Introduction
Gliomas account for 80% of all primary brain and
central nervous system malignancies (1). Gliomas are difficult to be cured by
surgical resection or radiotherapy, and the median survival of
patients with gliomas is only 12–15 months (2). Therefore, it is necessary to elucidate
the underlying mechanisms involved in glioma development and to
discover new targets for the treatment of gliomas.
Accumulating evidence suggests that changes in the
expression of microRNAs (miRNAs) are associated with cancer
development (3), and a number of
miRNAs have been identified to be important regulators of
tumorigenesis (4,5).
Previous research has shown that miR-124 is
abundantly expressed in normal brain tissue (6); however, only a few reports have
focused on the biological impact of miR-124 on glioma cells
(7) and the underlying mechanisms
need to be elucidated.
In the present study, we investigated the role of
miR-124a in glioma proliferation and invasion. The in vivo
study demonstrated that expression of miR-124a was downregulated in
glioma tissues and in highly malignant glioma cells. Restoration of
miR-124a or the knockdown of IQGAP1 in vitro inhibited
glioma cell proliferation and invasion. Furthermore, we confirmed
that miR-124a could inhibit glioma cell proliferation and invasion
by blocking the expression of the IQGAP1 gene and downstream
β-catenin and cyclin D1.
Materials and methods
Cell culture
Human glioma cell lines (U251, U343, U87, SF126 and
SF76) were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA) and cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented
with 10% fetal bovine serum (Gibco) at 37°C in a humidified
atmosphere of 5% CO2.
Tissue samples
This study was approved by the Ethics Committee of
the Third Xiangya Hospital of Central South University. All
patients provided written informed consent in compliance with the
code of ethics of the World Medical Association (Declaration of
Helsinki). Human glioma samples and adjacent normal tissue samples
were collected from 20 glioma patients who underwent surgery at the
Neurosurgery Department of the Third Xiangya Hospital of Central
South University (Changsha, Hunan, China). At the time of
diagnosis, 10 patients had early stage (I, II) whereas the other 10
patients had stage III and IV glioma. The tissue samples were
obtained from surgery and immediately frozen in liquid
nitrogen.
Real-time PCR
Total RNAs were isolated from cells and tissues
using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Reverse transcription was performed
using a RevertAid™ First Strand cDNA Synthesis kit (Fermentas,
Vilnius, Lithuania). Real-time PCR reaction was carried out in a
7900 Sequence Detection System (Applied Biosystems, Foster City,
CA, USA) using a SYBR-Green PCR kit (Applied Biosystems). The
relative mRNA expression level was calculated by the comparative Ct
method.
Western blotting
Tissues and cells were lysed by ice-cold protein
extraction buffer (150 mM Tris-HCl, pH 7.4, 120 mM NaCl, 2 mM EDTA,
50 mM sodium fluoride, 0.2% SDS, 1% Nonidet P-40, 100 mM sodium
vanadate and 1 mM phenylmethylsulfonyl fluoride). Proteins were
quantitated using the BCA kit (Beyotime, Shanghai, China). The
protein samples were separated in 10% SDS-PAGE and transferred onto
a nitrocellulose membrane (Millipore, Billerica, MA, USA). The
membranes were blocked overnight in 5% non-fat milk and then
incubated with the primary antibody [IQGAP1 and β-catenin
antibodies (Abcam, Cambridge, MA, USA); p-β-catenin and cyclin D1
antibodies (Cell Signaling Technology, Inc., Beverly, MA, USA);
GAPDH antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA)] for 1 h at room temperature. After washing with PBS, the
membranes were incubated with the secondary antibody
[HRP-conjugated goat anti-mouse IgG and HRP-conjugated goat
anti-rabbit IgG (Santa Cruz Biotechnology, Inc.)] for 1 h at room
temperature. Detection was performed using a
chemiluminescence-based detection system (ECL western blotting kit;
Pierce Biotechnology, Inc., Rockford, IL, USA).
Immunofluorescence
Cells were washed with PBS and fixed in 4%
paraformaldehyde solution for 1 h. After permeabilization with 0.2%
Triton X-100 at 4°C for 1 h, cells were blocked with goat serum and
then incubated with the primary antibody (β-catenin antibody) for 1
h at 37°C. Subsequently, the secondary antibody [Alexa Fluor
488-labeled anti-rabbit IgG (Cell Signaling Technology Inc.)] was
added, and the cells were incubated at 37°C for 1 h. DAPI (Santa
Cruz Biotechnology, Inc.) was used to label the nucleus, and the
cells were visualized by fluorescence microscopy (Nikon, Tokyo,
Japan).
Transfection
The miR-124a mimic and siRNAs were synthesized by
Biomics Biotechnologies Inc. (Nantong, Jiangsu, China). Cells were
transfected with the miR-124a mimic or siRNAs using Lipofectamine
2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s
instructions. After 6 h, the cultures were replaced with fresh
medium.
