin accumulation in MCF-7/Adr cells, we tested the effect
of genistein on the intracellular accumulation of Rh123, a
fluorescent substrate of P-gp. Rh123 is transported by P-gp and
therefore can be used as a measure of drug extrusion by the plasma
membrane (17–19). The intracellular accumulation assay
showed that genistein had no effect on the intracellular
accumulation of Rh123 (Fig. 2).
Assays of Rh123 excretion by MCF7/S and MCF7/Adr cells also showed
that genistein had no effect on the excretion of Rh123 (Fig. 2). These results suggest that
genistein specifically affects the intracellular accumulation of
doxorubicin but not that of Rh123.
Genistein increases doxorubicin-induced
cell cycle arrest
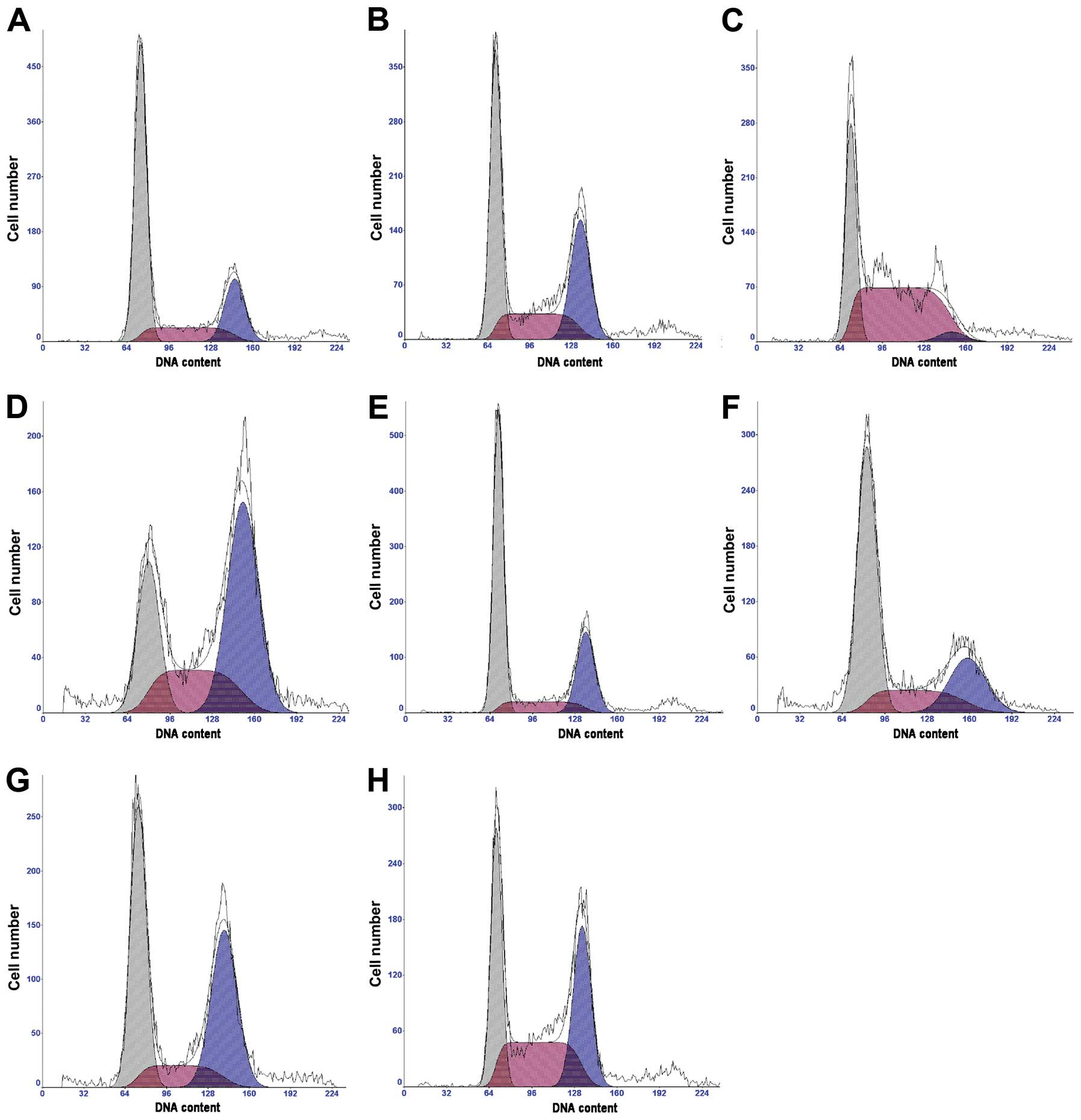
Doxorubicin intercalates into DNA and induces cell
cycle arrest. We tested whether genistein increases
doxorubicin-induced cell cycle arrest. Compared to the control,
both genistein and doxorubicin significantly induced MCF-7/Adr cell
cycle arrest at the G2/M phase (P<0.01; Table III and Fig. 3). Genistein treatment at 60 μmol/l
for 48 h and 72 h resulted in decreased percentages of cells in the
