Introduction
Rac1 is a member of the small RhoGTPase family, that
is activated by binding to GTP, and inactivated by binding to GDP.
It regulates a wide range of cellular properties, such as
proliferation, differentiation, apoptosis and migration (1). It is also crucial in regulation of the
cell cycle in human cancer cells (2). However, the mechanism is largely
unknown.
G1 to S phase transition is a pivotal step in the
cell cycle and plays a crucial role in various biological
processes, such as cell proliferation, terminal differentiation,
senescence or cell death (3).
Cyclin D1 is a key molecule that is required for S phase entry.
Overexpression of cyclin D1 accelerates G1/S transition (4). On the other hand, inhibition of cyclin
D1 induces cell cycle arrest in the G1 phase. In association with
cyclin D1, cyclin-dependent kinases (CDKs) CDK4 and CDK6
phosphorylate their substrates, such as retinoblastoma protein
(pRb), allowing the release of E2F transcription factors that
activate G1/S-phase gene expression (5). The level of cyclin D protein is
reduced through downregulation of protein expression or
phosphorylation-dependent degradation due to ubiquitination and
proteasome-mediated degradation (6).
Glycogen synthase kinase-3 (GSK3) is a critical
downstream element of the PI3K/AKT pathway. Therefore, its activity
can be inhibited by AKT-mediated phosphorylation at Ser21 of GSK3α
and Ser9 of GSK3β (7,8). GSK3β is reported to phosphorylate
cyclin D1 at Thr286. AKT positively regulates G1/S cell cycle
progression through inactivation of GSK3β, resulting in increased
cyclin D1. As a target of cyclin D1, E2F1 promotes cell cycle by
binding to different genes, such as myc and TK (9,10).
Rac1 has been shown to regulate the cell cycle by
regulation of cyclin D1 (11).
However, the intermediary molecules are unclear. Joyce et al
(12) reported that Rac1 regulates
cyclin D1 transcription through NF-κB-dependent signaling in NIH3T3
cells. However, our previous study found that NF-κB was not altered
in Rac1-depleted keratinocytes compared to wild-type keratinocytes
in vivo and in vitro (13). Therfore, the downstream pathway of
Rac1 involved in the regulation of G1/S transition through cyclin
D1 seems to be variable in different cell types.
In the present study, we aimed to investigate the
mechanism involved in the regulation of G1/S transition by Rac1 in
cancer cells. We found that inhibition of Rac1 activity induced
G1/S phase arrest through the GSK3/cyclin D1 pathway.
Materials and methods
Cell culture
A431 human epithelial carcinoma cells, SW480 human
colon cancer cells and U2-OS human osteosarcoma cells were grown in
Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) supplemented with
10% fetal bovine serum (FBS), 100 U/ml penicillin and 0.1 mg/ml
streptomycin at 37°C in 5% CO2. Rac1 inhibitor NSC23766
(Calbiochem) and GSK3 inhibitor lithium (Sigma) in PBS at final
concentrations of 100 μM and 100 mM were used.
Plasmid constructs and gene transfer
shRNA against GSK3 and the GSK3-overexpressing
plasmid were purchased from Shanghai GenePharma Co., Ltd (Shanghai,
China). High cycling Rac1 plasmid was a gift from Professor Cord
Brakebusch at the University of Copenhagen, Denmark. A431, SW480
and U2-OS cells were transfected using MirusTransIT transfection
reagents according to the manufacturer’s instructions, and selected
by G418 for setting up stable transfection.
MTT assay
A431, SW480 and U2-OS cells were plated in
quintuplicate in 96-well plates (5×103/well). At 24 h
after incubation, NSC23766 or LiCl was added to a final
concentration of 100 μM or 100 mM, respectively, for 1, 2 or 3
days. Next, 20 μl of 5 mg/ml MTT (Sigma, St. Louis, MO, USA) was
added to each well for 4 h at 37°C. Dimethylsulfoxide (DMSO) (150
μl) was added to dissolve the crystals, and absorbance was measured
with an enzyme-linked immunosorbent assay reader (Bio-Rad
Laboratories, Hercules, CA, USA), using a measurement wavelength of
570 nm.
Cell cycle analysis
A431, SW480 and U2-OS cells were seeded in 6-well
plates at 3×105 cells/well, and incubated with NSC23766
or LiCl added to a final concentration of 100 μM or 100 mM,
respectively, for 24 h. Cells were harvested by centrifugation and
fixed with 75% ethanol. Fixed cells were incubated with propidium
iodide for at least 30 min. The DNA content of the cells was
measured on a FACScan cytometer (Becton-Dickinson).
