Introduction
The RET proto-oncogene encodes a tyrosine
kinase (TK) receptor protein that is expressed normally in neural
crest-derived cells (1–3). In papillary thyroid cancer (PTC), the
RET proto-oncogene fuses to sequences of the 5′ portion of
partner genes at its TK domain via chromosomal inversion or
translocation, resulting in rearranged and constitutively activated
chimeric oncogenes called RET/PTC (4–7). To
date, at least 15 different types of rearranged RET/PTC have
been found, with 12 different partner genes (8–10). The
majority of rearranged RET oncogenes found in PTC are
RET/PTC1 and RET/PTC3, which are formed by
paracentric inversions of chromosome 10. Most rare types of these
RET rearrangements have been isolated from
radiation-associated PTCs, such as those arising post-Chernobyl
(10–17). All the partner genes of rearranged
RET/PTC thus far identified are widely expressed in various
human tissue including thyroid. The chimeric products of
RET/PTC are constitutively expressed and form homo-dimers
mediated by the coiled-coil domains of the partner genes in PTC,
resulting in a ligand-independent activation of TK of the RET.
During molecular analyses of ~100 PTC cases from
atomic bomb (A-bomb) survivors, rearrangements of genes with kinase
domains, such as RET, NTRK1 and ALK, have
frequently been detected in survivors exposed to high radiation
doses (18,19). Among these, RET/PTC1 was the
most common rearranged gene in the PTC cases examined in A-bomb
survivors exposed to a radiation dose of >500 mGy. An improved
rapid amplification of cDNA ends (RACE) method was established to
identify unknown RET rearrangement with RNA extracted from
archival formalin-fixed, paraffin-embedded (FFPE) thyroid cancer
tissue specimens. Using this method, a PTC A-bomb survivor exposed
to a high radiation dose was found to possess RET/PTC8
(20), which had been identified in
thyroid cancer from post-Chernobyl children (14). In addition, a novel type of
RET rearrangement, i.e., acyl-coenzyme A binding domain
containing 5 (ACBD5)-RET, was found in a PTC from
another high-dose A-bomb survivor. In the present study, we
reported the identification and molecular characteristics of the
ACBD-RET rearrangement that was formed by fusion of
the tyrosine kinase domain of RET to the 5′ portion of
ACBD5 gene located in the short arm of chromosome 10, via
pericentric inversion inv(10)(p12.1;q11.2).
Materials and methods
Tissue specimens
FFPE PTC specimens from A-bomb survivors, preserved
at room temperature for 20–50 years, were collected and analyzed
with approval of the Human Investigation Committee, and the Ethics
Committee for Genome Research at the Radiation Effects Research
Foundation (RERF). A novel RET rearrangement
(ACBD5-RET) was discovered in PTC specimen from one
A-bomb survivor whose DS02 dose (21) was 1.8 Gy (weighted thyroid
dose).
RNA extraction
RNA was isolated from dissected tissue using the
High Pure RNA Paraffin kit according to the manufacturer’s
instructions (Roche Diagnostics GmbH, Mannheim, Germany) with some
modifications, as described in a previous study (22).
cDNA synthesis and improved switching
mechanism at 5′ end of RNA transcript (SMART) RACE
cDNA synthesis and improved SMART RACE with RNA from
archival FFPE PTC tissues was carried out as described in a
previous study (20).
Cloning and sequencing of cDNA fragments
of ACBD5-RET gene
Target candidate cDNA fragments derived from the
second SMART RACE-PCR products were eluted from 8% acrylamide gel
and cloned into HincII digested plasmid vector. Plasmid DNA
containing an insert of >70 bp was sequenced using DNA sequencer
CEQ8000 (Beckman Coulter Inc., Fullerton, CA, USA), since the total
length of the SMART adaptor and the 5′ portion of exon 12 of
RET was 55 bp.
RT-PCR detection of ACBD5-RET
RT-PCR was carried out as previously described
(20). RT-PCR products were cloned
in a cloning vector and confirmed as actual ACBD5-RET
products by sequencing.
Determination of ACBD5 gene expression
levels in normal tissue by real-time PCR
Human normal tissue RNAs were purchased from
BioChain Institute, Inc. (USA). Total RNA (2 μg) was reverse
transcribed with 100 units of ReverTra Ace (Toyobo Co., Japan) as
described in a previous study (20). Quantitative PCR amplifications were
carried out using ABsolute™ qPCR SYBR-Green Mixes according to the
manufacturer’s instructions (Thermo Fisher Scientific Inc., USA).
Amplification conditions optimized for the iCycler instrument
(Bio-Rad, Hercules, CA, USA) resulted in a single PCR product,
which was judged by using melting curve and electrophoretic
analysis. 18SrRNA was used as a reference. The assays were run in
duplicate using the following primer set: ACBD5-RT56F,
5′-GCCATGATTGCATATGTTGA AG-3′ and ACBD5-RT56R,
5′-AACTCCTGCCACTCTTTT TGTC-3′ for ACBD5 gene; and 18SrRNART-F12,
5′-GTAG TCGCCGTGCCTACCAT-3′ and 18SrRNART-R12,
5′-GTTTCTCAGGCTCCCTCTCC-3′ for 18SrRNA.
