Introduction
Lung cancer is the leading cause of cancer-related
deaths in males and second after breast cancer in females. Its
incidence is high and has a global age-standardized rate (ASR) of
47.4/100,000 and 18.6/100,000 for males and females, respectively
(1). In the Arab countries, the
incidence of lung cancer ranks after prostate, colon or bladder
cancer in males and after breast and cervical cancer in females
(2). The ASRs are 13.44/100,000 and
2.91/100,000 for males and females, respectively, but the highest
incidence is reported in Tunisia followed by Bahrain. However, the
largest number of cases occurs in North African countries, Egypt
(20.6%), Morocco (20.1%), Algeria (15.4%) and Tunisia (10%),
reflective of their high population. As for Lebanon, lung cancer
incidence ranks third after Tunisia and Bahrain in males and second
after Bahrain in females (3). The
reported ASRs for lung adenocarcinoma in Lebanon are 27.3/100,000
and 10.2/100,000 for males and females, respectively (4).
For several decades lung cancer has been treated as
a single disease, yet with greater knowledge of its molecular
biology lung cancer is now considered a heterogeneous disease. Most
important was the discovery of the association of epidermal growth
factor receptor (EGFR) gene mutations and anaplastic
lymphoma kinase (ALK) gene fusion with lung adenocarcinoma
histology. These genetic aberrations have been linked to specific
ethnicities and gender in never-smokers and have shown a good
response to tyrosine kinase inhibitors (5–9).
Consequently, mutational analysis of EGFR and EML-ALK
has become a cornerstone in the management of patients with lung
adenocarcinoma (10). Moreover,
downstream of the EGFR pathway is the Kirsten rat sarcoma
viral oncogene, or v-Ki-ras2 homolog (KRAS), known to occur
in a wide range of cancers, with the highest prevalence in
pancreatic (~90%), colorectal (~60%) and primary lung
adenocarcinomas (~60%), which can be mutated in lung cancer
(11). When this mutation is
detected, it is mutually exclusive of EGFR mutations but
only rare and exceptional cases demonstrate both mutations
(13), and a number of studies
correlate its presence with resistance to anti-EGFR therapy
and poor patient prognosis (11).
Lung cancer is notoriously correlated with smoking;
therefore, the US and a number of European countries have
implemented tobacco control programs and attained a decreased
incidence worldwide (14,15). Unfortunately, in the Arab countries
the incidence is increasing since smoking habits are increasing
particularly among females and youth (3). Furthermore, in the hope of decreasing
cancer-related mortality, almost all ethnic groups have been
investigated for EGFR and KRAS gene mutation
frequency in lung adenocarcinoma (16,17),
but such a study has not been conducted in any Arab population
including the Lebanese. Therefore, we aimed to evaluate the
prevalence of KRAS and EGFR gene mutations in two
Lebanese lung adenocarcinoma cohort groups and to correlate the
findings with the clinical and pathologic features, including age,
gender, smoking history and histological grade.
Materials and methods
The Institutional Review Boards of the American
University of Beirut Medical Center and Hammoud Hospital University
Medical Center approved this study. Both boards waived the need for
written patient informed consents.
Patient selection
Patient cases (242) diagnosed clinically with
primary lung adenocarcinoma (AC) and 150 with non-small cell
carcinoma-not otherwise specified (NSCLC-NOS) were retrieved from
the archives of the Pathology Departments at the American
University of Beirut Medical Center and Hammoud Hospital University
Medical Center in Lebanon from the year 2001 to 2010. Of the 150
NSCLC-NOS cases, 91 had an adequate tumor size (>1 mm) for
further subtyping by immunohistochemical staining and 37 cases were
diagnosed as ‘favoring primary lung AC’. Moreover, to ensure good
quality and quantity of extracted DNA for mutational analysis, all
279 primary lung AC cases were then evaluated microscopically for
tumor size. The cases with >200 tumor cells were included; thus,
106 primary lung AC cases were selected for EGFR and
KRAS mutational analysis. The clinicopathologic information
pertaining to patient age at diagnosis, gender, history of smoking,
grade and TNM stage was retrieved for all cases, and none of the
patients in this study received chemotherapy prior to specimen
resection (lobectomy) or biopsy.
Immunohistochemistry
Three histological sections of 3-μm thickness were
prepared from formalin-fixed paraffin-embedded (FFPE) tissue blocks
of the NSCLC-NOS cases. Immunomarker detection was performed using
antibodies against TTF-1 (thyroid transcription factor-1) (1:200
dilution; Novocastra; SPT24), napsin A (1:400 dilution; Novocastra;
IP64) and p63 (Ready-to-use; Novocastra; 7JUL). Positive staining
for TTF-1 and/or napsin A was used for identifying AC and p63 for
squamous cell carcinoma. The immunomarkers were considered positive
if at least 10% of the tumor cells stained. Cases with <10%
staining and no focal areas of positive staining were interpreted
as negative. Appropriate positive and negative controls were
included. Immunostaining by TTF-1 of entrapped normal lung
epithelium or napsin A of pulmonary macrophages was ignored.
