Introduction
Disease biomarkers are widely used in medicine, but
few biomarkers are useful for the diagnosis and monitoring of
gastric cancer. The expansion of new technologies for molecular
diagnostics and tumor-targeted therapy also increased the need to
develop highly specific targeting ligands for molecules that are
differentially expressed in gastric cancer cells and normal tissues
(1). Gastric cancer is an
aggressive disease often diagnosed at an advanced stage. Despite
the improvements in surgical and adjuvant treatment, gastric cancer
remains a global public health problem with a 5-year survival of
<25% (2,3). In the past two decades, new biomarkers
have been identified with the potential to ameliorate the diagnosis
and treatment of gastric cancer. However, initially promising
biomarkers have not been validated for clinical use. The main
challenge in identifying reliable predictive biomarkers is
individual genetic variation and tumor heterogeneity, which have
yet to be adequately determined. Biomarkers currently used in the
clinic such the carcinoembryonic antigen (CEA) and carbohydrate
antigen CA72-4 and CA19-9 are, not only elevated at late stages of
cancer development, but also expressed by other types of cancer and
even by non-cancerous tissues, providing limited information
(1,4). Thus, more reliable prognostic and
predictive biomarkers may be extremely valuable for diagnosing,
stratifying, and targeting gastric cancer and ultimately, improving
patient survival.
Recently, a new class of molecular probes termed
aptamers has emerged as promising molecular probes for disease
diagnosis and therapy (5). Aptamers
are single-stranded DNA (ssDNA), RNA or modified nucleic acids that
bind specifically to targets, which range from small organic
molecules to proteins (6). The
basis for target recognition is the tertiary structures formed by
the single-stranded oligonucleotides (7). Aptamers possess numerous advantageous
characteristics, including small size, lack of immunogenicity, easy
and reproducible synthesis, high-binding affinity and molecular
specificity, fast tissue penetration and low toxicity, tenability
in binding affinity and long-term stability (5,6).
Aptamers are generated by an iterative in vitro evolution
procedure known as systematic evolution of ligands by exponential
enrichment (SELEX) (8,9). This method has been extended to
develop a live cell-based SELEX (live cell-SELEX) for generating
cancer cell-specific aptamers (10). Accumulating evidence has
demonstrated that the live cell-SELEX is simple, fast,
straightforward, reproducible and most importantly, effective even
when there is only a minor difference between a cancer cell and an
untransformed cell of the same type of tissue (5,6,11).
Using cell-SELEX, a group of aptamers that specifically recognize
leukemia cells (12,13), breast (14), lung (15) and colorectal cancer cells (16) were generated. Results of those
studies suggested that the live cell-SELEX is a promising strategy
for identifying biomarkers for gastric cancer diagnosis and
targeting therapy.
In the present study, a specific DNA aptamer against
gastric carcinoma cells was selected using live cell-SELEX. The
aptamer was confirmed by a series of experiments as having the
potential to be used for the study and diagnosis of gastric
cancer.
Materials and methods
Cell lines and cell culture
Human normal gastric epithelial GES-1, gastric
carcinoma AGS, HepG2 liver hepatoma and SW620 colon carcinoma cell
lines were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA). GES-1, AGS and SW620 cells were
maintained and propagated in Dulbecco’s minimal essential medium
(DMEM) supplemented with 20% fetal bovine serum (FBS) (both from
HyClone, Logan, UT, USA) and 100 U/ml penicillin-streptomycin
(Sigma, St. Louis, MO, USA). HepG2 cells were maintained and
propagated in RPMI-1640 (Sigma) supplemented with 10% FBS and 100
U/ml penicillin-streptomycin. The cells were cultured in 100×20-mm
culture dishes at 37°C in a humidified atmosphere containing 5%
CO2. The experiments were carried out using cultures
that had reached >90% confluence.
DNA primers and libraries
Random DNA primers and a library were designed using
the Integrated DNA Technologies software (IDT, Coralville, IA,
USA), synthesized by standard phosphoramidite chemistry with an
automated DNA synthesizer (3400 DNA Synthesizer; Applied Biosystems
Inc., Foster City, CA, USA) and purified by reversed-phase
high-performance liquid chromatography (RP-HPLC; Shanghai Sangon
Biological Company, Shanghai, China). The purified library
contained a central randomized sequence of 52 nucleotides (nt)
flanked by two 18-nt primer hybridization sites
(ATACCAGCTTATTCAATT-52-nt-AGATAGTAAGTGCAATCT). The forward and
reverse primers used in the PCR performed in the process of
cell-SELEX were separately labeled with fluorescein isothiocyanate:
(FITC), (5′-FITC-ATACCAGCTTATTCAATT-3′) and biotin (Bio),
(5′-Bio-AGATAGTAAGTGCAATCT-3′) at the 5′ end in order to synthesize
double-labeled and double-stranded DNA molecules.
