Introduction
MicroRNAs (miRNAs) are small non-coding regulatory
RNA molecules, ~22 nucleotides in length, which suppress gene
expression based on post-transcriptional suppression of target
mRNAs by affecting mRNA translation or stability (1). It has been widely demonstrated that
mRNAs are involved in the regulation of many cellular processes,
such as cell differentiation, proliferation and apoptosis (2,3).
Recently, increasing evidence indicates that miRNAs are closely
associated with cancer, and aberrant expression of miRNAs is often
reported in cancers (4,5). miRNAs can function as oncogenes or
tumor suppressors, and play significant roles in tumorigenesis
(6,7). Those miRNAs, such as miR-21 and
miR-155, whose expression levels tend to be upregulated in tumors,
may be considered as oncogenes (8,9).
Meanwhile, some miRNAs, such as miR-143, miR-126 and miR-138, whose
expression levels are usually downregulated in tumors, may be
considered as tumor suppressors (10–12).
These data indicate that miRNAs may be promising targets for tumor
treatment.
Osteosarcoma is one of the most common primary
malignant bone tumors and accounts for approximately 60% of all
malignant bone tumors in childhood and adolescence (13). It is characterized by a high
potential of lung metastasis (14),
and for patients who develop lung metastasis, the survival rate is
less than 30% (15). Therefore, to
achieve a better prognosis, it is essential to identify the
molecular mechanisms of osteosarcoma metastasis and to identify new
therapeutic targets. Recent studies have confirmed the altered
expression profile of miRNAs in osteosarcoma (16). Dysregulation of miRNAs, such as
miR-183, miR-34a, miR-145 and miR-143, has been shown to be
involved in the migration and invasion of osteosarcoma cells
(17–20), which undoubtedly highlights the
significance of miRNAs in osteosarcoma development and may reveal
new insights into the mechanisms of metastasis.
MicroRNA-101 (miR-101) is a tumor-suppressor miRNA.
It has been proven that miR-101 is downregulated in various types
of cancer cells including lung cancer, gastric cancer, breast
cancer and glioblastoma, and functions as a suppressor of cell
migration and invasion (21–24).
However, little is known concerning the functions of miR-101 in
osteosarcoma. Therefore, it is of great importance to study the
biological role and mechanism of miR-101 in osteosarcoma
metastasis.
In the present study, we found that miR-101
expression in high-metastatic human osteosarcoma F5M2 cells was
lower than that in low-metastatic human osteosarcoma F4 cells.
Furthermore, ectopic overexpression of miR-101 inhibited migration
and invasion of osteosarcoma cells by downregulating the expression
level of EZH2, which was closely correlated with tumor metastatic
potential (25–27). Overall, miR-101 may play a
suppressive role in osteosarcoma metastasis through EZH2
downregulation, indicating that it may be a novel promising
therapeutic target for osteosarcoma.
Materials and methods
Cell culture
The human osteosarcoma F5M2 and F4 sublines, which
are respectively high-metastatic and low-metastatic cell lines and
originate from the same human osteosarcoma SOSP-9607 cell line,
were established and reserved by our laboratory (28). F5M2 and F4 cells were maintained in
RPMI-1640 medium (HyClone, USA) supplemented with 10% fetal bovine
serum (FBS) (Sijiqing Co., China) and incubated at 37°C with 5%
CO2. The human osteosarcoma MG63 cell line was
maintained under the same conditions except that MEM was used.
Transfection
Cells were transfected with miR-101 mimics and the
NC mimics (negative control) (GenePharma Co., China) at a
concentration of 100 nM, using Lipofectamine™ 2000 reagent
(Invitrogen Life Technologies, USA) according to the manufacturer’s
instructions. Efficiency of miR-101 transfection was detected by
quantitative real-time PCR (qRT-PCR).