Western blot analysis
Western blot analysis was performed as previously
described (14). Cell lysates were
prepared from cell monolayers incubated in RIPA buffer [50 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1 mM sodium
orthovanadate, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% sodium
dodecyl sulfate (SDS), 2 mM phenylmethylsulfonyl fluoride (PMSF)]
and protease inhibitor cocktail. Total cellular protein samples of
50 μg were resolved by SDS-PAGE. Blots were probed with anti-Rac1,
p-AKT (Ser473), GSK3α, p-GSK3α (Ser21), GSK3β, p-GSK3β (Ser9),
cyclin D1, p-cyclin D1 (Thr286), CDK4, CDK6 and E2F1 antibodies
purchased from Cell Signaling Technology. The same antibodies were
used for immunofluoresent staining. Anti-tubulin (Abcam) was used
as the loading control. Secondary antibodies were goat anti-rabbit
or goat anti-mouse coupled to horseradish peroxidase. Development
was carried out with enhanced chemiluminescence reagents
(Millipore, Billerica, MA, USA), and signals were detected by a
chemiluminescence detection system (Clinx Science Instruments Co.,
Ltd., Shanghai, China).
Reverse transcription PCR (RT-PCR)
Total RNA was isolated from cells using the TRIzol
reagent (Sigma) according to the manufacturer’s protocol. Reverse
transcription was performed using the RT-PCR kit (BD Biosciences)
following the manufacturer’s protocol. cDNA was then amplified. The
reaction condition was first heated at 95°C for 5 min, then 30
cycles at 95°C for 1 min, followed at 55°C for 1 min and 72°C for 1
min, then 72°C for 10 min. Primers were: cyclin D1, 5′-CACA
CGGACTACAGGGGAGT-3′ (forward) and 5′-CACAGGA GCTGGTGTTCCAT-3′
(reverse); E2F1, 5′-ATGTTTTCCTG TGCCCTGAG-3′ (forward) and
5′-ATCTGTGGTGAGGGA TGAGG-3′ (reverse); GAPDH, 5′-GAGTCCACTGGCGTC
TTC-3′ (forward) and 5′-GGGGTGCTAAGCAGTTGGT-3′ (reverse).
Statistical analysis
All data are presented as means ± standard deviation
(SD). Statistical significance was determined by the Student’s
t-test. P-values <0.01 were considered significant. Analyses
were performed using SPSS 11.0.
Results
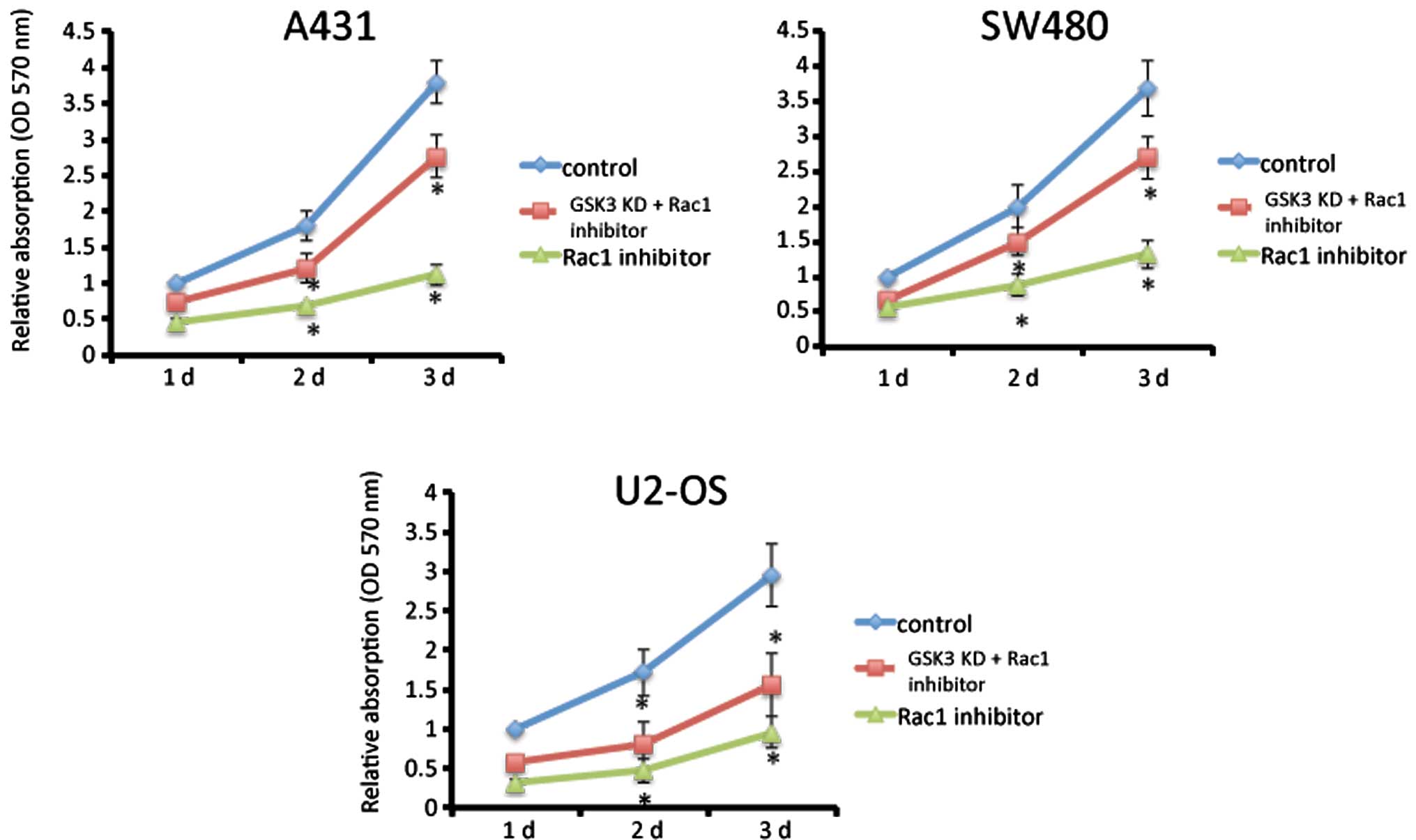
Rac1 activity is crucial for cancer cell
proliferation
The role of Rac1 activity was assessed using Rac1
inhibitor NSC23766 that has been confirmed to inhibit Rac1 activity
by interfering with the interaction between GEF and Rac1 (15), consequently inhibiting activation.