Procedure of cell-SELEX
AGS cells were used as target cells for the positive
selections and GES-1 as negative cells for the counter selections.
Prior to the selection, culture cells were harvested by
non-enzymatic cell dissociation solution and then washed twice with
washing buffer (4.5 g/l glucose and 5 mM MgCl2 in
Dulbecco’s phosphate-buffered saline with calcium chloride and
magnesium chloride) (both from Sigma). A total of 200 pmol library
or DNA pool was dissolved in 400 μl of binding buffer (500 nM/l).
The binding buffer was prepared by adding 0.1 mg/ml tRNA and 1
mg/ml of bovine serum albumin (BSA) (both from Sigma) into the
washing buffer. The library or DNA pool was denatured at 95°C for 5
min and quickly cooled on ice for 10 min. The DNA pool was then
incubated with 5×106 AGS cells at 4°C on a shaker for 40
min. Following incubation, the cells were washed three times with
washing buffer to remove unbound sequences. The cell-DNA complex
was resuspended in 400 μl binding buffer and heated at 95°C for 15
min and centrifuged at 14,000 rpm to elute the bound DNAs. The
eluted DNAs were incubated with 1×107 GES-1 cells for
counter selection at 4°C on a shaker for 40 min. The cells were
subsequently centrifuged at 14,000 rpm for 5 min. The supernatant
containing the ssDNA was recovered and amplified by polymerase
chain reaction (PCR) using FITC- and biotin-labeled primers.
Amplifications were carried out in an Eppendorf PCR Thermocycler
(Eppendorf GAC 22331; Hamburg, Germany). The PCR amplification
conditions of the primers and libraries were optimized prior to the
selections. The reaction system consisted of 50 mM KCl, 10 mM
Tris-HCl (pH 8.3), 1.5 mM MgCl2, 25 mM dNTPs (Takara),
0.5 mM of each primer, and 5 U/ml Hot start Taq DNA
Polymerase (Takara). The amplification steps included 35 cycles of
3 min at 95°C, 45 sec at 94°C, 30 sec at 63.5°C and 30 sec at 72°C,
followed by the final 10 min of extension at 72°C. The selected
sense ssDNA strands were separated from the biotinylated antisense
ssDNA strands by alkaline denaturation and purified by
streptavidin-coated sepharose beads (Amersham Biosciences). The
selected ssDNA was then dried and resuspended in binding buffer for
the subsequent round of selection. After 12 rounds of selections,
the final selected ssDNA pool was PCR-amplified and cloned into
Escherichia coli using the TOTO TA cloning kit (Invitrogen,
Carlsbad, CA, USA). Cloning of the PCR products and sequencing of
the selected sense ssDNA were performed by the Shanghai Sangon
Biological Company.
Gel electrophoresis of PCR products
To confirm the PCR products of each round of
selection containing the selected ssDNA strands from the library,
agarose gel electrophoresis was routinely performed. The nucleic
acid content was first determined at 260 nm using a UV
spectrophotometer (BioPhotometer; Eppendorf, Germany) and then
monitored by electrophoresis on a 2% agarose gel and visualized by
ethidium bromide staining. The correct molecular weight was
confirmed by a DNA molecular weight marker (Boehringer, Mannheim,
Germany).
Flow cytometric analysis
To assess the enrichment of specific aptamer
candidates and the binding capacity and affinity of the selected
aptamer candidates to AGS, cells were cultured at 90% confluence
were harvested by non-enzymatic cell dissociation solution and then
washed twice with washing buffer. Cells (5×105) were
incubated with varying concentrations of FITC-labeled selected
ssDNA in 200 μl binding buffer on ice for various time lengths.
Cells were then washed twice with washing buffer and suspended in
200 μl washing buffer. The fluorescence was analyzed by flow
cytometry (BD FACSCalibur; BD Biosciences). The FITC-labeled
unselected ssDNA to AGS and the FITC-labeled selected ssDNA to GES,
HepG2 and SW620 were used as controls. All the experiments were
repeated three times. The mean fluorescence intensity of target
cells labeled by selected ssDNA was calculated by subtracting the
mean fluorescence intensity produced by unselected ssDNA.
