Introduction
Gastric cancer has the second highest incidence and
mortality rates in the world, and the highest in China (1,2). In
terms of treatment, surgery remains the only effective treatment
for gastric cancer in resectable stages. However, approximately 84%
of patients with gastric cancer have advanced disease (3). Therefore, various adjuvant
chemotherapy and radiotherapy protocols have been compared with
surgery alone in advanced gastric cancer, but the 5-year survival
analysis suggested only a moderate improvement following adjuvant
treatments (4).
In our previous study, poor prognosis of patients
with gastric cancer was found to be correlated with elevated
expression of sphingosine kinase 1 (SphK1), one of the SphK
isoenzymes that generate the bioactive lipid mediator,
sphingosine-1-phosphate (S1P) (5).
The S1P, sphingosine (SPH), and sphingolipid metabolite ceramide
(Cer) play key roles in the determination of various cellular
functions, including cell proliferation, survival and mortality.
S1P is involved in stimulating growth and suppressing apoptosis,
and, in contrast, Cer and SPH inhibit proliferation and promote
apoptosis. Therefore, the relevance of S1P, Cer and SPH lead to a
proposal of ‘sphingolipid rheostat’, which is critical for
determination of cell fate (6,7).
Notably, SphK, the enzymes that phosphorylate SPH to form S1P,
plays a pivotal role in sphingolipid rheostat. The proliferative
and anti-apoptotic messenger S1P is produced by SphK, while SphK
also decreases levels of pro-apoptotic Cer and SPH (6–11).
Accumulating evidence further demonstrates the anti-apoptotic
effects of SphK1. For example, SphK1 could protect cancer cells
against apoptosis from apoptosis induced by TNF-a, ionizing
radiation or anticancer drugs, due to increased ceramide levels
(12,13). Meanwhile, ectopic overexpression of
SphK1 inhibited caspase cleavage and activated the pro-apoptotic
kinase JNK to inhibit PC12 cells from apoptosis caused by growth
factor withdrawal or exogenous Cer (14). Moreover, Bonhoure et al
demonstrated that the resistance to doxorubicin and
etoposide-induced cell death were conferred in SphK1-overexpressing
HL-60 leukemia cells (15). In
addition, Pchejetski et al found that the resistance to
camptothecin or docetaxel in prostate cancer cells was also
associated with elevated SphK1 activity (16). Taken together, these reports
indicate that SphK1 is involved in the regulation of cancer cell
apoptosis.
It has been well demonstrated that the
serine/threonine kinase Akt (also known as PKB) acts as one of the
most important protein kinases in various physiological and
pathological conditions, particularly in cancer (17). Many oncoproteins and tumor
suppressors are involved in the Akt pathway to exert their biologic
function. SphK1 acts as an oncoprotein and facilitates Akt
signaling activation in several human cancers, such as
glioblastoma, colon cancer and erythroleukemia (18–20).
Notably, several studies documented that Akt can phosphorylate
forkhead box O (FoxO) proteins and the regulation of FoxO proteins
is mainly due to the phosphatidylinositol 3-kinase (PI3K)-Akt
signaling pathway (21). Based on
these previous findings, we hypothesized that SphK1 may be involved
in gastric cancer tumorigenesis via regulation of the Akt/FoxO3a
signaling pathway.
In the present study, we reported the role of SphK1
in the sensitization of radiation-resistant MGC-803 gastric cancer
cells to UV-induced apoptosis. We also demonstrated that the
anti-apoptotic effect of SphK1 on gastric cancer cells is
associated with the activation of the Akt/FOX3a pathway, suggesting
that inhibition of SphK1 may represent a novel approach to the
treatment of gastric cancer.
Materials and methods
Cell line and retroviral infection
Gastric cancer cell line MGC-803 was maintained in
DMEM medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (FBS; HyClone, Logan, UT, USA). For ectopic
overexpression, an SphK1 expression construct from subcloning
PCR-amplified full-length human SphK1 cDNA was inserted into the
pMSCV plasmid. For depletion of SphK1, two human SphK1-targeting
siRNA sequences were cloned into pSuper-retro-puro to generate
pSuper-retro-SPHK1-RNAi(s), respectively, and the sequences were:
RNAi#1, GGCTGAAATCTCCTTCACG; RNAi#2, GGGCAAGGCCTTGCAGCTC.
Retroviral production and infection were performed as previously
described (22). Stable cell lines
expressing SphK1 or SphK1 shRNAs were selected for 10 days with 0.5
μg/ml puromycin.
