Introduction
Colorectal cancer remains the second most common
cause of cancer-related mortality in the United States. Of the
patients with colorectal cancer who die, most succumb with a
significant burden of metastatic disease (1). Colorectal cancer is an aggressive and
intractable human malignant tumor, despite the advances in the
diagnosis and treatment (2).
Therefore, it is highly important to seek novel targets for
therapeutic intervention.
Recently, increasingly more studies have shown that
tumor growth, development, invasion and metastasis depend on the
tumor microenvironment and tumor metabolism (3). Water molecules play a significant role
in the progression of malignant epithelial tumors, an understanding
of which is thus important in anticancer treatment strategy
(4). Aquaporins (AQPs) are a family
of membrane water channels that are required for the transport of
water through many secretory and absorptive epithelia. Some
subtypes of AQPs are also involved in the transport of other
molecules, such as glycerol and urea. There are currently 13 AQP
members which have been identified in mammals. Among them, AQP0,
AQP1, AQP2, AQP4, AQP5, AQP6 and AQP8 are primarily
water-selective, whereas AQP3, AQP7, AQP9, AQP10 and AQP12 also
transport glycerol and other small solutes (5). An altered expression of AQPs has been
revealed in several types of tumors based upon their specific
tissue localization. Expression of AQP1 is frequently associated
with brain tumors (6). In studies
involving AQP3-null mice, AQP3 gene deletion induced resistance to
carcinogen-induced skin tumors. Glycerol transport through AQP3
also contributes to the generation of ATP for cell proliferation
and tumorigenesis (7). AQP5 is
widely over-expressed in pancreatic cancer and appears to be
involved in cell proliferation (8).
In particular, AQP5 expression in colon cancer
tissues is associated with metastasis, suggesting that AQP5
overexpression plays a role in cancer progression (9). Ras signal transduction has been
suggested to enhance cell proliferation in AQP5-overexpressed
NIH3T3 cells (10). Moreover, a
molecular study revealed that AQP5 binds to the SH3 domains of
c-Src, a non-receptor cytoplasmic tyrosine kinase associated with
invasive and metastatic phenotypes in various tumors (11). However, the expression and clinical
significance of AQP5 in colorectal cancer, particularly the
correlation with circulating tumor cells (CTCs), has not been
elucidated. To evaluate the potential of AQP5 as a novel prognostic
marker of colorectal cancer, we used immunohistochemical, RT-PCR,
real-time PCR, western blotting and fluorescence in situ
hybridization (FISH) methods to detect the expression and
amplification of the AQP5 gene in clinical samples of colorectal
cancer, and immunofluorescence in situ hybridization
(imFISH) staining. We then analyzed the correlations between the
expression of AQP5 and clinicopathologic features, CTCs and
prognosis of colorectal cancer.
Materials and methods
Patient specimens
From January 2008 to December 2013, colorectal
cancer tissues (including adequately sized tumor tissue samples and
tissue samples obtained from areas within 2.0 cm around the tumor)
were obtained from 45 patients with colorectal cancer at the
Department of General Surgery, Second Affiliated Hospital of Xi’an
Jiaotong University. Samples were fixed with 4% formalin for
histological studies. Of the 45 patients, 24 were male and 21 were
female. Median age at the time of surgery was 58.3 years (range,
40–78 years). The histological type in all 45 patients was
colorectal adenocarcinoma. Tumor stage and histopathological
grading were recorded according to the classification of the
International Union Against Cancer. There were 3 stage I, 11 stage
II, 27 stage III, and 4 stage IV tumors. Histological grades for
the patients were as follows: 7 patients grade I, 20 grade II and
18 grade III. All patients were followed up and the median duration
of follow-up was 23 months (5–53 months). All the studies were
approved by the Human Subjects Committee of Xi’an Jiaotong
University, China. Consent forms signed by all patients recruited
in this study were approved by the Ethics Review Committee of the
Human Subjects Committee of the Xi’an Jiaotong University,
China.
Immunohistochemistry
AQP5 protein was detected immunohistochemically
using a standardized streptavidin-peroxidase (SP) method. Tissue
sections (4 μm) were incubated overnight with primary antibody at a
proper concentration. The next day, the slides were incubated for
30 min with biotinylated goat anti-rabbit IgG, followed by
incubation with peroxidase-conjugated streptavidin for 20 min at
room temperature. Color was developed using 0.02% 3,
3′-diaminobenzidine (DAB) in 50 mM Tris-HCl buffer (pH 7.6) for 5–7
min. Finally, the sections were counterstained with hematoxylin,
rinsed with water, dehydrated, cleared and coverslipped. Negative
controls for immunostaining replaced the primary antibody with
nonimmune goat or rabbit serum. The number of stained cells per
1,000 was determined under a microscope (Olympus Optical, Tokyo,
Japan) in three visual fields, at a ×400 magnification. When the
total number of cells observed under the microscope was <1,000,
all cells were counted. The staining was scored semiquantitatively
as negative (0, no staining); moderate (1, either diffuse weak
staining or strong staining in <30% of cells per core); or
strong (2, strong staining of ≥30% of the cells). The antibodies
against AQP5 and β-actin were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA, USA).
RT-PCR and real-time PCR
Total RNA was extracted from colorectal tumor,
peri-tumor and normal tissue using TRIzol reagent (Gibco-BRL).