Imaging of target cells stained with
apatamer candidates
The specificity of the aptamer candidate cy-apt 20
in recognizing AGS cells was then visualized by fluorescence
imaging. HepG2 and SW620 cells were used as controls. Prior to the
imaging, culture cells were washed twice with washing buffer in
flat-bottomed 6-well plates (Costar, Corning, NY, USA) and then
incubated with 400 nM of FITC-labeled cy-apt 20 in 200 μl binding
buffer on ice for 40 min. After washing, the stained cells were
viewed using an inverted fluorescence microscope (TE2000; Nikon)
using the standard-FITC filter set (excitation at 490 nm and
emission at 520 nm). Images of the stained cells were captured with
a DXM1200F digital camera (Nikon).
Data processing and statistical
analysis
Fluorescence was determined by flow cytometry by
counting 10,000 events/sample. Data were read and processed by
FlowJo software (version 7.6 for Windows; Tree Star, Ashland, OR,
USA). Relative intensity of gel bands and fluorescence signals of
stained tumor cells were quantified using ImageJ software (version
1.47 for Windows; NIH, Bethesda, MD, USA). Results were presented
as means ± standard error.
Results
Evolution of gastric carcinoma
cell-specific aptamers by cell-SELEX
In the present study, the entire live cell-SELEX
method was utilized to generate gastric cancer cell-specific
aptamers. Human gastric carcinoma AGS cells were used as target
cells for positive selections and human normal gastric epithelial
GES-1 cells as negative cells for counter selections. To avoid loss
of important cell surface molecules in the process of selection,
cultured cells were collected by non-enzymatic cell dissociation
solution. To ensure the exclusion of common or shared molecules,
the amount of cells used for counter selections was maintained at
>5-fold that for positive selections. The ssDNA pool collected
after each round of selection was amplified by PCR, and the product
was used to prepare ssDNA for the subsequent round of selection.
The enrichment of the selection ssDNA pool through successive
selection was monitored by agarose gel electrophoresis and flow
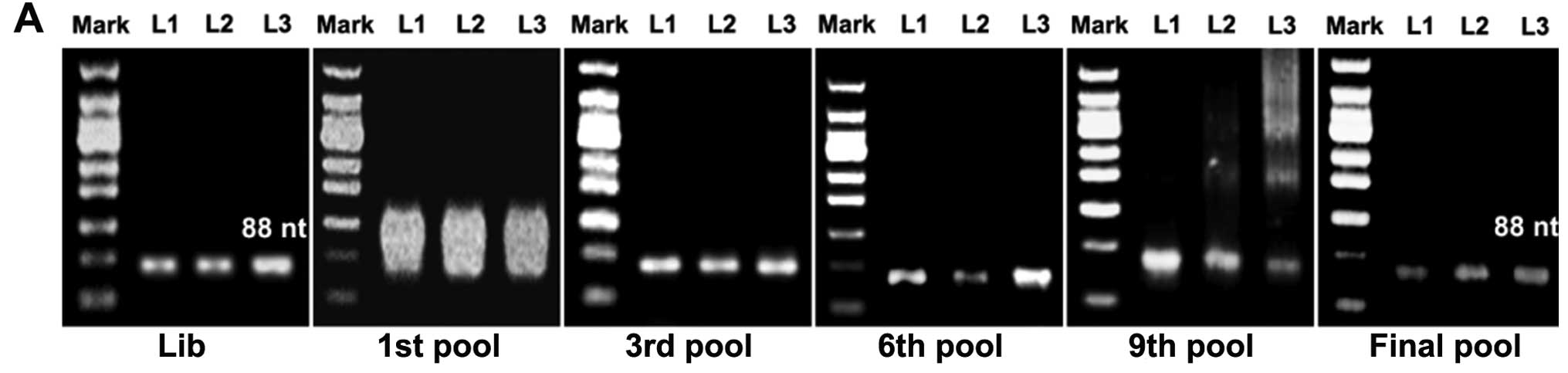
cytometry. Electrophoresis band position (Fig. 1A) and an increase in the
fluorescence intensity compared to the library ssDNA were
indications of successful selection (Fig. 1B). Increasing rounds of selection
denoted a steady increase in the fluorescence intensity of the
target cells, suggesting that the ssDNA sequences with a higher
binding affinity to the target cells were being enriched (Fig. 1C).