Western blot analysis
Western blot analyses were performed according to
the standard method as described previously (23), using anti-SphK1 antibody (Abgent,
San Diego, CA, USA); anti- Akt, anti-p-Akt, anti-cleaved caspase 3,
anti-PARP, anti-Bim, anti-bax, anti-Bcl-xL, anti-p-FoxO3a, and
anti-Bcl-2 antibodies (Cell Signaling, Danvers, MA, USA). The
membranes were stripped and re-blotted with an anti-α-tubulin Ab
(Sigma, St. Louis, MO, USA) as a loading control.
RNA extraction and real-time RT-PCR
Total RNA from cultured cells was extracted using
TRIzol reagent (Invitrogen) according to the manufacturer’s
instructions. Real-time PCR was performed according to standard
methods as described previously (23). Primer sequences were: SphK1 forward,
5′-CTTGCAGCTCTTCCGGAGTC-3′ and reverse, 5′-GCTC
AGTGAGCATCAGCGTG-3′; GAPDH forward, 5′-GACTC ATGACCACAGTCCATGC-3′
and reverse, 5′-AGAGGCAG GGATGATGTTCTG-3′. Expression data were
normalized to the housekeeping gene GAPDH as a loading control.
TUNEL assay
The DeadEnd™ Fluorometric TUNEL System (Promega,
Madison, WI, USA) was performed according to the manufacturer’s
instructions. Briefly, after incubation for 24 h, cells were
treated by UV irradiation (20 J/m2), and were then
washed with cold PBS. Fresh 4% formaldehyde solution was used to
fix for 25 min at 4°C, and the fixed slides were washed with PBS
for 5 min once, followed by 0.2% Triton X-100 in PBS for 5 min.
After a 5-min wash with PBS, 100 μl equilibration buffer was used
to cover cells for 5 min, followed by a 60-min incubation with 2X
SSC at 37°C to terminate the reaction. After washing with PBS 5 min
once, the samples were stained with 1 μg/ml propidium iodide (PI)
solution in the dark for 15 min, followed by 1 μg/ml DAPI solution
in the dark for 15 min. After a final wash with ddH2O
for 5 min at room temperature and air-drying, samples were
immediately analyzed under a fluorescence microscope. A standard
fluorescein filter was set to view the green fluorescence of
fluorescein at 520 nm, the red fluorescence of PI at 620 nm, and
the blue DAPI at 460 nm.
Annexin V binding assay
The ApopNexin™ FITC Apoptosis Detection Kit
(Millipore, Lake Placid, NY, USA) was performed to quantitate
apoptotic cells, according to the manufacturer’s instructions.
Briefly, cells were treated with UV irradiation (20
J/m2) followed by 6 h incubation. After a 5-min wash
with PBS, 150 μl of an Annexin V antibody in binding buffer was
added with incubation for 15 min at room temperature, followed by
addition of 1.5 μl of PI at 1 mg/ml and a further incubation for 5
min. Subsequently, after washing with the Annexin V Binding Buffer,
samples were immediately analyzed under a fluorescence microscope
equipped with a filter for fluorescein isothiocyanate (excitation,
490 nm; emission, 525 nm), and PI staining was assessed with the
filter for Texas red (excitation, 570 nm; emission, 610 nm).
Measurement of S1P levels in cellular
assays
An S1P competitive ELISA kit (Echelon Biosciences,
Salt Lake City, UT, USA) was used to analyze S1P level according to
the manufacturer’s instructions. In brief, cells were serum-starved
for 24 h and lysed in 400 μl of the lysis buffer. Diluted cell
lysate (1:10 in delipidized human sera) was analyzed with the
Echelon S1P ELISA using the anti-S1P antibody, and the absorbance
was measured at 450 nm using a microplate reader.
Statistical analysis
Comparisons between groups for statistical
significance were performed with a two-tailed Student’s t-test. A
P-value <0.05 (using a two-tailed paired t-test) was considered
to indicate a statistically significantly difference.