G1 and G2/M phases but increased the percentages of cells in the S
phase. The effect of combined genistein and doxorubicin treatment
on cell cycle arrest was much stronger than that of either
genistein or doxorubicin alone (Table
III and Fig. 3).
 | Table IIIEffect of genistein on
doxorubicin-induced MCF-7/Adr cell cycle arrest. |
Table III
Effect of genistein on
doxorubicin-induced MCF-7/Adr cell cycle arrest.
| | 48 h | 72 h |
|---|
| |
|
|
|---|
| Group | n | G1 | S |
G2/M | G1 | S |
G2/M |
|---|
| Control | 3 | 60.9 | 17.7 | 21.4 | 58.3 | 18.3 | 23.3 |
| 30 μmol/l Gen | 3 | 43.8 | 23.8 | 32.3a | 30.3 | 23.9 | 45.8a |
| 60 μmol/l Gen | 3 | 34.4 | 61.9a | 3.8 | 25.3 | 24.5 | 50.1a |
| 30 μmol/l Gen + 1
μM Dox | 3 | 58.1 | 14.3 | 27.6a | 53.3 | 16.2 | 30.5a |
| 60 μmol/l Gen + 10
μM Dox | 3 | 32.3 | 34.8 | 32.9a,c | 44.7 | 14.8 | 40.5a,b,c |
Genistein enhances doxorubicin-induced
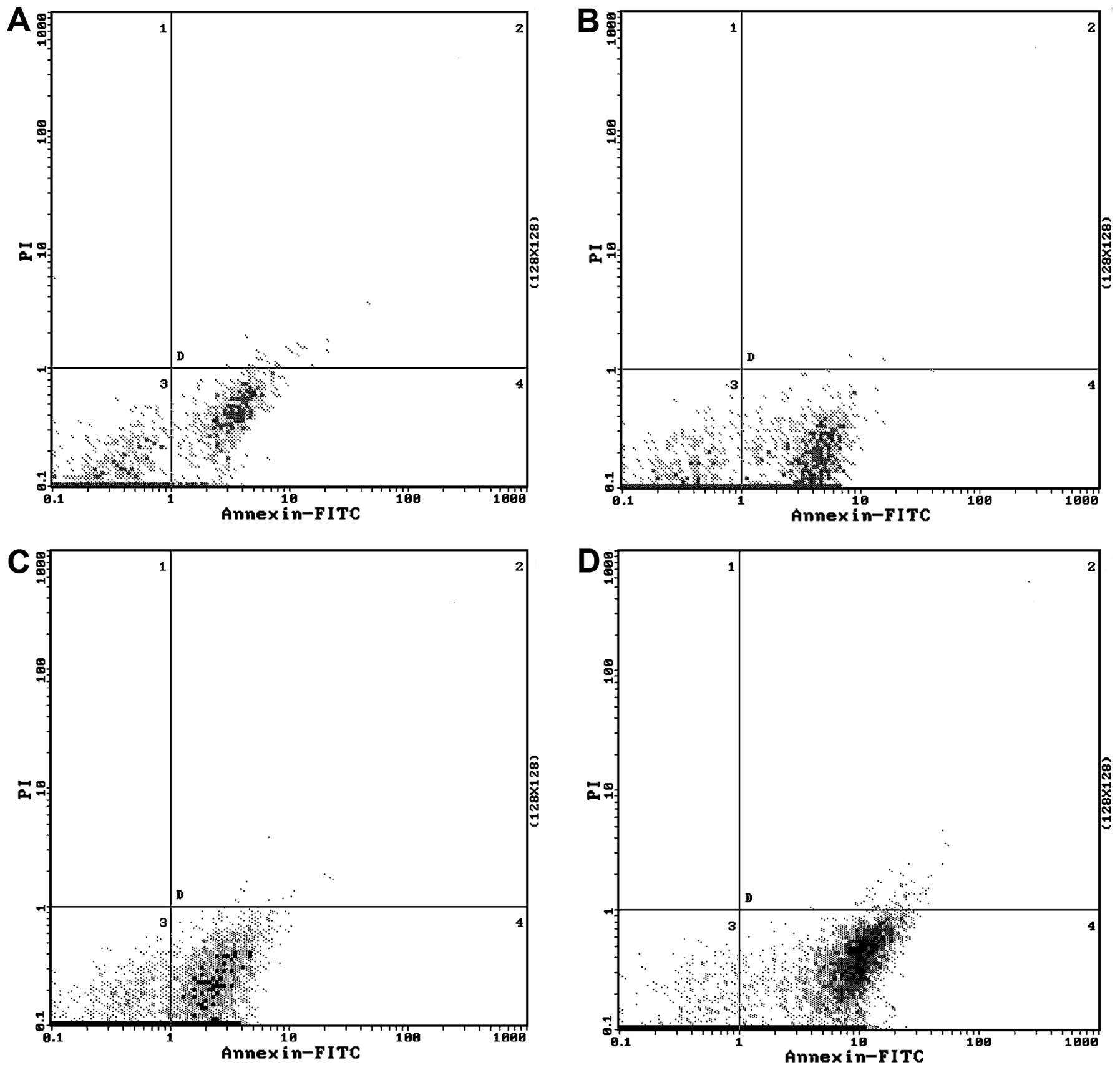
apoptosis
We tested whether genistein enhances
doxorubicin-induced apoptosis. Doxorubicin alone did not cause
obvious apoptosis in the MCF-7/Adr cells. However, when treated