Identification of a gastric cancer
cell-specific DNA aptamer from the final pool
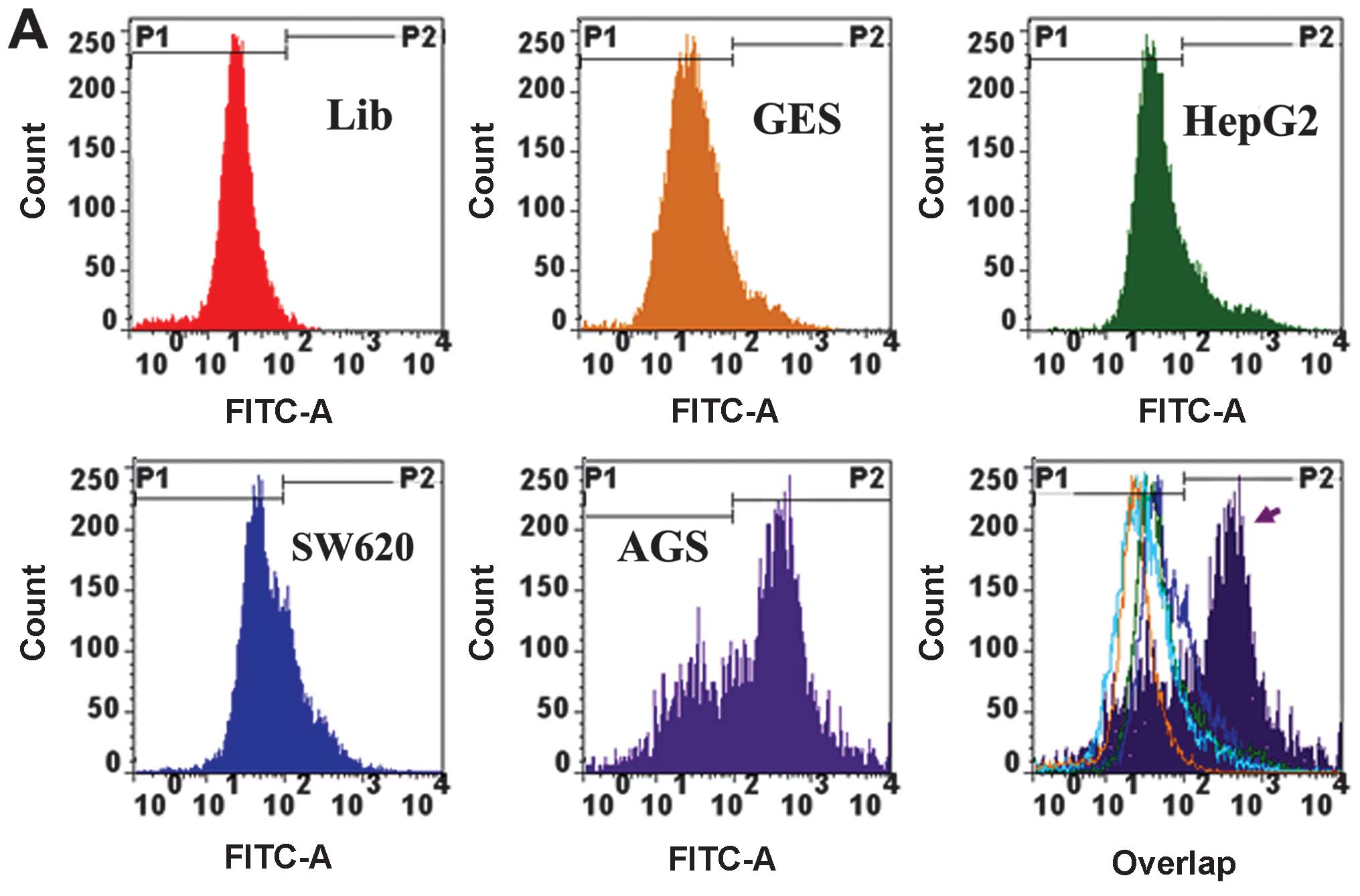
The final enriched ssDNA pool was analyzed by flow
cytometry in comparison with GES, HepG2 and SW620 cells. The
results showed that the final enriched ssDNA pool had significantly
higher binding affinity to AGS than non-AGS cells (Fig. 2A and B). The final selected ssDNA
pool was cloned and the positive clones were sequenced. Sequence
analysis identified 30 potential ssDNA sequences designated as
cy-apt 01–30 as potential aptamer candidates specific to AGS cells
(data not shown). A comparison of experiments were subsequently
performed to confirm their target binding specificity towards AGS
cells. Ten sequences had certain binding ability to AGS cells. Four
of the 10 sequences had binding rates >60% to AGS cells
(Fig. 2C), with only cy-apt 20
exhibiting >70% binding rate to AGS cells and <30% binding
affinity to non-gastric cancer cells (Fig. 2C and D).