Results
Overexpression and silencing of SphK1
expression in gastric cancer cells
To investigate the effect of SphK1 expression in
gastric cancer cells, retrovirally mediated overexpression and
silencing of SphK1 were conducted in this study. In order to
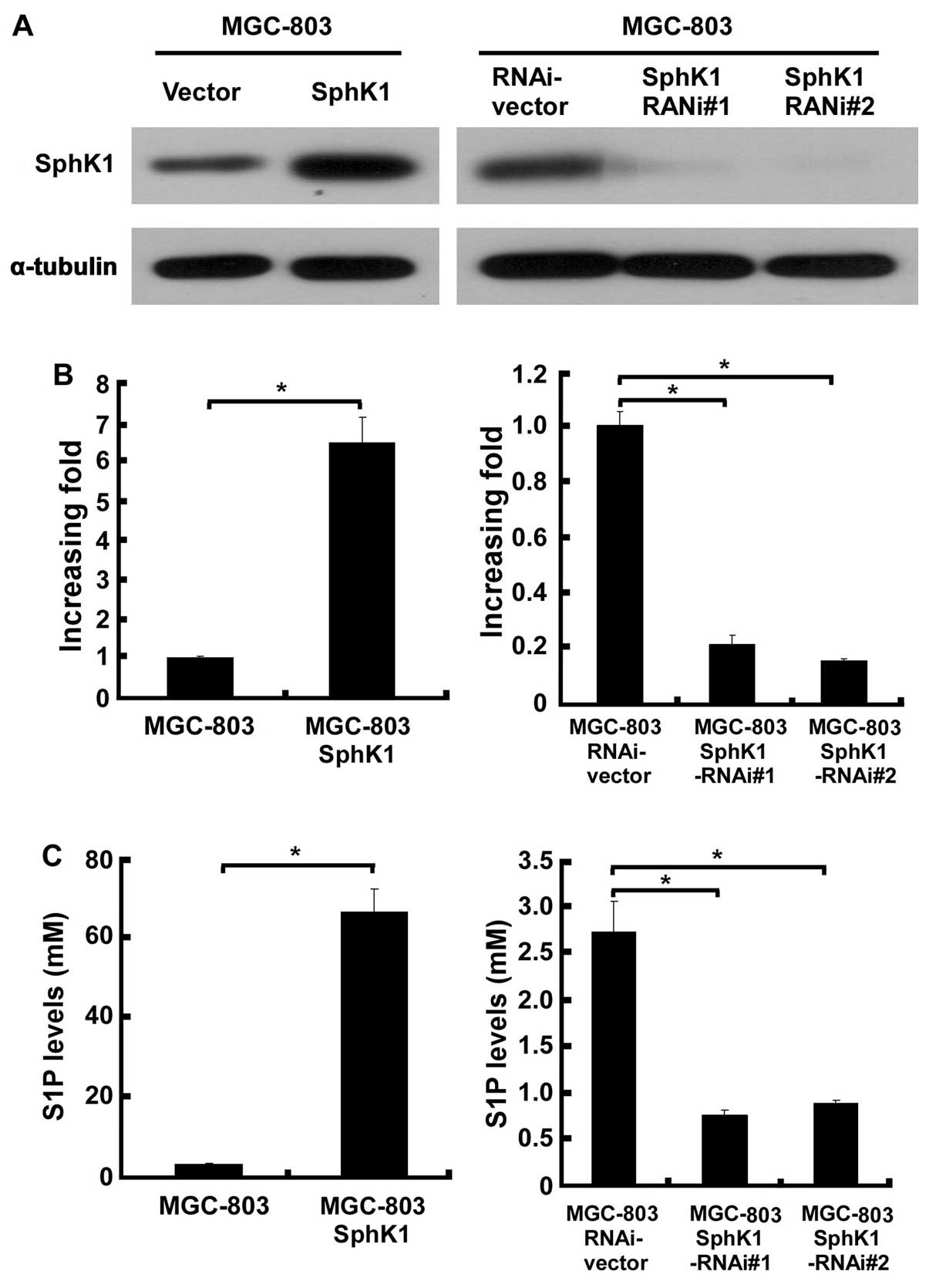
demonstrate the expression levels of SphK1 in the engineered
MGC-803 gastric cancer cells, western blotting and real-time PCR
analysis were performed. After retroviral transduction and
puromycin selection, SphK1 protein and mRNA levels were markedly
increased or reduced by, respectively, pMSCVSphK1 and
pSuper-shSphK1, as compared to the vector-control cells (Fig. 1A and B). As it is widely known that
activation of SphK1 requires post-translational steps, we examined
the cellular S1P level in the above engineered MGC-803 cells, and
as shown in Fig. 1C, overexpressing
SphK1 starkly increased 66-fold, and knockdown of SphK1 reduced
3-fold, respectively, the cellular S1P level in MGC-803 cells as
compared with the control cells.
