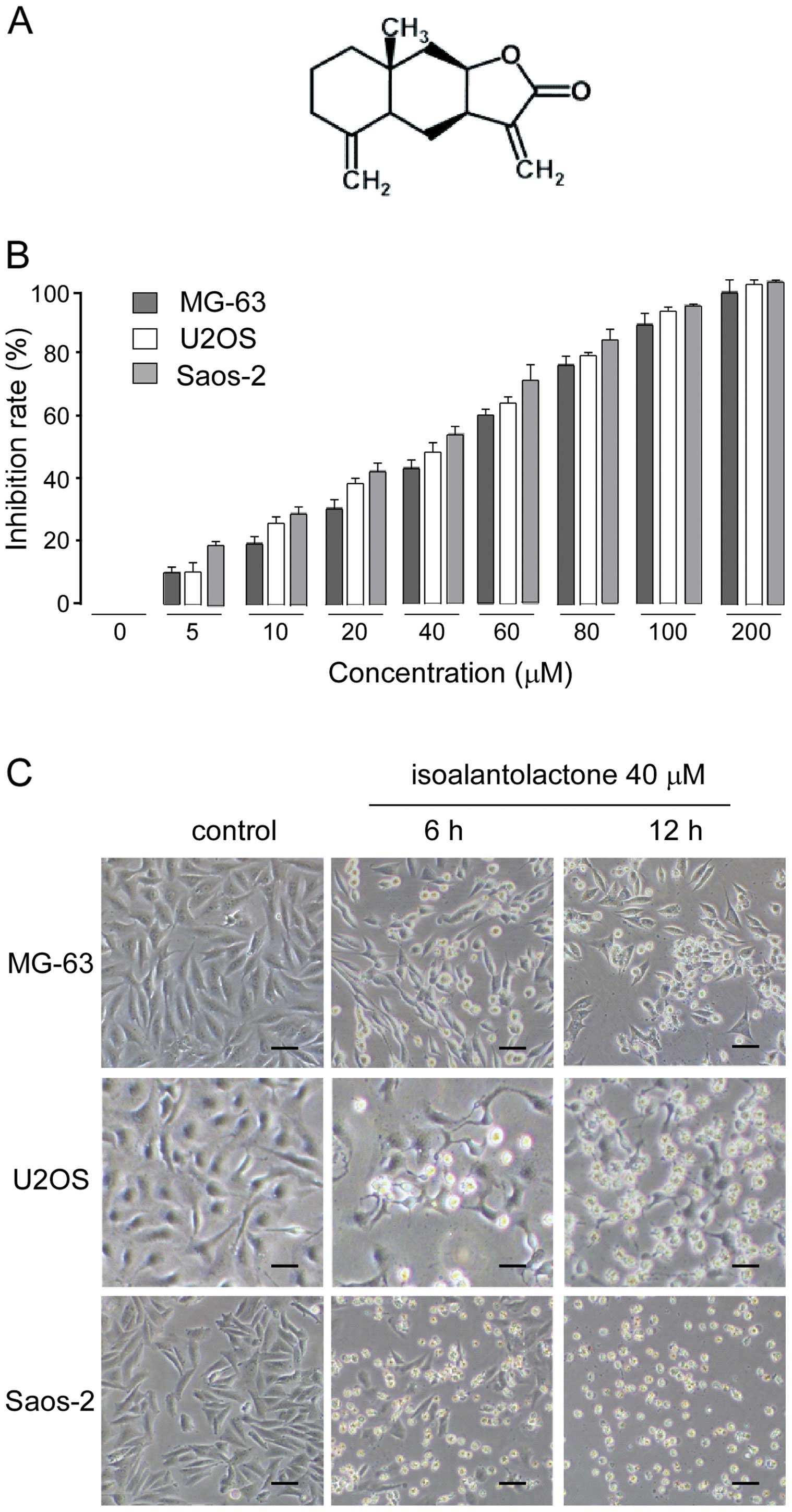
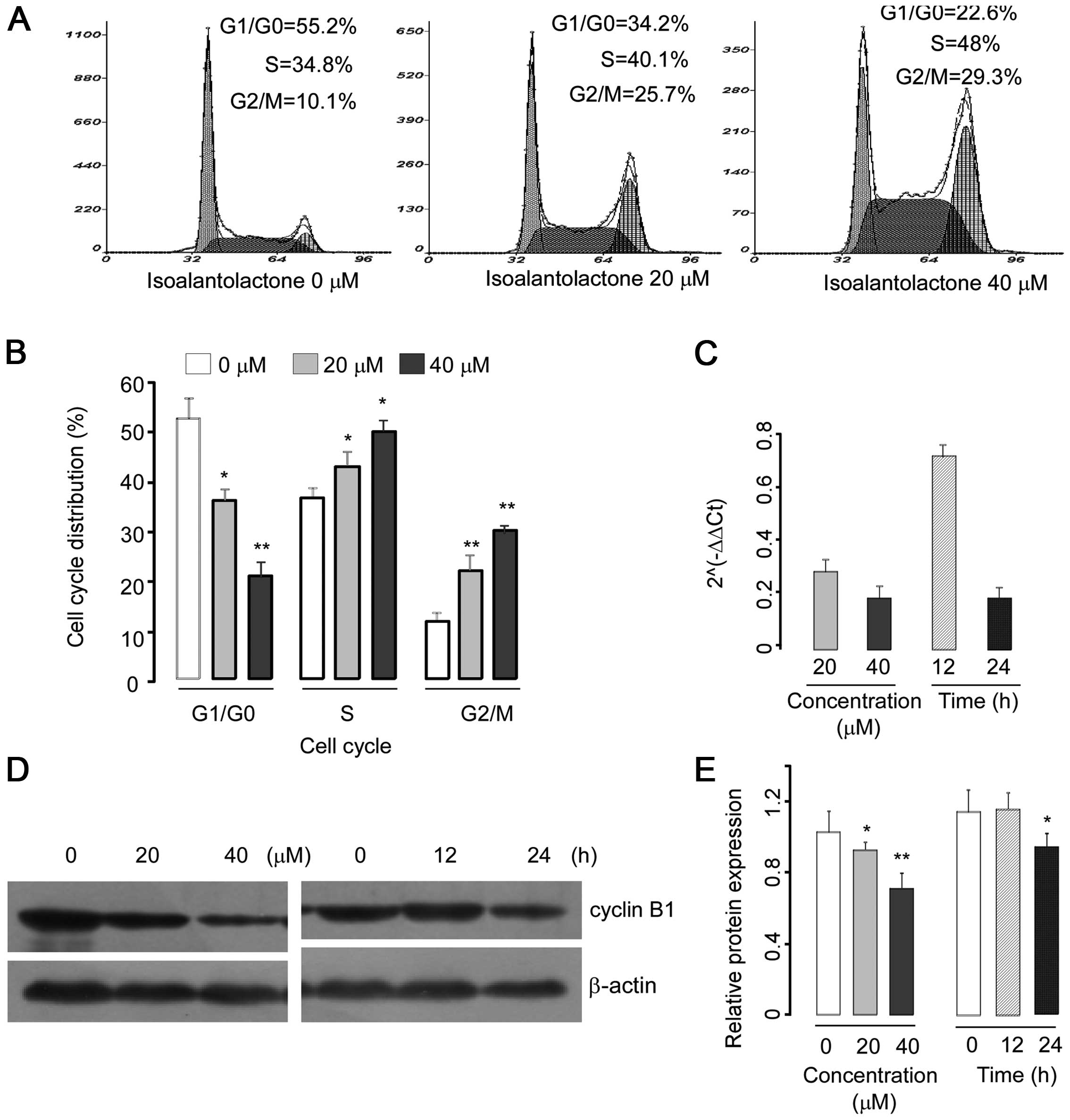
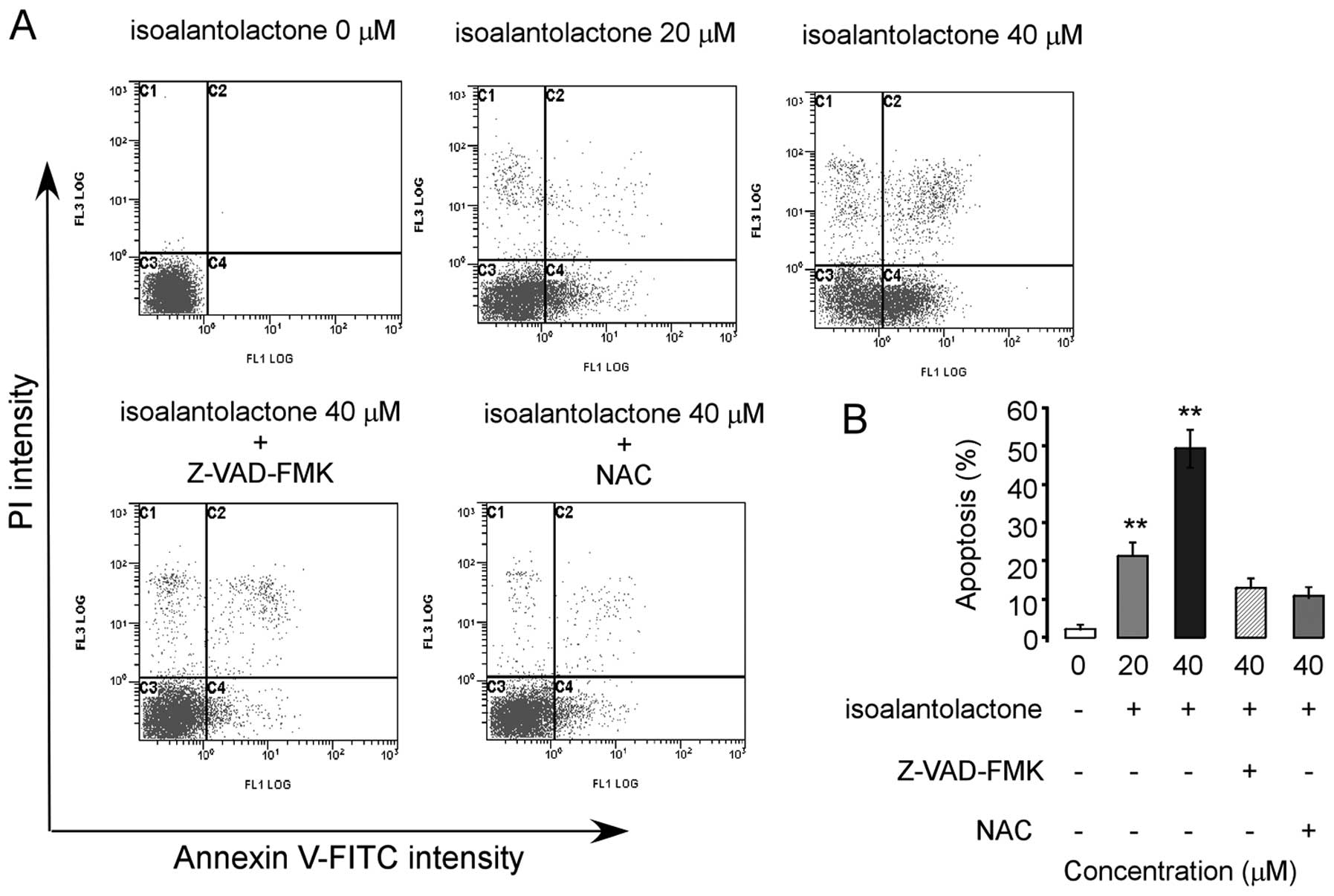
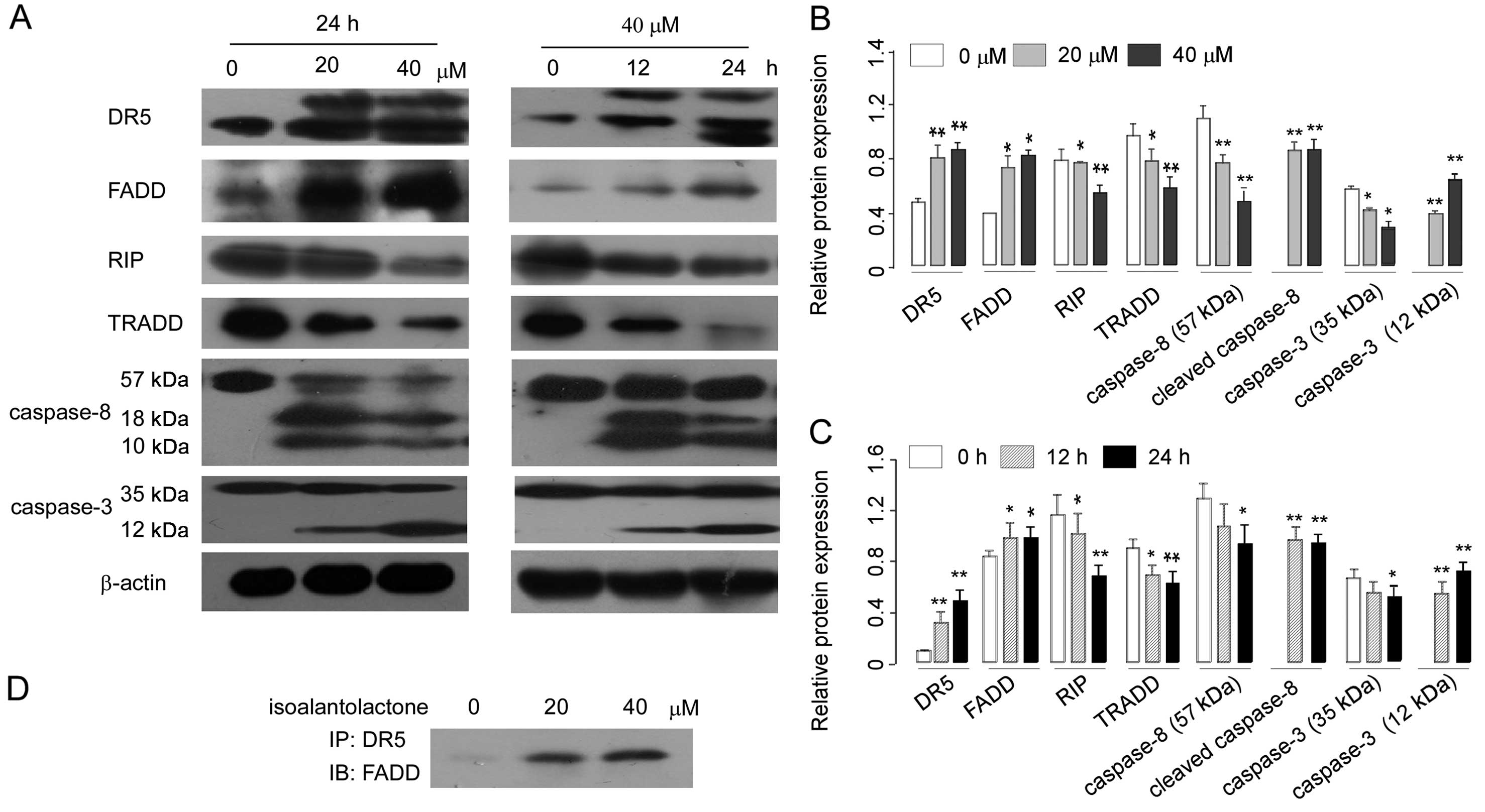
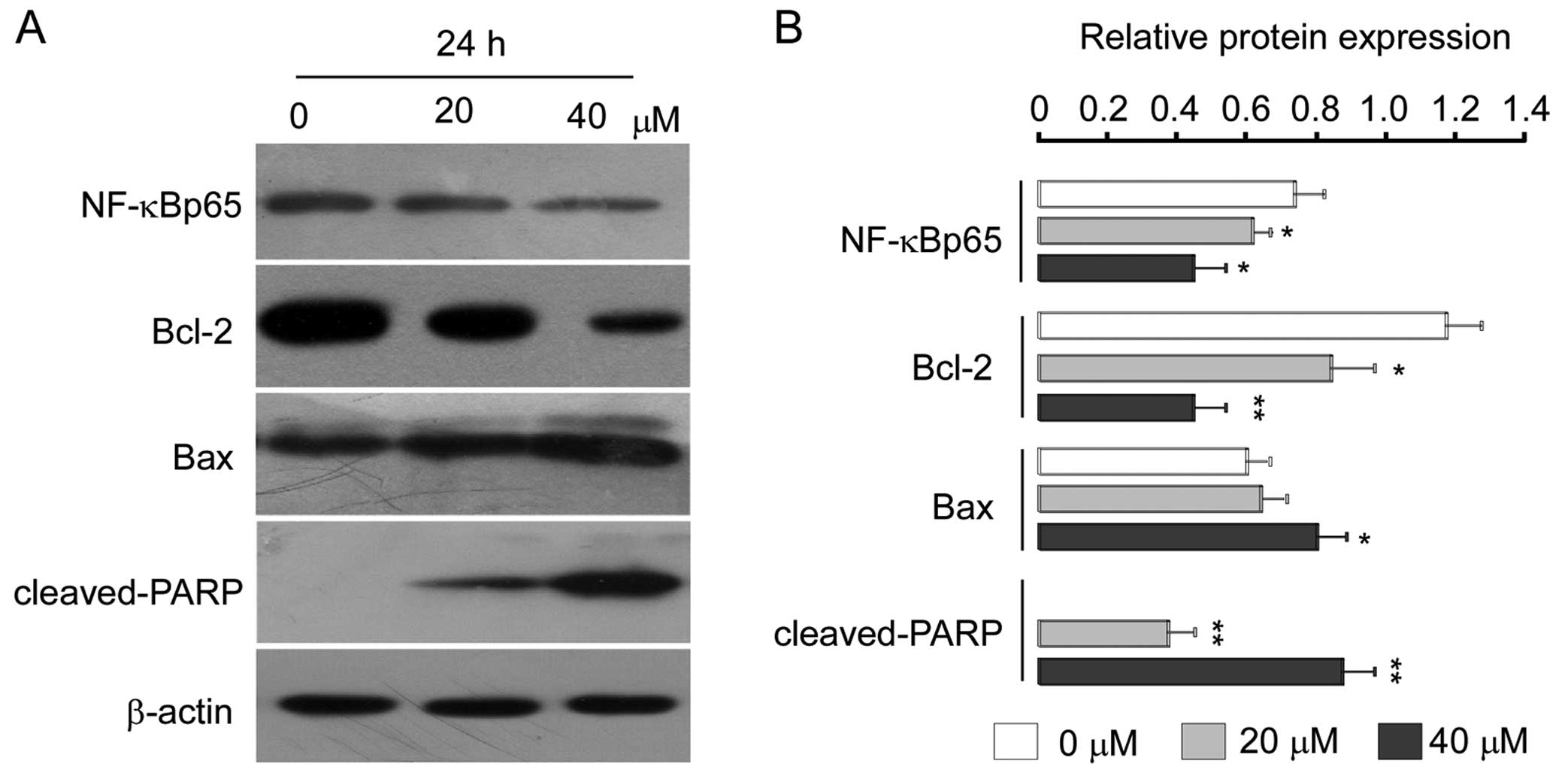
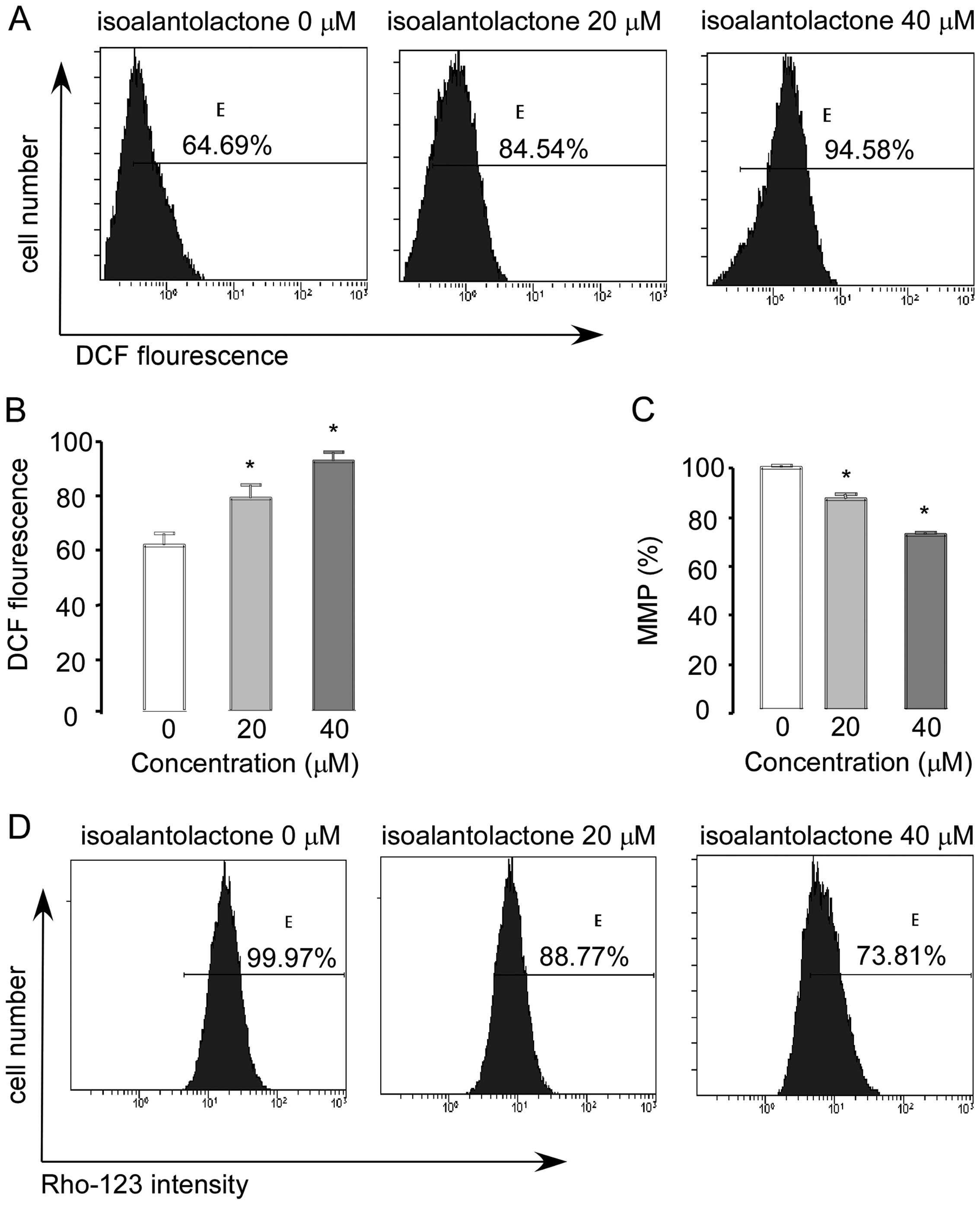
|
1
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: a