First-strand cDNA was synthesized from 2 μg of total RNA using the
RevertAid Kit (Fermentas MBI, Amherst, NY, USA). The PCR primer
sets were designed: i) for AQP5, forward, TGACGAGGACTGGGAGG AGC-3′
and reverse, 5′-GGCGGCATTCAATGAACCA-3′; ii) for β-actin, forward,
5′-ATCGTGCGTGACATTAAGGAG AAG-3′ and reverse, 5′-AGGAAGGAAGGCTGGAAGA
GTG-3′. The PCR conditions included an initial denaturation step
for 5 min at 95 °C followed by 22 cycles of amplification: 30 sec
at 95°C, 30 sec at 57°C and 30 sec at 72°C. After the last cycle, a
final extension was performed at 72°C for 10 min. The housekeeping
gene β-actin was used as an internal control.
Real-time quantitative PCR was carried out with
Platinum SYBR-Green qPCR SuperMix UDG (Invitrogen, Carlsbad, CA,
USA) using the Rotor-Gene RG-3000 (Corbett Research, Doncaster
Victoria, Australia). For each amplicon, the amount of AQP5 and
β-actin was determined from a standard curve generated by serial
dilution. Prior to amplification, the samples were incubated at
95°C for 10 min, and each amplification cycle consisted of
denaturation for 45 sec at 95°C, annealing for 30 sec at 57°C and
extension for 30 sec at 73°C. The amount of target genes in the
cDNA samples was calculated based on the threshold cycle (Ct). The
PCR signals were quantitated by densitometric analysis using
Quantity One® analysis software.
Western blotting
Colorectal tumor tissues were minced and incubated
on ice for 30 min in 0.5 ml of ice-cold whole-cell lysate buffer
(10% NP-40, 5 M NaCl, 1 M HEPES, 0.1 M EGTA, 0.5 M EDTA, 0.1 M
PMSF, 0.2 M sodium orthovanadate, 1 M NaF, 2 μg/ml aprotinin, 2
μg/ml leupeptin). The minced tissue was homogenized using a Dounce
homogenizer and centrifuged at 16,000 × g at 4°C for 10 min. The
protein was separated by 10% SDS-PAGE and electro-transferred onto
nitrocellulose membranes. After being blocked with 5% non-fat milk
in TBST (20 mM Tris, 150 mM NaCl, 0.2% Tween-20, pH 7.6), the
membranes were incubated with primary antibodies at 4°C overnight,
followed by 1:2,000 horseradish peroxidase (HRP)-conjugated
secondary antibody for 2 h. Immunoreactive bands were visualized
using an enhanced chemiluminescence kit (Amersham Pharmacia
Biotech, Piscataway, NJ, USA). The western blotting signals were
quantitated by densitometric analysis using Total Lab Nonlinear
Dynamic Image® a nalysis software ( Nonlinear, USA).
Final histogram results = Target gene absolute value/β-actin
absolute value.
Isolation of normal colon cells and
culture of colorectal cancer cells
Normal colon cells were isolated from colorectal
site in normal colorectal specimens. Briefly, each specimen was
collected and transferred to the laboratory. After several washings
with sterile phosphate-buffered saline (PBS), 1-cm2
pieces of tissues were placed into the wells of culture flasks.
Once the tissue appeared to attach flasks (5–6 h), Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% FBS was added gently
to the tissue pieces. Specimens were inspected daily and the medium
was exchanged after 24 h for the first time and every third day
thereafter. Tissue samples were then removed from the cultures and
cells were transferred to larger tissue culture vessels once they
had reached 70% confluence, after approximately 2 weeks.
The COLO 205 and SW480 colorectal cancer cell lines
(the American Tissue Type Collection, USA) were maintained in DMEM,
(Gibco-BRL) supplemented with penicillin (100 U/ml), streptomycin
(100 μg/ml), 0.1 mM non-essential amino acids, 0.2 mM glutamine, 1
mM pyruvate, and 10% heat-inactivated fetal bovine serum (FBS) and
incubated in a 5% CO2 humidified atmosphere at 37°C.
Immunofluorescence assay
Exponentially growing cells were seeded on 25-mm
square glass cover slips placed in 35-mm diameter culture dishes.
The cells were fixed with 4% formaldehyde for 5 min, permeabilized
with 0.2% solution of Triton X-100 in PBS, and blocked with 2%
bovine serum albumin-PBS 30 min. Slides were incubated with
anti-AQP5 for overnight. Fluorescent imaging was obtained with a
confocal laser scanning microscope (Carl Zeiss Micro Imaging).
FISH
AQP5 gene amplification was detected with dual-color
FISH using a Passvision AQP5 DNA probe kit (Vysis Inc., Downers
Grove, IL, USA), according to the manufacturer’s instructions.
Tissue sections (4-μm thick) were baked overnight at 56°C, and were
handled with deparaffinization, enzyme digestion and fixation. The
slides were then denatured in 70% formamide/2X standard saline
citrate (SSC), at 72°C for 5 min. After a buffer wash, 10 μl of a
mixture of two directly labelled probes (AQP5 specific sequence
probe) were added to the tissue sections and hybridization was
carried out at 37°C for 14–18 h. The slides were then washed in a
post-hybridization wash at 72°C, counterstained with DAPI, mounted
and stored in dark before signal enumeration. AQP5-spectrum red
probe contains a DNA sequence specific for the AQP5 gene.
Chromosome enumeration probe 17 (CEP17)/spectrum green probe
containing α-satellite DNA that hybridizes to the D17Z1 locus
(centromere region of chromosome 17) was used as a control. The
slides were observed under a fluorescence microscope equipped with
a digital camera (DP50; Olympus, Tokyo, Japan). For each specimen,
gene amplification was scored when a minimum of 20 cancer cell
nuclei exhibited AQP5/CEP17 ratio ≥2, or when AQP5 signal cluster
was observed.