Sensitization of gastric cancer cells to
pro-apoptotic stimuli
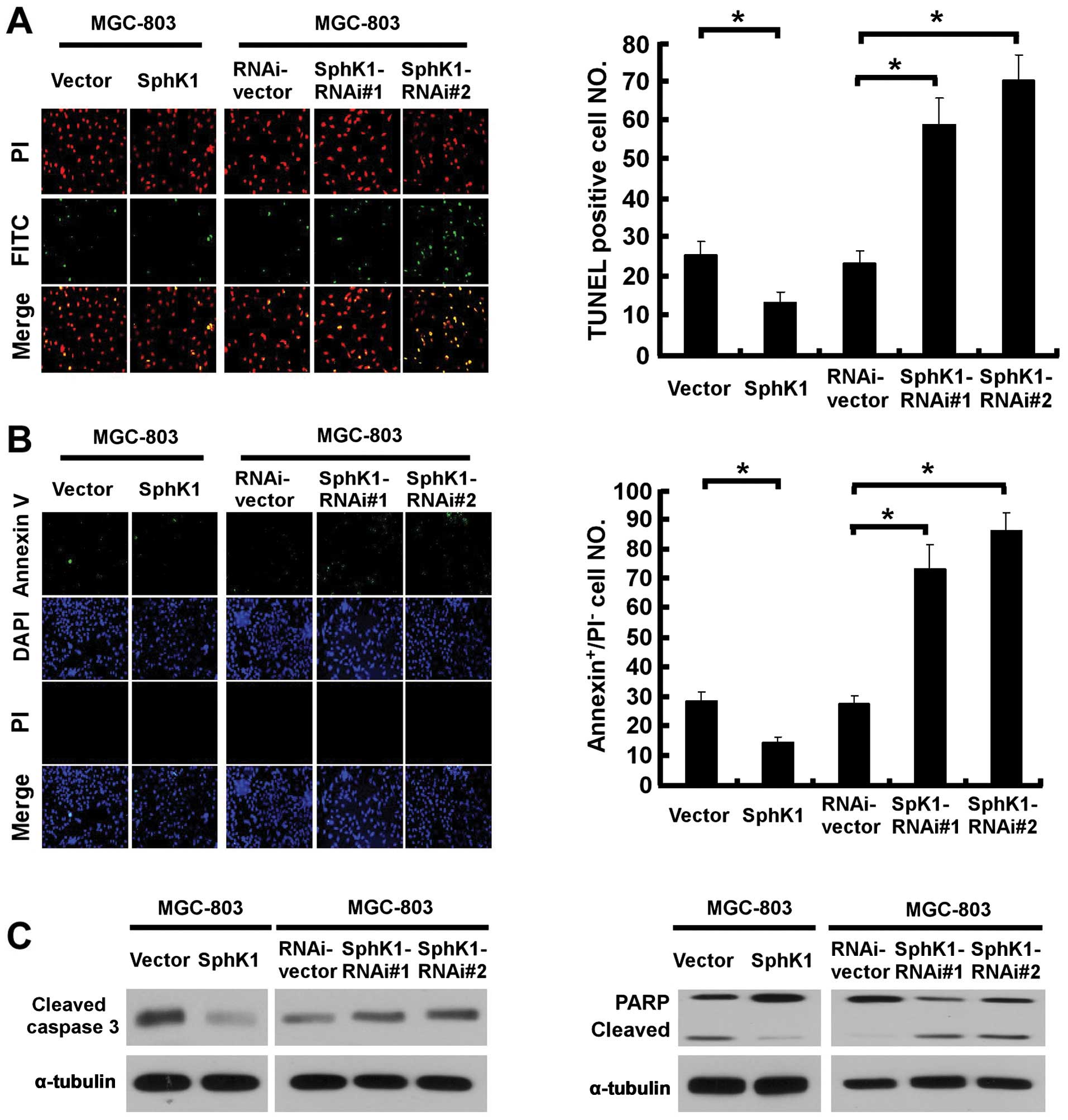
We then attempted to further understand and
characterize the anti-apoptotic activity of SphK1 to pro-apoptotic
stimuli. As shown in Fig. 2A, the
number of apoptotic cells induced by UV irradiation decreased
approximately 2-fold in SphK1-overexpressing cells, and increased
3-fold in SphK1-knockdown MGC-803 cells, as compared to the control
cells. Similarly, the result of Annexin V-binding assay was
consistent with the TUNEL assay (Fig.