Expression of variant 1 and 2 of ACBD5
gene in normal tissue by RT-PCR
RT-PCR of variant 1 and 2 of ACBD5 gene was
carried out as described elsewhere (20). Briefly, the primer sets used were:
ACBD5ex2F2, 5′-TCTTCTGCTAGACATGCTC TTCCT-3′ and ACBD5ex3R1,
5′-CCCAAGAGCCTGCATG AAAC-3′ for variant 1; and ACBD5ex1F2,
5′-GACAGGCCT TGTCCGGCAATA-3′ and ACBD5ex3R1, 5′-CCCAAGAGC
CTGCATGAAAC-3′ for variant 2. The cDNA derived from 2 ng of total
RNA was used as a template for RT-PCR. PCR conditions consisted of
initial denaturation (95°C for 2 min), followed by 36 cycles
(denaturation at 95°C for 30 sec, annealing at 60°C for variant 2
or 62°C for variant 1, for 30 sec, extension at 72°C for 30 sec),
and final extension at 72°C for 3 min.
Construction of ACBD5-RET
Total RNA from the PTC cell line TPC1 was used to
amplify RET/PTC1 using a sense primer (H4PRTF1,
TTAAGCTTCTGCTGCTCCTCCTCCT TTCCA)
located in the 5′ flanking region of H4 gene and an
antisense primer (RET-3′ end R3, AATCTAGATCAGGGGTA GTGGCTGCTCAGTA)
located in the 3′ untranslated region of RET gene. After
digestion of amplified fragments with HindIII and
XbaI, a fragment of ~2,400 bp was inserted into
HindIII and XbaI-digested pBluescript II SK(−). To
replace the partner of RET/PTC1 with 5′ region of
ACBD5 gene, a fragment of ~1,200 bp covering the region from
exon 5 to exon 12 of the ACBD5 gene was amplified with total
RNA from human normal thymus using sense primer ACBD5F2,
TTAA
GCTTAATGGGATGCTTGGAGTTCACTG, located in the joint region of
exons 4 and 5 of ACBD5 gene, and antisense primer
ACBD5-RETR1, CTTTGGATCCTCCTGTGAGG
TGGGCTGAGGAGCA, which corresponds to regions of 3′ end of exon 12
of ACBD5 and of 5′ end of exon 12 of RET. Following
digestion with HindIII and BamHI, a fragment of
~1,200 bp was inserted into HindIII and
BamHI-digested pBluescript II SK(−) containing
BamHI-XbaI fragment of RET kinase domain. To
complete the construction of ACBD5-RET, a fragment of
~500 bp containing the region from initiation codon to exon 5 of
ACBD5 gene was amplified with total RNA from human normal
thymus using sense primer ACBD5F1, ACAACAAGCTTTCATGCAGGCTCTTGGGAAAGC,
and antisense primer ACBD5R2, CGGTTTTGGCGTTCGGA GTAGA. After
digestion of the amplified fragments with HindIII and
NdeI, whose recognition site is located in exon 5 of
ACBD5 gene, the digested fragments were ligated to
HindIII- and NdeI-digested pBluescript II SK(−)
containing regions of exons 4–12 of ACBD5 and exons 12–20 of
RET. Underlined primers indicate the restriction enzyme
sites that were artificially created to make the construct of
ACBD5-RET.
DNA transfection
NIH3T3 cells (5×104) were transfected
with 10 μg of empty vector, ACBD5-RET or
RET/PTC3 expression vectors (pcDNA3.1) (Invitrogen,
Carlsbad, CA, USA) using FuGENE6 Transfection Reagent (Roche
Diagnostics GmbH). After 2 days, cells were further incubated in
DMEM containing 10% calf serum and 500 μg/ml of G418.
G418-resistant colonies were screened for transfected cDNA
expression by RT-PCR. Selected clones were then analyzed for
phosphorylation of ERK protein by western blotting.
Western blot analysis
After scraping out the cells from 100 mm dishes,
1×106 stable transfectants were lysed in 100 μl of RIPA
lysis buffer (Merck Millipore Co., Germany). After adding 2X SDS
buffer to cell lysate, and heating at 95°C, an aliquot (~2 μg of
proteins) was separated by 8% SDS-polyacrylamide gel and then
transferred to a PVDF membrane. Specific bands were detected by
incubating with primary antibodies and then with horseradish
peroxidase-conjugated anti-rabbit antibody (Cell Signaling
Technology Co., USA), and finally by treatment with enhanced
chemiluminescence reagent (Amersham Biosciences). For analysis of
RET protein, Ret (C31B4) rabbit monoclonal antibody (Cell Signaling
Technology Co.) was used as the primary antibody. Phospho-p44/42
MAP kinase antibody and p44/42 MAP kinase antibody (Cell Signaling
Technology Co.) were used as primary antibodies for analysis of
phosphorylation of ERK1/2.