Blood sampling and enrichment of
CTCs
Peripheral blood (7.5 ml) collected in a BD
Vacutainer tube (Becton, Dickinson and Co., Franklin Lakes, NJ,
USA) was washed with PBS. In order to avoid epithelial cell
contamination during veni-puncture, all samples were collected
after discarding the first 2 ml blood. Red blood cells (RBCs) were
mixed with 45 ml lysis buffer (155 mM NH4Cl, 10 mM
KHCO3, 0.1 mM EDTA), followed by rotation for 8 min and
centrifugation (600 × g for 5 min) to remove RBCs. Resulting cell
pellet was resuspended in PBS and subsequently incubated with 0.5
ml of antileukocyte surface marker CD45 monoclonal antibody coated
magnetic beads for 30 min, followed by separation of magnetic beads
using a magnetic stand (Promega, Madison, WI, USA). Supernatants
were transferred into a new tube, and subsequently centrifuged at
800 × g for 3 min. Cell pellets were spotted on glass slides,
followed by imFISH staining.
imFISH staining (CEP8-CD45-DAPI)
Tumor cells were negative enriched by the
immunomagnetic beads method, followed by identification with
cytology analysis. FISH was performed using centromere DNA probes
of chromosome 8 (yellow) (Vysis), and immunofluorescence assay was
performed using anti-CD45 (red) (Santa Cruz, CA, USA). The slides
were washed three times with TBS (10 mM Tris, 2.8 mM KCl, 137 mM
NaCl, pH 7.4) containing 0.2% BSA for 3 min, and subsequently
rinsed with TBS once. Cells were mounted with mounting medium
containing the nuclear dye DAPI. A blinded review of the
fluorescent images by three technicians confirmed the identity of
the CTCs from 3-color fluorescent images that were magnified ×400.
Evaluation criteria for CTC identification from fluorescent images
included both CEP8 ≥3 and CD45 (−) staining pattern overlying the
DAPI staining of the nucleus.
Statistical analysis and patient
outcome
Data were analyzed by the χ2 or two-sided
Fisher’s exact test, as appropriate. Pearson’s correlation
coefficient was used to measure the strength of the association
among AQP5 gene amplification, enumeration of CTCs and AQP5
expression levels. Survival rate was calculated by the Kaplan-Meier
method, and differences were examined by the log-rank test. Factors
found to be significant were then selected for a stepwise Cox’s
multivariate proportional hazard model to determine their
prognostic values. Gene SNP was analyzed using the software of