Quantitative real-time PCR analysis
Total RNA was isolated from cell lines with TRIzol
reagent (Invitrogen Life Technologies) following the manufacturer’s
protocol. The miR-specific primers (Sangon Biotech Co., China) were
designed for miR-101 qRT-PCR. A miR-specific primer
(5′-ctcaactggtgtcgtggagtcggcaattcagttgagttcagttat-3′) was used for
reverse transcription. A pair of miR-specific primers (forward,
5′-acactccagctgggtacagtactgtgata-3′ and reverse,
5′-tggtgtcgtggagtcg-3′) were used on the ABI-Prism 7500 real-time
PCR system (Applied Bioscience, USA) for amplification, compared
with the normalization control U6 snRNA. A pair of primers
(forward, 5′-gccagactgggaagaaatctg-3′ and reverse,
5′-tgtgctggaaaatccaagtca-3′) were designed for EZH2 qRT-PCR. To
normalize the expression level of EZH2, GAPDH was used as an
internal control. The relative expression level of mRNA was
analyzed using the comparative 2−ΔΔCt method.
Migration and invasion assays
A total of 1×106 cells in low-serum media
containing 1% FBS was added to the upper chamber of a Transwell
system (24-well insert; pore size, 8 μm; Corning, USA). Six hundred
microliters of complete media containing 20% FBS was added to the
lower chamber. In the migration assay, cells that migrated to the
outer surface of the membrane were fixed with 95% ethanol and
stained with crystal violet after 24 h of incubation. In the
invasion assay, the upper chamber was precoated with 50 μl of
Matrigel (1:8 dilution; BD Biosciences, USA). After 48 h
incubation, the invaded cells were fixed and stained as described
above. The cell numbers in 5 random fields were counted for each
insert under a microscope (magnification, ×200).
Wound healing assay
Adherent cell monolayers in 6-well plates were
scratched with a 200-μl pipette tip. The cells were then cultured
in complete culture media at 37°C with 5% CO2. Wound
healing ability was observed under microscopy after 0, 24 and 48 h
(magnification, ×200).
MTT assay
Cell proliferation capacity was detected using the
standard MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide] procedure, which was performed in 96-well plates. In
brief, at 48 h after transfection, F5M2 cells were harvested and
seeded at a density of 4,000 per well with 200 μl complete culture
medium. Each group contained 8 wells, and the wells without cells
were used as blanks. Cell proliferation was documented every 24 h
for 4 days; 20 μl of 5 g/l MTT (Sigma, USA) was added into each
well. After 4 h of incubation at 37°C, the media were removed and
150 μl dimethyl sulfoxide (DMSO; Sigma) was added into each well.
The optical density (OD) was evaluated according to the absorbance
value detected at 490 nm with a reference of 630 nm.
Western blot analysis
The western blot assay was carried out to detect the
protein expression level of EZH2 following the standard method and
this assay was performed with the EZH2 rabbit monoclonal antibody
and the anti-β-actin mouse monoclonal antibody (Abcam, UK).