with genistein alone or in combination with doxorubicin, MCF-7/Adr
cells became apoptotic (P<0.01; Table IV and Fig. 4). The genistein and doxorubicin
combination group showed the highest percentage of apoptotic cells
(Table IV and Fig. 4).
 | Table IVEffect of genistein on
doxorubicin-induced apoptosis. |
Table IV
Effect of genistein on
doxorubicin-induced apoptosis.
| | Apoptosis rate
(%) |
|---|
| |
|
|---|
| Group | n | Annexin (+) PI
(−) | Annexin (+) PI
(+) |
|---|
| Control | 3 | 5.92±0.22 | 0.24±0.02 |
| Dox (10 μM) | 3 | 13.8±0.85 | 0.02±0.01 |
| Gen (μmol/l) | 3 | 26.0±0.99a | 0.11±0.01 |
| Gen (60 μmol/l +
Dox (10 μM) | 3 | 35.8±1.57a,b | 0.57±0.04 |
Genistein suppresses Her2/neu mRNA
expression but not mdr-1 mRNA expression
To better understand the molecular mechanism of
genistein’s effects, we tested the influence of genistein on the
expression of mdr-1 and Her2/neu mRNA. Genistein and doxorubicin
alone or in combination had no effect on mdr-1 mRNA expression
(P>0.05; Fig. 5A). However,
genistein downregulated Her2/neu mRNA expression in a
dose-dependent manner (P<0.01; Fig.