MTT assay was performed to test cell proliferation. Fig. 1 shows that NSC23766 at the
concentration of 100 μM inhibited proliferation in A431 skin cancer
cells, SW480 colon cancer cells and U2-OS osteosarcoma cells
(P<0.01). However, suppression of GSK3 partially rescued this
inhibition in all three cancer cell lines.
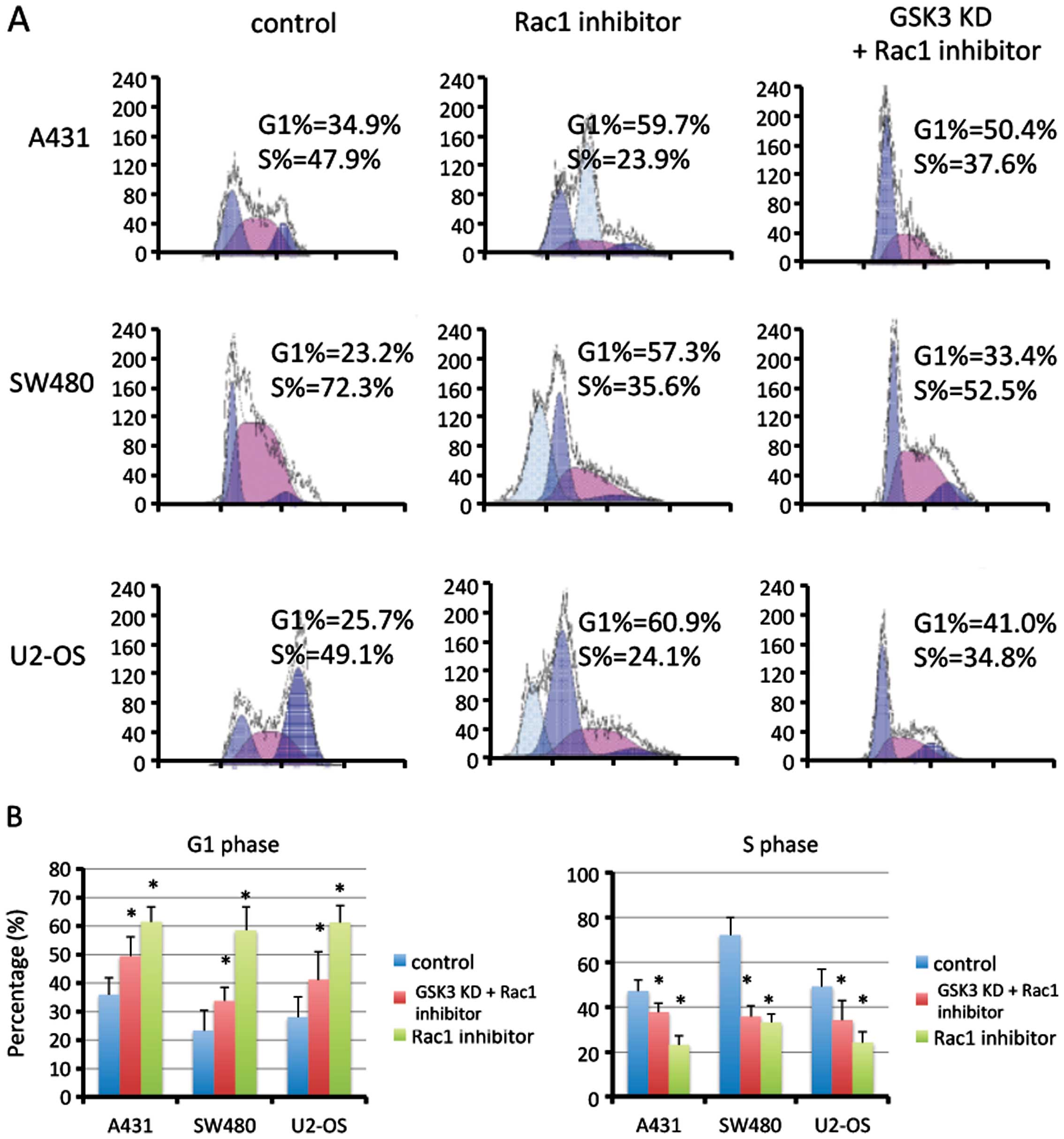
Inhibition of Rac1 induces G1/S phase
arrest
The cell cycle is a crucial effector of
proliferation. Using flow cytometry, we found that inhibition of
Rac1 activation by NSC23766 markedly induced cell cycle arrest in
the G1 phase in the A431, SW480 and U2-OS cells (P<0.01;
Fig. 2). Suppression of GSK3
partially rescued this effect in all three cancer cell lines.