Immunohistochemistry was performed using the Leica Bond-Max
autostainer (Leica Microsystems Inc., Buffalo Grove, IL, USA) with
the manufacturer’s preset timed reagents.
Mutational analysis for KRAS and
EGFR
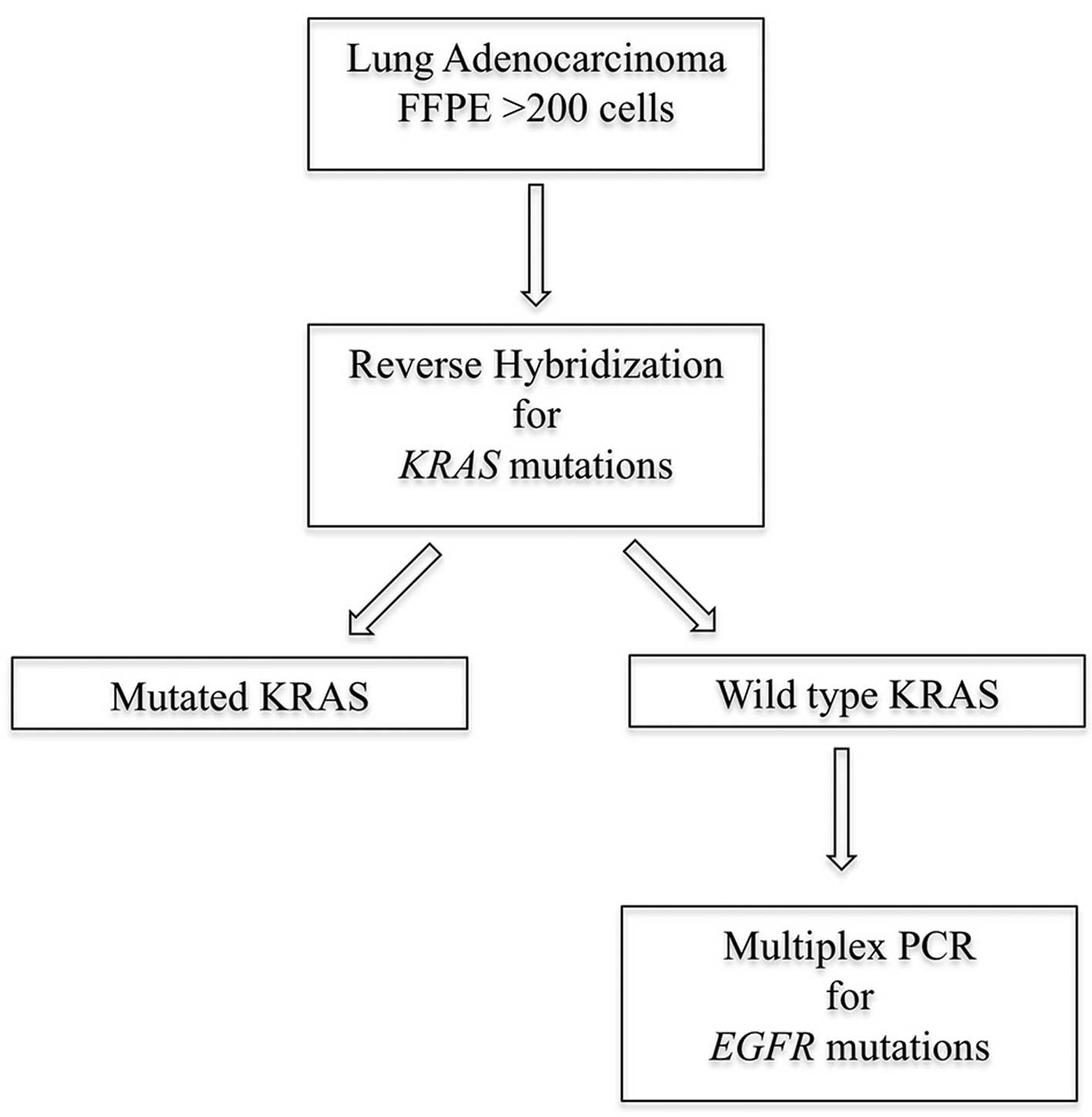
FFPE tissue blocks of the included 106 lung
adenocarcinoma cases were collected for KRAS and EGFR
mutational analysis according to the algorithm (Fig. 1).
DNA extraction from FFPE
Eight 10-μm FFPE tissue ribbons were obtained from
each block, and lysis was allowed overnight at 65°C with 1.5 μl
proteinase K (20 mg/ml) and 400 μl cell lysis solution (Qiagen;
158906). DNA was extracted following a standard protocol and
quantified using the NanoDrop ND-1000 spectrophotometer. The
resultant DNA was stored at 4°C until utilized for reverse
hybridization and multiplex real-time polymerase chain reaction
(PCR) procedures.