5A). The combination of genistein and doxorubicin also
significantly suppressed Her2/neu mRNA expression (P<0.01;
Fig. 5A).
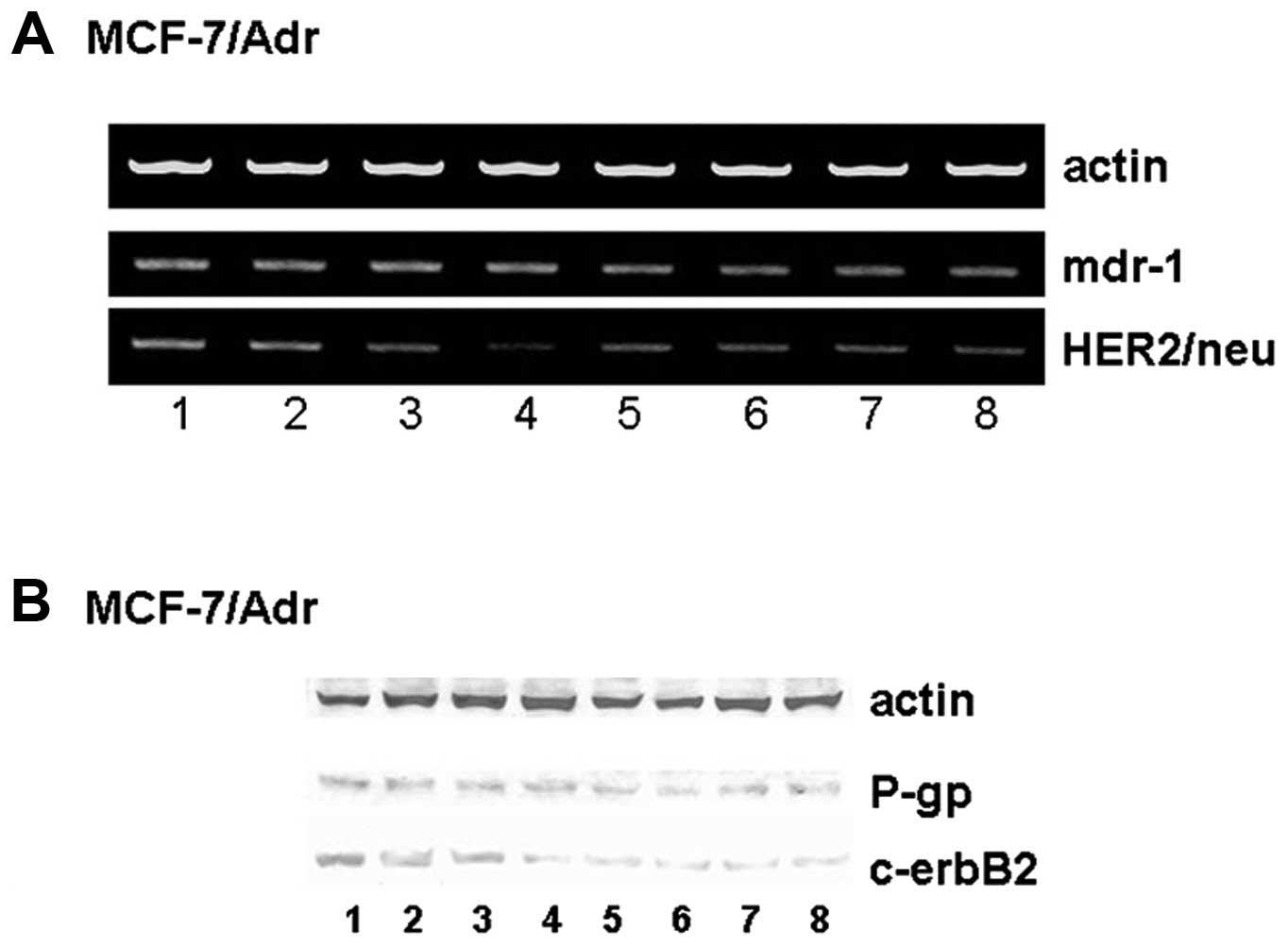 | Figure 5Effect of genistein on Her2/neu,
mdr-1, c-erbB2, and P-gp expression. (A) Genistein suppresses mRNA
expression of Her2/neu but not mdr-1. Lane 1, control; lane 2, 10
μM doxorubicin; lane 3, 1 μM doxorubicin; lane 4, 10 μM doxorubicin
+ 60 μmol/l genistein; lane 5, 30 μmol/l genistein; lane 6, 60
μmol/l genistein; lane 7, 1 μmol/l genistein; lane 8, 1 μM
doxorubicin + 30 μmol/l genistein. (B) Genistein suppresses the
protein expression of c-erbB2 but not P-gp. Lane 1, control; lane
2, 10 μM doxorubicin; lane 3, 1 μM doxorubicin; lane 4, 10 μM
doxorubicin + 60 μmol/l genistein; lane 5, 30 μmol/l genistein;
lane 6, 60 μmol/l genistein; lane 7, 1 μmol/l genistein; lane 8, 1
μM doxorubicin + 30 μmol/l genistein. |
Genistein suppresses c-erbB2 but not P-gp
expression
We examined the expression of P-gp and c-erbB2
proteins. Genistein and doxorubicin alone or in combination had no
effect on the expression of P-gp (P>0.05; Fig. 5B). However, genistein downregulated
the expression of c-erbB2 (P<0.01; Fig. 5B).
Discussion
It is well recognized that chemotherapy can
significantly improve the prognosis of breast cancer patients;
however, the development of multi-drug resistance is the main cause
of failure of chemotherapy for long therapeutic durations (23–25).
Therefore, reversing and overcoming chemoresistance are urgent and
clinically relevant issues that should be resolved by basic