pyrosequencing equipment (PyroMark Q24, Qiagen, Germany). P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using SPSS® ver.
13.0 statistical software (SPSS, Chicago, IL, USA).
Results
Protein expression and gene amplification
of AQP5 in colorectal cancer
It is known that AQP5 is expressed on the membrane
and in the cytoplasm of cells. To determine the expression status
of AQP5 in colorectal cancer, we first used immunohistochemistry to
evaluate patient specimens in the colorectal cancer, peri-tumor and
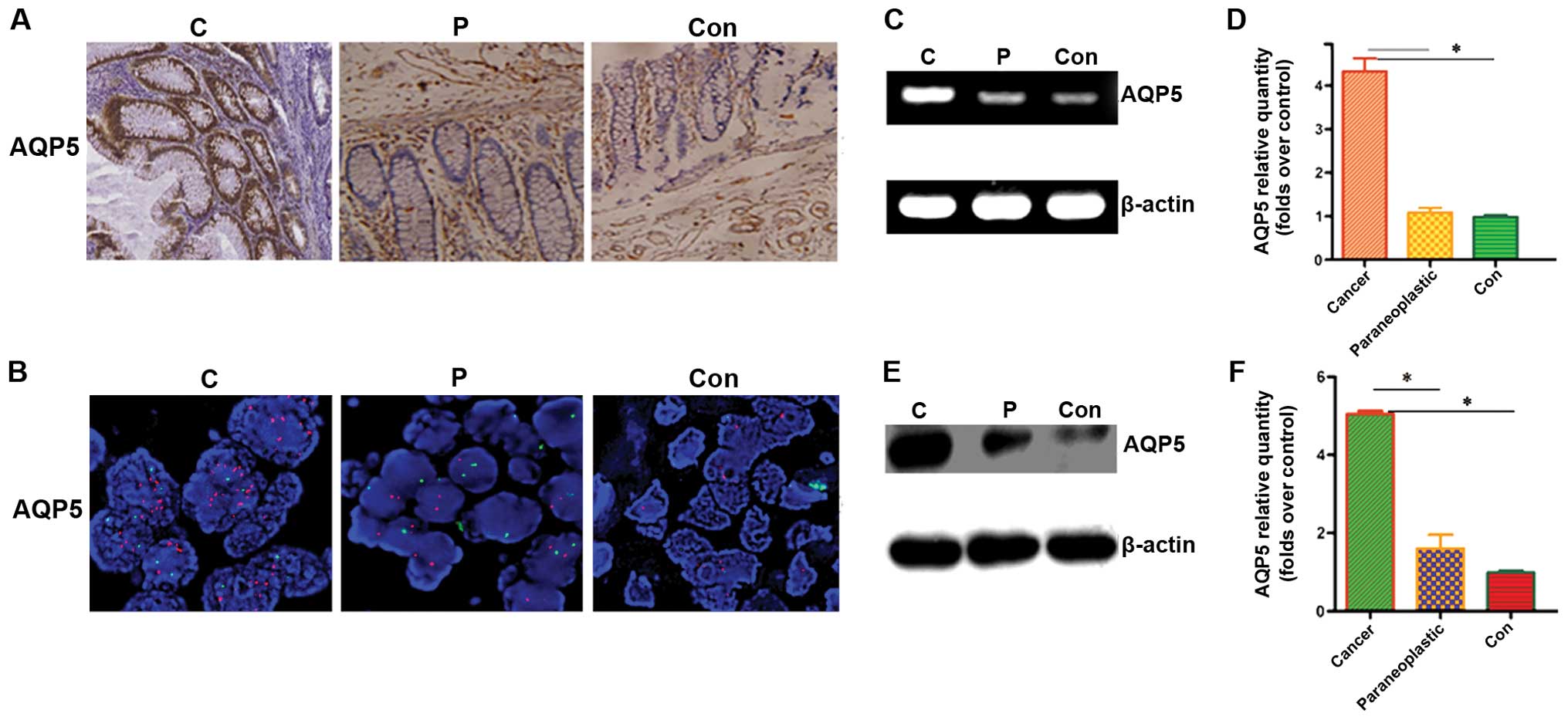
normal groups. The results highlighted the difference observed in
AQP5 immunostaining in the compartment (Fig. 1A); specifically, 14/45 (31.1%) of
the patients had strong AQP5 expression and 29/45 (64.4%) of the
patients had moderate AQP5 expression in the cancer group. AQP5 was
only occasionally detected in the peri-tumor [3/45, (6.67%)] and
normal tissues [3/45, (6.67%)].
Since FISH is a more accurate and sensitive assay,
and is generally regarded as the gold standard for detecting gene
amplification compared to gene expression using
immunohistochemistry (12), we
further detected AQP5 amplification status using FISH. Notably,
strong expression of AQP5 had a positive correlation with AQP5 gene
amplification (r = 0.712, P=0.000; Fig.
2B; Table I). In contrast, the
expression of AQP5 was low or absent in peri-tumor and normal
samples, while the AQP5 gene was not amplified.
 | Table IRelationship between expression of
AQP5 and amplification. |
Table I
Relationship between expression of
AQP5 and amplification.
| | AQP5 | |
|---|
| |
| |
|---|
| Gene | Case | 0
n (%) | 1
n (%) | 2
n (%) | P-value |
|---|
| Total | 45 | 2 | 29 | 14 | |
| AQP5 | | | | | 0.000a |
| Amplification | 15 | 0 (0.0) | 2 (6.9) | 13 (92.9) | |
| Normal | 30 | 2 (6.7) | 27 (90.0) | 1 (3.3) | |
Finally, we continued to determine the expression
status of AQP5 in samples by RT-PCR and real-time PCR. The AQP5
mRNA level was significantly upregulated in tumor samples when
compared to peri-tumor and normal tissues (Fig. 1C and D; P<0.05). Similarly,
western blotting results showed that AQP5 protein was upregulated
compared to peritumor and normal tissues (Fig. 1E and F; P<0.05).
Expression of AQP5 in colorectal cancer
and normal colon cells
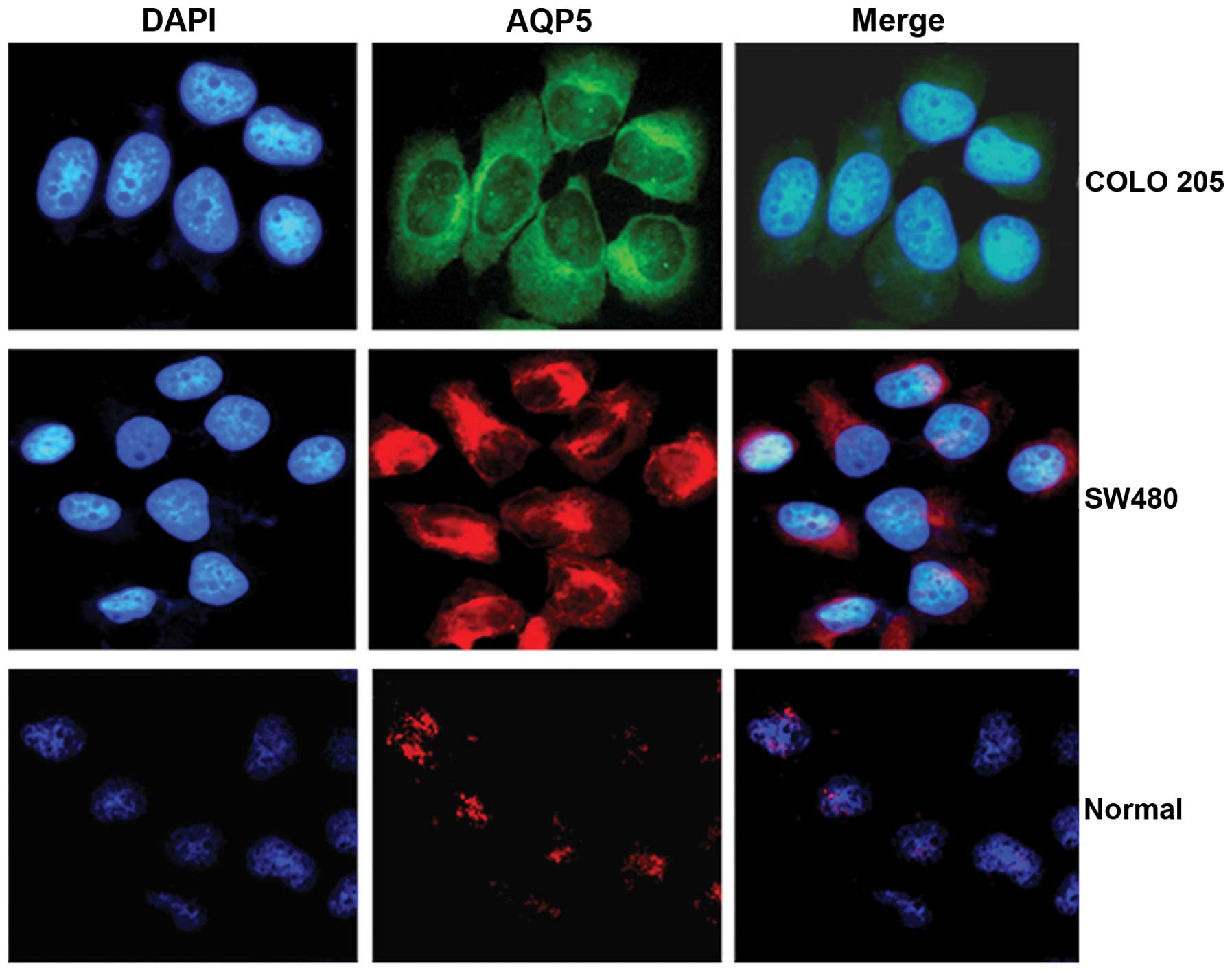
We further determined AQP5 protein expression status
in colorectal cancer cells (COLO 205 and SW480) and colon normal
cells using an immunofluorescence assay and analyzed by confocal