Transformation assay
Stable transfectants were suspended at a cell
concentration of 2×106/ml in culture media and injected
subcutaneously into the flank of BALB/c nu/nu female mice. Three
clones of stable transfectants with ACBD5-RET and one
clone of stable transfectants with RET/PTC3 and
G418-resistant NIH3T3 cells with empty vector were injected into
one flank of the mice at different cell densities
(1×106, 5×105 and 2.5×105 cells).
Animal experiments in this study were conducted on the basis of
approval from the RERF’s Experimental Animal Care Committee.
Results and Discussion
Identification and expression of
ACBD5-RET gene in PTC of one A-bomb survivor exposed to high-dose
radiation
During analysis of RET/PTC rearrangements in
PTC specimens among A-bomb survivors, a cDNA fragment of a new type
of RET/PTC, with a size of 102 bp, was isolated from one
A-bomb survivor exposed to 1.8 Gy using the improved RACE method
(20) in PTC. Sequence analysis
revealed that the isolated fragments contained a portion of exon 12
of the ACBD5 gene that was fused in frame with exon 12 of
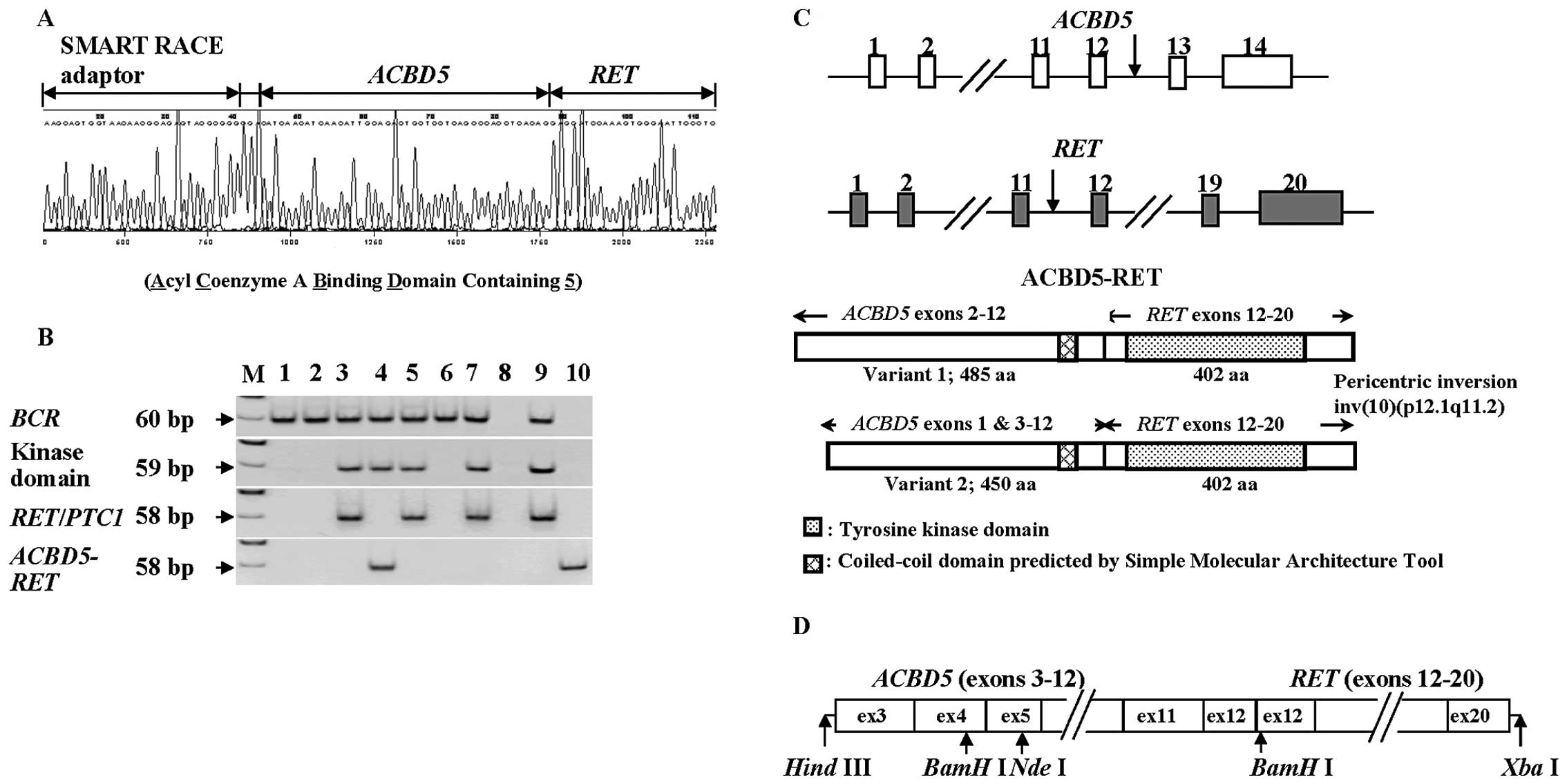
the RET gene (Fig. 1A). The
fragment obtained from the 5′ SMART RACE method contained an
unknown insert of 3 bp (GGA) between the adaptor for RACE and
ACBD5; RT-PCR-amplified fragments covering the corresponding region
did not include any 3 bp-insert (GGA), and were coincident with
sequences of the reported ACBD5 (data not shown). The 3
bp-insert was presumably derived from the template-independent
addition of a few nucleotides by terminal transferase activity of
reverse transcriptase in the process of SMART RACE.