Reverse hybridization for detection of
KRAS mutations
Detection of KRAS mutations in exon 2, codon
12 and 13 and exon 3 codon 61 was performed by reverse
hybridization using the KRAS 12/13/61 StripAssay®
(ViennaLab Diagnostics GmbH, Vienna, Austria). This technique
involves three steps: PCR amplification using biotinylated
oligonucleotide primers, hybridization of the amplified products to
a strip containing allele-specific oligonucleotide probes
immobilized as an array of parallel lines and placed in a shaking
water bath at 45°C and identification of bound biotinylated
sequences using streptavidin-alkaline phosohatase and color
substrates performed at room temperature. Bands were then analyzed
visually. Cases that did not exhibit these mutations were then
submitted for EGFR exons 18–21 mutational analysis, knowing
that KRAS and EGFR mutations are predominantly
mutually exclusive.
Multiplex real-time PCR for detection of
EGFR mutations
EGFR mutational analysis was performed using
the EGFR RGQ PCR kits (Qiagen, Valencia, CA, USA), that
enables detection of 29 somatic mutations in the EGFR gene
including: 19 deletions in exon 19, T790M-exon 20, L858R-exon 21,
L861Q-exon 21, G719X-exon 18, S768I-exon 20 and 3 insertions in
exon 20. The kit utilizes two technologies, amplification
refractory mutation system (ARMS) technology and Scorpions dual
primer probes available for detection of EGFR mutations
using real-time PCR on the Rotor-Gene Q 5-plex HRM instrument
(Qiagen). This analysis is performed in two steps: the first step
is to perform a control assay in order to assess the total DNA in
the sample and the second step is to complete the assay for the
presence or absence of mutated DNA. This procedure was completed as
per the manufacturer’s instructions manual. We considered this
technology because of its high sensitivity particularly for samples
with low levels of tumor DNA, such as in small lung biopsy
samples.
Statistical analysis
Age, gender, smoking history, grade and TNM stage
were summarized using means and standard deviation for age and
frequency distribution for the other 4 variables. Those variables
were compared between patients with KRAS mutation and those
without KRAS mutation using the independent t-test for age
(or Wilcoxon rank sum test for small sample size) and the
Chi-squared test or Fisher’s exact test (when cell counts were
small) for the other 4 variables. Similar comparisons were made
between patients with an EGFR mutation and those without
mutations.
Results
The overall clinical and pathological data of the
106 primary lung adenocarcinoma cases demonstrated a mean age of 62
years and a male to female ratio of 2:1. The majority of patients
were smokers (55.7%), although we could not determine the smoking
history data for 27.7%. A total of 56.2% of the tumors were poorly
differentiated when applying the three-tier grading system. TNM
staging data were available for 45% of the cases, which limited our
statistical analysis, but more tumors had a size larger than 3 cm,
while approximately half of the cases had lymph node and/or distant
metastasis (Table I).
 | Table IOverall clinical characteristics and
association with KRAS and EGFR mutations. |
Table I
Overall clinical characteristics and
association with KRAS and EGFR mutations.
| Variables | Overall n (%) | KRAS
mutation n (%) | No KRAS
mutation n (%) | P-value | EGFR
mutation n (%) | No EGFR
mutation n (%) | P-value |
|---|
| Age (years) | | | | 0.172 | | | 0.232 |
| Mean ± SD | 62.1±10.4 | 64.0±8.7 | 61.0±11.2 | | 57.8±8.8 | 62.3±10.4 | |
| Gender | | | | 0.942 | | | 0.005a |
| Female | 34 (32.1) | 13 (32.5) | 21 (31.8) | | 7 (77.8) | 27 (28.4) | |
| Male | 72 (67.9) | 27 (67.5) | 45 (68.2) | | 2 (22.2) | 70 (71.6) | |
| Tumor
differentiation | | | | 0.207 | | | <0.001a |
| Poor | 66 (62.3) | 25 (62.5) | 41 (62.1) | | 5 (55.6) | 61 (62.9) | |
| Moderate | 35 (33.0) | 15 (37.5) | 20 (30.3) | | 0 (0.0) | 35 (36.1) | |
| Well | 5 (4.7) | 0 (0.0) | 5 (7.6) | | 4 (44.4) | 1 (1.0) | |
| Smoking | | | | 0.286 | | | 0.003a |
| Yes | 59 (55.7) | 23 (57.5) | 36 (54.6) | | 1 (11.1) | 58 (59.8) | |