2B), suggesting that SphK1 promoted resistance of gastric
cancer cells against radiation-induced apoptosis. Furthermore, the
effect of SphK1 on apoptosis was associated with decreased
caspase-3 and PARP activation in UV radiation-treated
SphK1-overexpressing cells, and a contrary effect was observed in
SphK1-knockdown cells (Fig.
2C).
SphK1 regulates the expression of Bim to
induce gastric cancer cell survival through the Akt/FoxO3a pathway
in gastric cancer cells
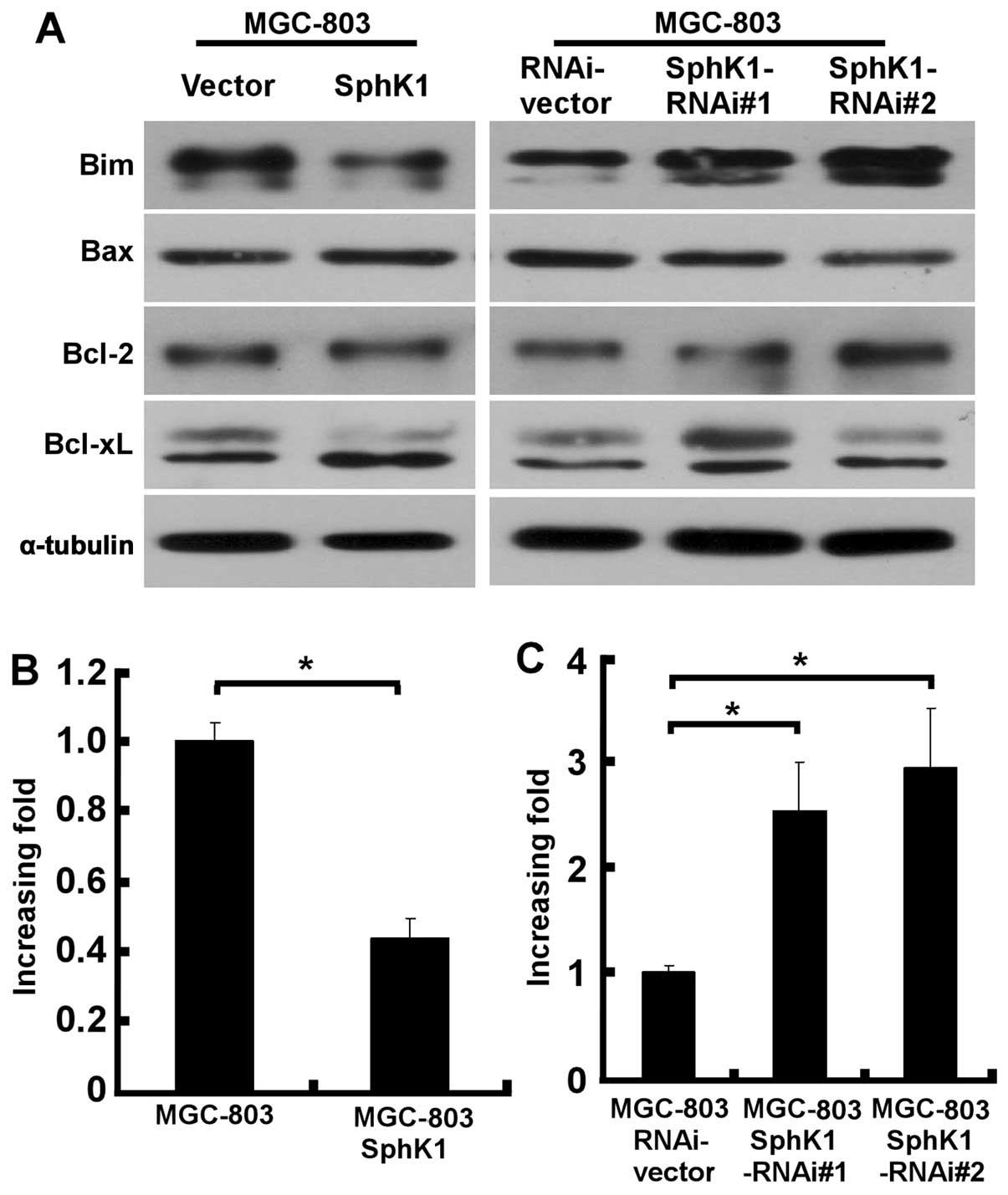
We next examined the possible involvement of
signaling molecules in the effect of SphK1 on apoptosis. Notably,
ectopic overexpression of SphK1 did not affect the expression of
apoptosis regulators Bax, Bcl-2 and Bcl-xL, but, instead,
significantly suppressed the expression of Bim (Fig. 3A). In contrast, Bim level was
significantly upregulated in SphK1-knockdown gastric cancer cells
(Fig. 3A), suggesting a specific
regulatory role of SphK1 in cell apoptosis. To understand at which
level SphK1 is involved in the regulation of Bim expression,
realtime PCR was performed to examine the mRNA levels of Bim in
SphK1-overexpressing, SphK1-knockdown and vector control gastric
cancer cells. As exhibited in Fig.
