Introduction
Nasopharyngeal carcinoma (NPC) is an endemic disease
in southern China and Southeast Asia, with a global incidence of
84,400 new cases annually and a mortality of 51,600 in 2008
(1). After primary treatment with
radiotherapy or chemoradiotherapy, a significant proportion of
endemic NPC patients, particularly those with stage III or IV,
relapsed locoregionally and/or systemically (2,3). The
median overall survival after recurrence is generally poor and
ranges from 7.2 to 22 months (4–6).
Therefore, new therapeutic strategies are required.
Cancer derives from the progressive accumulation of
abnormalities in cellular DNA which provides growth advantages to
cancer cells (7). Somatic mutations
become useful targets and biomarkers in selecting personalized
therapy for many solid tumors. For example, molecular driven
therapeutic targets such as epidermal growth factor receptor
(EGFR) and abnormal fusion of echinoderm
microtubule-associated protein-like 4 and anaplastic lymphoma
kinase (EML4-ALK) genes have resulted in a paradigm shift in
the treatment of lung adenocarcinoma (8,9).
Furthermore, KRAS (Kirsten rat sarcoma viral oncogene
homolog) and BRAF (v-raf murine sarcoma viral oncogene
homolog B) oncogene mutations are positively associated with
resistance to anti-EGFR drugs (10,11).
Hence, current anticancer therapy depends more on the knowledge of
genetic alterations in specific tumors.
Lifestyle exposure such as salted fish, EBV
infection, smoking and drinking has consistently been linked to NPC
risk (12), predicate on the
hypothesis that genomic alternations as a result of lifestyle
exposure may be a reason for NPC oncogenesis. Despite the fact that
chromosomal abnormalities (13)
together with amplification of certain oncogenes have been
identified in NPC (14,15), information regarding oncogene
mutations in NPC is limited (16).
Several studies demonstrated that oncogene phosphatidylinositol-4,
5-bisphosphate 3-kinase catalytic subunit α (PIK3CA)
mutation was an uncommon event in NPC patients (17–19).
No EGFR kinase domain mutation was found in 60 Moroccan NPC
patients (20). BRAF and
RAS mutants were observed to be absent in 65 NPC samples
(17). KIT (v-kit
Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog) intron
mutation was reported in NPC cell lines (21). Therefore, hotspot mutations in a
group of actionable oncogenes remain to be investigated in larger
studies.
In the present study, a high throughput OncoCarta™
ver. 1.0 mutation profiling panel was used to determine the
prevalence of 238 hotspot mutations across 19 oncogenes in 8 NPC
cell lines and 160 NPC patients. This panel interrogates with
oncogenes with known targeted drugs or genes that interact with
oncogenic pathways (22–24). Furthermore, the association between
oncogene mutations and clinicopathological factors of NPC patients
was also investigated in our study.
Materials and methods
Patient samples
The present study included 160 formalin-fixed
paraffin-embedded (FFPE) tumor samples and matched peripheral blood
cell samples from adult patients with newly diagnosed NPC. All
samples were obtained from the Sun Yat-sen University Cancer Center
between January 2006 and December 2009 and were collected before
patients underwent treatment (radiotherapy or chemotherapy). All
tissue slides were pathologically diagnosed by at least two
independent pathologists according to the World Health Organization
(WHO) classification (J.Z. and J.Y.).
The clinicopathological characteristics of all
patients, including age, gender and clinical staging were collected
and summarized in Table I. Clinical
staging was classified according to the criteria of the 7th edition
of the AJCC Cancer Staging Manual. The follow-up duration was
calculated from the first day of treatment to either the day of
death or the day of last examination. The median follow-up time was
40.0 months (range, 1.87–67.33). This study was approved by the
Institutional Ethics Review Boards of Sun Yat-sen University Cancer
Center and written informed consent was obtained from all
patients.
 | Table IClinicopathological characteristics
of NPC patients (n=160). |
Table I
Clinicopathological characteristics
of NPC patients (n=160).
| | Mutation | |
|---|
| |
| |
|---|
|
Characteristics | No. of patients
(n=160) | Absent n (%) | Present n (%) | P-valuea |
|---|
| Age (years)b | 160 | 47.4±12.1 | 47.9±11.5 | 0.86c |
| Gender |
| Female | 36 | 32 (88.9) | 4 (11.1) | 1.00 |
| Male | 124 | 111 (89.5) | 13 (10.5) | |
| Histology |
|
Differentiated | 6 | 5 (83.3) | 1 (16.7) | 1.00 |
|
Undifferentiated | 154 | 138 (89.6) | 16 (10.4) | |
| Smokers |
| Yes | 81 | 73 (90.1) | 8 (9.9) | 0.58 |
| No | 71 | 62 (87.3) | 9 (12.7) | |
| Alcohol
consumption |
| Yes | 29 | 28 (96.6) | 1 (3.4) | 0.25 |
| No | 123 | 107 (87.0) | 16 (13.0) | |
| VCA-IgA |
| <1:80 | 18 | 15 (83.3) | 3 (16.7) | 0.64 |
| ≥1:80 | 141 | 127 (90.1) | 14 (9.9) | |
| EA-IgA |
| <1:10 | 23 | 19 (82.6) | 4 (17.4) | 0.45 |
| ≥1:10 | 136 | 123 (90.4) | 13 (9.6) | |
| T stage |
| T1-2 | 37 | 30 (81.1) | 7 (18.9) | 0.12 |
| T3-4 | 123 | 113 (91.9) | 10 (8.1) | |
| N stage |
| N0-1 | 71 | 62 (87.3) | 9 (12.7) | 0.62 |
| N2-3 | 89 | 81 (91.0) | 8 (9.0) | |
| TNM stage |
| I–II | 15 | 10 (66.7) | 5 (33.3) | 0.01 |
| III–IV | 145 | 133 (91.7) | 12 (8.3) | |
Cell lines
The NPC cell lines SUNE-1, CNE-1, C666-1, CNE-2,
HONE-1, HNE-1, 5-8F and 6-10B were obtained and maintained in
RPMI-1640 (Invitrogen, Beijing, China) supplemented with 10% fetal
bovine serum (Gibco, Montevideo, Uruguay) as previously described
(25). The immortalized
nasopharyngeal epithelial cell line (NP69) was cultured in
keratinocyte serum-free medium (Invitrogen, NY, USA) supplemented
with bovine pituitary extract (BD Biosciences, San Jose, CA, USA)
(25). All cell lines were passaged
for less than ten generations and incubated at 37°C in a 5%
CO2 incubator.