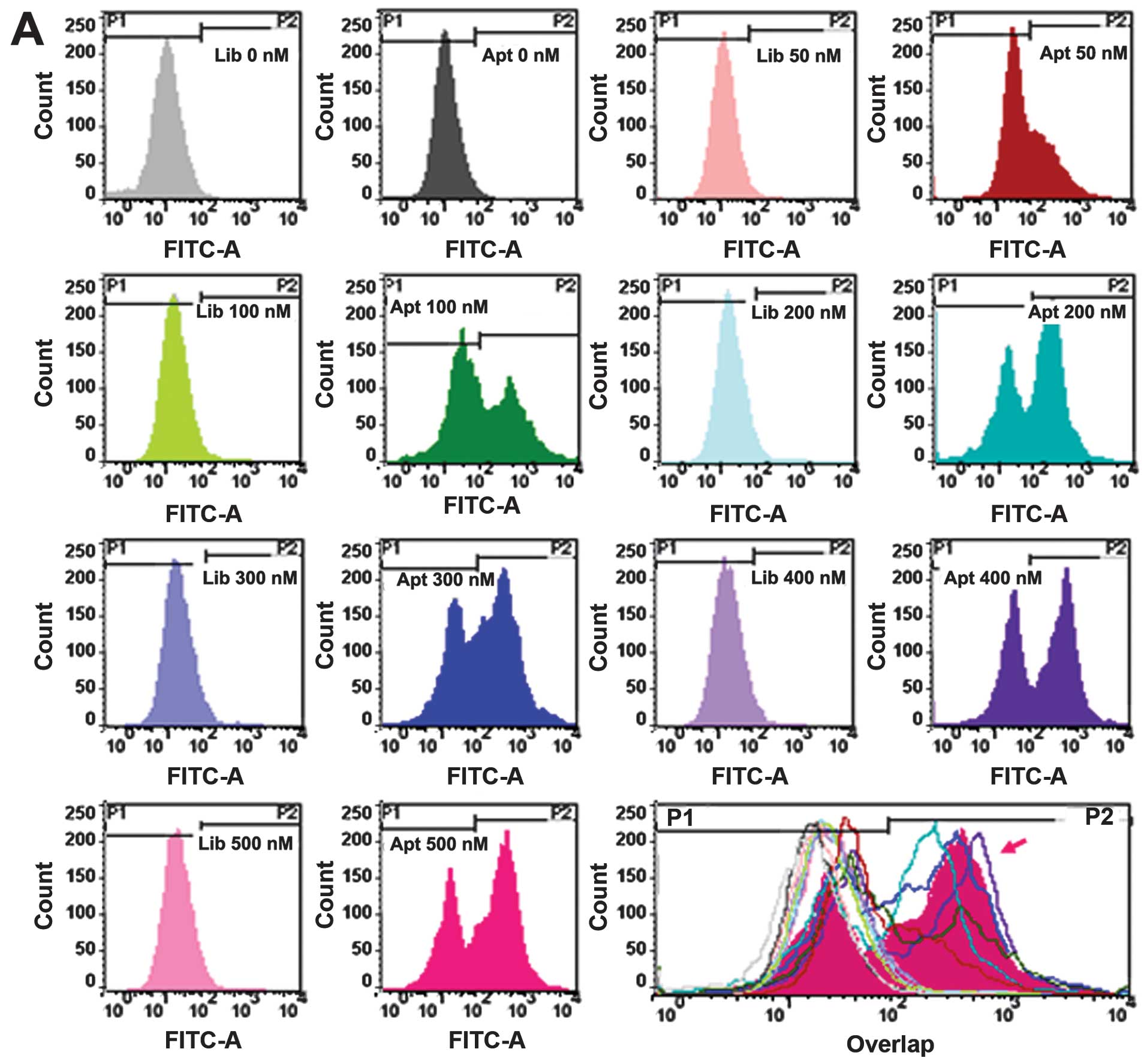
Characterization of aptamer cy-apt 20 in recognizing
target cells. The binding affinity and capacity of aptamer cy-apt
20 to AGS cells was also assessed by flow cytometry. Equivalent
library ssDNA was used as controls under the same conditions as
cy-apt 20. The concentrations of FITC-cy-apt 20 were initially
varied from 0 to 500 nM, and then the time lengths of incubation
varied from 0 to 50 min. The data showed that the fluorescence
intensity of AGS cells was steadily increased after 40 min of
incubation with increasing concentrations of FITC-cy-apt 20
(Fig. 3A and B) and peaked at the
concentration of 400 nM. The fluorescence intensity of AGS cells
were also steadily elevated with increasing time length of
incubation of AGS cells with FITC-cy-apt 20 and peaked at the time
point of 40 min (Fig. 3C and
D).