The ACBD5-RET chimera gene was
detected only in PTC from this A-bomb survivor by RT-PCR with
gene-specific primers (Fig. 1B).
Since the tissue samples used were specimens that had been fixed
with unbuffered formalin, embedded in paraffin and preserved for
>40 years (resulting in vigorous degradation of RNA), we were
unable to clone a full-length cDNA of the ACBD5-RET
gene using either 5′ RACE method or RT-PCR. However, the
ACBD5 gene is ubiquitously expressed in various normal human
tissues including thyroid, with two major products, variants 1 and
2 (Fig. 2A and B). The former
starts from exon 2, while the latter starts at exon 1 and skips
exon 2 (Fig. 1C). When we compared
the expression levels of variants 1 and 2 of the ACBD5 gene
in various human tissues, we found that expression levels of
variant 2 of ACBD5 gene were higher than those of variant 1
in normal thyroid (Fig. 2B).
Assuming that the ACBD5-RET fusion gene utilized an
initiation codon in exon 3 of the ACBD5 gene, as is the case
for variant 2 of ACBD5, we prepared a construct of the
ACBD5-RET gene (Fig.
1D), as described in the Materials and methods section.
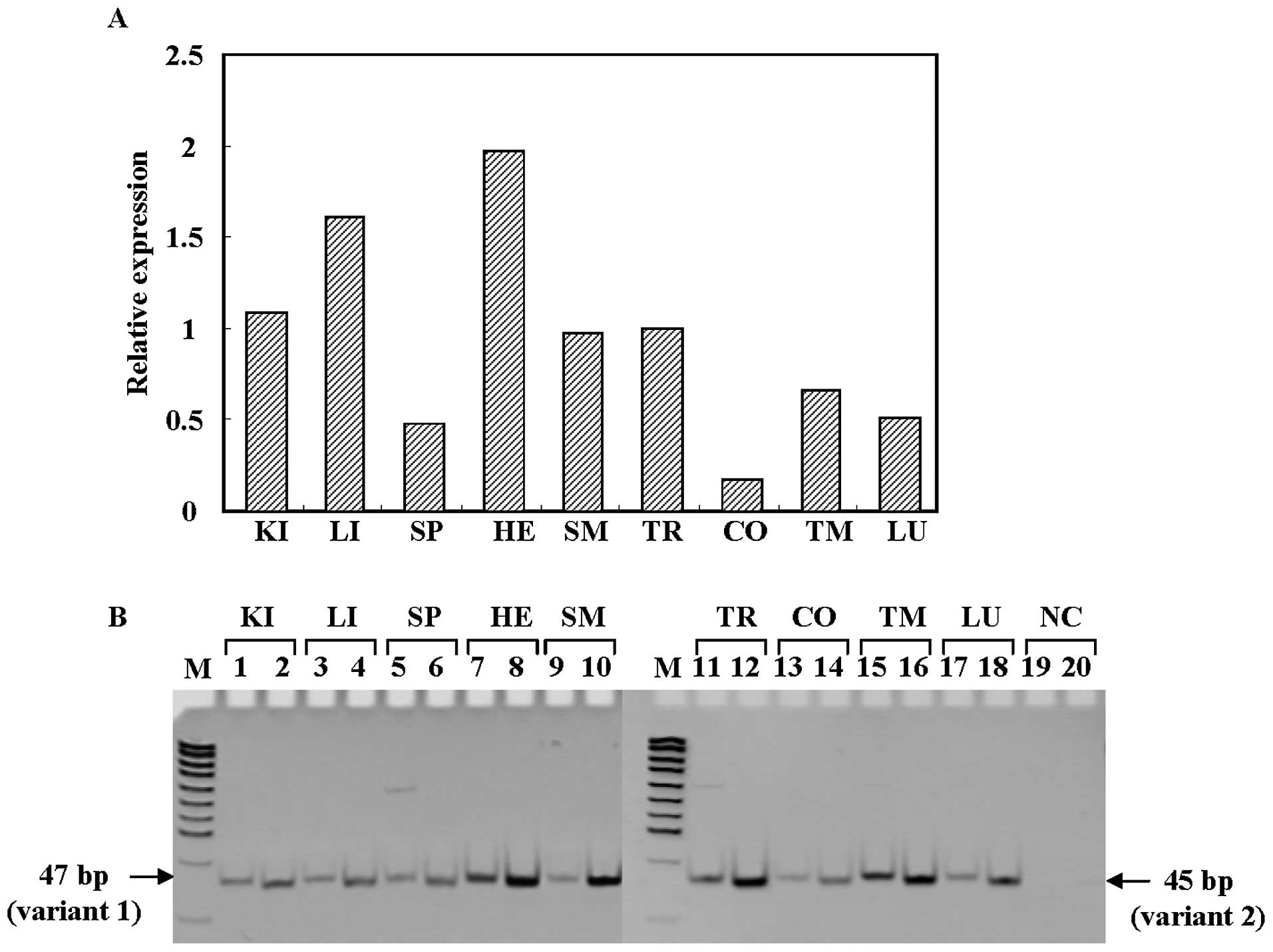 | Figure 2Expression of ACBD5 mRNA
(variants 1 and 2) in various human normal tissue. (A) Relative
ACBD5 mRNA expression levels determined by quantitative