microscopy. The AQP5 fluorescence signal in colorectal cancer cells
was increased compared to normal colon cells (Fig. 2). These findings further indicate
that AQP5 is upregulated in colorectal cancer.
Relationship between AQP5 expression and
clinicopathologic parameters
Table II summarizes
the associations between AQP5 protein expression and
clinicopathologic parameters in colorectal cancer. AQP5 was
strongly expressed in 1/14 stage I-II cases (7.1%), which was
significantly lower than stage III [33.3%, (9/27)] and stage IV
[100.0% (4/4)] cases (P=0.002). AQP5 was also associated with lymph
node (P=0.016) and distant metastases (P=0.000). We also determined
the relationships between age, gender, histologic grade, and tumor
size with expression of AQP5, however no significant relationships
were observed (P>0.05).
 | Table IIAssociation between AQP5 protein
expression and clinicopathologic factors in colon cancer. |
Table II
Association between AQP5 protein
expression and clinicopathologic factors in colon cancer.
| | AQP5 | |
|---|
| |
| |
|---|
| Variables | Case | 0
n (%) | 1
n (%) | 2
n (%) | P-value |
|---|
| Total | 45 | 2 | 29 | 14 | |
| Age (years) | | | | | 0.848 |
| >60 | 28 | 1 (3.6) | 18 (64.3) | 9 (32.1) | |
| ≤60 | 17 | 1 (5.9) | 11 (64.7) | 5 (29.4) | |
| Gender | | | | | 1.000 |
| Female | 21 | 1 (4.8) | 14 (66.7) | 7 (33.3) | |
| Male | 24 | 1 (4.2) | 15 (62.5) | 7 (29.2) | |
| Histologic grade | | | | | 0.913 |
| I | 7 | 2 (28.6) | 4 (57.1) | 2 (28.6) | |
| II | 20 | 0 (0.0) | 12 (60.0) | 6 (30.0) | |
| III | 18 | 0 (0.0) | 13 (72.2) | 6 (33.3) | |
| Tumor size
(cm) | | | | | 0.263 |
| ≤2 | 15 | 1 (6.7) | 10 (66.7) | 4 (26.7) | |
| 2–5 | 26 | 1 (3.8) | 18 (69.2) | 8 (30.8) | |
| >5 | 4 | 0 (0.0) | 1 (25.0) | 2 (50.0) | |
| Lymph node
metastasis | | | | | 0.016 |
| Negative | 17 | 2 (11.8) | 8 (47.0) | 3 (17.6) | |
| Positive | 28 | 0 (0.0) | 19 (67.9) | 11 (39.3) | |
| Distant
metastasis | | | | | 0.000 |
| Negative | 39 | 2 (5.1) | 29 (74.3) | 8 (20.5) | |
| Positive | 6 | 0 (0.0) | 0 (0.0) | 6 (100.0) | |
| TNM stage | | | | | 0.002 |
| I/II | 14 | 1 (7.1) | 12 (85.7) | 1 (7.1) | |
| III | 27 | 1 (3.7) | 17 (62.9) | 9 (33.3) | |
| IV | 4 | 0 (0.0) | 0 (0.0) | 4 (100.0) | |
Prognostic value of AQP5 in patients with
colorectal cancer
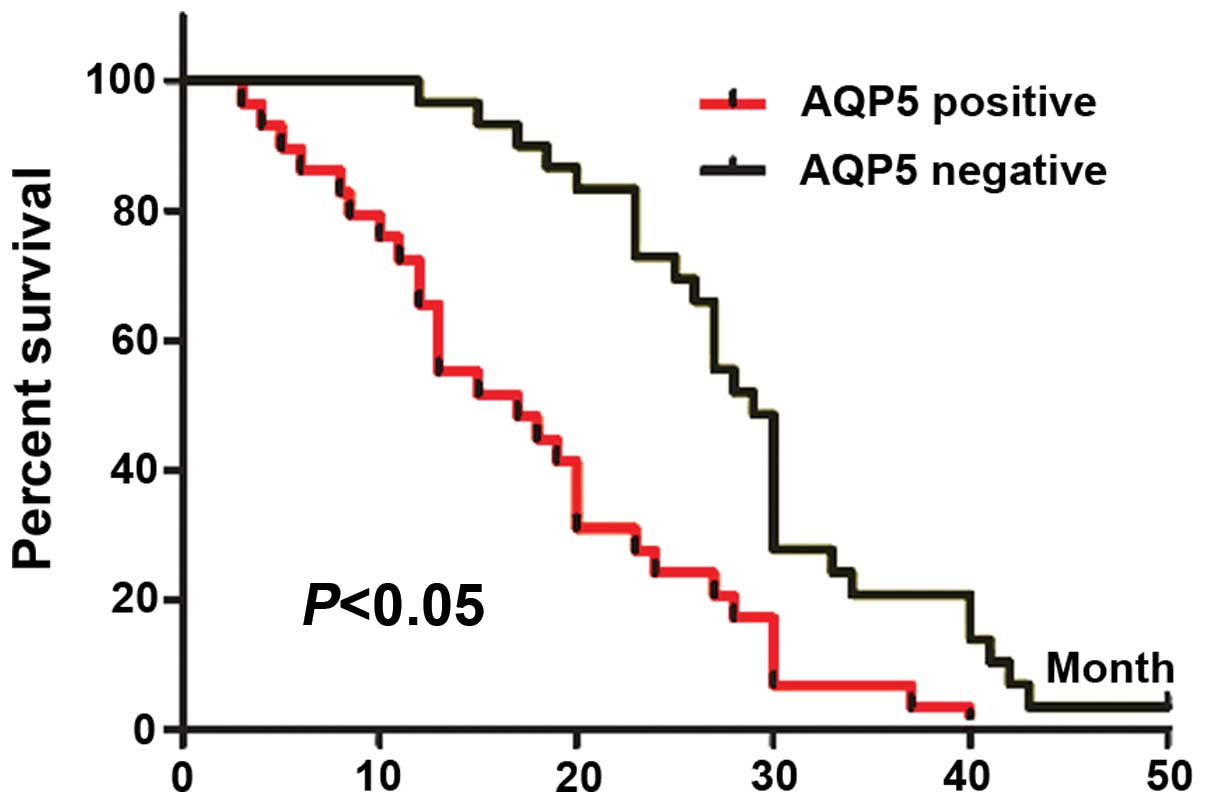
To determine the prognostic value of AQP5 for
colorectal cancer, we analyzed the cumulative survival of patients