| No | 18 (17.0) | 4 (10.0) | 14 (21.2) | | 5 (55.6) | 13 (13.4) | |
| Not available | 29 (27.4) | 13 (32.5) | 16 (24.2) | | 3 (33.3) | 26 (26.8) | |
| Tumor size (T)
(cm) | | | | 0.389 | | | 0.881 |
| ≤3 | 12 (11.3) | 6 (15.0) | 6 (9.1) | | 1 (11.1) | 11 (11.3) | |
| >3 | 31 (29.3) | 9 (22.5) | 22 (33.3) | | 2 (22.2) | 29 (29.9) | |
| Not available | 63 (59.4) | 25 (62.5) | 38 (57.6) | | 6 (66.7) | 57 (58.8) | |
| Lymph node (N) | | | | 0.879 | | | 0.424 |
| Yes | 19 (17.9) | 7 (17.5) | 12 (18.2) | | 0 (0.0) | 19 (19.6) | |
| No | 21 (19.8) | 7 (17.5) | 14 (21.2) | | 2 (22.2) | 19 (19.6) | |
| Not available | 66 (62.3) | 26 (65.0) | 40 (60.6) | | 7 (77.8) | 59 (60.8) | |
| Metastasis (M) | | | | 0.658 | | | 0.792 |
| Yes | 20 (18.9) | 6 (15.0) | 14 (21.2) | | 2 (22.2) | 18 (18.6) | |
| No | 23 (21.7) | 10 (25.0) | 13 (19.7) | | 1 (11.1) | 22 (22.7) | |
| Not available | 63 (59.4) | 24 (60.0) | 39 (59.1) | | 6 (66.7) | 57 (58.8) | |
The prevalence of KRAS mutations was detected
in 37.7% of the primary lung AC cases. The majority (85%) had a
G>T substitutions in codon 12 of exon 2 and 6 cases had this
substitution in codon 13 (Table
II). An A>G substitution in codon 61 of exon 3 was detected
in one case. All of the KRAS mutations were single except
for 5 cases that exhibited double mutations. The KRAS
mutations were predominant in males (67.5 vs. 32.5% in females) and
in smokers (57.5 vs. 10.0% in non-smokers); however, no statistical
significance could be concluded (p=0.942 and p=0.286,
respectively). The majority of the KRAS-mutated tumors were
poorly differentiated (62.5%) and had a tumor size larger than 3
cm, but the frequency of lymph node or distant metastasis was not
significantly higher when compared to the lung adenocarcinoma cases
with no KRAS mutation (Table
I).
 | Table IISummary of KRAS mutations in
exon 2. |
Table II
Summary of KRAS mutations in
exon 2.
| KRAS
mutation | No. of cases |
|---|
| c.34G>T,
p.G12C | 19 |
| c.34G>A,
p.G12C | 1 |
| c.35G>C,
p.G12A | 11 |
| c.35G>A,
p.G12A | 2 |
| c.35G>A,
p.G12D | 5 |
| c.35G>T,
p.G12V | 2 |
| c.37G>T,
p.G13C | 2 |
| c.38G>A,
p.G13A | 2 |
| c.38G>A,
p.G13D | 2 |
EGFR mutations were detected in 9 (8.5%) of
the lung adenocarcinoma patients with no KRAS mutations. The
most common mutations (8 cases, 88.9%) were deletion in exon 19
while one case had a substitution L858R in exon 21 (Table III). Predominance of EGFR
mutations was significant in females (p=0.005), non-smokers
(p=0.003) and well differentiated tumors (p<0.001). TNM data
were not available for all the 9 cases; therefore, we could not
draw a significant correlation (Table
I).
 | Table IIISummary of EGFR mutations. |
Table III
Summary of EGFR mutations.
| EGFR
mutation | No. of cases |
|---|
| Exon 18 | 0 |
| Exon 19
deletions | 8 |
| Exon 20 | 0 |
| L858R-exon 21 | 1 |
In summary, we identified mutations in 46.2% of the
lung adenocarcinoma cases distributed into 37.7% with KRAS
mutations and 8.5% with EGFR mutations (Table IV).
 | Table IVSummary of the EGFR and
KRAS mutations in primary adenocarcinoma. |
Table IV
Summary of the EGFR and
KRAS mutations in primary adenocarcinoma.
| Mutations |
|---|
|
|
|---|
|
EGFR
n (%) |
KRAS
n (%) | Total
n (%) |
|---|
| Cases | 9 (8.5) | 40 (37.7) | 49 (45.2) |
Discussion
Molecular genetic analysis of lung adenocarcinoma
has become an integral part of lung cancer diagnosis and present
treatment (18,19). The most commonly detected driver
mutations are those involving KRAS, EGFR and
EML4-ALK genes. Moreover, EGFR tyrosine kinase
inhibitors (TKIs), gefitinib, erlotinib and afatinib, are
considered as first-line targeted therapy for advanced (locally or
metastatic) lung adenocarcinoma. However, the 3prevalence of these
aberrations varies with gender, ethnicity and smoking history
(20). Consequently, lung
adenocarcinomas have been extensively profiled for genetic
aberrations in most ethnic groups. In the Arab population, only one
study evaluated EGFR genetic aberrations in 34 Saudi lung
cancer patients (21). In the
present study, 106 cases of lung adenocarcinomas from Lebanon were
analyzed for mutations in KRAS and EGFR.