research. P-gp-mediated drug resistance is considered a main
mechanism by which cancer cells avoid the effects of
chemotherapeutic drugs (26,27).
Overexpression of the Her2/neu oncogene has been observed in
approximately 30% of breast cancer patients and is believed to
contribute to the failure of chemotherapy (28). Genistein not only inhibits the
growth of many types of cancers, but also has a similar structure
to drugs that can reverse chemoresistance (29,30).
This suggests that genistein may be able to reverse
chemoresistance, and indeed, several reports have shown that
genistein reverses chemoresistance (29,30).
However, the molecular mechanisms of genistein’s action are not
fully understood. Several mechanisms have been proposed: i)
genistein enhances apoptosis by direct inhibition of CYP24 enzyme
activity (31); ii) genistein
upregulates expression of cell cycle regulators
P21WAF1/CIPl and Bax (32); and iii) genistein induces apoptosis
via upregulation of PTEN and Bax and enhancement of Bcl-2 and
Bcl-XL expression (33). It has
been reported that genistein enhances the cytotoxic effect of
gefitinib in T790M non-small cell lung cancer cells by inhibiting
p-EGFR, p-Akt and p-mTOR expression and promoting caspase-3 and
PARP activity (34). Consistent
with previous reports, we also found that genistein significantly
improved chemotherapeutic efficacy.
Genistein derived from soybeans and other food
sources is less expensive than conventional chemotherapeutic drugs.
This is significant as most cancer patients are under tremendous
economic pressure due to the expensive cost of chemotherapeutic
drugs.