DNA extraction
For all FFPE tumor samples, hematoxylin and eosin
(H&E) stained slides were reviewed by two pathologists (Z.J.
and J.Y.) to ensure a percentage of tumor cells >70% as
previously described (26,27). Eight 10 μm unstained FFPE tissue
sections of each sample were deparaffinized by xylene wash (20 min)
followed by two 100% ethanol washes. DNA was extracted from the
pellets, cell lines and matched peripheral blood cell samples using
the Qiagen DNA extraction Kits (Qiagen, Valencia, CA, USA)
according to the manufacturer’s instructions. The quality and
quantity of DNA was determined using the NanoDrop ND1000
Spectrophotometer and gel agarose electrophoresis.
Oncogene mutation detection and
analysis
DNA samples were amplified using the OncoCarta™ v1.0
Kit (Sequenom, San Diego, CA, USA) containing 24 pools of PCR
primers and extension primers that allow the detection of 238
pathogenic mutations in 19 oncogenes (ABL1, AKT1, AKT2, BRAF,
CDK4, EGFR, ERBB2, FGFR1, FGFR3, FLT3, JAK2, KIT, MET, HRAS, KRAS,
NRAS, PDGFA, PIK3CA and RET) (Table II) (23,28).
The extension products were analyzed based on the matrix-assisted
laser desorption ionization-time of flight mass spectrometry
(MALDI-TOF) technology on the Sequenom MassArray platform (28). The experiments were conducted
according to the manufacturer’s instructions, as previously
described (28,29). The spectra were analyzed by
MassArray Typer Analyzer® ver. 4.0.4.20 Software
(Sequenom) which automates the identification of mutants by
comparing ratios of wild-type (WT) peaks to all suspected mutants
and adjusting these peaks when adducts are detected in the
spectrum. All mutations detected were manually reviewed by three
different persons (N.J., N.L. and F.Y.). Mutation peaks that
appeared in both tumor and matched blood cell DNA were not
considered as somatic mutations and were excluded from further
analysis.
 | Table IIMutations detected with the
OncoCarta™ ver. 1.0 kit. |
Table II
Mutations detected with the
OncoCarta™ ver. 1.0 kit.
| No. | Genes | Targeted
mutations |
|---|
| 1 | ABL1 | D276G, E255K,
E255V, F311L, F317L, F359V, G250E, H396R, M351T, Q252H, T315I,
Y253F, Y253H |
| 2 | AKT1 | E17del, E319G,
L357P, P388T, Q43X, V167A, V167A, V461L |
| 3 | AKT2 | R371H, S302G |
| 4 | BRAF | D594V, D594G,
F468C, F595L, G464R, G464V, G464E, G466R, G469S, G469E, G469A,
G469V, G469R, G596R, K601E, K601N, L597Q, L597V, L597S, L597R,
T599I, V600E, V600K |
| 5 | CDK4 | R24C, R24H |
| 6 | EGFR | A289V,
D770_N771>AGG, D770_N771insG, E709A, E709G, E709V, E709K, E709H,
E746_A750del, E746_A750del, V ins, E746_A750del, T751A,
E746_T751del, I ins, E746_T751del, S752D, E746_T751del, V ins,
G598V, G719A, G719S, G719C, H773_V774insH, H773_V774insNPH,
H773>NPY, L747_E749del, A750P, L747_S752del, P753S,
L747_S752del, Q ins, L747_T750del, P ins, L747_T751del, L858R,
L861Q, M766_A767insAI, N771_P772>SVDNR, P772_H773insV, R108K,
S752_I759del, S768I, SNP C2255T, T263P, T751A, T790M,
V769_D770insASV, V769_D770insCV, V774_C775insHV |
| 7 | ERBB2 | A775_G776 insYVMA,
G776S, G776LC, G776VC, L755P, P780_Y781 insGSP, S779_P780
insVGS |
| 8 | FGFR1 | P252T, S125L |
| 9 | FGFR3 | A391E, G370C,
K650Q, K650E, K650T, K650M, Y373C |
| 10 | FLT3 | D835H, D835Y,
I836del |
| 11 | HRAS | G12V, G12D, G13C,
G13R, G13S, Q61H, Q61H, Q61K, Q61L, Q61R, Q61P |
| 12 | JAK2 | V617F |
| 13 | KIT | D52N, D579del,
D816H, D816Y, D816V, E561K, E839K, F584S, K550_K558del,
K558_E562del, K558_V560del, K642E, L576P, M552L, P551_V555del,
P585P, V559_V560del, V559D, V559A, V559G, V559del, V559I, V560D,
V560G, V560del, V825A, W557R, W557R, W557G, Y503_F504insAY,
Y553_Q556del, Y568D, Y570_L576del |
| 14 | KRAS | A59T, G12A, G12C,
G12D, G12F, G12R, G12S, G12V, G13V, G13D, Q61E, Q61K, Q61H, Q61H,
Q61L, Q61R, Q61P |
| 15 | MET | M1250T, R970C,
T992I, Y1230C, Y1235D |
| 16 | NRAS | A18T, G12C, G12R,
G12S, G12V, G12A, G12D, G13C, G13R, G13S, G13V, G13A, G13D, Q61E,
Q61K, Q61H, Q61L, Q61R, Q61P |
| 17 | PDGFRA | D1071N,
D842_H845del, D842V, D846Y, F808L, I843_D846del, I843_S847>T,
N870S, S566_E571>K, T674I, V561D |
| 18 | PIK3CA | C420R, C901F,
E542K, E545K, H1047R, H1047L, H701P, M1043I, M1043I, N345K, P539R,
Q546K, R38H, R88Q |
| 19 | RET | A664D, C634R,
C634W, C634Y, E632_L633del, M918T |
Statistical analysis
Statistical analyses were conducted using SPSS ver.
20 Software. The χ2 test and Fisher’s exact test were
used to assess differences in the distribution of clinical
variables and oncogene mutation status. Kaplan-Meier analysis was
used to determine survival; the differences between genotypes were
compared using the log-rank test. HR values were calculated using
univariate Cox regression analysis. Multivariate Cox regression
analysis was used to test the independent significance, in which
KIT mutation, age, gender, T and N stages were used as
covariates. All tests were two-tailed, and P-values <0.05 were
considered to indicate a statistically significant difference.
Results
Oncogene mutations detected in NPC
Of the 160 NPC patients, 17 (10.6%) had at least one
oncogene mutation and 4 (23.5%) of them had two or more mutations.