Luciferase assay
The pRL-TK Renilla luciferase reporter
vector, pmirGLO luciferase reporter vector and Dual-luciferase
reporter assay system were purchased from Promega (Madison, WI,
USA). Reporter plasmids containing the 3′UTR of IQGAP1
(pmirGLO-IQGAP1) were co-transfected with the NC mimic or the
miR-124a mimic into cells. pRL-TK Renilla luciferase
reporter vector was used as an internal control. Luciferase
activity was measured by the Dual-luciferase reporter assay system.
Results were expressed as the firefly luciferase activity
normalized to Renilla luciferase activity.
MTT assay
The MTT assay was used to assess cell proliferation.
Cells were seeded in 96-well plates and allowed to grow for 24 h.
After transfection, 50 μl MTT solution was added and incubated at
37°C for 4 h. After dissolving the formazine granulars with 150 μl
DMSO, the optical density (OD) at 570 nm was measured using a
microplate reader (Ascent 354; Thermo Labsystems, Waltham, MA,
USA).
Transwell-Matrigel invasion assay
Transwell inserts (Corning) were coated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) at 37°C for 30
min. The cells were suspended in serum-free medium at a final
density of 5×104 cells/ml and seeded to the upper
chambers. Cell medium containing 10% FBS was added to the lower
chambers. After incubation at 37°C for 12 h, non-invaded cells were
removed by a cotton swab. The invaded cells were fixed in 95%
ethanol for 20 min, followed by staining with hematoxylin for 10
min. The number of invaded cells was counted under an inverted
microscope (Nikon).
Statistical analysis
The Student’s t-test was used to analyze differences
between two groups. Data are expressed as the mean ± SD of at least
three independent experiments. P<0.05 was defined as
statistically significant.
Results
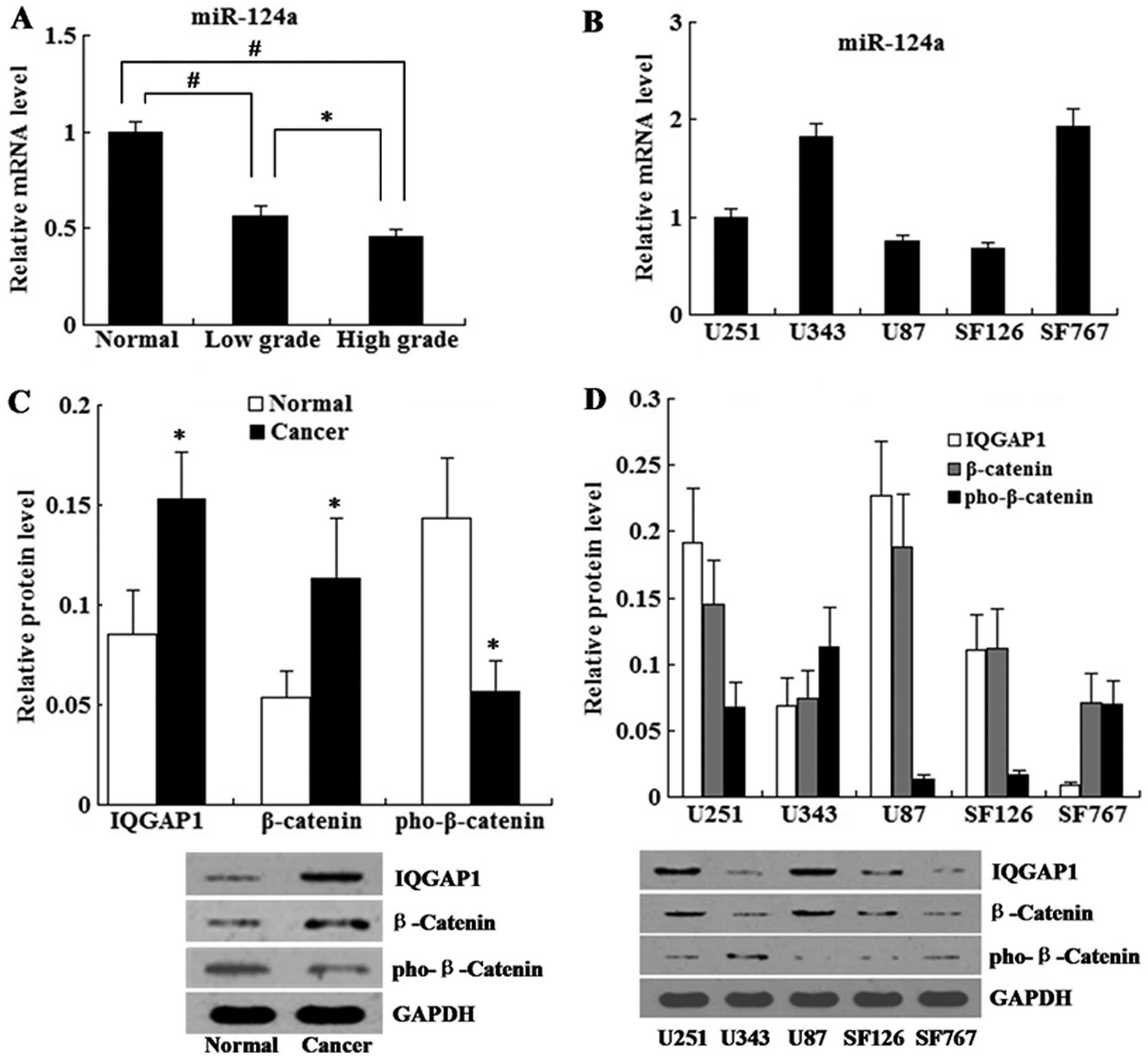
Expression of miR-124a in glioma tissue
samples and glioma cell lines