EGFR mutations were detected in 8.5% of the
lung adenocarcinomas, a frequency higher than that in the Saudi
population, comparable to that of some European countries, but much
lower than that of Asian countries. The highest reported frequency
of EGFR mutations is in Asian, non-smoker females (Taiwan,
57.3%) (22). However, the
Europeans reported much lower frequencies and Szumera-Ciećkiewicz
et al reviewed the results from 19 European studies and
showed a range of 2.6% (in Italy) to 39% (in Germany) (23). In the US, a study of 3026 cases by
Dogan et al demonstrated a frequency of 20% (24) and Reinersman et al reported a
frequency of 19% in African-Americans (25). In Latin America, the average
frequency is 33.2% with the lowest in Argentina (19.3%) and the
highest in Peru (67%). This reported frequency is correlated with
the high rate of Asian migration (26). To the best of our knowledge, in the
Middle East and the Levant countries, only one study from Saudi
Arabia has been published. This study applied direct DNA sequencing
to 34 lung cancer cases and detected the EGFR mutation in
one lung adenocarcinoma (3%) (21).
The analysis of EGFR mutation subtypes
demonstrated a predominance of deletions in exon 19 and only one of
the 9 cases had an L858R substitution in exon 21. These findings
are comparable to the reported frequency of EGFR mutation
subtypes: in-frame deletions in exon 19 (44% of all mutations),
missense point mutations (L858R substitution) in exon 21 (41% of
all mutations), in-frame duplications/insertions in exon 20 (5% of
all mutations) and G719X (X indicates A, C or S) substitution in
exon 18 (4% of all mutations) (27). It is important to subtype these
mutations since they respond differently to EGFR TKIs
(28). For example, the T790M
mutation in exon 20 correlates with drug resistance and relapse
(29). In addition, we demonstrated
a significant correlation between EGFR mutations and
females, non-smokers and well differentiation of the tumor. These
findings are in concordance with the reported prevalence of these
mutations in females more than in males and in never-smokers than
in smokers and to their correlation with a better prognosis
(30).
KRAS mutations were detected in 37.7% of the
lung adenocarcinomas. Similar to EGFR mutations, the
prevalence of KRAS mutations in lung adenocarcinoma varies
with ethnic groups as well, but is generally mutually exclusive
with EGFR mutations (13,31).
An overview of KRAS and EGFR mutation frequency from
different countries presented in Table
V demonstrates an inverse EGFR mutation frequency as
compared to that of KRAS. The lowest values were reported in
Asians (Taiwan: KRAS, 5.03%; EGFR, 57.3%), while the
highest KRAS prevalence was reported in Europeans (The
Netherlands: KRAS, 36.9%; EGFR, 10.6%).
 | Table VFrequency of KRAS and
EGFR mutations reported from different countries and
ethnicities. |
Table V
Frequency of KRAS and
EGFR mutations reported from different countries and
ethnicities.
| Country | KRAS
(%) | EGFR
(%) | Smoking
history | Na | Year (ref.) |
|---|
| Australia | 17.0 | | | 108 | 1998 (38) |
| Saudi Arabia | | 2.94 | | 34 | 2006 (21) |
| Hong Kong | 9.8 | 54.0 | Correlated | 215 | 2006 (39) |
| Korea | 7.3 | 17.4 | | 55 | 2007 (40) |
| USA | 21.2 | | Not correlated | 482 | 2008 (31) |
| Taiwan | 5.03 | 57.3 | Not correlated | 159 | 2008 (22) |
| Japan | 12.6 | 49.4 | | 254 | 2009 (41) |
| Korea | 9.6 | 24.0 | | 94 | 2009 (42) |
| Italy | 17.9 | 12.6 | | 411 | 2009 (43) |
| USA | 23.0 | 10.0 | Correlated | 345 | 2010 (36) |
| Argentina | | 19.3 | Correlated | 244 | 2011 (26) |
| Columbia | 17.1 | 24.8 | Correlated | 322 | 2011 (26) |
| Mexico | 16.0 | 31.2 | Correlated | 381 | 2011 (26) |
| Peru | 16.8 | 67.0 | Correlated | 381 | 2011 (26) |
| China | 8.0 | 41.0 | Correlated | 861 | 2012 (44) |
| USA | 26.0 | 20.0 | Correlated | 2529/3026b | 2012 (24) |
| Japan | 16.5 | 41.7 | Correlated | 182 | 2012 (45) |
| Netherlands | 36.9 | 10.6 | Correlated | 662 | 2012 (46) |
| Brazilian | 14.6 | 30.4 | Correlated | 207 | 2012 (47) |
| China | 5.9 | | | 1935 | 2013 (48) |
| Czech Republic | 21.0 | | | 233 | 2013 (49) |
| Western Turkey | | 42.6 | Correlated | 48 | 2013 (50) |
| Europe | | 2.6–39c | Correlated | 23–147 | 2013 (23) |
| Korea | | 39.0 | Correlated | 502 | 2013 (51) |
| Lebanon | 37.7 | 8.5 | Not correlated | 106 | Present study |
In addition, we found that KRAS mutations
were predominantly in exons 2 and 3 with 85% involving codon 12,
thus concordant with that reported by Bos, where 97% of KRAS
mutation subtypes are substitutions of G→T and
G→C in codon 12 of exon 2 in addition to the missense
point mutations in codons 61 that prevent GTP cleaving, thus
transforming the gene into an oncogene (32). However, we could not demonstrate a
correlation between smoking and the type of substitution and the
subtype of the mutation. Such a correlation was described by Riely
et al where they demonstrated that the G→T and
G→C transversions are present in both smokers and