Doxorubicin is a first-line chemotherapeutic drug in
many cancer types, including breast cancer (35). Therefore, elucidating the molecular
mechanisms of chemoresistance and overcoming such resistance are
critically important in the clinic. MCF-7/Adr is a well established
doxorubicin-resistant cell line and has been widely used to study
chemoresistance (36,37). Using MCF-7/Adr cells as a model, we
found that genistein greatly increased the intracellular
accumulation of doxorubicin, leading to doxorubicin-induced cell
death. The intracellular accumulation of doxorubicin was not
dependent on P-gp; we did not observe a functional change in p-Gp
protein using Rhl23, a lipophilic cationic fluorescent dye that can
specifically bind to P-gp. This was further confirmed by our RT-PCR
and western blot data, which revealed that genistein did not
influence the expression of P-gp. These results suggest that
genistein increases the intracellular accumulation of doxorubicin
by a P-gp-independent mechanism. However, the exact molecular
mechanism requires further investigation.
Her2 (also known as Neu) is a member of the
epidermal growth factor receptor (EGFR/ErbB) family (38). Thirty percent of breast cancer
patients show amplification or overexpression of the Her2/neu gene,
and overproduction of this gene contributes to chemoresistance.
Overexpression of Her2/neu results in activation of downstream
oncogenic pathways, such as the Ras/MAPK and PI3K/Akt pathways.
Genistein inhibits receptor tyrosine kinase (RTK) activation and
subsequently blocks Her2/neu/PI3K/Akt-mediated chemoresistance
(39). Seo et al reported
that genistein and quercetin inhibit the growth of MCF-7 human
breast cancer cells and MCF-7/Her2 vascular endothelial cell
proliferation by inhibition of NF-κB activation (40). Choi and Kim showed that soy
isoflavone aglucones and genistein exhibit anticancer effects by
affecting ERα and c-erbB2 receptor expression (41). In addition, genistein was found to
strongly inhibit ERα and c-erbB2 expression in a dose-dependent
manner in breast cancer SK-BR 3 and ZR-75 cells. Genistein was
found to reduce survivin as well as EGFR, Her2 and ERα expression
(42). Consistent with previous
reports, we found that genistein suppressed both mRNA and protein
expression of c-erbB2. Taken together, these results suggest that
genistein overcomes chemoresistance by targeting multiple targets
and multiple mechanisms.
The results presented here along with those of
previous studies demonstrate that genistein at concentrations of
20–30 μM or greater can significantly inhibit tumor growth. This
concentration is far below the IC50 value for cultured
bone marrow stromal progenitor cells (CFU-F) and
granulocyte-macrophage progenitor cells (CFU-GM). This suggests
that the drug’s toxic effects on the bone marrow are very minimal.
Genistein may be a promising multidrug-resistance reversal agent in
the clinical treatment of breast cancer.
Acknowledgements
This study was supported by the Science Career
Development Foundation of Hubei Medical College (2007ZQB14). The
authors are grateful to Professor Huang Tao and the Department of
Surgery Laboratory of Xiehe Hospital at the Huazhong University of
Science and Technology for the valuable technical assistance.
References
|
1
|
Trapé AP and Gonzalez-Angulo AM: Breast
cancer and metastasis: on the way toward individualized therapy.
Cancer Genomics Proteomics. 9:297–310. 2012.PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
3
|
Harbeck N: Never too late: reducing late
breast cancer relapse risk. Curr Med Res Opin. 24:3295–3305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simstein R, Burow M, Parker A, Weldon C
and Beckman B: Apoptosis, chemoresistance, and breast cancer:
insights from the MCF-7 cell model system. Exp Biol Med.
228:995–1003. 2003.PubMed/NCBI
|
|
5
|
Payne KK and Manjili MH: Adaptive immune
responses associated with breast cancer relapse. Arch Immunol Ther
Exp. 60:345–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joerger M and Thürlimann B: Chemotherapy
regimens in early breast cancer: major controversies and future
outlook. Expert Rev Anticancer Ther. 13:165–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Redden MH and Fuhrman GM: Neoadjuvant
chemotherapy in the treatment of breast cancer. Surg Clin North Am.