The expression level of miR-124a in the clinical
tissue specimens was determined by real-time PCR. The clinical
glioma tissue samples were divided into two groups: low grade
gliomas (grades I–II) and high grade gliomas (grades III–IV). As
shown in Fig. 1A, the expression
level of miR-124a in the glioma tissues was decreased when compared
with the level in the normal tissues, and the expression level of
miR-124a in the high grade gliomas was lower than that in the low
grade gliomas.
We detected the expression level of miR-124a in a
series of human glioma cell lines (U251, U343, U87, SF126, SF767).
The results revealed that the expression level of miR-124a was
lowest in the highly malignant glioma cell line U87 (Fig. 1B).
Expression of IQGAP1, β-catenin and
phospho-β-catenin in glioma tissue samples and glioma cell
lines
Expression of IQGAP1, β-catenin and
phospho-β-catenin in the glioma tissue samples was determined by
western blot analysis. The results revealed that the relative
protein levels of IQGAP1 and β-catenin were increased in the glioma
tissues when compared with the levels in the normal tissues.
However, the relative protein level of phospho-β-catenin in the
glioma tissues was lower than that in the normal tissues (Fig. 1C).
In addition, expression levels of IQGAP1, β-catenin
and phospho-β-catenin were examined in a series of human glioma
cell lines. As shown in Fig. 1D,
U87 cells had high levels of IQGAP1 and β-catenin, but a low
expression level of phospho-β-catenin. Based on the low expression
level of miR-124a and high expression level of IQGAP1, we conducted
miR-124a restoration and IQGAP1 knockdown on U87 cells for the
following study in vitro.
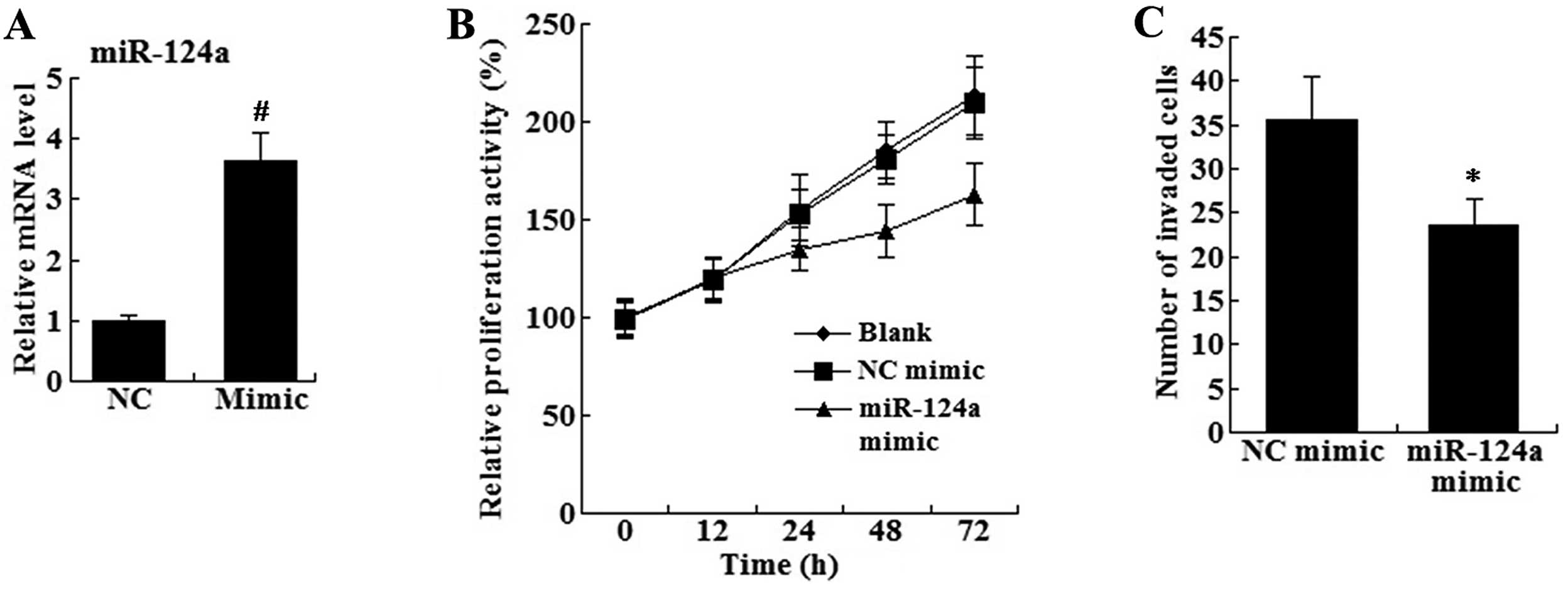
miR-124a restoration inhibits cell
proliferation and invasion
To investigate the effects of miR-124a restoration
on glioma cells, the miR-124a mimic was transfected into U87 cells.