non-smokers, while a G→A transversion is more common
in non-smokers (12). Moreover, we
detected more KRAS mutations in males, smokers and poorly
differentiated tumors, but this observation did not demonstrate a
statistically significant correlation. Several other studies have
shown that KRAS mutations are higher in males and smokers as
compared to females and never-smokers (33), predict a worse prognosis (34,35),
and show a low response to adjuvant chemotherapy and
EGFR-TKI treatment in metastatic lung adenocarcinoma
(7). Unfortunately, because it
leads to loss of enzymatic activity, effective targeted therapy is
more difficult to achieve as compared to a gain of functional
mutation. Currently, clinical trials targeting downstream effector
molecules are being conducted, but no effective targeted therapy
against KRAS mutations has been achieved thus far.
Two mutational analysis techniques were used in this
study. The Scorpion amplified refractory mutation system (ARMS)
multiplex real-time PCR was used for the detection of EGFR
mutations. Although this technique does not detect mutations
comprehensively, it covers all the common 29 EGFR mutations
in exons 18–21. It is highly sensitive (1%) as compared to direct
Sanger sequencing (25%) of mutant DNA (36). For KRAS mutations we used
reverse hybridization (StripAssay, ViennaLab), which is considered
most analytically sensitive when compared to direct sequencing,
pyrosequencing, high resolution melting analysis and the
TheraScreen DxS kit (37). This
technique can detect 13 mutations in the KRAS codon 12, 13
and 61. The high sensitivity of both techniques was necessary to
overcome the tissue limitation of small lung biopsy material that
constituted the majority of our cases. However, some biopsy
material was too scant to be included in this study thus
constituting a limitation for this study. In addition, the
unavailability of adequate smoking history data for all cases, and
being retrospective in nature are additional limitations.
In summary, this is the first study in an Arab
population to report the prevalence of both EGFR and
KRAS gene mutations in lung adenocarcinoma using sensitive
mutational analysis techniques. We showed that the frequency of
EGFR in lung adenocarcinoma was 8.5% and that it was more
common in women, non-smokers and well-differentiated tumors. In
addition, we found that KRAS was highly prevalent in the
Lebanese population, but we could not significantly correlate it
with smoking; however, it remains a possible major cause.
Therefore, we conclude that EGFR reflex testing should be
implemented as per the recommendation of the College of American
Pathologists, International Association for the Study of Lung
Cancer and Association for Molecular Pathology (10). While KRAS testing is useful
for understanding the molecular biology of adenocarcinoma, the
clinical utilization of this information must await the
identification of effective targeted therapy.
Acknowledgements
The authors are grateful for the generous funding
support provided by the Lebanese National Council for Scientific
Research (LNCSR), grant no. PALM. GZ.09.
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Salim EI, Moore MA, Al-Lawati JA, et al:
Cancer epidemiology and control in the Arab world - past, present
and future. Asian Pac J Cancer Prev. 10:3–16. 2009.PubMed/NCBI
|
|
3
|
Salim EI, Jazieh AR and Moore MA: Lung
cancer incidence in the Arab league countries: risk factors and
control. Asian Pac J Cancer Prev. 12:17–34. 2011.PubMed/NCBI
|
|
4
|
Ministry of Public Health (MOPH) L.
Literature review report on selected diseases in Lebanon. 2004.
|
|
5
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paez JG, Janne PA, Lee JC, et al:
EGFR mutations in lung cancer: correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar
|
|
7
|
Eberhard DA, Johnson BE, Amler LC, et al:
Mutations in the epidermal growth factor receptor and in KRAS are
predictive and prognostic indicators in patients with
non-small-cell lung cancer treated with chemotherapy alone and in
combination with erlotinib. J Clin Oncol. 23:5900–5909. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Schmid-Bindert G and Zhou C:
Erlotinib in the treatment of advanced non-small cell lung cancer:
an update for clinicians. Ther Adv Med Oncol. 4:19–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmed SM and Salgia R: Epidermal growth
factor receptor mutations and susceptibility to targeted therapy in
lung cancer. Respirology. 11:687–692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lindeman NI, Cagle PT, Beasley MB, et al:
Molecular testing guideline for selection of lung cancer patients
for EGFR and ALK tyrosine kinase inhibitors: guideline from the
College of American Pathologists, International Association for the
Study of Lung Cancer, and Association for Molecular Pathology. Arch
Pathol Lab Med. 137:828–860. 2013.
|
|
11
|
Kiaris H and Spandidos D: Mutations of
ras genes in human tumours (Review). Int J Oncol. 3:413–421.