real-time RT-PCR. The tissue used is: KI, kidney; LI, liver; SP,
spleen; HE, heart; SM, skeletal muscle; TR, thyroid; CO, colon; TM,
thymus; LU, lung. NC shows H2O for negative control, and
M indicates pUC19-MspI digest for DNA size marker. (B)
Detection of the ACBD5 transcripts by RT-PCR. Odd and even
numbers indicate variants 1 and 2, respectively. |
Activation of MAPK pathway in
ACBD5-RET-introduced NIH3T3 stable transfectants
Since RET/PTC fusion gene products are
involved in cell growth through phosphorylation of ERK protein, we
evaluated the ability of the ACBD5-RET protein to phosphorylate ERK
protein using stable transfectants of NIH3T3 cells constitutively
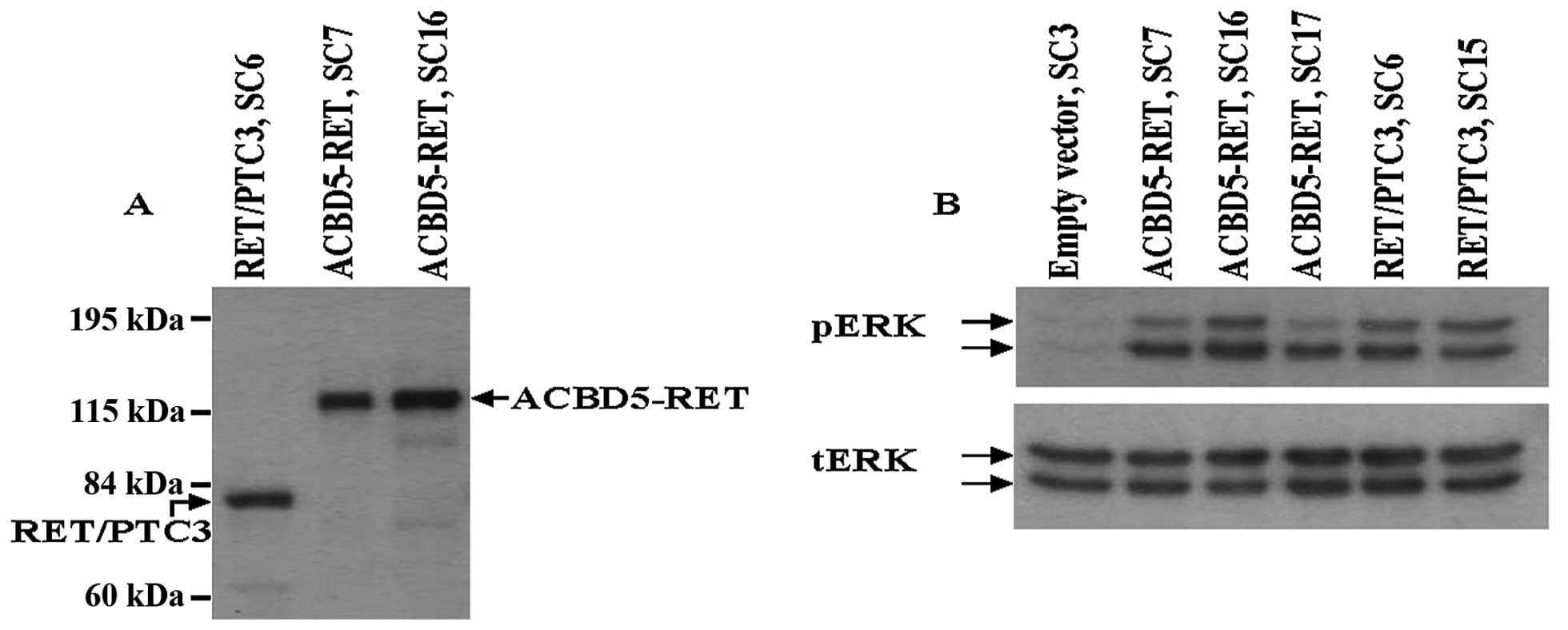
expressing ACBD5-RET. Expression of ACBD5-RET or
RET/PTC3 proteins in these stable transfectants is shown in
Fig. 3A. Enhanced ERK protein
phosphorylation was observed in three different transfectants: SC7,
SC16 and SC17; and phosphorylation levels were comparable to those
of two NIH3T3 stable transfectants with RET/PTC3, SC6
and SC15 (Fig. 3B). In RET/PTC
fusion proteins, oligomerization is mediated by coiled-coil domains
of the partner gene of RET/PTC genes, resulting in
constitutive activation of tyrosine kinase through
autophosphorylation of critical tyrosine residues in the
RET/PTC genes (23).