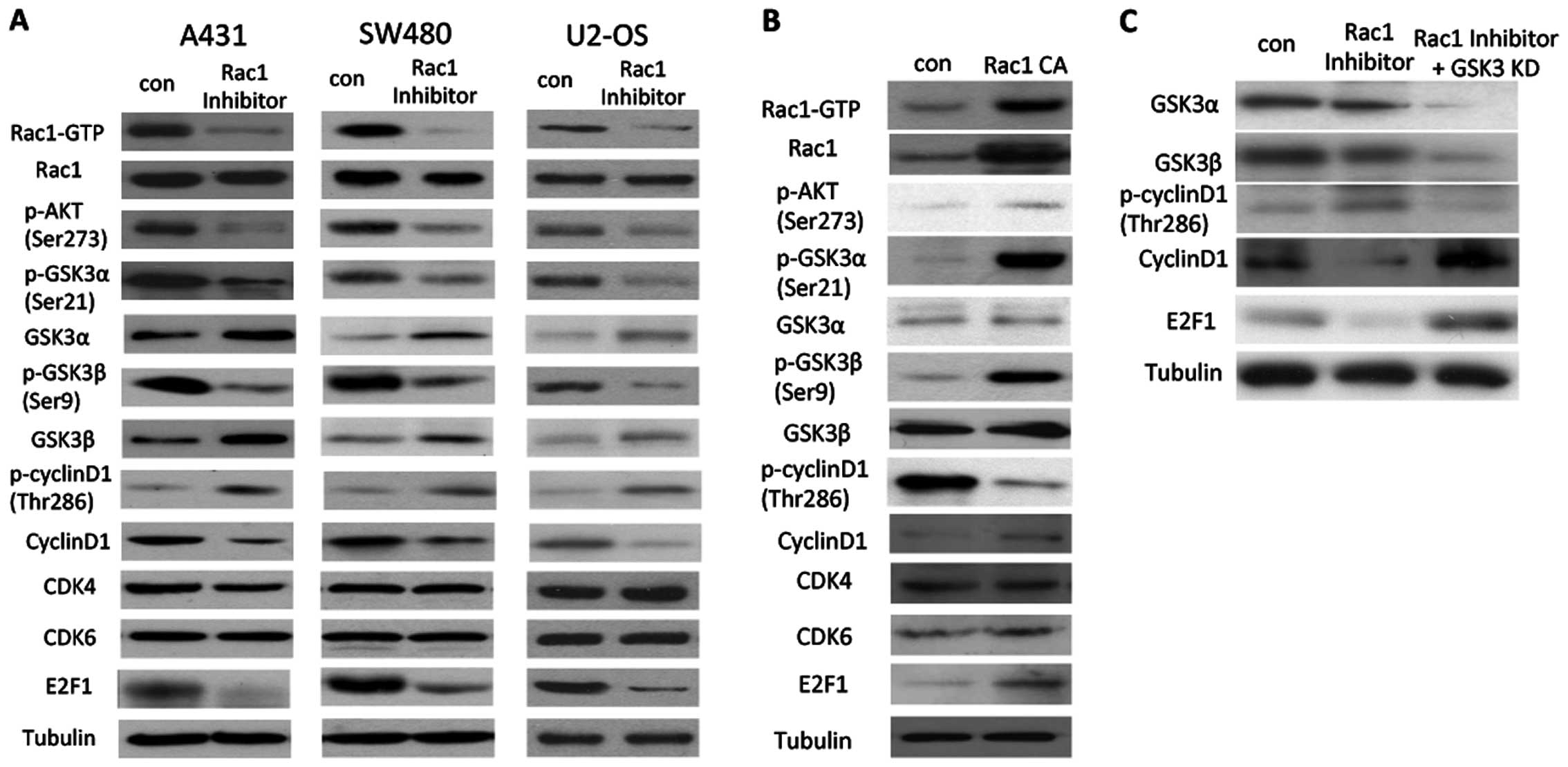
Inhibition of Rac1 suppresses cyclin D1
through GSK3
In A431 cells, following incubation of NSC23766,
phosphorylation of AKT at ser473 was decreased. Consequently,
phosphorylation of both GSK3α and GSK3β was reduced as well. Total
expression of GSK3α and GSK3β was slightly increased after
incubation of NSC23766. Higher levels of p-cyclin D1 and a lower
level of total cyclin D1 were observed in the Rac1-inhibited cells,
while CDK4 and CDK6 expression was not altered. The transcription
factor E2F1, a target of cyclin D1, was repressed following
inhibition of Rac1 activation. The changes in the above proteins
were similar in the SW480 and U2-OS cells (Fig. 3A). In contrast, constitutive active
Rac1 in SW480 cells induced a higher level of p-AKT, p-GSK3α and
p-GSK3β. A lower level of p-cyclin D1 and higher levels of cyclin
D1 and E2F1 were observed in the Rac1-activated cells (Fig. 3B). Moreover, suppression of GSK3
reduced p-cyclin D1, increased total levels of cyclin D1 and E2F1,
even in the presence of the Rac1 inhibitor (Fig. 3C).