As shown in Fig. 2A, the expression
level of miR-124a was higher in the miR-124a mimic group than that
in the control group.
The proliferation rate of the U87 cells was
determined using the MTT assay. As shown in Fig. 2B, the cells transfected with the
miR-124a mimic proliferated at a significantly lower rate compared
with the blank and NC mimic-transfected cells. In addition, the
cell invasion assay revealed that the number of invaded cells was
significantly decreased in the miR-124a mimic group compared with
the number in the NC mimic group (Fig.
2C).
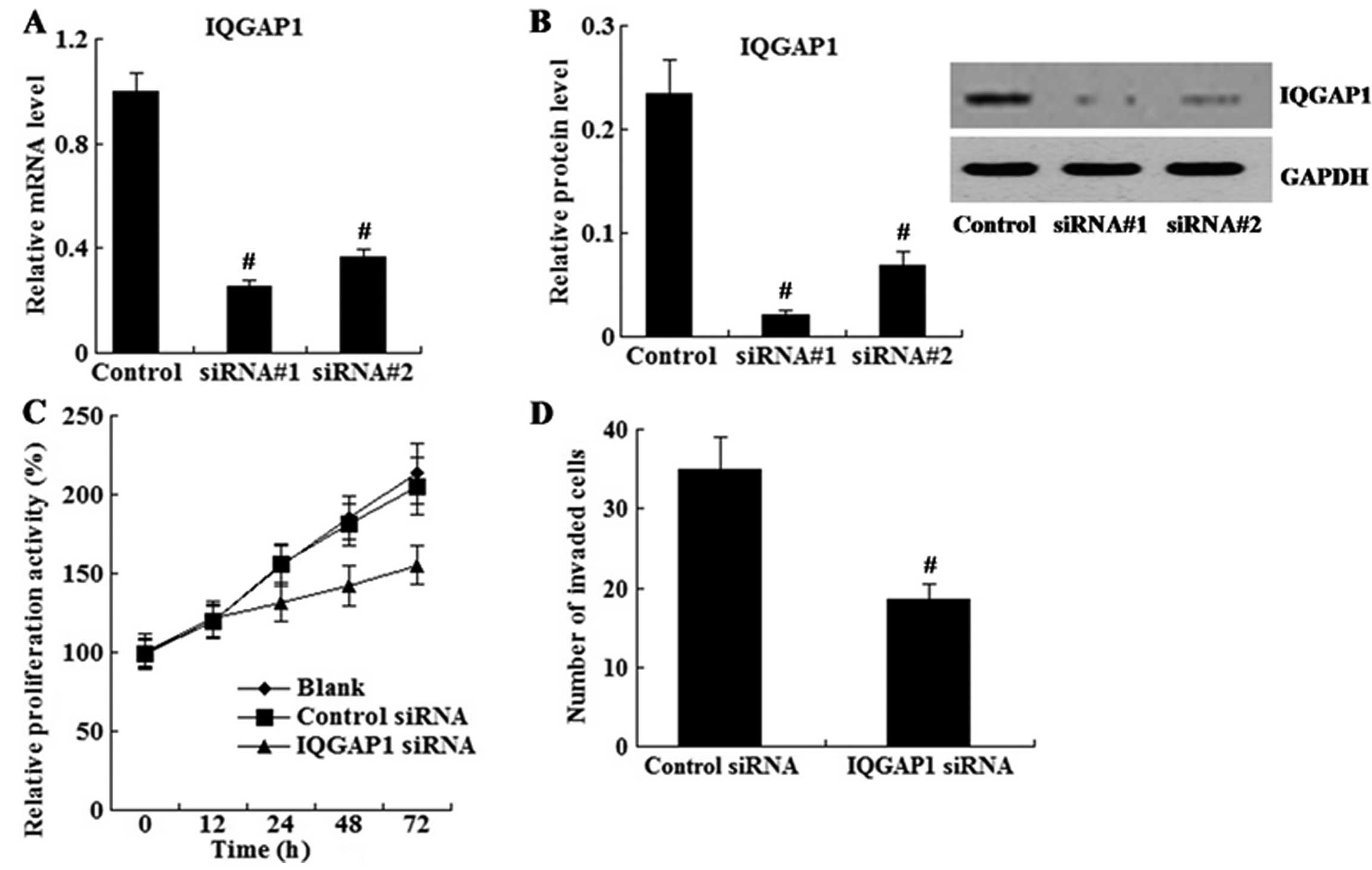
Effect of IQGAP1 knockdown on cell
proliferation and invasion
U87 cells were transfected with siRNA to knock down
IQGAP1 expression. As shown in Fig. 3A
and B, IQGAP1 expression was successfully reduced by siRNAs as
determined using real-time PCR and western blot analyses.