In total, we identified 24 mutations located in 11 genes by
Sequenom OncoCarta kit (Table
III). EGFR variants were detected in 5 tumors, followed
by CDK4, KIT and PDGFRA mutations in 3
patients and KRAS, BRAF and MET (MET
proto-oncogene) mutations in 2 patients. FGFR3 (fibroblast
growth factor receptor 3), AKT1 (v-akt murine thymoma viral
oncogene homolog 1), PIK3CA and NRAS mutations were
only detected in one sample (Table
III). We did not detect any mutations in the remaining eight
oncogenes. Patients with BRAF mutations were WT in
KRAS, which was consistent with previous findings in colon
cancer (30). Direct sequencing was
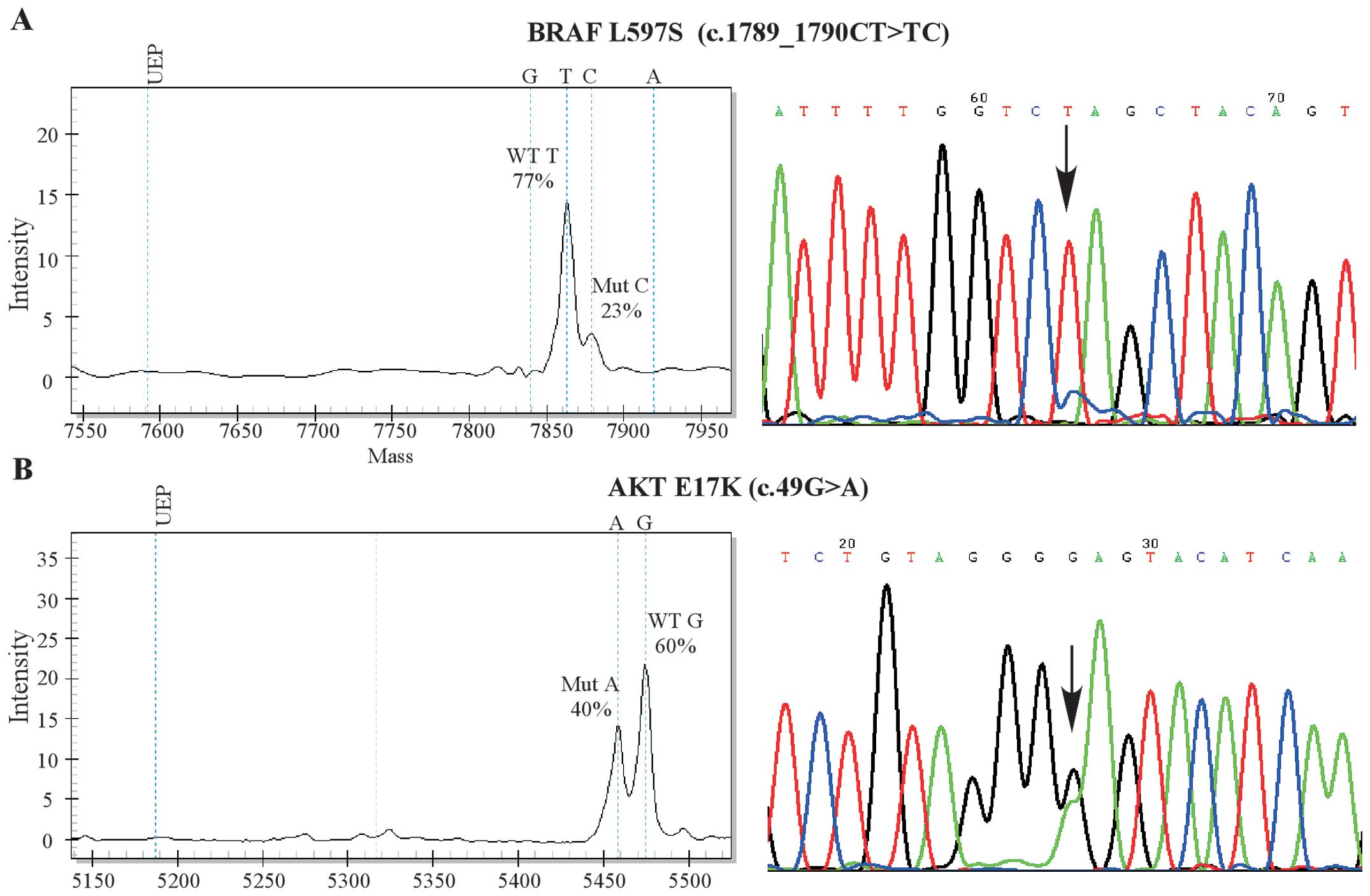
adopted to validate the MassArray findings. Representative figures
of detected mutations are shown in Fig.
1A and B.
 | Table IIISummary of specific mutations
detected in NPC samples using MassArray. |
Table III
Summary of specific mutations
detected in NPC samples using MassArray.
| | | Alele
frequency | |
|---|
| | |
| |
|---|
| Gene | Mutation | Allele | WT | Mut | Sample |
|---|
| AKT1 | E17K | G | 0.6 | 0.4 | 56 |
| BRAF | L597S | G | 0.77 | 0.23 | 17 |
| G469R | T | 0.911 | 0.089 | 104 |