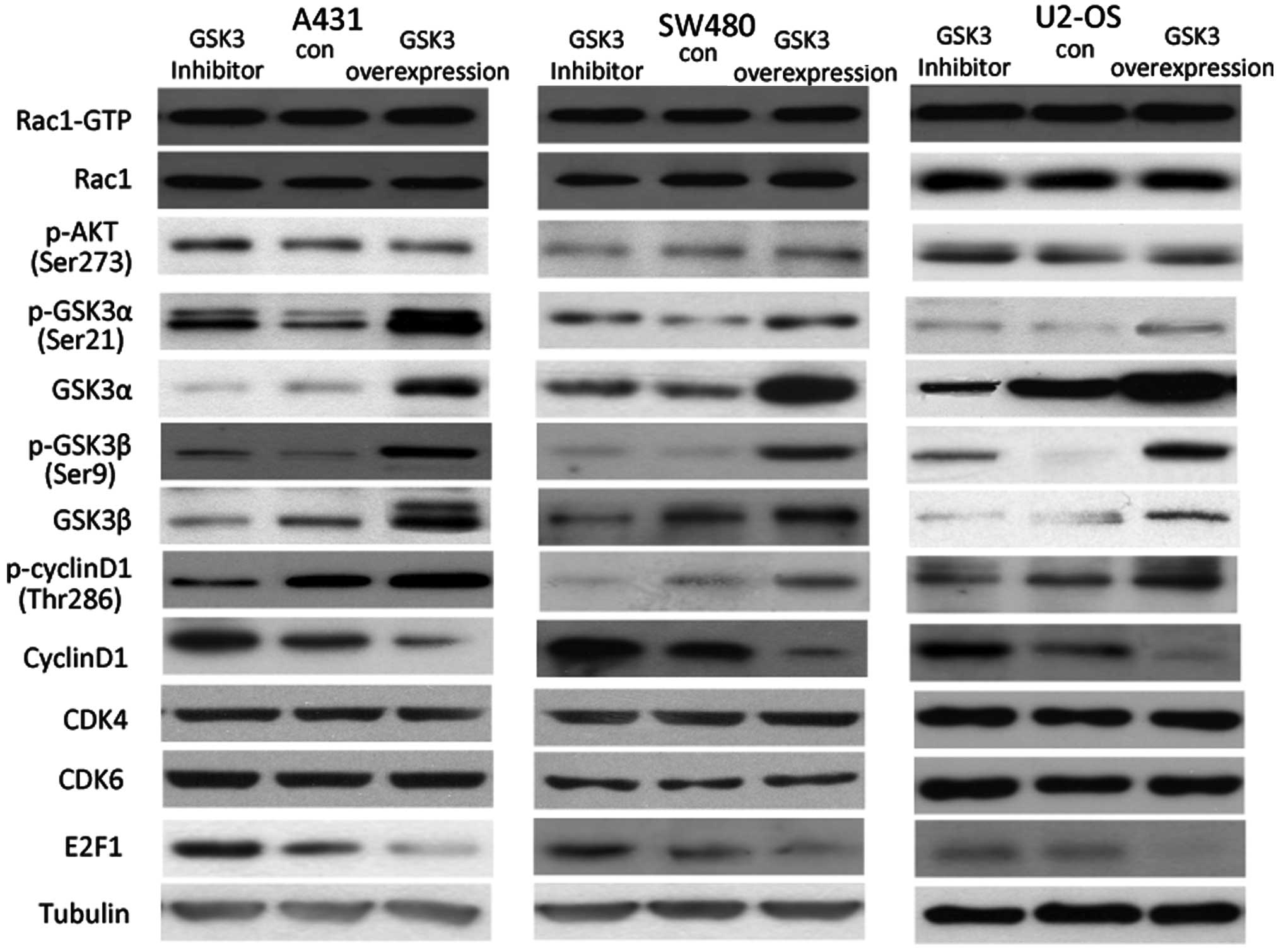
Inhibition of GSK3 suppresses cyclin D1
and E2F1
In the A431 cells, following incubation with LiCl, a
GSK3 inhibitor, phosphorylation of both GSK3α and GSK3β was
elevated, while total expression of GSK3α and GSK3β was reduced.