Next, cells transfected with siRNA#1 were subjected
to the MTT and cell invasion assays. As shown in Fig. 3C and D, U87 cells with reduced
IQGAP1 showed decreased cell proliferation and invasion ability in
comparison to the control cells.
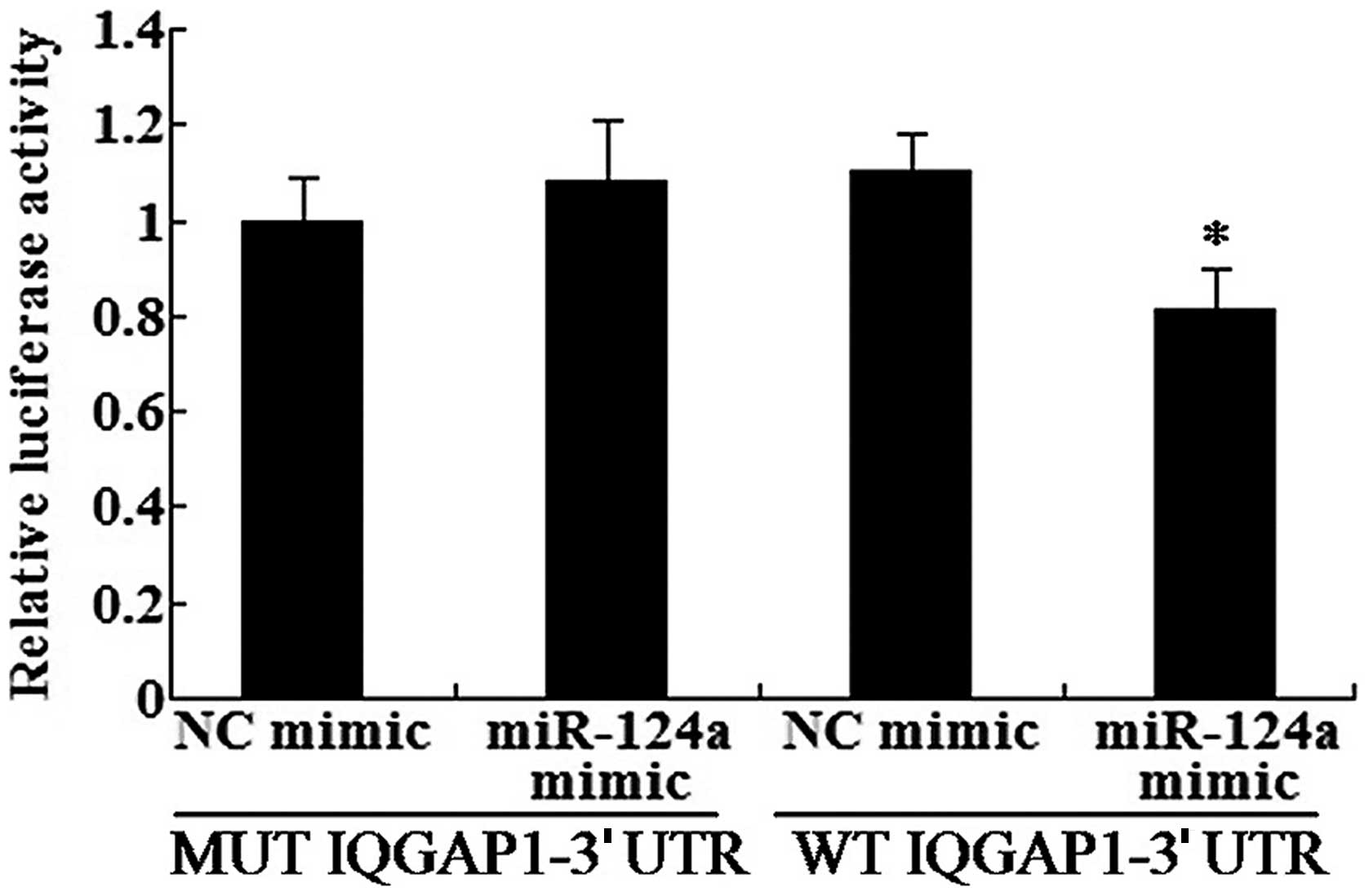
IQGAP1 is a direct target of miR-124a in
the U87 cells
In order to further validate the relationship
between miR-124a and IQGAP1, we carried out the IQGAP1 3′UTR
reporter assay in U87 cells. The results revealed that luciferase
activity was significantly decreased in the U87 cells transfected
with the miR-124a mimic and wild-type IQGAP1-3′UTR, but no
reduction was observed in the mutant IQGAP1-3′UTR (Fig. 4). The results demonstrated that
IQGAP1 is a direct target of miR-124a.