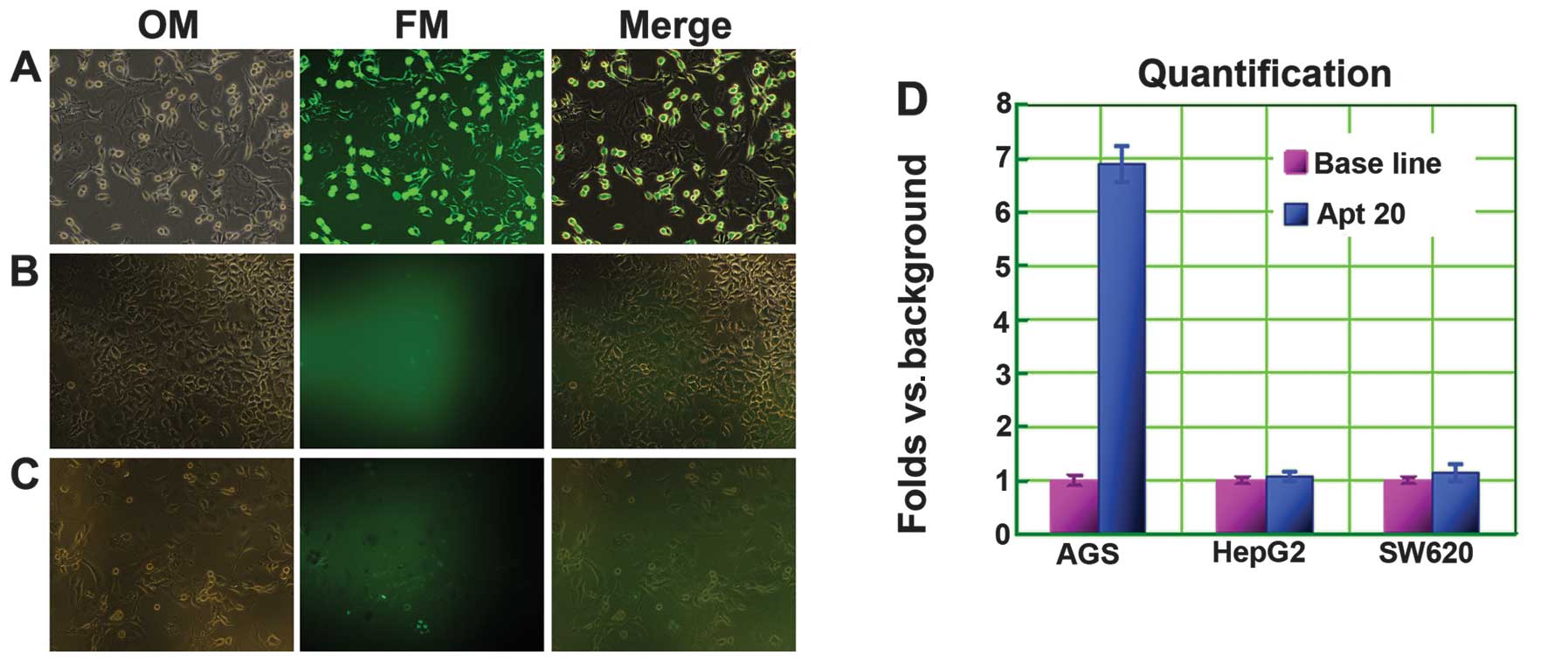
Fluorescence imaging of cancer cells using cy-apt
20. The specificity of aptamer cy-apt 20 in recognizing AGS cells
was ascertained by fluorescence imaging, with HepG2 and SW620 cells
being used as controls. The three tumor cells were separately
incubated with 400 nM of FITC-labeled cy-apt 20. After 40 min of
incubation, the stained cells were viewed using an inverted
fluorescence microscope. Most of the AGS cells were stained by
FITC-labled cy-apt 20 (Fig. 4A and
D). By contrast, few viable HepG2 (Fig. 4B and D) and SW620 (Fig. 4C and D) cells exhibited detectable
fluorescence.