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kempf-Bielack B, Bielack SS, Jürgens H, et
al: Osteosarcoma relapse after combined modality therapy: an
analysis of unselected patients in the Cooperative Osteosarcoma
Study Group (COSS). J Clin Oncol. 23:559–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu M, Zhang H, Hu J, et al:
Isoalantolactone inhibits UM-SCC-10A cell growth via cell cycle
arrest and apoptosis induction. PLoS One. 8:e760002013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan M, Ding C, Rasul A, et al:
Isoalantolactone induces reactive oxygen species mediated apoptosis
in pancreatic carcinoma PANC-1 cells. Int J Biol Sci. 8:533–547.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konishi T, Shimada Y, Nagao T, Okabe H and
Konoshima T: Antiproliferative sesquiterpene lactones from the
roots of Inula helenium. Biol Pharm Bull. 25:1370–1372.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rasul A, Khan M, Yu B, et al:
Isoalantolactone, a sesquiterpene lactone, induces apoptosis in
SGC-7901 cells via mitochondrial and phosphatidylinositol
3-kinase/Akt signaling pathways. Arch Pharm Res. 36:1262–1269.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rasul A, Di J, Millimouno FM, et al:
Reactive oxygen species mediate isoalantolactone-induced apoptosis
in human prostate cancer cells. Molecules. 18:9382–9396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seo JY, Park J, Kim HJ, et al:
Isoalantolactone from Inula helenium caused Nrf2-mediated
induction of detoxifying enzymes. J Med Food. 12:1038–1045.