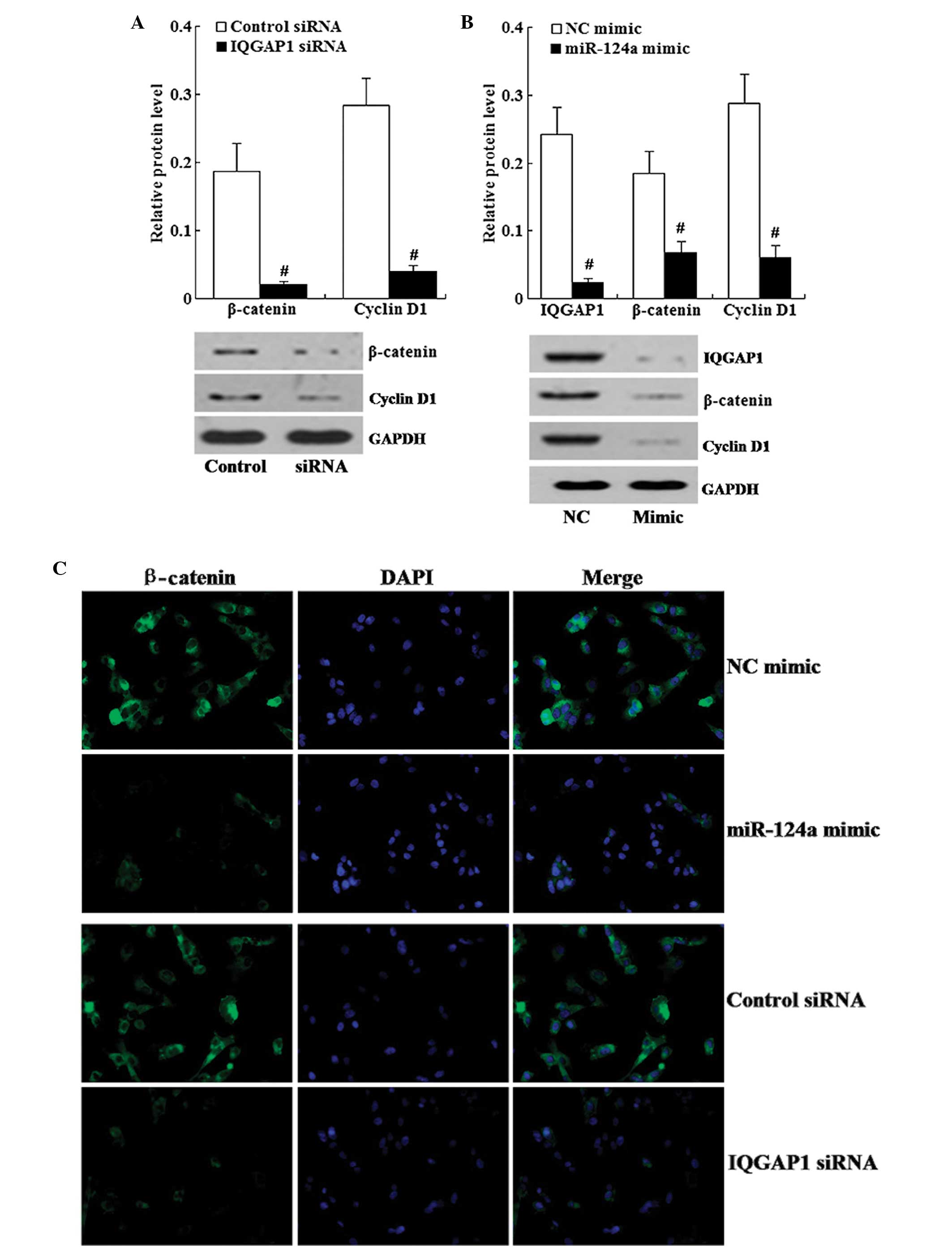
Effect of IQGAP1 knockdown on the
expression of β-catenin and cyclin D1
We tested whether IQGAP1 knockdown could affect the
expression of β-catenin and cyclin D1. As shown in Fig. 5A, western blot analysis revealed
that IQGAP1 knockdown resulted in decreased expression of β-catenin
and cyclin D1.