Discussion
The entire live cell-SELEX procedure has been proven
to be a simple, but effective, reproducible and widely applicable
procedure in generating high-affinity aptamers without previous
knowledge of target molecules on tumor cells (5,6). A
large number of useful aptamers generated by this method are
applied in the study of tumor biology, and even in the diagnosis
and therapeutics of various types of cancer (12–21).
The encouraging results obtained with aptamers combined with their
intrinsic properties and the versatility of the live cell-SELEX
procedure have led to the application of this technical method in
the development of gastric cancer-specific DNA aptamers.
In the process of the culture cell-based selections,
we used human gastric carcinoma AGS cells as target cells for
positive selections and human normal gastric epithelial GES-1 cells
as negative cells for counter selections. To avoid loss of
important cell surface molecules in the process of selection,
cultured cells were collected by non-enzymatic cell dissociation
solution (10). To ensure the
elimination of common or shared molecules on tumor and normal
cells, the amount of cells used for counter selections was
maintained at >5-fold that of positive selections (9,10,22).
Through 12 rounds of successive selections, as indicated by
electrophoresis band position (Fig.
1A) and increased fluorescence intensity compared to the
library ssDNA (Fig. 1B), a pool of
ssDNA containing sequences with higher binding affinity to the
target cells was enriched (Fig.
1C).
The final enriched ssDNA pool was subsequently
confirmed by flow cytometric binding assays having high-binding
capacity to the target cells, but with minimal recognition to the
controls (Fig. 2A and B). By
cloning and sequencing, we identified 30 ssDNA sequences termed
cy-apt 01–30 as potential aptamer candidates specific to AGS cells
(data not shown). A comparison of experiments subsequently
identified 10 sequences from these candidates that bound to AGS
cells, four of which had binding rates >60% to AGS cells
(Fig. 2C), whereas only cy-apt 20
had >70% binding rate to AGS cells and <30% binding affinity
to non-gastric cancer cells (Fig. 2C
and D). These results suggest that cy-apt 20 is a useful
molecular tool for the recognition of gastric cancer cells.
The binding affinity and stability of cy-apt 20 in
the recognition of AGS cells were assessed by flow cytometry in a
dose- and time-dependent manner, since a molecular tool for
detecting target cancer cells should possess tenable binding
affinity and stability in addition to high specificity (5,6,11,12).
An equivalent library ssDNA was used as controls under the same
conditions as those for cy-apt 20. The results show that the
fluorescence intensity of AGS cells was steadily increased after 40
min of incubation with increasing concentrations of FITC-cy-apt 20
and peaked at the concentration of 400 nM (Fig. 3A and B). Similarly, the fluorescence
intensity of AGS cells was enhanced with the increasing time length
of incubation with 400 nM of FITC-cy-apt 20, which peaked at 40 min
(Fig. 3C and D). The results
suggest that targeting recognition can be established by using a
minimal dose of cy-apt 20 that continued over a long period of
time.
The feasibility of using aptamer cy-apt 20 for
detecting gastric cancer cells was ascertained by fluorescence
imaging, as an optimal biomarker-based diagnosis should be produced
in direct, simplified and visualized ways (1,2,4,23).
The three tumor cells were separately incubated with 400 nM of
FITC-labeled cy-apt 20 for the comparisons made. After 40 min of
incubation, the stained cells were viewed using an inverted
fluorescence microscope. The imaging showed that the majority of
AGS cells were stained by FITC-labeled cy-apt 20 (Fig. 4A), whereas, few HepG2 cells
(Fig. 4B) and SW620 cells (Fig. 4C) were stained by FITC-labeled
cy-apt 20. The fluorescence intensity of AGS cells was ~7-fold that
of HepG2 and SW620 cells (Fig. 4D).
The results suggest that cy-apt 20 is a useful molecular tool for
detecting gastric cancer cells.