| CDK4 | R24H | T | 0.73 | 0.27 | 17 |
| R24C | A | 0.57 | 0.43 | 26 |
| R24C | A | 0.868 | 0.132 | 116 |
| EGFR |
N771_P772>SVDNR | GCGT | 0.91 | 0.09 | 48 |
|
N771_P772>SVDNR | GCGT | 0.83 | 0.17 | 106 |
| T790M | T | 0.924 | 0.076 | 100 |
|
H773_V774insNPH | AA..AC | 0.909 | 0.091 | 104 |
| R108K | A | 0.91 | 0.09 | 149 |
| FGFR3 | Y373C | G | 0.85 | 0.15 | 26 |
| KIT | V559I | A | 0.724 | 0.276 | 12 |
| E839K | A | 0.901 | 0.099 | 104 |
| K558_V560del | DEL | 0.899 | 0.101 | 157 |
| KRAS | A59T | T | 0.89 | 0.11 | 13 |
| G12D | T | 0.639 | 0.287 | 105 |
| MET | R970C | T | 0.92 | 0.08 | 17 |
| R970C | T | 0.895 | 0.105 | 129 |
| NRAS | A18T | T | 0.89 | 0.12 | 13 |
| PDGFRA | T674I | T | 0.85 | 0.15 | 14 |
| T674I | T | 0.69 | 0.31 | 17 |
| T674I | T | 0.94 | 0.06 | 96 |
| PIK3CA | E545K | A | 0.894 | 0.106 | 153 |
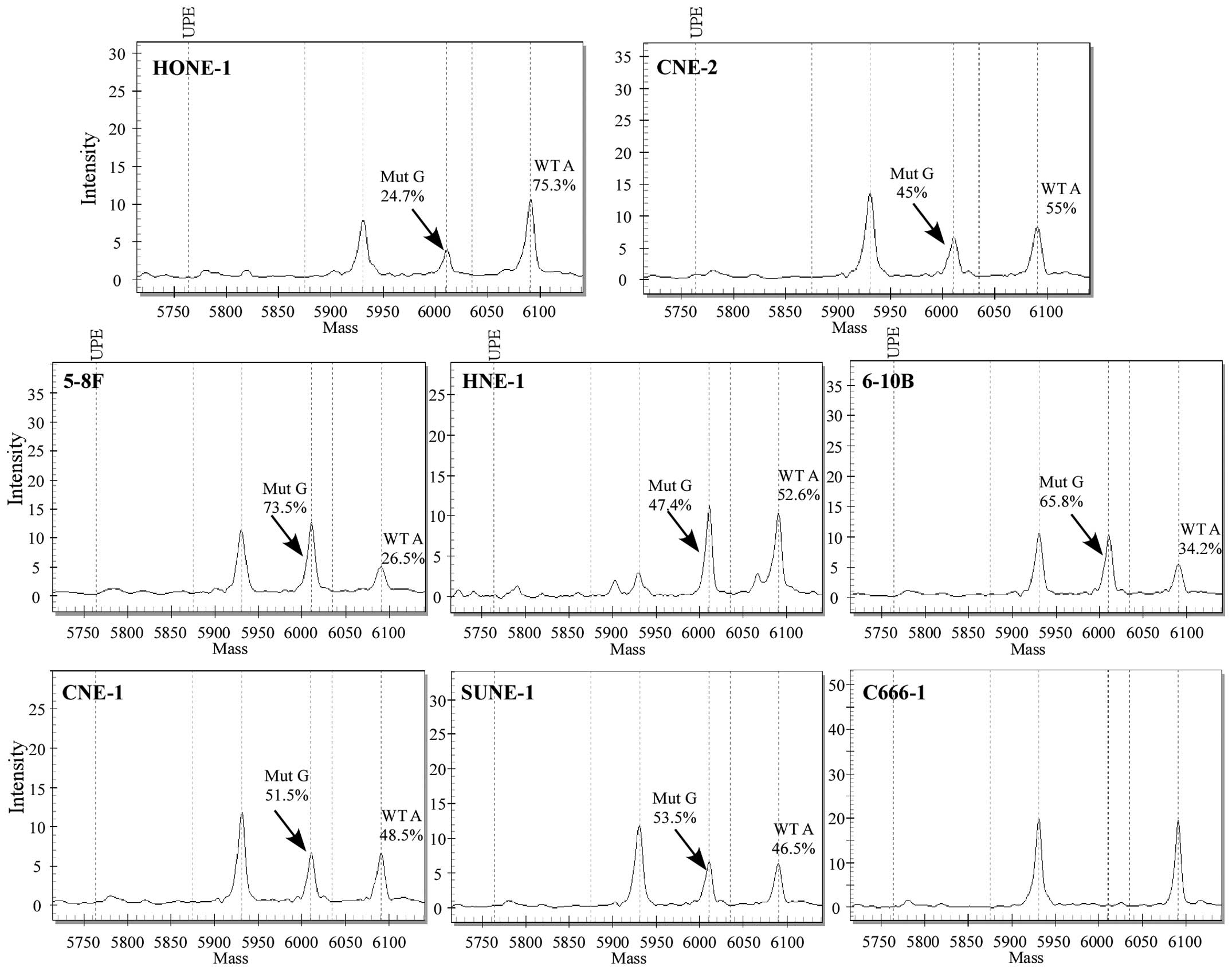
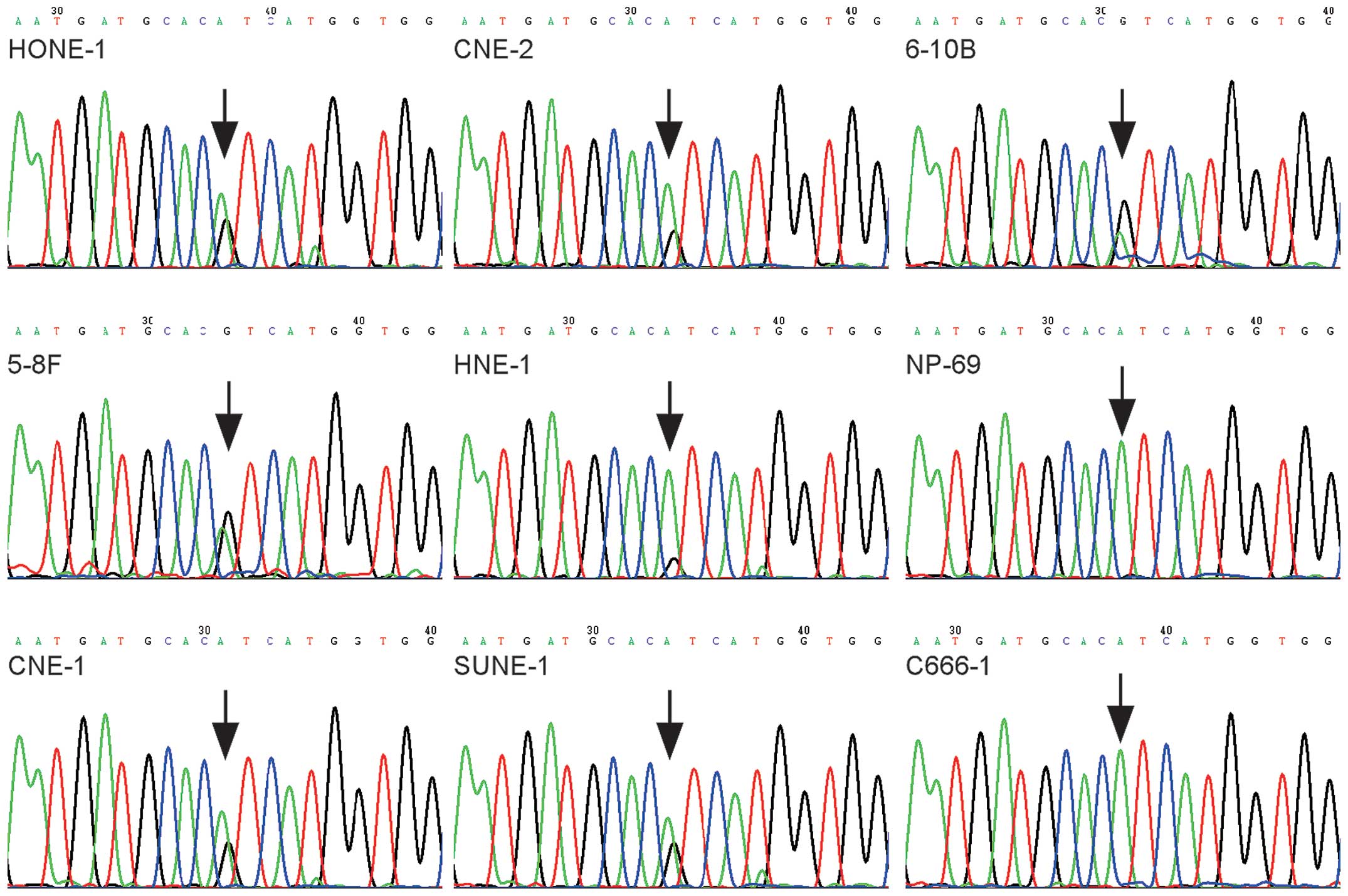
We next determined the 238 hotspot mutations in 8
NPC cell lines: 5-8F, 6-10B, SUNE-1, CNE-1, C666-1, CNE-2, HONE-1
and HNE-1. PIK3CA c.3140A>G (H1047R) was the only
mutation observed in 7 NPC cell lines, but not in C666-1 (Fig. 2). The results were confirmed by
direct sequencing (Fig. 3).