Neither Rac1 activity nor AKT phosphorylation was altered when GSK3
activity was inhibited, indicating that GSK3 functions downstream
of Rac1 and AKT. Followed by decrease in the GSK3 level following
incubation with LiCl, cyclin D1 phosphorylation was reduced and
total cyclin D1 was increased, possibly resulting in a higher level
of E2F1. Furthermore, overexpression of GSK3 in the A431 cells
further increased p-cyclin D1 and decreased the total amount of
cyclin D1 and E2F1 protein, suggesting that GSK3 is a molecule that
regulates cyclin D1 phosphorylation and expression downstream of
Rac1 and AKT. Similar results were observed in the SW480 and U2-OS
cells (Fig. 4).
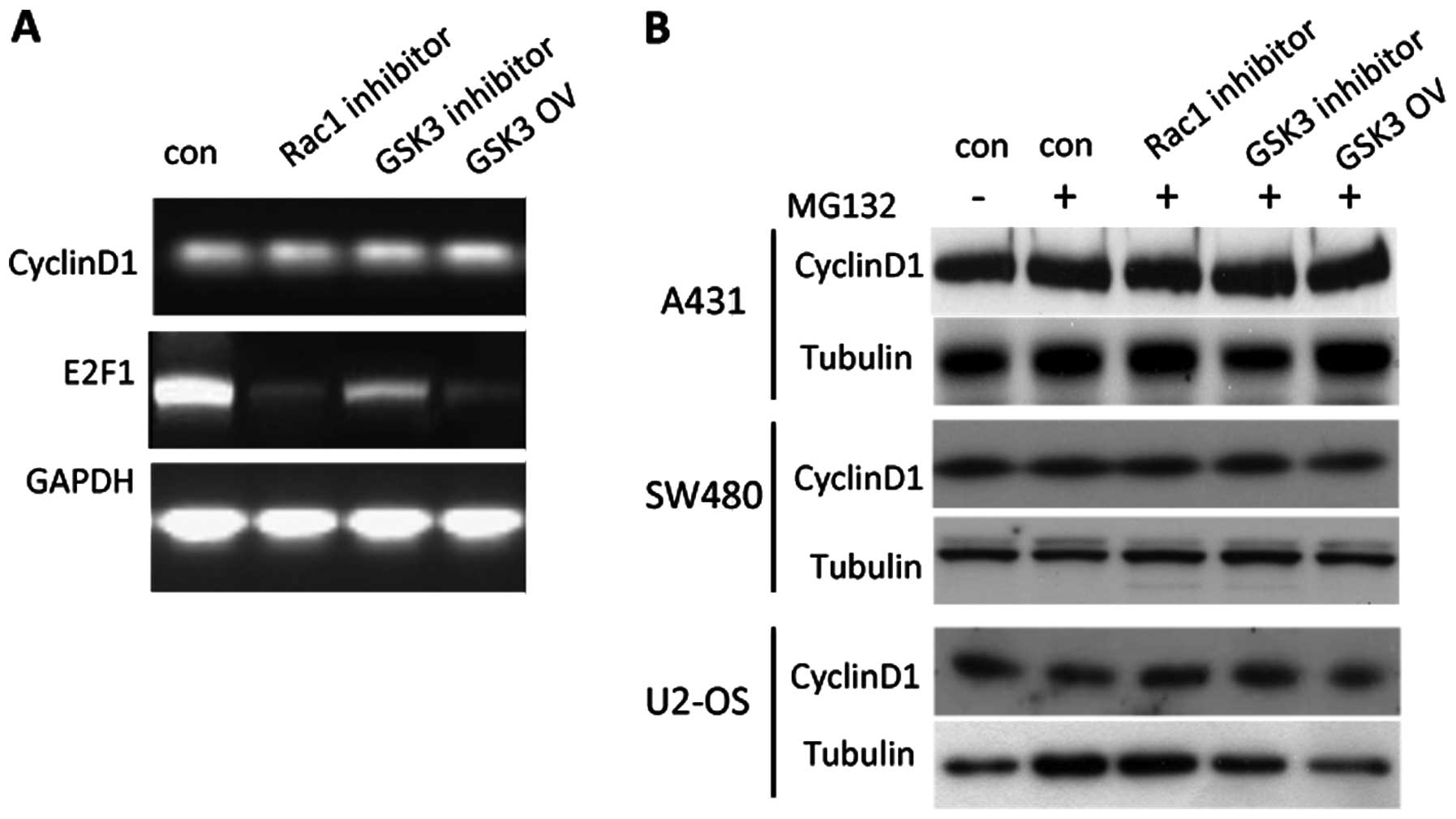
Notably, neither inhibition of Rac1 and GSK3
activity nor elevated GSK3 expression altered cyclin D1 expression
at the RNA level. Moreover, expression of cyclin D1 at the protein
level remained constant in the presence of the proteasome inhibitor
MG132 at the concentration of 50 μM (Fig. 5). These results indicate that Rac1
and GSK3 may change cyclin D1 protein expression at the
post-transcription level. E2F1 expression was markedly reduced at
the RNA level when Rac1 or GSK3 was inactivated, suggesting that
Rac1 and GSK3 regulate E2F1 expression at the transcription
level.