In addition, immunofluorescence staining showed that
in the IQGAP1 siRNA group, β-catenin was localized in the
cytoplasm, and the staining of β-catenin was weaker in the IQGAP1
knockdown cells compared with the staining in the control cells
(Fig. 5C).
miR-124a restoration downregulates the
expression of IQGAP1, β-catenin and cyclin D1
We examined the effects of miR-124a restoration on
the expression of IQGAP1, β-catenin and cyclin D1. Western blot
analysis demonstrated that miR-124a restoration led to decreased
expression of IQGAP1, β-catenin and cyclin D1 (Fig. 5B).
As shown in Fig. 5C,
immunofluorescence staining of β-catenin revealed that compared
with the miR-124a NC-transfected cells, β-catenin was localized in
the cytoplasm and the staining of β-catenin was weaker in the
miR-124a mimic-transfected cells.
Discussion
MicroRNAs (miRNAs) are a class of ~22 nt long
non-coding RNAs which regulate target mRNAs by interacting with the
3′UTR (8). They play important
roles in numerous biological processes, such as proliferation,
differentiation, development, immunology and cell death (9–14). An
increasing number of studies have revealed that aberrant miRNA
expression is related to cancer biology, acting as either tumor
suppressors or oncogenes (4,5,15).
microRNA-124 (miR-124) has been classified as a
tumor suppressor in several types of human cancers (16–18).
Previous research has shown that miR-124 is abundantly expressed in
normal brain tissue (6), is
involved in embryonic neuronal differentiation (19), and is an important regulator of
adult neurogenesis in the subventricular zone stem cell niche
(20). The present study
demonstrated that miR-124a expression is downregulated in human
glioma tissues and its expression level is negatively correlated
with the pathological grade of glioma. Furthermore, the in
vitro study revealed that miR-124a restoration inhibited glioma
cell proliferation and invasion. This indicates that miR-124a acts
as a tumor suppressor in gliomas.
IQ motif containing GTPase activating protein 1
(IQGAP1) is a member of the IQGAP family. It regulates actin
dynamics and cell motility (21,22) by
interacting with cytoskeleton components, cell adhesion molecules
and several signaling molecules (23). IQGAP1 is important for normal
cellular function and homeostasis. Amplification and overexpression
of IQGAP1 were reported to be associated with certain malignancies
(24–28). IQGAP1 localizes to sites of
cell-cell contact (29) and
regulates cell-cell adhesion via interacting with E-cadherin and
β-catenin. β-catenin is the central denominator of the Wnt pathway
and plays roles in various types of cancer (30). Cytoplasmic β-catenin is associated
with APC and Axin and forms a destruction complex on which
β-catenin is phosphorylated by the kinases CK1α and GSK3β, leading
to β-catenin degradation by the ubiquitin-proteasome mechanism.
Disruption of β-catenin degradation inhibits β-catenin
phosphorylation and leads to the translocation of β-catenin into
nuclei, where it activates an array of target genes and cell cycle
regulators such as c-myc and cyclin D1 (31,32).
Given the important role of β-catenin in cancer, it is of
particular interest to identify regulators that may interfere with
β-catenin signaling and thereby lead to cancer development, in
particular those miRNAs that can simultaneous interact with
multiple regulators of the β-catenin pathway. In the present study,
examination of clinical samples of gliomas showed that IQGAP1 and
β-catenin were upregulated in the glioma tissues, while
phospho-β-catenin was downregulated in the glioma tissues.
Furthermore, the in vitro study revealed that IQGAP1
knockdown led to decreased cell proliferation and invasion. Next,
molecular mechanisms underlying glioma cell proliferation and
invasion were studied. RNA interference assay showed that IQGAP1
regulated the expression of β-catenin in glioma cells. IQGAP1
knockdown resulted in the downregulation of β-catenin and cyclin
D1. Luciferase assay demonstrated that IQGAP1 is a direct target of
miR-124a. Overall, the present study demonstrated that miR-124a
inhibits cell proliferation and invasion in glioma cells by
targeting IQGAP1, consequently suppressing β-catenin and downstream
cyclin D1.
Our study uncovered a novel molecular mechanism
underlying glioma cell proliferation and invasion and may provide a
useful molecular therapy for gliomas.