Notably, the PIK3CA c.3140A>G mutation was also found
absent in an immortalized nasopharyngeal epithelial cell line NP69
by direct sequencing (Fig. 3).
Taken together, hotspot oncogene mutations which are
common in other solid tumors are infrequent events in NPC.
Correlation between mutational profile
and clinicopathological characteristics
The relationship between clinicopathological factors
and mutation patterns were assessed. As shown in Table I, oncogene mutations were
significantly associated with the TNM stage of NPC patients
(P=0.01). However, there was no association between oncogene
mutations and clinical characteristics such as age, gender,
histopathological grade, EBV-related antigen levels, tumor (T) and
lymph node (N) stage of NPC patients (P>0.05, Table I). We also did not find any
difference in risk habits (smoking or alcohol consumption) in
patients with or without oncogene mutations (P>0.05, Table I).
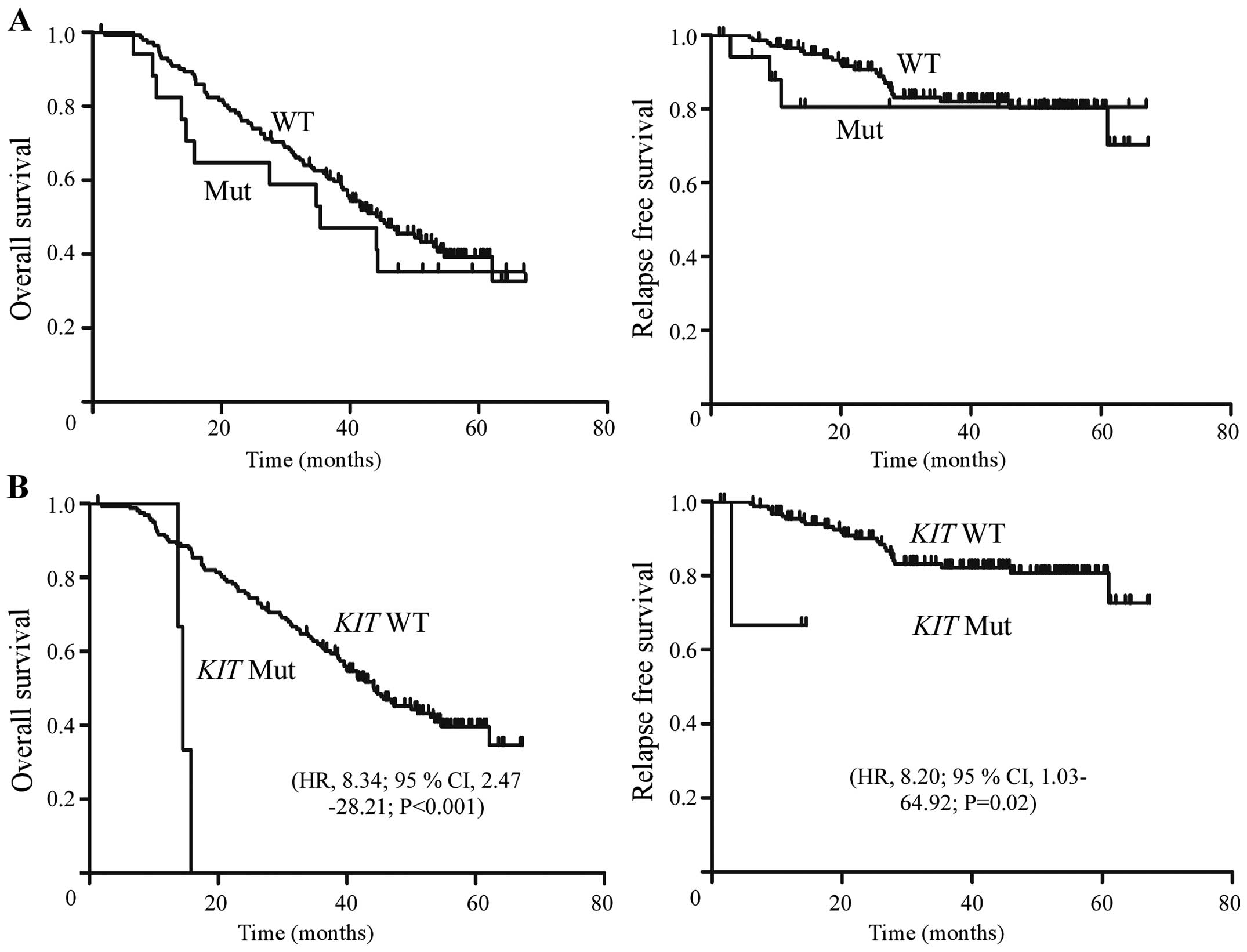
Furthermore, Kaplan-Meier analysis did not show
significant differences in overall survival (OS) and relapse-free
survival (RFS) between patients with and without oncogene mutations
(P>0.05) (Fig. 4A). Patients
with KIT mutation were associated with poorer OS (HR, 8.34;
95% CI, 2.47–28.21; P<0.001) and RFS (HR, 8.20; 95% CI,
1.03–64.92; P=0.02) (Fig. 4B).