based on AQP5 status (Fig 3). AQP5
weak and no staining were merged as negative, while strong staining
was regarded as positive. The cumulative survival rate in
AQP5-negative patients (n=31) at 3 years was 24.3% (median time,
21.2 months). In contrast, the cumulative survival rate in
AQP5-positive patients (n=14) was 7.4% (median time, 7.7 months), a
difference that was highly statistically significant
(P<0.05).
Multivariate analysis revealed that lymph node
metastasis (P=0.018) and TNM stage (P=0.031) were independent
prognostic factors for overall survival in patients with colorectal
cancer; tumor diameter and other clinical parameters were not
independent prognostic factors.
Expression of AQP5 correlates with CTC
enumeration
CTCs are tumor cells that are shed from the primary
tumor into the circulation. The presence of CTCs in the peripheral
blood of patients has long been associated with metastasis and poor
survival, although some authorities debate the biologic
significance of CTCs due to tumor genomic instability and potential
metastatic inefficiency. In view of the obvious clinical relevance,
CTCs have been recently recommended by the American Society of
Clinical Oncology as an acceptable cancer marker. To further
determine whether or not AQP5 is an adverse prognostic biomarker,
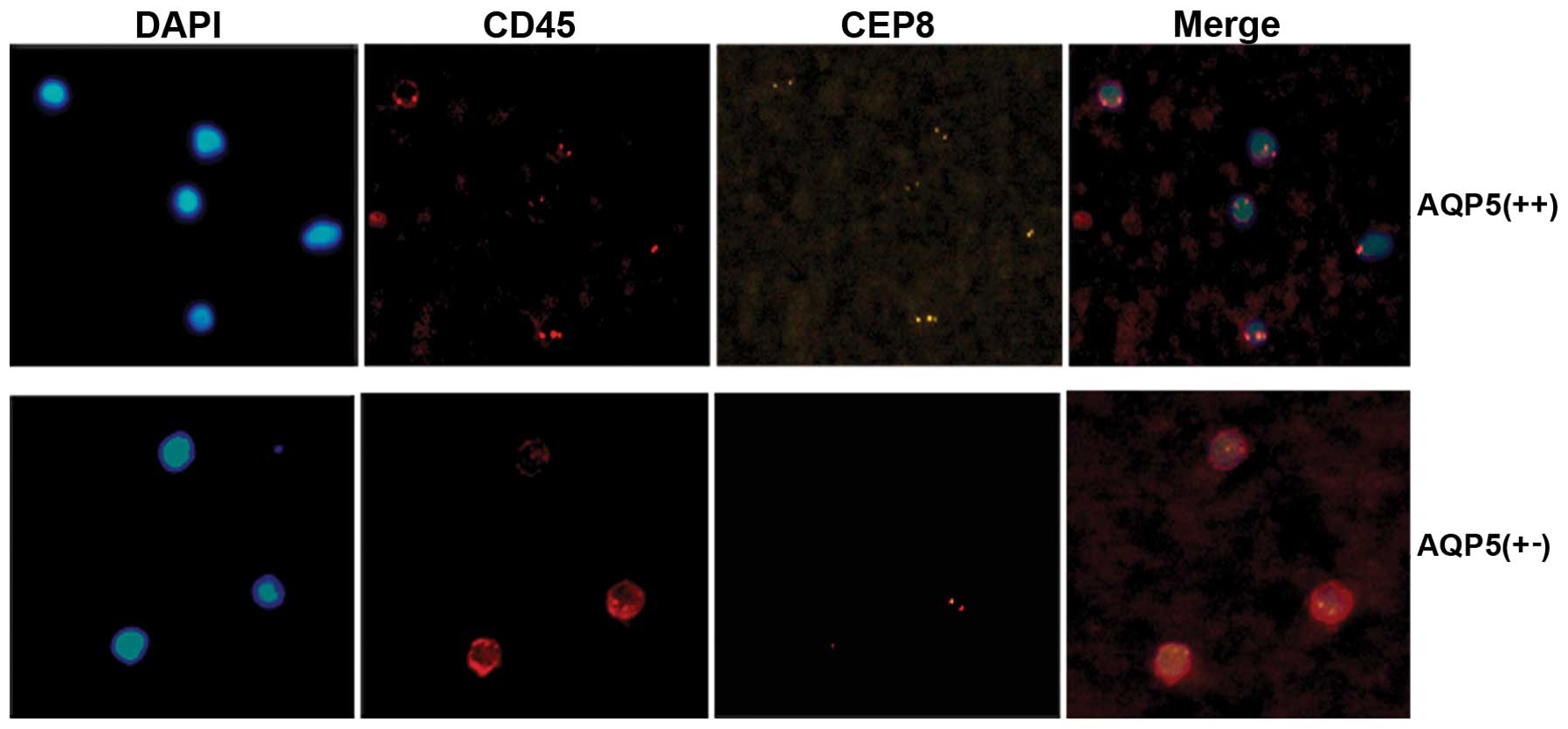
the correlation between AQP5 expression and the number of CTCs was
analyzed. CTCs were detected in 78.57% (11/14) of colorectal cancer
patients with strong AQP5 expression vs. 45.16% (14/31) with low
and absent AQP5 expression (P<0.05; Fig. 4); Moreover, a significantly greater
number of CTC enumeration was observed in patients with strong
expression of AQP5 compared to patients with low and absent
expression of AQP5 (19.5±2.0 in 7.5 ml blood vs. 5.5±1.5 in 7.5 ml
blood). In conclusion, AQP5 is positively correlated with the
number of CTCs.
Discussion
Colorectal cancer is characterized by early lymph
node metastasis and poor prognosis (1). In the present study, we determined the
clinical significance of AQP5 expression in colorectal cancer
patients, and thereby better defined the potential role for AQP5 in
novel therapeutic modalities. Our results revealed that AQP5 is
upregulated in colorectal cancer compared with peri-tumor and
normal tissues accompanied by AQP5 DNA amplification. Furthermore,
AQP5 was closely correlated with advanced TNM stage and poor