3B, upregulation of SphK1 in MGC-803 cells was found to
decrease the mRNA levels of Bim, whereas Bim mRNA was significantly
elevated in SphK1-knockdown MGC-803 cells, as compared with those
in vector-control cells (Fig.
3C).
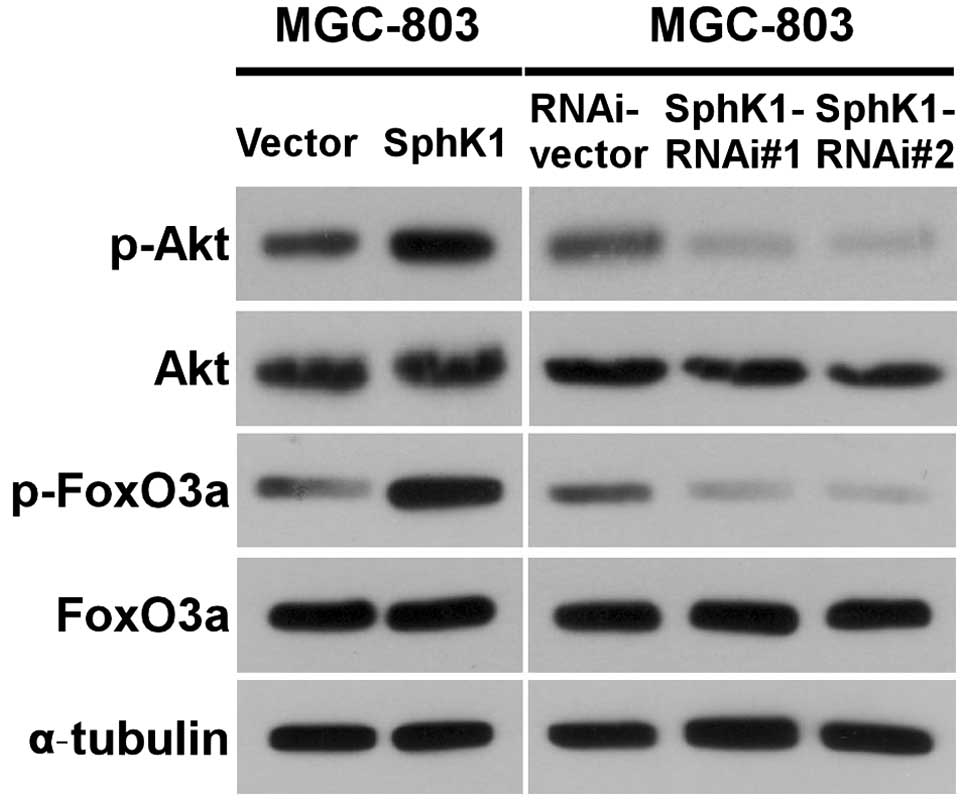
Given that the expression of Bim could be
transcriptionally regulated by FoxO3a and the transcriptional
activity of FoxO3a could be modulated by Akt phosphorylation, we
examined whether upregulating SphK1 expression could activate
Akt/FoxO3a signaling. As shown in Fig.
4, the phosphorylation levels of Akt and FoxO3a were indeed
increased in ectopically SphK1-transduced gastric cancer cells. In
contrast, the expression levels of phosphorylated FoxO3a and
phosphorylated Akt in SphK1-knockdown cells were decreased
(Fig. 4), indicating that SphK1
regulates the expression of Bim via Akt/FoxO3a signaling.
Discussion
Several lines of evidence have indicated that
increased resistance to apoptosis is a hallmark of most types of
cancer (24). Deregulation of
apoptotic or pro-apoptotic pathways is one of the most important
events for tumor development and progression. Moreover, the failure
of treatment by chemotherapy or radiotherapy may be associated with
apoptotic programming. Thus, understanding the mechanisms of
apoptosis/survival process in a specific type of cancer may provide
new insights into developing more effective therapeutic strategies.