Furthermore, we performed multivariate analyses with KIT
mutation, age, gender, T and N stages as covariates (P<0.05). We
found that the KIT mutation (HR, 5.94; 95% CI, 1.73–20.42;
P<0.01), age (HR, 1.69; 95% CI, 1.11–2.58; P=0.01) and N stage
(HR, 1.56; 95% CI, 1.02–2.41; P=0.04) were independent prognostic
factors associated with OS in NPC patients (Table IV).
 | Table IVSummary of the multivariable analysis
of prognostic factors for overall survival and risk score in
NPC. |
Table IV
Summary of the multivariable analysis
of prognostic factors for overall survival and risk score in
NPC.
| Variables | P-value | HR | 95% CI |
|---|
| Age group (years)
(≤47 vs. >47) | 0.01 | 1.69 | 1.11–2.58 |
| Gender (Male vs.
female) | 0.06 | 0.58 | 0.32–1.02 |
| T stage (T3-T4 vs.
T1-T2) | 0.18 | 1.42 | 0.85–2.37 |
| N stage (N2-N3 vs.
N1-N0) | 0.04 | 1.56 | 1.02–2.41 |
| KIT
mutation | <0.01 | 5.94 | 1.73–20.42 |
Discussion
In recent years, identification of somatic mutations
as key perturbations that promote tumorigenesis has become an
essential component in determining the management of certain
malignancies. For example, oncogenic mutations in EGFR,
KRAS have been clinically used as target and sensitivity
biomarkers in the treatment of non-small cell lung carcinoma
(31). However, knowledge regarding
oncogene mutational patterns in NPC remains limited, especially in
patients from NPC prevalent southern China. Thus, a high-throughput
OncoCarta panel was adopted in the current study to determine 238
hotspot mutations across 19 oncogenes in NPC. This panel provides a
cost effective and efficient technology for detecting known hotspot
mutations in FFPE samples (23,32).
Moreover, information concerning 10 of the 19 oncogenes
investigated by the OncoCarta™ ver. 1.0 assay is either limited or
absent in COSMIC database in NPC (http://www.sanger.ac.uk/cosmic).
The present study demonstrated that oncogene
mutations were uncommon in NPC with 10.6% of patients carrying one
or more mutations across 11 oncogenes. There was no difference
between patients with or without oncogene mutations in risk habits
(tobacco and alcohol consumption) and EBV infection status, both of
which are historically associated with NPC. Consistent with
previous reports, known PIK3CA mutations were detected in
both NPC cell lines and tissues with similar mutation frequency
compared to COSMIC database (Table
V) (17,33). Furthermore, we found a correlation
between KIT mutation and poorer survival in NPC patients.
KIT mutation together with age and N stage were independent
prognostic factors for NPC. The identification of mutated oncogenes
in NPC is encouraging as it may provide insight into the etiology
of NPC and influence future clinical management.
 | Table VMutations detected in the present
study compared with the COSMIC database. |
Table V
Mutations detected in the present
study compared with the COSMIC database.
| Genes | COSMIC
database
Mut/total cases (%)a | Present
study
Mut cases (%; n=168)a |
|---|
| AKT1 | N | 1 (0.6) |
| BRAF | 0/65 (0) | 2 (1.2) |
| CDK4 | N | 3 (1.8) |
| EGFR | 0/78 (0) | 5 (3.0) |
| FGFR3 | N | 1 (0.6) |
| KIT | 0/3 (0) | 3 (1.8) |
| KRAS | 0/74 (0) | 2 (1.2) |
| MET | 0/5 (0) | 2 (1.2) |
| NRAS | 0/18 (0) | 1 (0.6) |
| PDGFRA | 0/3 (0) | 3 (1.8) |
| PIK3CA | 8/105 (7.6) | 9 (5.4) |
PIK3CA, BRAF, EGFR, KIT, KRAS, HRAS, NRAS,
PDGFRA and MET (34)
oncogenes have been previously reported to be absent in NPC
according to the COSMIC database, whereas mutations in gene
AKT1, CDK4, FGFR3 and ABL1 have not yet been studied
in NPC (Table V) (35). In the present study, mutations in
these oncogenes were reported in NPC for the first time. This could
be explained by the use of a more sensitive MALDI-TOF technology
which is as sensitive as next generation sequencing, and more
sensitive than Sanger sequencing, as Su et al demonstrated
(36). Secondly, according to the
COSMIC database, most of the previous studies were based on cohorts
<100 samples (Table V).
Furthermore, this difference may also be due to different
pathological characteristics or population background.
The PIK3CA gene encodes the 110 kDa catalytic
subunit of PI3K. Upon activation, PIK3CA generates an activating
signaling cascade involved in cell growth, survival, proliferation,
motility and morphology (37,38). A
study by Or et al previously reported a one base
substitution of c.3140A>G (H1047R) in the PIK3CA gene in
CNE-2 and HONE-1, but not in C666-1 cells line (33). We confirmed the same mutant in 7 NPC
cell lines, including CNE-2 and HONE-1. However, EBV positive
C666-1 and an immortalized nasopharyngeal epithelial cell line NP69
showed absence of this mutation. Since the c.3140A>G mutant acts
as a gain-of-function mutation in PIK3CA kinase domain
(39) and appears only in EBV
negative NPC cell lines, we speculate that this mutation may play
an important role in the transformation of EBV-negative NPC cell
lines, which deserves further investigation. We did not find this
mutant in 160 patient samples among which 98.1% of patients showed
detectable EBV-related antigens. Instead, a PIK3CA
c.1633G>A (E545K) mutation was detected in one NPC patient. This
mutant was previously reported by Chou et al in 4 NPC
patients (17). Moreover, in
comparison with the mutation rate of 7.6% (8/105) reported by
COSMIC database (including cell line data), this study found a
similar mutation frequency of 5.6% (8/168) in PIK3CA
oncogene (Table V) (18,33,40).
The proto-oncogene c-KIT encodes a
transmembrane tyrosine kinase receptor which plays important roles
in the hematogenous system, placenta, heart, lung, and
midgestational kidney (41,42). Gain-of-function mutations of the
c-KIT gene promote constitutive phosphorylation of
KIT and, consequently, activation of downstream PI3K/AKT,
Src family kinases and MAPK pathways (42). In our study, we found KIT
K558_V560del, E839K and V559I mutations in three NPC patients.
K558_V560del and V559I are juxtamembrane mutations located in exon
11 of KIT oncogene which have been observed in GISTs
(43,44) and aggressive systemic mastocytosis
(ASM), respectively (44). These
two mutants showed spontaneous KIT phosphorylation (45) and could transform IL-3-dependent
Ba/F3 cells into IL-3-independent growth in the absence of KIT
ligand stem cell factor (SCF) (46). In contrast, KITE839K was
not spontaneously phosphorylated in response to exogenous SCF and
thus lacked cell transforming ability (45). KIT phosphorylated mutants could be
inhibited by KIT inhibitors such as imatinib and dasatinib
(47). These data together with our
findings that KIT mutation correlated with poor survival in
NPC, suggest that targeting KIT could be a potential therapeutic
strategy in the treatment of NPC. We did not find any KIT
mutation in NPC cell lines. This is consistent with a study by
Huang et al (21) in which
no KIT exon 9–21 hotspot mutation was found in 5 NPC cell
lines. However, the fact that KIT mutation was only detected
in 3/160 NPC patients requires broader studies in the future.