Discussion
Rac1 is either overexpressed or overactivated in
many cancer types, such as colon, testicular, gastric, breast, oral
and skin squamous cell carcinoma (1). Rac1 has been demonstrated to be
crucial for tumor formation by control of transformation,
proliferation and survival of tumor cells. We inhibited Rac1
activity in A341 skin cancer cells, SW480 colon cancer cells and
U2-OS osteosarcoma cells, and proliferation of the three cell lines
was significantly reduced, indicating the indispensible role of
Rac1 in cancer cell proliferation. However, suppression of GSK3
partially rescued this inhibition of proliferation, suggesting that
Rac1 regulates cell proliferation via GSK3.
Cell cycle progression is the process of cell
division, which decides the speed of cell proliferation (16). In early G1, cyclin D1 associates
with CDK4 and CDK6 to form active cyclin D/CDK4/CDK6 complexes
(17). This complex is responsible
for targeting nuclear transcription factors. In the present study,
inhibition of Rac1 or GSK3 activity induced cell cycle arrest in
the G1 phase in all three cancer cell lines. This cell cycle arrest
was partially rescued by suppression of GSK3, suggesting that Rac1
regulates the cell cycle through GSK3.
GSK3 functions to phosphorylate cyclin D1 at Thr286,
subsequently inducing its proteasomal degradation, thereby
triggering cyclin D1 turnover (18). Inhibition of Rac1 activity reduced
the phosphorylation of AKT, consistent with previous results that
phosphorylation of AKT was decreased in Rac1-depleted epidermis
treated by TPA (15). In contrast,
constitutive active Rac1 induced higher levels of p-AKT, p-GSK3α
and p-GSK3β, a lower level of p-cyclin D1, as well as a higher
level of cyclin D1, indicating that the GSK3/cyclin D1 pathway is
regulated by Rac1.
GSK3 activity can be phosphorylated and inhibited by
the PI3K/AKT pathway (7). GSK3α and
GSK3β phosphorylation was reduced when Rac1 was inhibited, possibly
due to the inactivation of AKT, resulting in the increase in the
amount of GSK3α and GSK3β protein. GSK3 is known to phosphorylate
and degrade cyclin D1. Suppression of GSK3 reduced p-cyclin D1 and
increased the total level of cyclin D1 (Fig. 3C). Moreover, the level of p-cyclin
D1 was increased, possibly due to the higher protein level of GSK3
in the Rac1-inhibited cancer cells, correlating with a lower cyclin
D1 protein level, possibly due to increased cyclin D1 degradation
after phosphorylation. To confirm this hypothesis, we performed
RT-PCR to assess the expression of cyclin D1 at the RNA level in
the Rac1-inhibited cancer cells. Results showed that cyclin D1
expression at the RNA level remained constant in the
Rac1-inhibited, GSK3-inhibited and overexpressing cells. We next
inhibited the proteasome to inhibit ubiquitination and degradation
of cyclin D1. Expression of cyclin D1 at the protein level remained
unchanged in the Rac1-inhibited, GSK3-inhibited and overexpressing
cells. These results suggest that Rac1 and GSK3 regulate cyclin D1
expression at the post-transcription level, possibly by the effect
of ubiquitination and degradation.
E2F1, known as a downstream target of cyclin D1 and
a member of the E2F family that induces G1 phase entry to S phase,
is a potent stimulator of cell cycle entry (19,20).
It binds to specific DNA sequences and regulates transcription of
E2F target genes. E2F1 was suppressed following inhibition of Rac1
activity or GSK3 activity, but was overexpressed by constitutive
active Rac1 or suppression of GSK3, followed by increased or
decreased cyclin D1.
In conclusion, in the cancer cell lines, inhibition
of Rac1 reduced AKT phosphorylation, thus inhibiting GSK3
phosphorylation, resulting in an elevated level of GSK3. This
subsequently induced a higher level of p-cyclin D1, which induced
degradation of cyclin D1, leading to suppression of E2F1 resulting
in induction of G1/S phase arrest (Fig.
6).
Acknowledgements
The present study was sponsored by the National
Natural Science Foundation of China (nos. 81071689 and 81202119)
and the Key Foundation of Shaanxi Province for International
Communication (2013KW30-01).
References
|
1
|
Karlsson R, Pedersen ED, Wang Z and
Brakebusch C: RhoGTPases function in tumor. Biochim Biophys Acta.