Enhanced phosphorylation of ERK in the stable transfectants with
ACBD5-RET would result from the ligand-independent
activation of tyrosine kinase by homodimerization of this
chimera-protein through coiled-coil region located on exon 10 of
ACBD5 gene, which was predicted by simple molecular
architecture tool (EMBL).
Transforming activity of ACBD5-RET in
NIH3T3 stable transfectants
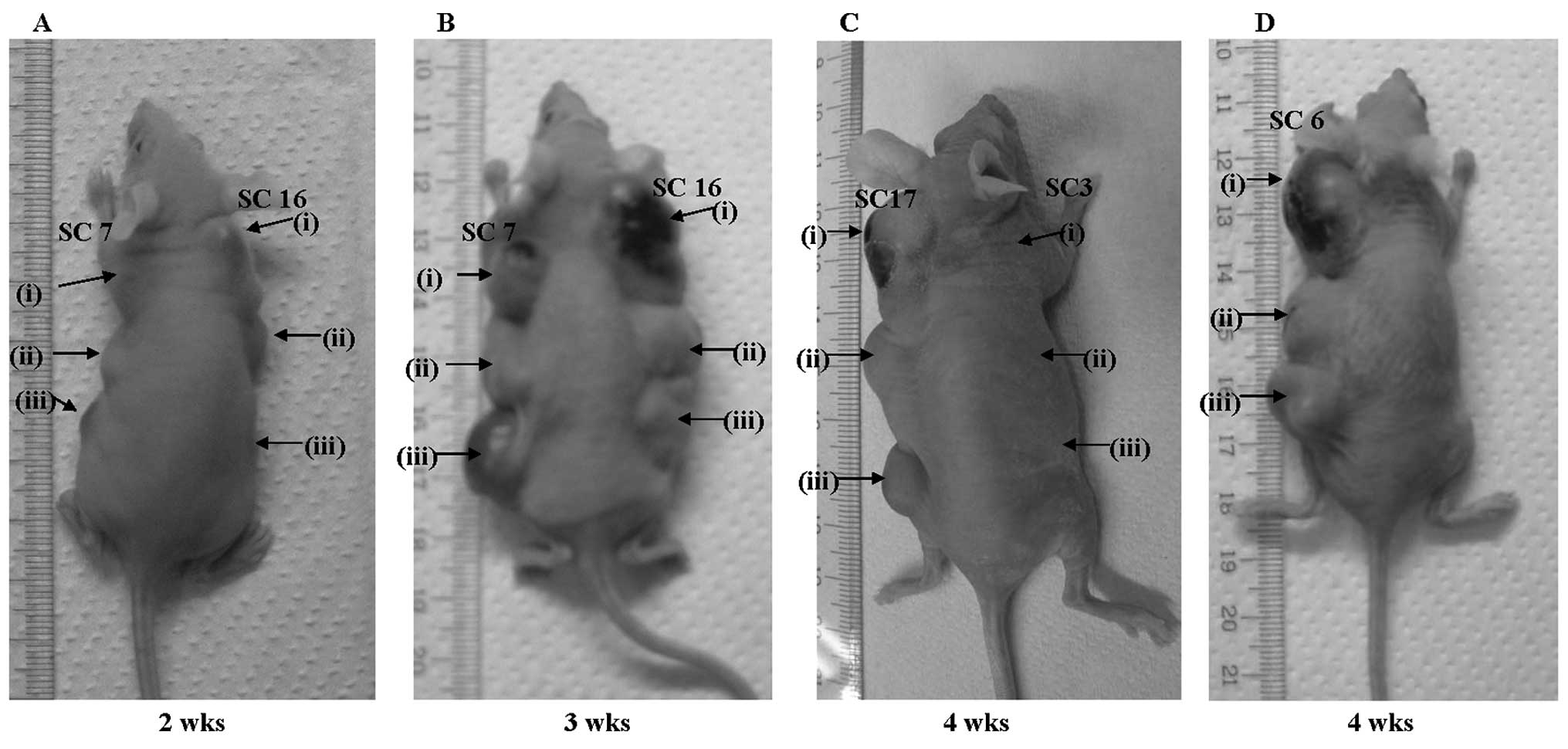
To assess the tumorigenic activity of
ACBD5-RET, we conducted a tumor formation assay in
nude mice, using three clones (SC7, SC16 and SC17) from
ACBD5-RET-introduced NIH3T3 stable transfectants.