Mounting evidence has shown that SphK1 plays an important role in
regulating tumor cell apoptosis. For example, Bonhoure et al
found that HL-60 acute myeloid leukemia cells resist doxorubicin
and etoposide-induced cell death due to SphK1 overexpression
(15). Resistance to camptothecin
and docetaxel in PC-3 and LNCaP prostate cancer cells,
respectively, is associated with stimulation of SphK1 activity
(16). Here, we demonstrated that
SphK1 indeed plays an important role in the anti-apoptotic state of
gastric cancer cells. Ectopic expression of SphK1 in MGC-803 cells
markedly enhanced their resistance to apoptosis induced by UV
irradiation, a commonly used model to study radiotherapy, whereas
suppressing SphK1 expression with shRNAs markedly abrogated the
ability of MGC-803 cells to resist UV-induced cell death,
suggesting that SphK1 contributes to sustaining the unwanted
survival of gastric cancer cells under radiotherapy.
FoxO transcription factors including FoxO1, FoxO3a,
FoxO4 and FoxO6 contribute to the regulation of downstream gene
expression which modulates several biologic phenomena, such as
apoptosis, proliferation and DNA repair (25–27).
Multiple reports have revealed that phosphorylation of FoxO
proteins by Akt can promote apoptosis in cancer cells (28–30).
It is of note that phosphorylation of FoxO3a triggers apoptosis
through a mechanism that depends on a pro-apoptotic gene Bim that
encodes a member of the BH3-only subgroup of Bcl-2 family proteins
(31). Bim has been shown to be
transcriptionally regulated by FoxO proteins (32,33).
Furthermore, two functional FRE sites present in the Bim promoter
support that FOXO transcription factors directly activate Bim gene
expression and promote apoptosis (34). Consistent with these results, the
present study demonstrated that the expression level of FoxO3a, an
upstream regulator of Bim, is suppressed in gastric cancer cells
expressing high level of SphK1, whereas it was upregulated in
SphK1-knockdown gastric cancer cells. Furthermore, we showed that
downregulation of FoxO3a by SphK1 is associated with Akt
phosphorylation. Collectively, the present study suggests a novel
signaling cascade that links SphK1 to the antiapoptotic property of
cancer cells and provides novel therapeutic targets against gastric
cancer. These results also warrant further investigation into how
SphK1 activates the PI3K/Akt pathway, currently being carried out
in our laboratory.
Acknowledgements
This study was supported by the Key Medical
Disciplines and Specialties’ Program of Guangzhou, a grant from the
Natural Science Foundation of China (nos. 81272417, 81370076) and
the National Science and Technique Major Project (2012ZX10004213,
311030, 201305017, 2012ZX09102101-017).
References
|
1
|
Ajani J, D’Amico TA, Hayman JA, Meropol NJ
and Minsky B; National Comprehensive Cancer Network. Gastric
cancer. Clinical practice guidelines in oncology. J Natl Compr
Cancer Netw. 1:28–39. 2003.
|
|
2
|
Chen XM, Chen GY, Wang ZR, Zhu FS, Wang XL
and Zhang X: Detection of micrometastasis of gastric carcinoma in
peripheral blood circulation. World J Gastroenterol. 10:804–808.
2004.PubMed/NCBI
|
|
3
|
Rivera F, Vega-Villegas ME and Lopez-Brea
MF: Chemotherapy of advanced gastric cancer. Cancer Treat Rev.
33:315–324. 2007. View Article : Google Scholar
|
|
4
|
Falcone A: Future strategies and adjuvant
treatment of gastric cancer. Ann Oncol. 14:ii45–ii47. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li W, Yu CP, Xia JT, et al: Sphingosine
kinase 1 is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 15:1393–1399. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maceyka M, Payne SG, Milstien S and
Spiegel S: Sphingosine kinase, sphingosine-1-phosphate, and
apoptosis. Biochim Biophys Acta. 1585:193–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cuvillier O, Pirianov G, Kleuser B, et al:
Suppression of ceramide-mediated programmed cell death by
sphingosine-1-phosphate. Nature. 381:800–803. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hannun YA and Luberto C: Ceramide in the
eukaryotic stress response. Trends Cell Biol. 10:73–80. 2000.
View Article : Google Scholar
|
|
9
|
Kolesnick R and Hannun YA: Ceramide and
apoptosis. Trends Biochem Sci. 24:224–225. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spiegel S and Milstien S:
Sphingosine-1-phosphate: signaling inside and out. FEBS Lett.