prognosis. Markedly, AQP5-overexpressing cancer was shown to be
prone to metastasis; clearly, there are more CTCs circulating in
patients with AQP5 overexpression. These findings extend our
understanding of the role of AQP5 as a diagnostic marker. To our
knowledge, this is the first study to show AQP5 in colorectal
cancer and its association with CTCs.
Aquaporins play a crucial role in maintaining water
homeostasis and modulating a variety of physiologic and pathologic
processes (13). Notably, the
present study suggested that AQPs, including AQP5, are involved in
tumorigenesis. AQP5, a 21–24 kDa protein, was initially described
as the main structural protein in caveolae and was believed to be a
key molecule involved in oncogenic transformation and malignant
progression (14,15). AQP5 plays an important regulatory
role in several signaling pathways leading to cellular
transformation, including those mediated by the Src family of
tyrosine kinases, epidermal growth factor receptor, Wnt, and Erk1/2
(10,16,17).
Recently, Lee et al (11)
reported that AQP5 overexpression is significantly associated with
lymph node involvement and a poorer prognosis in patients with
breast cancer, suggesting the value of AQP5 as a prognostic marker
in breast cancer. Huang et al (18) also verified that AQP5 promotes the
proliferation and migration of human gastric carcinoma cells. Wang
et al (19) performed a
study to show that AQP5 was mainly expressed in colorectal
carcinoma cells and barely expressed in paraneoplastic normal
tissues. The clinical significance of AQP5 in colorectal carcinoma
is unknown. Our results showed that AQP5 is mainly expressed in
colorectal cancer cells and minimally expressed in peritumor and
normal tissues. The expression patterns of AQP5 in colorectal
tissues detected herein are consistent with the results of previous
studies. Furthermore, we analyzed the correlations of AQP5
expression with the clinicopathologic features of colorectal cancer
and showed that AQP5 expression is not significantly associated
with the gender or age of patients with colorectal cancer, but is
closely associated with the differentiation, TNM stage and distant
lymph node metastasis of colorectal cancer.
CTCs are tumor cells shed from the primary tumor
into the circulation. The presence of CTCs in the peripheral blood
of patients has long been associated with metastasis and poor
survival and is now considered an acceptable cancer marker.