References
|
1
|
Dolecek TA, Propp JM, Stroup NE and
Kruchko C: CBTRUS statistical report: primary brain and central
nervous system tumors diagnosed in the United States in 2005–2009.
Neuro Oncol. 14(Suppl 5): v1–v49. 2012.PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, et al:
The 2007 WHO classification of tumours of the central nervous
system. Acta Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
5
|
Hammond SM: MicroRNAs as oncogenes. Curr
Opin Genet Dev. 16:4–9. 2006. View Article : Google Scholar
|
|
6
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
An L, Liu Y, Wu A and Guan Y: microRNA-124
inhibits migration and invasion by down-regulating ROCK1 in glioma.
PLoS One. 8:e694782013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Q, Bian S, Hong J, et al: Timing
specific requirement of microRNA function is essential for
embryonic and postnatal hippocampal development. PLoS One.
6:e260002011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CZ, Li L, Lodish HF and Bartel DP:
MicroRNAs modulate hematopoietic lineage differentiation. Science.
303:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brennecke J, Hipfner DR, Stark A, Russell
RB and Cohen SM: bantam encodes a developmentally regulated
microRNA that controls cell proliferation and regulates the
proapoptotic gene hid in Drosophila. Cell. 113:25–36.
2003. View Article : Google Scholar
|
|
12
|
Chen CH, Guo M and Hay BA: Identifying
microRNA regulators of cell death in Drosophila. Methods Mol
Biol. 342:229–240. 2006.PubMed/NCBI
|
|
13
|
Fu LL, Wen X, Bao JK and Liu B:
MicroRNA-modulated autophagic signaling networks in cancer. Int J
Biochem Cell Biol. 44:733–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Medina PP and Slack FJ: microRNAs and
cancer: an overview. Cell Cycle. 7:2485–2492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia J, Wu Z, Yu C, et al: miR-124 inhibits
cell proliferation in gastric cancer through down-regulation of
SPHK1. J Pathol. 227:470–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lang Q and Ling C: MiR-124 suppresses cell
proliferation in hepatocellular carcinoma by targeting PIK3CA.
Biochem Biophys Res Commun. 426:247–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lv XB, Jiao Y, Qing Y, et al: miR-124
suppresses multiple steps of breast cancer metastasis by targeting
a cohort of pro-metastatic genes in vitro. Chin J Cancer.
30:821–830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao X, Pfaff SL and Gage FH: A functional
study of miR-124 in the developing neural tube. Genes Dev.
21:531–536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng LC, Pastrana E, Tavazoie M and
Doetsch F: miR-124 regulates adult neurogenesis in the
subventricular zone stem cell niche. Nat Neurosci. 12:399–408.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Watanabe T, Wang S, Noritake J, et al:
Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin
filaments during cell polarization and migration. Dev Cell.
7:871–883. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mataraza JM, Briggs MW, Li Z, Entwistle A,
Ridley AJ and Sacks DB: IQGAP1 promotes cell motility and invasion.
J Biol Chem. 278:41237–41245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nabeshima K, Shimao Y, Inoue T and Koono
M: Immunohistochemical analysis of IQGAP1 expression in human
colorectal carcinomas: its overexpression in carcinomas and
association with invasion fronts. Cancer Lett. 176:101–109. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong P, Nabeshima K, Nishimura N, Kawakami
T, Hachisuga T, Kawarabayashi T and Iwasaki H: Overexpression and
diffuse expression pattern of IQGAP1 at invasion fronts are
independent prognostic parameters in ovarian carcinomas. Cancer
Lett. 243:120–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Balenci L, Clarke ID, Dirks PB, et al:
IQGAP1 protein specifies amplifying cancer cells in glioblastoma
multiforme. Cancer Res. 66:9074–9082. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson M, Sharma M and Henderson BR:
IQGAP1 regulation and roles in cancer. Cell Signal. 21:1471–1478.
2009. View Article : Google Scholar
|
|
28
|
Nakamura H, Fujita K, Nakagawa H, Kishi F,
Takeuchi A, Aute I and Kato H: Expression pattern of the scaffold
protein IQGAP1 in lung cancer. Oncol Rep. 13:427–431.
2005.PubMed/NCBI
|
|
29
|
Kuroda S, Fukata M, Nakagawa M, et al:
Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in
regulation of E-cadherin-mediated cell-cell adhesion. Science.
281:832–835. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.
|
|
31
|
Breuhahn K, Longerich T and Schirmacher P:
Dysregulation of growth factor signaling in human hepatocellular
carcinoma. Oncogene. 25:3787–3800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee HC, Kim M and Wands JR: Wnt/Frizzled
signaling in hepatocellular carcinoma. Front Biosci. 11:1901–1915.
2006. View Article : Google Scholar : PubMed/NCBI
|