2009.
|
|
11
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mühlethaler-Mottet A, Bourloud KB,
Auderset K, Joseph JM and Gross N: Drug-mediated sensitization to
TRAIL-induced apoptosis in caspase-8-complemented neuroblastoma
cells proceeds via activation of intrinsic and extrinsic pathways
and caspase-dependent cleavage of XIAP, Bcl-xL and RIP.
Oncogene. 23:5415–5425. 2004.
|
|
13
|
Kamachi M, Le TM, Kim SJ, Geiger ME,
Anderson P and Utz PJ: Human autoimmune sera as molecular probes
for the identification of an autoantigen kinase signaling pathway.
J Exp Med. 196:1213–1225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang D, Lahti JM and Kidd VJ: Caspase-8
activation and bid cleavage contribute to MCF7 cellular execution
in a caspase-3-dependent manner during staurosporine-mediated
apoptosis. J Biol Chem. 275:9303–9307. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan M, Yi F, Rasul A, et al:
Alantolactone induces apoptosis in glioblastoma cells via GSH
depletion, ROS generation, and mitochondrial dysfunction. IUBMB
Life. 64:783–794. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu YJ, Yang SH, Chien CM, et al: Induction
of G2/M phase arrest and apoptosis by a novel enediyne derivative,
THDB, in chronic myeloid leukemia (HL-60) cells. Toxicol In Vitro.
21:90–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torres K and Horwitz SB: Mechanisms of
Taxol-induced cell death are concentration dependent. Cancer Res.
58:3620–3626. 1998.PubMed/NCBI
|
|
18
|
Gamet-Payrastre L, Li P, Lumeau S, et al:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.PubMed/NCBI
|
|
19
|
Murray AW: Recycling the cell cycle:
cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Orren DK, Petersen LN and Bohr VA:
Persistent DNA damage inhibits S-phase and G2
progression, and results in apoptosis. Mol Biol Cell. 8:1129–1142.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bodmer JL, Holler N, Reynard S, et al:
TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat
Cell Biol. 2:241–243. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghantous A, Gali-Muhtasib H, Vuorela H,
Saliba NA and Darwiche N: What made sesquiterpene lactones reach
cancer clinical trials? Drug Discov Today. 15:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lyss G, Knorre A, Schmidt TJ, Pahl HL and
Merfort I: The anti-inflammatory sesquiterpene lactone helenalin
inhibits the transcription factor NF-κB by directly targeting p65.
J Biol Chem. 273:33508–33516. 1998.
|
|
24
|
Chaudhary PM, Eby M, Jasmin A, Bookwalter
A, Murray J and Hood L: Death receptor 5, a new member of the TNFR
family, and DR4 induce FADD-dependent apoptosis and activate the
NF-κB pathway. Immunity. 7:821–830. 1997.PubMed/NCBI
|
|
25
|
Juan ME, Wenzel U, Daniel H and Planas JM:
Resveratrol induces apoptosis through ROS-dependent mitochondria
pathway in HT-29 human colorectal carcinoma cells. J Agric Food
Chem. 56:4813–4818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schumacker PT: Reactive oxygen species in
cancer cells: live by the sword, die by the sword. Cancer Cell.
10:175–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu JQ, Gills JJ, Park EJ, et al:
Sesquiterpenoids from Tithonia diversifolia with potential
cancer chemopreventive activity. J Nat Prod. 65:532–536.