93:493–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fuksa L, Micuda S, Grim J, Ryska A and
Hornychova H: Predictive biomarkers in breast cancer: their value
in neoadjuvant chemotherapy. Cancer Invest. 30:663–678. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weiss RB: The anthracyclines: will we ever
find a better doxorubicin? Semin Oncol. 19:670–686. 1992.PubMed/NCBI
|
|
10
|
Prados J, Melguizo C, Ortiz R, et al:
Doxorubicin-loaded nanoparticles: new advances in breast cancer
therapy. Anticancer Agents Med Chem. 12:1058–1070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brunello A, Roma A, Falci C and Basso U:
Chemotherapy and targeted agents for elderly women with advanced
breast cancer. Recent Pat Anticancer Drug Discov. 3:187–201. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alm El-Din MA, El-Badawy SA and Taghian
AG: Breast cancer after treatment of Hodgkin’s lymphoma: general
review. Int J Radiat Oncol Biol Phys. 72:1291–1297. 2008.
|
|
13
|
Khalil MY, Mapa M, Shin HJ and Shin DM:
Advances in the management of malignant mesothelioma. Curr Oncol
Rep. 5:334–341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giai M, Biglia N and Sismondi P:
Chemoresistance in breast tumors. Eur J Gynaecol Oncol. 12:359–373.
1991.
|
|
15
|
Sakamoto T, Horiguchi H, Oguma E and
Kayama F: Effects of diverse dietary phytoestrogens on cell growth,
cell cycle and apoptosis in estrogen-receptor-positive breast
cancer cells. J Nutr Biochem. 21:856–864. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Lemos ML: Effects of soy phytoestrogens
genistein and daidzein on breast cancer growth. Ann Pharmacother.
35:1118–1121. 2001.PubMed/NCBI
|
|
17
|
Usui T: Pharmaceutical prospects of
phytoestrogens. Endocr J. 53:7–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarkar FH, Adsule S, Padhye S, Kulkarni S
and Li Y: The role of genistein and synthetic derivatives of
isoflavone in cancer prevention and therapy. Mini Rev Med Chem.
6:401–407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ravindranath MH, Muthugounder S, Presser N
and Viswanathan S: Anticancer therapeutic potential of soy
isoflavone, genistein. Adv Exp Med Biol. 546:121–165. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanagasabai R, Krishnamurthy K, Druhan LJ
and Ilangovan G: Forced expression of heat shock protein 27 (Hsp27)
reverses P-glycoprotein (ABCB1)-mediated drug efflux and MDR1 gene
expression in adriamycin-resistant human breast cancer cells. J
Biol Chem. 286:33289–33300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu L, Zhou D, Jiang X, Song K, Li K and
Ding W: Loss of E-cadherin in multidrug resistant breast cancer
cell line MCF-7/ Adr: possible implication in the enhanced invasive
ability. Eur Rev Med Pharmacol Sci. 16:1271–1279. 2012.PubMed/NCBI
|
|
22
|
Zhang HC, Zhang F, Wu B, et al:
Identification of the interaction between P-glycoprotein and Anxa2
in multidrug-resistant human breast cancer cells. Cancer Biol Med.
9:99–104. 2012.PubMed/NCBI
|
|
23
|
Gampenrieder SP, Rinnerthaler G and Greil
R: Neoadjuvant chemotherapy and targeted therapy in breast cancer:
past, present, and future. J Oncol. 2013:7320472013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steward WP and Brown K: Cancer
chemoprevention: a rapidly evolving field. Br J Cancer. 109:1–7.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bartlett J, Canney P, Campbell A, et al:
Selecting breast cancer patients for chemotherapy: the opening of
the UK OPTIMA trial. Clin Oncol. 25:109–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loo TW and Clarke DM: Location of the
rhodamine-binding site in the human multidrug resistance
P-glycoprotein. J Biol Chem. 277:44332–44338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruefli AA, Tainton KM, Darcy PK, Smyth MJ
and Johnstone RW: P-glycoprotein inhibits caspase-8 activation but
not formation of the death inducing signal complex (disc) following
Fas ligation. Cell Death Differ. 9:1266–1272. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Menard S, Pupa SM, Campiglio M and
Tagliabue E: Biologic and therapeutic role of HER2 in cancer.