1995.
|
|
12
|
Riely GJ, Marks J and Pao W: KRAS
mutations in non-small cell lung cancer. Proc Am Thorac Soc.
6:201–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roberts PJ, Stinchcombe TE, Der CJ and
Socinski MA: Personalized medicine in non-small-cell lung cancer:
is KRAS a useful marker in selecting patients for epidermal
growth factor receptor-targeted therapy? J Clin Oncol.
28:4769–4777. 2010.PubMed/NCBI
|
|
14
|
Centers for Disease Control and Prevention
(CDC). Lung cancer rates decline nationwide. 2011, http://www.cdc.gov/media/releases/2011/p0915_lung_cancer.html.
|
|
15
|
Cancer Research UK. Lung cancer incidence
statistics. 2013, http://www.cancerresearchuk.org/cancer-info/cancerstats/types/lung/incidence/uk-lung-cancer-incidence-statistics.
|
|
16
|
Underwood JM, Townsend JS, Tai E, et al:
Racial and regional disparities in lung cancer incidence. Cancer.
118:1910–1918. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou W and Christiani DC: East meets West:
ethnic differences in epidemiology and clinical behaviors of lung
cancer between East Asians and Caucasians. Chin J Cancer.
30:287–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Greulich H: The genomics of lung
adenocarcinoma: opportunities for targeted therapies. Genes Cancer.
1:1200–1210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou C, Wu YL, Chen G, et al: Erlotinib
versus chemotherapy as first-line treatment for patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase
3 study. Lancet Oncol. 12:735–742. 2011.PubMed/NCBI
|
|
20
|
Travis WD, Brambilla E, Noguchi M, et al:
International Association for the Study of Lung Cancer/American
Thoracic Society/European Respiratory Society International
Multidisciplinary Classification of Lung Adenocarcinoma. J Thorac
Oncol. 6:244–285. 2011. View Article : Google Scholar
|
|
21
|
Al-Kuraya K, Siraj AK, Bavi P, et al: High
epidermal growth factor receptor amplification rate but low
mutation frequency in Middle East lung cancer population. Hum
Pathol. 37:453–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu CC, Hsu HY, Liu HP, et al: Reversed
mutation rates of KRAS and EGFR genes in
adenocarcinoma of the lung in Taiwan and their implications.
Cancer. 113:3199–3208. 2008.PubMed/NCBI
|
|
23
|
Szumera-Cieckiewicz A, Olszewski WT,
Tysarowski A, et al: EGFR mutation testing on cytological
and histological samples in non-small cell lung cancer: a Polish,
single institution study and systematic review of European
incidence. Int J Clin Exp Pathol. 6:2800–2812. 2013.
|
|
24
|
Dogan S, Shen R, Ang DC, et al: Molecular
epidemiology of EGFR and KRAS mutations in 3,026 lung
adenocarcinomas: higher susceptibility of women to smoking-related
KRAS-mutant cancers. Clin Cancer Res. 18:6169–6177.
2012.
|
|
25
|
Reinersman JM, Johnson ML, Riely GJ, et
al: Frequency of EGFR and KRAS mutations in lung
adenocarcinomas in African Americans. J Thorac Oncol. 6:28–31.
2011.PubMed/NCBI
|
|
26
|
Arrieta O, Cardona AF, Federico Bramuglia
G, et al: Genotyping non-small cell lung cancer (NSCLC) in Latin
America. J Thorac Oncol. 6:1955–1959. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gazdar AF, Shigematsu H, Herz J and Minna
JD: Mutations and addiction to EGFR: the Achilles ‘heal’ of
lung cancers? Trends Mol Med. 10:481–486. 2004.PubMed/NCBI
|
|
28
|
Kim YT, Kim TY, Lee DS, et al: Molecular
changes of epidermal growth factor receptor (EGFR) and KRAS and
their impact on the clinical outcomes in surgically resected
adenocarcinoma of the lung. Lung Cancer. 59:111–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Godin-Heymann N, Bryant I, Rivera MN, et
al: Oncogenic activity of epidermal growth factor receptor kinase
mutant alleles is enhanced by the T790M drug resistance mutation.
Cancer Res. 67:7319–7326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pao W, Miller V, Zakowski M, et al: EGF
receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
|
|
31
|
Riely GJ, Kris MG, Rosenbaum D, et al:
Frequency and distinctive spectrum of KRAS mutations in
never smokers with lung adenocarcinoma. Clin Cancer Res.