2002.PubMed/NCBI
|
|
28
|
Koch E, Klaas CA, Rüngeler P, et al:
Inhibition of inflammatory cytokine production and lymphocyte
proliferation by structurally different sesquiterpene lactones
correlates with their effect on activation of NF-κB. Biochem
Pharmacol. 62:795–801. 2001.PubMed/NCBI
|
|
29
|
Nam NH: Naturally occurring NF-κB
inhibitors. Mini Rev Med Chem. 6:945–951. 2006.
|
|
30
|
Lee SM, Kwon JI, Choi YH, Eom HS and Chi
GY: Induction of G2/M arrest and apoptosis by water extract of
Strychni Semen in human gastric carcinoma AGS cells.
Phytother Res. 22:752–758. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and
Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric
adenocarcinoma cells via simultaneous induction of apoptosis and
autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI
|
|
32
|
Scott FL, Stec B, Pop C, et al: The
Fas-FADD death domain complex structure unravels signalling by
receptor clustering. Nature. 457:1019–1022. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guicciardi ME and Gores GJ: Life and death
by death receptors. FASEB J. 23:1625–1637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gaeta ML, Johnson DR, Kluger MS and Pober
JS: The death domain of tumor necrosis factor receptor 1 is
necessary but not sufficient for Golgi retention of the receptor
and mediates receptor desensitization. Lab Invest. 80:1185–1194.
2000. View Article : Google Scholar
|
|
35
|
Sheikh MS and Fornace AJ Jr: Death and
decoy receptors and p53-mediated apoptosis. Leukemia. 14:1509–1513.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ramaswamy M, Efimova EV, Martinez O,
Mulherkar NU, Singh SP and Prabhakar BS: IG20 (MADD splice
variant-5), a proapoptotic protein, interacts with DR4/DR5 and
enhances TRAIL-induced apoptosis by increasing recruitment of FADD
and caspase-8 to the DISC. Oncogene. 23:6083–6094. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
MacEwan DJ: TNF ligands and receptors - a
matter of life and death. Br J Pharmacol. 135:855–875. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stanger BZ, Leder P, Lee TH, Kim E and
Seed B: RIP: a novel protein containing a death domain that
interacts with Fas/APO-1 (CD95) in yeast and causes cell death.
Cell. 81:513–523. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hsu H, Shu HB, Pan MG and Goeddel DV:
TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF
receptor 1 signal transduction pathways. Cell. 84:299–308. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bharti AC, Donato N, Singh S and Aggarwal
BB: Curcumin (diferuloylmethane) down-regulates the constitutive
activation of nuclear factor-κB and IκBα kinase in human multiple
myeloma cells, leading to suppression of proliferation and
induction of apoptosis. Blood. 101:1053–1062. 2003.PubMed/NCBI
|
|
41
|
Li Q, Yu YY, Zhu ZG, et al: Effect of
NF-κB constitutive activation on proliferation and apoptosis of
gastric cancer cell lines. European surgical research. Eur Surg
Res. 37:105–110. 2005.
|
|
42
|
Shen HM and Tergaonkar V: NFκB signaling
in carcinogenesis and as a potential molecular target for cancer
therapy. Apoptosis. 14:348–363. 2009.
|
|
43
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar
|
|
44
|
Danial NN: BCL-2 family proteins: critical
checkpoints of apoptotic cell death. Clin Cancer Res. 13:7254–7263.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frenzel A, Grespi F, Chmelewskij W and
Villunger A: Bcl2 family proteins in carcinogenesis and the
treatment of cancer. Apoptosis. 14:584–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wei W, Huang H, Zhao S, et al:
Alantolactone induces apoptosis in chronic myelogenous leukemia
sensitive or resistant to imatinib through NF-κB inhibition and
Bcr/Abl protein deletion. Apoptosis. 18:1060–1070. 2013.
|
|
47
|
Felty Q, Xiong WC, Sun D, et al:
Estrogen-induced mitochondrial reactive oxygen species as
signal-transducing messengers. Biochemistry. 44:6900–6909. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jabs T: Reactive oxygen intermediates as
mediators of programmed cell death in plants and animals. Biochem
Pharmacol. 57:231–245. 1999. View Article : Google Scholar : PubMed/NCBI
|