Administration of 1×106 of each stable transfectant with
ACBD5-RET resulted in tumor formation in nude mice as
early as 10 days after injection. Detectable tumors formed in nude
mice two weeks after injection of 5×105 or
2.5×105 cells (Fig. 4A).
In all three clones, tumors grew to >15 mm 3–4 weeks after
injection of each cell dilution (Fig.
4B and C). NIH3T3 stable transfectants, with
RET/PTC3 used as positive control, also formed
detectable tumors 2–4 weeks after injection (Fig. 4D). By contrast, no tumors were
detected even 4 weeks after injection of 0.25–1×106 of
NIH3T3 cells with empty vectors (Fig.
4C). These results suggest that ACBD5-RET gene
products may function as oncogene by constitutively activating MAP
kinase through their homodimerization, as do products of other
RET/PTC genes (23–25).
Acknowledgements
The Radiation Effects Research Foundation (RERF),
Hiroshima and Nagasaki, Japan, is a public interest foundation
funded by the Japanese Ministry of Health, Labour and Welware
(MHLW), and the US Department of Energy (DOE). This study was
funded in part through DOE award DE-HS0000031 to the National
Academy Sciences, and in part by a Grant-in-Aid for Science
Research from the Ministry of Education, Culture, Sports, Science
and Technology, and a Grant-in Aid for Cancer Research from the
Ministry of Health, Labour and Welfare of Japan, as well as a
Research Grant for the Princess Takamatsu Cancer Research Fund
(08-24015). This publication was supported by RERF Research
Protocols RP 5-02. The views of the authors do not necessarily
reflect those of the two governments.
References
|
1
|
Pachinis V, Mankoo B and Costatini F:
Expression of the c-ret proto-oncogene during mouse
embryogenesis. Development. 19:1005–1017. 1993.
|
|
2
|
Avantaggiato V, Dathan NA, Griero M,
Fabien N, Lazzaro D, Fusco A, Simeone A and Santoro M:
Developmental expression of the RET protooncogene. Cell
Growth Differ. 5:305–311. 1994.
|
|
3
|
Schuchardt A, D’Agati V, Larsson-Blomberg
L, Costantini F and Pachnis V: Defects in the kidney and enteric
nervous system of mice lacking the tyrosine kinase receptor Ret.
Nature. 367:380–383. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grieco M, Santoro M, Berlingieri MT,
Melillo RM, Donghi R, Bongarzone I, Pierotti MA, Della Porta G,
Fusco A and Vecchio G: PTC is a novel rearranged form of the
ret proto-oncogene and is frequently detected in vivo in
human thyroid papillary carcinomas. Cell. 60:557–563.
1990.PubMed/NCBI
|
|
5
|
Bongarzone I, Monzini N, Borrello MG,
Carcano C, Ferraresi G, Arighi E, Mondellini P, Della Porta G and
Pierotti MA: Molecular characterization of a thyroid tumor-specific
transforming sequence formed by the fusion of ret tyrosine kinase
and the regulatory subunit RI alpha of cyclic AMP-dependent protein
kinase A. Mol Cell Biol. 13:358–366. 1993.
|
|
6
|
Bongarzone I, Butti MG, Coronelli S,
Borrello MG, Santoro M, Mondellini P, Pilotti S, Fusco A, Della
Porta G and Pierotti MA: Frequent activation of ret
protooncogene by fusion with a new activating gene in papillary
thyroid carcinomas. Cancer Res. 54:2979–2985. 1994.PubMed/NCBI
|
|
7
|
Santoro M, Dathan N, Berlingieri MT,
Bongarzone I, Paulin C, Grieco M, Pierotti MA, Vecchio G and Fusco
A: Molecular characterization of RET/PTC3; a novel
rearranged version of the RET proto-oncogene in a human
thyroid papillary carcinoma. Oncogene. 9:509–516. 1994.
|
|
8
|
Tallini G and Asa SL: RET oncogene
activation in papillary thyroid carcinoma. Adv Anat Pathol.