KIT DNA amplification, protein overexpression and their
clinical relevance also warrants further investigation in NPC.
Mutations in exon 18–21 of EGFR tyrosine
kinase domain are present in lung cancer, and some of them are
related to response to anti-EGFR agents such as gefitinib or
erlotinib (8,24). EGFR tyrosine kinase domain mutations
have been previously found absent in 60 Moroccan patients (20) and four NPC cell lines (48). In our series, we detected five NPC
cases carrying four EGFR mutants (Table III). EGFR T790M, H773_V774insNPH
and N771_P772>SVDNR mutations located in EGFR exon 20 could
change the crystal structures of EGFR which lead to resistance to
EGFR inhibitors (49). EGFR R108K
is a gain-of-function mutation in EGFR exon 3 which has been
reported in glioma. It would be of interest to determine whether
these mutants affect the response to EGFR-TKIs in NPC patients in
the future.
In the present study, we first reported three
CDK4 mutations in NPC. Cyclin-dependent kinase 4 (CDK4) is
the chief catalytic subunit of the regulatory cyclin D that governs
G1-to-S phase cell cycle progression (50). Dominant activating mutations
affecting codon 24 of the CDK4 gene (R24H or R24C) render
CDK4 insensitive to p16INK4 inhibition and are responsible for
multiple neoplasia developing (50,51).
Collectively, these data indicated that CDK4 mutation may
play a role in oncogenesis in a subset of NPC patients. Moreover,
mutations affect the RAS/RAF pathway and growth factor receptors
such as MET, PDGFRA, FGFR3 were also found in NPC in our study.
Since all these molecules are popular targets in anticancer
treatment, NPC patients with these mutations may benefit from
therapies targeting these oncogenes
In summary, this study provided evidence for
understanding oncogenic mutational patterns in NPC. Our data showed
lower oncogene mutation frequencies in NPC compared to other solid
tumors (52–55). The presence of mutations in a few
key oncogenes may ultimately be important in clinical management of
NPC and requires future verification.
Acknowledgements
The authors thank Professor Feng Chen (Department of
Epidemiology and Biostatistics, School of Public Health, Nanjing
Medical University, Nanjing, Jiangsu, China) for helpful discussion
and assistance. This research was supported by grants from the
Guangdong Province Universities and Colleges Pearl River Scholar
Funded Scheme (2010), the National Natural Science Foundation of
China (no. 81230056), the Innovation Team Development Plan of the
Ministry of Education (no. IRT1297), the Science and Technology
Project of Guangzhou City, China (no. 12BppZXaa2060002) and
Guangdong Translational Medicine Public Platform (no. 4202037).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Lee AW, Sze WM, Au JS, et al: Treatment
results for nasopharyngeal carcinoma in the modern era: the Hong
Kong experience. Int J Radiat Oncol Biol Phys. 61:1107–1116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wee J, Tan EH, Tai BC, et al: Randomized
trial of radiotherapy versus concurrent chemoradiotherapy followed
by adjuvant chemotherapy in patients with American Joint Committee
on Cancer/International Union against cancer stage III and IV
nasopharyngeal cancer of the endemic variety. J Clin Oncol.
23:6730–6738. 2005. View Article : Google Scholar
|
|
4
|
Bensouda Y, Kaikani W, Ahbeddou N, et al:
Treatment for metastatic nasopharyngeal carcinoma. Eur Ann
Otorhinolaryngol Head Neck Dis. 128:79–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caponigro F, Longo F, Ionna F and Perri F:
Treatment approaches to nasopharyngeal carcinoma: a review.
Anticancer Drugs. 21:471–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma BB and Chan AT: Systemic treatment
strategies and therapeutic monitoring for advanced nasopharyngeal
carcinoma. Expert Rev Anticancer Ther. 6:383–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shaw AT, Yeap BY, Mino-Kenudson M, et al:
Clinical features and outcome of patients with non-small-cell lung
cancer who harbor EML4-ALK. J Clin Oncol. 27:4247–4253. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Misale S, Yaeger R, Hobor S, et al:
Emergence of KRAS mutations and acquired resistance to anti-EGFR
therapy in colorectal cancer. Nature. 486:532–536. 2012.PubMed/NCBI
|
|
11
|
Bardelli A and Janne PA: The road to
resistance: EGFR mutation and cetuximab. Nat Med. 18:199–200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klein G: Nasopharyngeal carcinoma (NPC) is
an enigmatic tumor. Semin Cancer Biol. 12:415–418. 2002.PubMed/NCBI
|
|
13
|
Hui AB, Lo KW, Leung SF, et al: Detection
of recurrent chromosomal gains and losses in primary nasopharyngeal
carcinoma by comparative genomic hybridisation. Int J Cancer.
82:498–503. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan CS, Wong N, Leung SF, et al: Frequent
c-myc and Int-2 over-representations in nasopharyngeal carcinoma.
Hum Pathol. 31:169–178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hui AB, Lo KW, Teo PM, To KF and Huang DP:
Genome wide detection of oncogene amplifications in nasopharyngeal
carcinoma by array based comparative genomic hybridization. Int J
Oncol. 20:467–473. 2002.PubMed/NCBI
|
|
16
|
Forbes SA BG, Bamford S, Dawson E, Kok C,
Clements J, Menzies A, Teague JW, Futreal PA and Stratton MR: The
Catalogue of Somatic Mutations in Cancer (COSMIC). Curr Protoc Hum
Genet. Chapter 10:2008.
|
|
17
|
Chou CC, Chou MJ and Tzen CY: PIK3CA
mutation occurs in nasopharyngeal carcinoma but does not
significantly influence the disease-specific survival. Med Oncol.
26:322–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yip WK, Leong VC, Abdullah MA, Yusoff S
and Seow HF: Overexpression of phospho-Akt correlates with
phosphorylation of EGF receptor, FKHR and BAD in nasopharyngeal
carcinoma. Oncol Rep. 19:319–328. 2008.PubMed/NCBI
|
|
19
|
Fendri A, Khabir A, Mnejja W, et al:
PIK3CA amplification is predictive of poor prognosis in Tunisian
patients with nasopharyngeal carcinoma. Cancer Sci. 100:2034–2039.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Naji F, Attaleb M, Laantri N, et al:
Identification of G2607A mutation in EGFR gene with a significative
rate in Moroccan patients with nasopharyngeal carcinoma. Cell Mol
Biol. 56:OL1442–OL1446. 2010.PubMed/NCBI
|
|
21
|
Huang PY, Hong MH, Zhang X, Mai HQ, Luo DH
and Zhang L: C-KIT overexpression and mutation in nasopharyngeal
carcinoma cell lines and reactivity of Imatinib on these cell
lines. Chin J Cancer. 29:131–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding L, Getz G, Wheeler DA, et al: Somatic
mutations affect key pathways in lung adenocarcinoma. Nature.
455:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fumagalli D, Gavin PG, Taniyama Y, et al:
A rapid, sensitive, reproducible and cost-effective method for
mutation profiling of colon cancer and metastatic lymph nodes. BMC
Cancer. 10:1012010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu N, Tang LL, Sun Y, et al: MiR-29c
suppresses invasion and metastasis by targeting TIAM1 in
nasopharyngeal carcinoma. Cancer Lett. 329:181–188. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wetterskog D, Lopez-Garcia MA, Lambros MB,
et al: Adenoid cystic carcinomas constitute a genomically distinct
subgroup of triple-negative and basal-like breast cancers. J
Pathol. 226:84–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duprez R, Wilkerson PM, Lacroix-Triki M,
et al: Immunophenotypic and genomic characterization of papillary
carcinomas of the breast. J Pathol. 226:427–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thomas RK, Baker AC, Debiasi RM, et al:
High-throughput oncogene mutation profiling in human cancer. Nat
Genet. 39:347–351. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pearce M, Hogg G, Hosseni D and Ehrich M:
Mutation profiling in tumor samples using the Sequenom OncoCarta™
Panel. Nature Methods. 6:vii–viii. 2009.
|
|
30
|
Jhawer M, Goel S, Wilson AJ, et al: PIK3CA
mutation/PTEN expression status predicts response of colon cancer
cells to the epidermal growth factor receptor inhibitor cetuximab.
Cancer Res. 68:1953–1961. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cappuzzo F, Ciuleanu T, Stelmakh L, et al:
Erlotinib as maintenance treatment in advanced non-small-cell lung
cancer: a multicentre, randomised, placebo-controlled phase 3
study. Lancet Oncology. 11:521–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tie J, Lipton L, Desai J, et al: KRAS
mutation is associated with lung metastasis in patients with
curatively resected colorectal cancer. Clin Cancer Res.
17:1122–1130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Or YY, Hui AB, To KF, Lam CN and Lo KW:
PIK3CA mutations in nasopharyngeal carcinoma. Int J Cancer.
118:1065–1067. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qian CN, Guo X, Cao B, et al: Met protein
expression level correlates with survival in patients with
late-stage nasopharyngeal carcinoma. Cancer Res. 62:589–596.
2002.PubMed/NCBI
|
|
35
|
Forbes SA, Bindal N, Bamford S, et al:
COSMIC: mining complete cancer genomes in the Catalogue of Somatic
Mutations in Cancer. Nucleic Acids Res. 39:D945–D950. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Su KY, Chen HY, Li KC, et al: Pretreatment
epidermal growth factor receptor (EGFR) T790M mutation predicts
shorter EGFR tyrosine kinase inhibitor response duration in
patients with non-small-cell lung cancer. J Clin Oncol. 30:433–440.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shayesteh L, Lu Y, Kuo WL, et al: PIK3CA
is implicated as an oncogene in ovarian cancer. Nat Genet.
21:99–102. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang S, Bader AG and Vogt PK:
Phosphatidylinositol 3-kinase mutations identified in human cancer
are oncogenic. Proc Natl Acad Sci USA. 102:802–807. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abers GA, Ferris A, Craig M, et al: Mantle
compensation of active metamorphic core complexes at Woodlark rift
in Papua New Guinea. Nature. 418:862–865. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qiu FH, Ray P, Brown K, et al: Primary
structure of c-kit: relationship with the CSF-1/PDGF receptor
kinase family - oncogenic activation of v-kit involves deletion of
extracellular domain and C terminus. EMBO J. 7:1003–1011.
1988.PubMed/NCBI
|
|
42
|
Phung B, Sun J, Schepsky A, Steingrimsson
E and Ronnstrand L: C-KIT signaling depends on
microphthalmia-associated transcription factor for effects on cell
proliferation. PLoS One. 6:e240642011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Orfao A, Garcia-Montero AC, Sanchez L and
Escribano L; REMA. Recent advances in the understanding of
mastocytosis: the role of KIT mutations. Br J Haematol. 138:12–30.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nakagomi N and Hirota S:
Juxtamembrane-type c-kit gene mutation found in aggressive systemic
mastocytosis induces imatinib-resistant constitutive KIT
activation. Lab Invest. 87:365–371. 2007.PubMed/NCBI
|
|
45
|
Longley BJ Jr, Metcalfe DD, Tharp M, et
al: Activating and dominant inactivating c-KIT catalytic domain
mutations in distinct clinical forms of human mastocytosis. Proc
Natl Acad Sci USA. 96:1609–1614. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Growney JD, Clark JJ, Adelsperger J, et
al: Activation mutations of human c-KIT resistant to imatinib
mesylate are sensitive to the tyrosine kinase inhibitor PKC412.
Blood. 106:721–724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lennartsson J and Rönnstrand L: Stem cell
factor receptor/c-Kit: from basic science to clinical implications.
Physiol Rev. 92:1619–1649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma BB, Lui VW, Poon FF, et al: Preclinical
activity of gefitinib in non-keratinizing nasopharyngeal carcinoma
cell lines and biomarkers of response. Invest New Drugs.
28:326–333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yasuda H, Kobayashi S and Costa DB: EGFR
exon 20 insertion mutations in non-small-cell lung cancer:
preclinical data and clinical implications. Lancet Oncol.
13:e23–e31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rane SG, Cosenza SC, Mettus RV and Reddy
EP: Germ line transmission of the Cdk4(R24C) mutation facilitates
tumorigenesis and escape from cellular senescence. Mol Cell Biol.
22:644–656. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vax VV, Bibi R, Diaz-Cano S, et al:
Activating point mutations in cyclin-dependent kinase 4 are not
seen in sporadic pituitary adenomas, insulinomas or Leydig cell
tumours. J Endocrinol. 178:301–310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Brose MS, Volpe P, Feldman M, et al: BRAF
and RAS mutations in human lung cancer and melanoma. Cancer Res.
62:6997–7000. 2002.PubMed/NCBI
|
|
53
|
Davies H, Bignell GR, Cox C, et al:
Mutations of the BRAF gene in human cancer. Nature. 417:949–954.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kobayashi S, Boggon TJ, Dayaram T, et al:
EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Paez JG, Jänne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|