However, current techniques are limited (20,21).
The only commercially available CTC test (CellSearch; Veridex,
Raritan, NJ, USA) has a detection rate of 50% in late-stage
patients (22,23). In the present study, we detected
CTCs harboring negative enrichment using an immunomagnetic beads
method, followed by identification with cytologic analysis,
immunofluorescence and imFISH. This combination of methods resulted
in detection rates up to 78.57% in patients with AQP5
overexpression, suggesting that imFISH staining could be used as a
detection method for CTCs in future studies. In addition, our study
verified that AQP5 overexpression is associated with the
possibility of metastasis compared to lower expression of AQP5.
In summary, the AQP5 protein is upregulated in
colorectal cancer and is closely related to advanced TNM stage,
lymph node metastasis and poor prognosis. AQP5 strong
overexpression is highly correlated with gene amplification. Thus,
AQP5 may be used as a novel biomarker for colorectal cancer
aggressiveness and metastasis.
Acknowledgements
The authors thank the staff of the Biology and
Genetics Laboratory of Xi’an Jiaotong University for their
technical assistance in these studies.
References
|
1
|
American Cancer Society. Cancer facts and
figures. 2004, http://acs.org.
|
|
2
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koontongkaew S: The tumor microenvironment
contribution to development, growth, invasion and metastasis of
head and neck squamous cell carcinomas. J Cancer. 4:66–83. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sekine S, Shimada Y, Nagata T, Moriyama M,
Omura T, et al: Prognostic significance of aquaporins in human
biliary tract carcinoma. Oncol Rep. 27:1741–1747. 2012.PubMed/NCBI
|
|
5
|
Moon C, Soria JC, Jang SJ, Lee J, Obaidul
HM, et al: Involvement of aquaporins in colorectal carcinogenesis.
Oncogene. 22:6699–6703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang D and Owler BK: Expression of AQP1
and AQP4 in paediatric brain tumours. J Clin Neurosci. 18:122–127.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hara-Chikuma M and Verkman AS: Prevention
of skin tumorigenesis and impairment of epidermal cell
proliferation by targeted aquaporin-3 gene disruption. Mol Cell
Biol. 28:326–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burghardt B, Elkaer ML, Kwon TH, Racz GZ,
Varga G and Steward MC: Distribution of aquaporin water channels
AQP1 and AQP5 in the ductal system of the human pancreas. Gut.
52:1008–1016. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang SK, Chae YK, Woo J, Kim MS, Park JC,
et al: Role of human aquaporin 5 in colorectal carcinogenesis. Am J
Pathol. 173:518–525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Woo J, Lee J, Kim MS, Jang SJ, Sidransky D
and Moon C: The effect of aquaporin 5 overexpression on the Ras
signaling pathway. Biochem Biophys Res Commun. 367:291–298. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SJ, Chae YS, Kim JG, Kim WW, Jung JH,
et al: AQP5 expression predicts survival in patients with early
breast cancer. Ann Surg Oncol. 21:375–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shpiz S, Lavrov S and Kalmykova A:
Combined RNA/DNA fluorescence in situ hybridization on whole-mount
Drosophila ovaries. Methods Mol Biol. 1093:161–169. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCoy E and Sontheimer H: Expression and
function of water channels (aquaporins) in migrating malignant
astrocytes. Glia. 55:1034–1043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung HJ, Park JY, Jeon HS and Kwon TH:
Aquaporin-5: a marker protein for proliferation and migration of
human breast cancer cells. PLoS One. 6:e284922011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Z, Chen Z, Song Y, Zhang P, Hu J and
Bai C: Expression of aquaporin 5 increases proliferation and
metastasis potential of lung cancer. J Pathol. 221:210–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen XJ, Yang JH and Zheng W: Effect of
topotecan on expression of aquaporin protein 5 and nuclear
factor-kappaB in ovarian cancer SKOV3 cells. Ai Zheng. 28:856–860.
2009.(In Chinese).
|
|
17
|
Choi JH, Wu HG, Jung KC, Lee SH and Kwon
EK: Apoptosis and expression of AQP5 and TGF-beta in the irradiated
rat submandibular gland. Cancer Res Treat. 41:145–154. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang YH, Zhou XY, Wang HM, Xu H, Chen J
and Lv NH: Aquaporin 5 promotes the proliferation and migration of
human gastric carcinoma cells. Tumour Biol. 34:1743–1751. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Li Q, Yang T, Bai G, Li D, Li Q
and Sun H: Expression of AQP5 and AQP8 in human colorectal
carcinoma and their clinical significance. World J Surg Oncol.
10:2422012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Onstenk W, Gratama JW, Foekens JA and
Sleijfer S: Towards a personalized breast cancer treatment approach
guided by circulating tumor cell (CTC) characteristics. Cancer
Treat Rev. 39:691–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Attard G, Crespo M, Lim AC, Pope L, Zivi
A, et al: Reporting the capture efficiency of a filter-based
microdevice: a CTC is not a CTC unless it is CD45 negative -
letter. Clin Cancer Res. 17:3048–3050. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coumans FA, Ligthart ST, Uhr JW and
Terstappen LW: Challenges in the enumeration and phenotyping of
CTC. Clin Cancer Res. 18:5711–5718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lustberg M, Jatana KR, Zborowski M and
Chalmers JJ: Emerging technologies for CTC detection based on
depletion of normal cells. Recent Results Cancer Res. 195:97–110.
2012. View Article : Google Scholar : PubMed/NCBI
|