8:345–354. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arighi E, Borrello MG and Sariola H: RET
tyrosine signaling in development and cancer. Cytokine Growth
Factor Rev. 16:441–467. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ciampi R, Giordano TJ, Wikenheiser-Brokamp
K, Koenig RJ and Nikiforov YE: HOOK3-RET: a novel type of
RET/PTC rearrangement in papillary thyroid carcinoma. Endocr
Relat Cancer. 14:445–452. 2007. View Article : Google Scholar
|
|
11
|
Fugazzola L, Pierotti MA, Vigano E, Pacini
F, Vorontsova TV and Bongarzone I: Molecular and biochemical
analysis of RET/PTC4, a novel oncogenic rearrangement between RET
and ELE1 genes, in a post-Chernobyl papillary thyroid cancer.
Oncogene. 13:1093–1097. 1996.
|
|
12
|
Klugbauer S, Demidchik EP, Lengfelder E
and Rabes HM: Detection of a novel type of RET rearrangement
(PTC5) in thyroid carcinomas after Chernobyl and analysis of the
involved RET-fusion gene RFG5. Cancer Res.
58:198–203. 1998.PubMed/NCBI
|
|
13
|
Klugbauer S and Rabes HM: The
transcription coactivator HTIF1 and a related protein are
fused to the RET receptor tyrosine kinase in childhood
papillary thyroid carcinomas. Oncogene. 18:4388–4393. 1999.
|
|
14
|
Klugbauer S, Jauch A, Lengfelder E,
Demidchik E and Rabes HM: A novel type of RET rearrangement
(PTC8) in childhood papillary thyroid carcinomas and
characterization of the involved gene (RFG8). Cancer Res.
60:7028–7032. 2000.
|
|
15
|
Salassidis K, Bruch J, Zitzelsberger H,
Lengfelder E, Kellerer AM and Bauchinger M: Translocation
t(10;14)(q11.2:q22.1) fusing the kinetin to the RET
gene creates a novel rearranged form (PTC8) of the RET
proto-oncogene in radiation-induced childhood papillary thyroid
carcinoma. Cancer Res. 60:2786–2789. 2000.
|
|
16
|
Corvi R, Berger N, Balczon R and Romeo G:
RET/PCM-1: a novel fusion gene in papillary thyroid cancer.
Oncogene. 19:4236–4242. 2000. View Article : Google Scholar
|
|
17
|
Saenko V, Rogounovitch T, Shimizu-Yoshida
Y, Abrosimov A, Lushnikov E, Roumiantsev P, Matsumoto N, Nakashima
M, Merimanov S, Ohtsuru A, Namba H, Tsyb A and Yamashita S: Novel
tumorigenic rearrangement, Δ rfp/ret, in a papillary thyroid
carcinoma from externally irradiated patient. Mutat Res. 527:81–90.
2003.
|
|
18
|
Hamatani K, Eguchi E, Ito R, Mukai M,
Takahashi K, Taga M, Imai K, Cologne J, Soda M, Arihiro K, Fujihara
M, Abe K, Hayashi T, Nakashima M, Sekine I, Yasui W, Hayashi Y and
Nakachi K: RET/PTC rearrangements preferentially occurred in
papillary thyroid cancer among atomic bomb survivors exposed to
high radiation dose. Cancer Res. 68:7176–7182. 2008. View Article : Google Scholar
|
|
19
|
Hamatani K, Mukai M, Takahashi K, Hayashi
Y, Nakachi K and Kusunoki Y: Rearranged anaplastic lymphoma kinase
(ALK) gene in adult-onset papillary thyroid cancer amongst
atomic bomb survivors. Thyroid. 22:1153–1159. 2012.PubMed/NCBI
|
|
20
|
Hamatani K, Eguchi H, Taga M, Mukai M,
Koyama K, Hayashi Y and Nakachi K: Improved method for analysis of
RNA present in long-term preserved thyroid cancer tissue of
atomic-bomb survivors. Thyroid. 20:43–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Young RW and Kerr GD: Reassessment of the
Atomic Bomb Radiation Dosimetry for Hiroshima and Nagasaki -
Dosimetry System 2002. 1. Radiation Effects Research Foundation;
Hiroshima, Japan: 2005
|
|
22
|
Hamatani K, Eguchi H, Takahashi K, Koyama
K, Mukai M, Ito R, Taga M, Yasui W and Nakachi K: Improved RT-PCR
amplification for molecular analyses with long-term preserved
formalin-fixed, paraffin-embedded tissue specimens. J Histochem
Cytochem. 54:773–780. 2006. View Article : Google Scholar
|
|
23
|
Tong Q, Xing S and Jhiang SM: Leucine
zipper-mediated dimerization is essential for the PTC1
oncogenic activity. J Biol Chem. 272:9043–9047. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alberti L, Carniti C, Miranda C, Roccato E
and Pierotti M: RET and NTRK1 proto-oncogenes in human diseases. J
Cell Physiol. 195:168–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kodama Y, Asai N, Kawai K, Jijiwa M,
Murakumo Y, Ichihara M and Takahashi M: The RET
proto-oncogene: a molecular therapeutic target in thyroid cancer.
Cancer Sci. 96:143–148. 2005.PubMed/NCBI
|