14:5731–5734. 2008.PubMed/NCBI
|
|
32
|
Bos JL: ras oncogenes in human cancer: a
review. Cancer Res. 49:4682–4689. 1989.PubMed/NCBI
|
|
33
|
Okudela K, Woo T and Kitamura H: KRAS gene
mutations in lung cancer: particulars established and issues
unresolved. Pathol Int. 60:651–660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Siegfried JM, Gillespie AT, Mera R, et al:
Prognostic value of specific KRAS mutations in lung
adenocarcinomas. Cancer Epidemiol Biomarkers Prev. 6:841–847.
1997.PubMed/NCBI
|
|
35
|
Johnson ML, Sima CS, Chaft J, et al:
Association of KRAS and EGFR mutations with survival
in patients with advanced lung adenocarcinomas. Cancer.
119:356–362. 2013.
|
|
36
|
Dacic S: EGFR assays in lung cancer. Adv
Anat Pathol. 15:241–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jancik S, Drabek J, Berkovcova J, et al: A
comparison of direct sequencing, pyrosequencing, high resolution
melting analysis, TheraScreen DxS, and the K-ras StripAssay for
detecting KRAS mutations in non small cell lung carcinomas.
J Exp Clin Cancer Res. 31:792012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fong KM, Zimmerman PV and Smith PJ: KRAS
codon 12 mutations in Australian non-small cell lung cancer. Aust
NZ J Med. 28:184–189. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tam IY, Chung LP, Suen WS, et al: Distinct
epidermal growth factor receptor and KRAS mutation patterns
in non-small cell lung cancer patients with different tobacco
exposure and clinicopathologic features. Clin Cancer Res.
12:1647–1653. 2006.PubMed/NCBI
|
|
40
|
Bae NC, Chae MH, Lee MH, et al:
EGFR, ERBB2, and KRAS mutations in Korean
non-small cell lung cancer patients. Cancer Genet Cytogenet.
173:107–113. 2007. View Article : Google Scholar
|
|
41
|
Kosaka T, Yatabe Y, Onozato R, Kuwano H
and Mitsudomi T: Prognostic implication of EGFR,
KRAS, and TP53 gene mutations in a large cohort of
Japanese patients with surgically treated lung adenocarcinoma. J
Thorac Oncol. 4:22–29. 2009.
|
|
42
|
Jang TW, Oak CH, Chang HK, Suo SJ and Jung
MH: EGFR and KRAS mutations in patients with adenocarcinoma of the
lung. Korean J Intern Med. 24:48–54. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Boldrini L, Ali G, Gisfredi S, et al:
Epidermal growth factor receptor and K-RAS mutations in 411 lung
adenocarcinoma: A population-based prospective study. Oncol Rep.
22:683–691. 2009.PubMed/NCBI
|
|
44
|
Xu J, He J, Yang H, et al: Somatic
mutation analysis of EGFR, KRAS, BRAF and PIK3CA in 861 patients
with non-small cell lung cancer. Cancer Biomark. 10:63–69.
2011.PubMed/NCBI
|
|
45
|
Kakegawa S, Shimizu K, Sugano M, et al:
Clinicopathological features of lung adenocarcinoma with
KRAS mutations. Cancer. 117:4257–4266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Smits AJ, Kummer JA, Hinrichs JW, et al:
EGFR and KRAS mutations in lung carcinomas in the
Dutch population: increased EGFR mutation frequency in
malignant pleural effusion of lung adenocarcinoma. Cell Oncol.
35:189–196. 2012. View Article : Google Scholar
|
|
47
|
Bacchi CE, Ciol H, Queiroga EM, Benine LC,
Silva LH and Ojopi EB: Epidermal growth factor receptor and
KRAS mutations in Brazilian lung cancer patients. Clinics.
67:419–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guan JL, Zhong WZ, An SJ, et al: KRAS
mutation in patients with lung cancer: a predictor for poor
prognosis but not for EGFR-TKIs or chemotherapy. Ann Surg Oncol.
20:1381–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fiala O, Pesek M, Finek J, Benesova L,
Belsanova B and Minarik M: The dominant role of G12C over other
KRAS mutation types in the negative prediction of efficacy
of epidermal growth factor receptor tyrosine kinase inhibitors in
non-small cell lung cancer. Cancer Genet. 206:26–31.
2013.PubMed/NCBI
|
|
50
|
Unal OU, Oztop I, Calibasi G, et al:
Relationship between epidermal growth factor receptor gene
mutations and clinicopathological features in patients with
non-small cell lung cancer in western Turkey. Asian Pac J Cancer
Prev. 14:3705–3709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Choi YL, Sun JM, Cho J, et al: EGFR
mutation testing in patients with advanced non-small cell lung
cancer: a comprehensive evaluation of real-world practice in an
East Asian tertiary hospital. PloS One. 8:e560112013. View Article : Google Scholar
|