1796:91–98. 2009.PubMed/NCBI
|
|
2
|
Qiu RG, Chen J, McCormick F and Symons M:
A role for Rho in Ras transformation. Proc Natl Acad Sci USA.
92:11781–11785. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richard-Parpaillon L, Cosgrove RA, Devine
C, Vernon AE and Philpott A: G1/S phase cyclin-dependent kinase
overexpression perturbs early development and delays
tissue-specific differentiation in Xenopus. Development.
131:2577–2586. 2004. View Article : Google Scholar
|
|
4
|
Quelle DE, Ashmun RA, Shurtleff SA, Kato
JY, Bar-Sagi D, Roussel MF and Sherr CJ: Overexpression of mouse
D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes
Dev. 1559–1571. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nevins JR: E2F, a link between the Rb
tumor suppressor protein and viral oncoproteins. Science.
258:424–429. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsieh TC, Yang CJ, Lin CY, Lee YS and Wu
JM: Control of stability of cyclin D1 by quinone reductase 2 in
CWR22Rv1 prostate cancer cells. Carcinogenesis. 33:670–677. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahmani M, Aust MM, Attkisson E, Williams
DC Jr, Ferreira-Gonzalez A and Grant S: Dual inhibition of Bcl-2
and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in
human myeloid leukemia cells through a GSK3- and Bim-dependent
mechanism. Cancer Res. 73:1340–1351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moore SF, van den Bosch MT, Hunter RW,
Sakamoto K, Poole AW and Hers I: Dual regulation of glycogen
synthase kinase 3 (GSK3)α/β by protein kinase C (PKC)α and Akt
promotes thrombin-mediated integrin αIIbβ3 activation
and granule secretion in platelets. J Biol Chem. 288:3918–3928.
2013.
|
|
9
|
Rhee K, Ma T and Thompson EA: The
macromolecular state of the transcription factor E2F and
glucocorticoid regulation of c-myc transcription. J Biol Chem.
269:17035–17042. 1994.PubMed/NCBI
|
|
10
|
Sahin F and Sladek TL: E2F-1 has dual
roles depending on the cell cycle. Int J Biol Sci. 6:116–128. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olson MF, Ashworth A and Hall A: An
essential role for Rho, Rac, and Cdc42 GTPases in cell cycle
progression through G1. Science. 269:1270–1272. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joyce D, Bouzahzah B, Fu M, Albanese C,
D’Amico M, Steer J, Klein JU, Lee RJ, Segall JE, Westwick JK, Der
CJ and Pestell RG: Integration of Rac-dependent regulation of
cyclin D1 transcription through a nuclear factor-κB-dependent
pathway. J Biol Chem. 274:25245–25249. 1999.PubMed/NCBI
|
|
13
|
Peterson E, Wang Z, Stanley A, Peyrollier
K, Quondamatteo F and Brakebusch C: Rac1 in keratinocytes regulates
crosstalk to immune cells by Arp2/3 control of STAT1. J Cell Sci.
125:5379–5390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Zhu S, Min Shen, Liu J, Wang M, Li
C, Wang Y and Mei Q: STAT3 is involved in esophageal carcinogenesis
through regulation of Oct-1. Carcinogenesis. 34:678–688. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Pedersen E, Basse A, Lefever T,
Karine P, Kapoor S, Mei Q, Karlsson R and Brakebusch C: Rac1 is
crucial for Ras-dependent skin tumor formation by controlling
Pak1-Mek-Erk hyperactivation and hyperproliferation in vivo.
Oncogene. 29:3362–3373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang D, Sun SQ, Yu YH, Wu WZ, Yang SL and
Tan JM: Suppression of SCIN inhibits human prostate cancer cell
proliferation and induces G0/G1 phase arrest. Int J Oncol.
44:161–166. 2014.PubMed/NCBI
|
|
17
|
Chiron D, Martin P, Di Liberto M, Huang X,
Ely S, Lannutti BJ, Leonard JP, Mason CE and Chen-Kiang S:
Induction of prolonged early G1 arrest by CDK4/CDK6 inhibition
reprograms lymphoma cells for durable PI3Kδ inhibition through
PIK3IP1. Cell Cycle. 12:1892–1900. 2013.PubMed/NCBI
|
|
18
|
Diehl JA, Cheng M, Roussel MF and Sherr
CJ: Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and
subcellular localization. Genes Dev. 12:3499–3511. 1998.
|
|
19
|
Peng J and Jordan VC: Expression of
estrogen receptor alpha with a Tet-off adenoviral system induces
G0/G1 cell cycle arrest in SKBr3 breast cancer cells. Int J Oncol.
36:451–458. 2010.PubMed/NCBI
|
|
20
|
Subtil-Rodríguez A, Vázquez-Chávez E,
Ceballos-Chávez M, Rodríguez-Paredes M, Martín-Subero JI, Esteller
M and Reyes JC: The chromatin remodeller CHD8 is required for
E2F-dependent transcription activation of S-phase genes. Nucleic
Acids Res. 42:2185–2196. 2014.PubMed/NCBI
|