Oncogene. 22:6570–6578. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Banerjee S, Li Y, Wang Z and Sarkar FH:
Multi-targeted therapy of cancer by genistein. Cancer Lett.
269:226–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tarkowski M, Kokocinska M and Latocha M:
Genistein in chemoprevention and treatment. Pol Merkur Lekarski.
34:54–57. 2013.(In Polish).
|
|
31
|
Swami S, Krishnan AV, Peehl DM and Feldman
D: Genistein potentiates the growth inhibitory effects of
1,25-dihydroxyvitamin D3 in DU145 human prostate cancer cells: role
of the direct inhibition of CYP24 enzyme activity. Mol Cell
Endocrinol. 241:49–61. 2005. View Article : Google Scholar
|
|
32
|
Yu Z, Tang Y, Hu D and Li J: Inhibitory
effect of genistein on mouse colon cancer MC-26 cells involved
TGF-beta1/Smad pathway. Biochem Biophys Res Commun. 333:827–832.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pabona JM, Dave B, Su Y, et al: The
soybean peptide lunasin promotes apoptosis of mammary epithelial
cells via induction of tumor suppressor PTEN: similarities and
distinct actions from soy isoflavone genistein. Genes Nutr.
8:79–90. 2013. View Article : Google Scholar
|
|
34
|
Zhu H, Cheng H, Ren Y, Liu ZG, Zhang YF
and De Luo B: Synergistic inhibitory effects by the combination of
gefitinib and genistein on NSCLC with acquired drug-resistance in
vitro and in vivo. Mol Biol Rep. 39:4971–4979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tacar O, Sriamornsak P and Dass CR:
Doxorubicin: an update on anticancer molecular action, toxicity and
novel drug delivery systems. J Pharm Pharmacol. 65:157–170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y, Du F, Chen W, Yao M, Lv K and Fu P:
Knockdown of dual specificity phosphatase 4 enhances the
chemosensitivity of MCF-7 and MCF-7/ADR breast cancer cells to
doxorubicin. Exp Cell Res. 319:3140–3149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi R, Peng H, Yuan X, et al:
Down-regulation of c-fos by shRNA sensitizes adriamycin-resistant
MCF-7/ADR cells to chemotherapeutic agents via P-glycoprotein
inhibition and apoptosis augmentation. J Cell Biochem.
114:1890–1900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Coussens L, Yang-Feng TL, Liao YC, et al:
Tyrosine kinase receptor with extensive homology to EGF receptor
shares chromosomal location with neu oncogene. Science.
230:1132–1139. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harari D and Yarden Y: Molecular
mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene.
19:6102–6114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seo HS, Choi HS, Choi HS, et al:
Phytoestrogens induce apoptosis via extrinsic pathway, inhibiting
nuclear factor-kappaB signaling in HER2-overexpressing breast
cancer cells. Anticancer Res. 31:3301–3313. 2011.PubMed/NCBI
|
|
41
|
Choi EJ and Kim GH: Antiproliferative
activity of daidzein and genistein may be related to ERα/c-erbB-2
expression in human breast cancer cells. Mol Med Rep. 7:781–784.
2013.PubMed/NCBI
|
|
42
|
Mai Z, Blackburn GL and Zhou JR: Genistein
sensitizes inhibitory effect of tamoxifen on the growth of estrogen
receptor-positive and HER2-overexpressing human breast cancer
cells. Mol Carcinog. 46:534–542. 2007. View
Article : Google Scholar : PubMed/NCBI
|