476:55–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pyne S and Pyne NJ: Sphingosine
1-phosphate signalling in mammalian cells. Biochem J. 349:385–402.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hait NC, Oskeritzian CA, Paugh SW,
Milstien S and Spiegel S: Sphingosine kinases, sphingosine
1-phosphate, apoptosis and diseases. Biochim Biophys Acta.
1758:2016–2026. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olivera A, Kohama T, Edsall L, et al:
Sphingosine kinase expression increases intracellular
sphingosine-1-phosphate and promotes cell growth and survival. J
Cell Biol. 147:545–558. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edsall LC, Cuvillier O, Twitty S, Spiegel
S and Milstien S: Sphingosine kinase expression regulates apoptosis
and caspase activation in PC12 cells. J Neurochem. 76:1573–1584.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bonhoure E, Pchejetski D, Aouali N, et al:
Overcoming MDR-associated chemoresistance in HL-60 acute myeloid
leukemia cells by targeting sphingosine kinase-1. Leukemia.
20:95–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pchejetski D, Golzio M, Bonhoure E, et al:
Sphingosine kinase-1 as a chemotherapy sensor in prostate
adenocarcinoma cell and mouse models. Cancer Res. 65:11667–11675.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kapitonov D, Allegood JC, Mitchell C, et
al: Targeting sphingosine kinase 1 inhibits Akt signaling, induces
apoptosis, and suppresses growth of human glioblastoma cells and
xenografts. Cancer Res. 69:6915–6923. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nemoto S, Nakamura M, Osawa Y, et al:
Sphingosine kinase isoforms regulate oxaliplatin sensitivity of
human colon cancer cells through ceramide accumulation and Akt
activation. J Biol Chem. 284:10422–10432. 2009. View Article : Google Scholar
|
|
20
|
Radeff-Huang J, Seasholtz TM, Chang JW,
Smith JM, Walsh CT and Brown JH: Tumor necrosis
factor-alpha-stimulated cell proliferation is mediated through
sphingosine kinase-dependent Akt activation and cyclin D
expression. J Biol Chem. 282:863–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tran H, Brunet A, Griffith EC and
Greenberg ME: The many forks in FOXO’s road. Sci STKE.
2003:RE52003.
|
|
22
|
Hahn WC, Dessain SK, Brooks MW, et al:
Enumeration of the simian virus 40 early region elements necessary
for human cell transformation. Mol Cell Biol. 22:2111–2123. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Zhang N, Song LB, et al: Astrocyte
elevated gene-1 is a novel prognostic marker for breast cancer
progression and overall patient survival. Clin Cancer Res.
14:3319–3326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
25
|
Huang H and Tindall DJ: Dynamic FoxO
transcription factors. J Cell Sci. 120:2479–2487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Accili D and Arden KC: FoxOs at the
crossroads of cellular metabolism, differentiation, and
transformation. Cell. 117:421–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barthel A, Schmoll D and Unterman TG: FoxO
proteins in insulin action and metabolism. Trends Endocrinol Metab.
16:183–189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Biggs WH III, Meisenhelder J, Hunter T,
Cavenee WK and Arden KC: Protein kinase B/Akt-mediated
phosphorylation promotes nuclear exclusion of the winged helix
transcription factor FKHR1. Proc Natl Acad Sci USA. 96:7421–7426.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brunet A, Bonni A, Zigmond MJ, et al: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kops GJ, de Ruiter ND, De Vries-Smits AM,
Powell DR, Bos JL and Burgering BM: Direct control of the Forkhead
transcription factor AFX by protein kinase B. Nature. 398:630–634.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sunters A, Fernandez de Mattos S, Stahl M,
et al: FoxO3a transcriptional regulation of Bim controls apoptosis
in paclitaxel-treated breast cancer cell lines. J Biol Chem.
278:49795–49805. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dijkers PF, Medema RH, Lammers JW,
Koenderman L and Coffer PJ: Expression of the pro-apoptotic Bcl-2
family member Bim is regulated by the forkhead transcription factor
FKHR-L1. Curr Biol. 10:1201–1204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stahl M, Dijkers PF, Kops GJ, et al: The
forkhead transcription factor FoxO regulates transcription of
p27Kip1 and Bim in response to IL-2. J Immunol. 168:5024–5031.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gilley J, Coffer PJ and Ham J: FOXO
transcription factors directly activate bim gene expression and
promote apoptosis in sympathetic neurons. J Cell Biol. 162:613–622.
2003. View Article : Google Scholar : PubMed/NCBI
|