In summary, the results of the present study have
demonstrated that the entire live cell-SELEX procedure was simple,
but effective in generating cancer cell-specific aptamers, as
previously reported (12–20). By using this method, we have
identified a DNA aptamer termed cy-apt 20 with tenable binding
affinity and stability to AGS cells. Additional investigations are
required to determine the feasibility of using cy-apt 20 for
detecting gastric cancer in vivo.
Acknowledgements
The present study was supported by a grant from the
Nanjing Medical Science and Technology Development Foundation. We
would like to thank the members of the Experiment Center of Basic
Medicine of Nanjing Medical University, Jie Ling, Xian-Bo Zhu and
Xiang Zhu, for valuable scientific discussions, cell culture and
flow cytometric analysis. We also thank Professor Marie-Davis Du
for her kind assistance in the writing of the manuscript.
References
|
1
|
Pietrantonio F, De Braud F, Da Prat V, et
al: A review on biomarkers for prediction of treatment outcome in
gastric cancer. Anticancer Res. 33:1257–1266. 2013.PubMed/NCBI
|
|
2
|
Takahashi T, Saikawa Y and Kitagawa Y:
Gastric cancer: current status of diagnosis and treatment. Cancers.
5:48–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
4
|
Yang S and Chung HC: Novel biomarker
candidates for gastric cancer. Oncol Rep. 19:675–680.
2008.PubMed/NCBI
|
|
5
|
Lassalle HP, Marchal S, Guillemin F, et
al: Aptamers as remarkable diagnostic and therapeutic agents in
cancer treatment. Curr Drug Metab. 13:1130–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Radom F, Jurek PM, Mazurek MP, et al:
Aptamers: molecules of great potential. Biotechnol Adv.
31:1260–1274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hermann T and Patel DJ: Adaptive
recognition by nucleic acid aptamers. Science. 287:820–825. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tuerk C and Gold L: Systematic evolution
of ligands by exponential enrichment: RNA ligands to bacteriophage
T4 DNA polymerase. Science. 249:505–510. 1990. View Article : Google Scholar
|
|
9
|
Stoltenburg R, Reinemann C and Strehlitz
B: SELEX - a (r)evolutionary method to generate high-affinity
nucleic acid ligands. Biomol Eng. 24:381–403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sefah K, Shangguan D, Xiong X, et al:
Development of DNA aptamers using Cell-SELEX. Nat Protoc.
5:1169–1185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cerchia L and de Franciscis V: Targeting
cancer cells with nucleic acid aptamers. Trends Biotechnol.
28:517–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shangguan D, Li Y, Tang Z, et al: Aptamers
evolved from live cells as effective molecular probes for cancer
study. Proc Natl Acad Sci USA. 103:11838–11843. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sefah K, Tang ZW, Shangguan DH, et al:
Molecular recognition of acute myeloid leukemia using aptamers.
Leukemia. 23:235–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang K, Sefah K, Tang L, et al: A novel
aptamer developed for breast cancer cell internalization. Chem Med
Chem. 7:79–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunii T, Ogura S, Mie M and Kobatake E:
Selection of DNA aptamers recognizing small cell lung cancer using
living cell-SELEX. Analyst. 136:1310–1312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sefah K, Meng L, Lopez-Colon D, et al: DNA
aptamers as molecular probes for colorectal cancer study. PLoS One.
5:e142692010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu G, Ye M, Donovan MJ, et al: Nucleic
acid aptamers: an emerging frontier in cancer therapy. Chem Commun.
48:10472–10480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Chen Y, Han D, et al: Aptamers
selected by cell-SELEX for application in cancer studies.
Bioanalysis. 2:907–918. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sefah K, Bae KM, Phillips JA, et al:
Cell-based selection provides novel molecular probes for cancer
stem cells. Int J Cancer. 132:2578–2588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zueva E, Rubio LI, Ducongé F and Tavitian
B: Metastasis-focused cell-based SELEX generates aptamers
inhibiting cell migration and invasion. Int J Cancer. 128:797–804.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berezovski MV, Lechmann M, Musheev MU, et
al: Aptamer-facilitated biomarker discovery (AptaBiD). J Am Chem
Soc. 130:9137–9143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang X and Tan W: Aptamers generated from
cell-SELEX for molecular medicine: a chemical biology approach. Acc
Chem Res. 43:48–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pavlou MP, Diamandis EP and Blasutig IM:
The long journey of cancer biomarkers from the bench to the clinic.
Clin Chem. 59:147–157. 2013. View Article : Google Scholar : PubMed/NCBI
|