Statistical analysis
All experiments were performed at least in
triplicate. The results are expressed as the mean values ± SD and
performed using SPSS 19.0 software. The Student’s t-test was
performed to determine the differences between groups, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
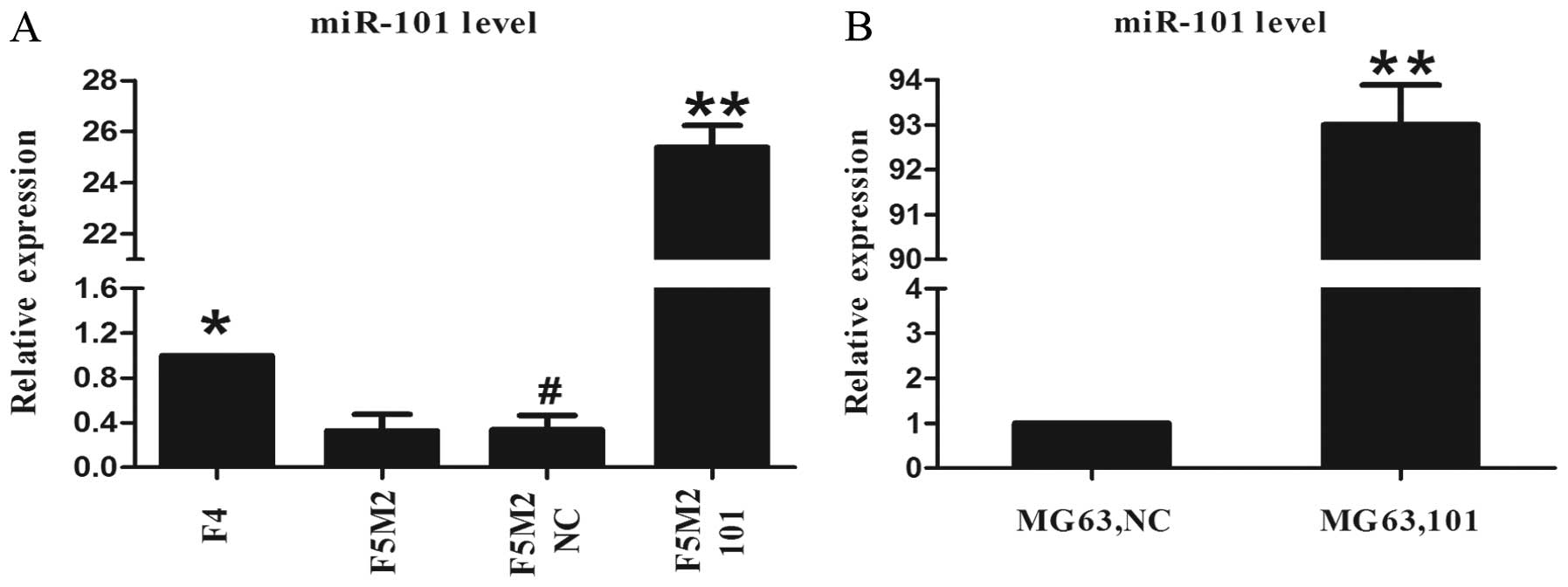
Expression of miR-101 in the F5M2 cells
is lower than that in the F4 cells
To investigate the potential role of miR-101 in
osteosarcoma metastasis, qRT-PCR analysis was carried out to
compare miR-101 expression between the F5M2 and F4 cell lines.
miR-101 expression in the F5M2 cells with high-metastatic potential
was lower than that in the F4 cells with low-metastatic potential.
The difference was statistically significant (P<0.05, Fig. 1A).
Osteosarcoma cells transfected with
miR-101 mimics show significant overexpression of miR-101
SOSP-9607 and MG63 cells were separately transfected
with the miR-101 mimics at a concentration of 100 nM. qRT-PCR
analysis was performed to detect the expression level of miR-101 at
48 h after transfection. The expression level of miR-101 was
significantly upregulated in both the F5M2 and MG63 cells after
treatment with the miR-101 mimics, compared with the F5M2 or MG63
cells treated with the NC mimics, respectively (P<0.01, Fig. 1A and B). Meanwhile, there was no
significant difference in miR-101 expression between the F5M2 cells
treated with the NC mimics and untreated cells (Fig. 1A).
miR-101 significantly inhibits the
migratory and invasive capacities of osteosarcoma cells
We used the Transwell assay to measure the migratory
and invasive capacities of SOSP-9607 and MG63 cells. The results
showed that osteosarcoma cells treated with the miR-101 mimics
displayed significantly lower Transwell migration capacity,
compared with the cells untreated or treated with the NC mimics
(P<0.01, Fig. 2A and E). In the
invasion assay, ectopic expression of miR-101 led to significantly
decreased invasion of the osteosarcoma cells (P<0.01, Fig. 2B and F). These results indicate a
functional role for miR-101 in downregulating the migration and
invasion of osteosarcoma cells.
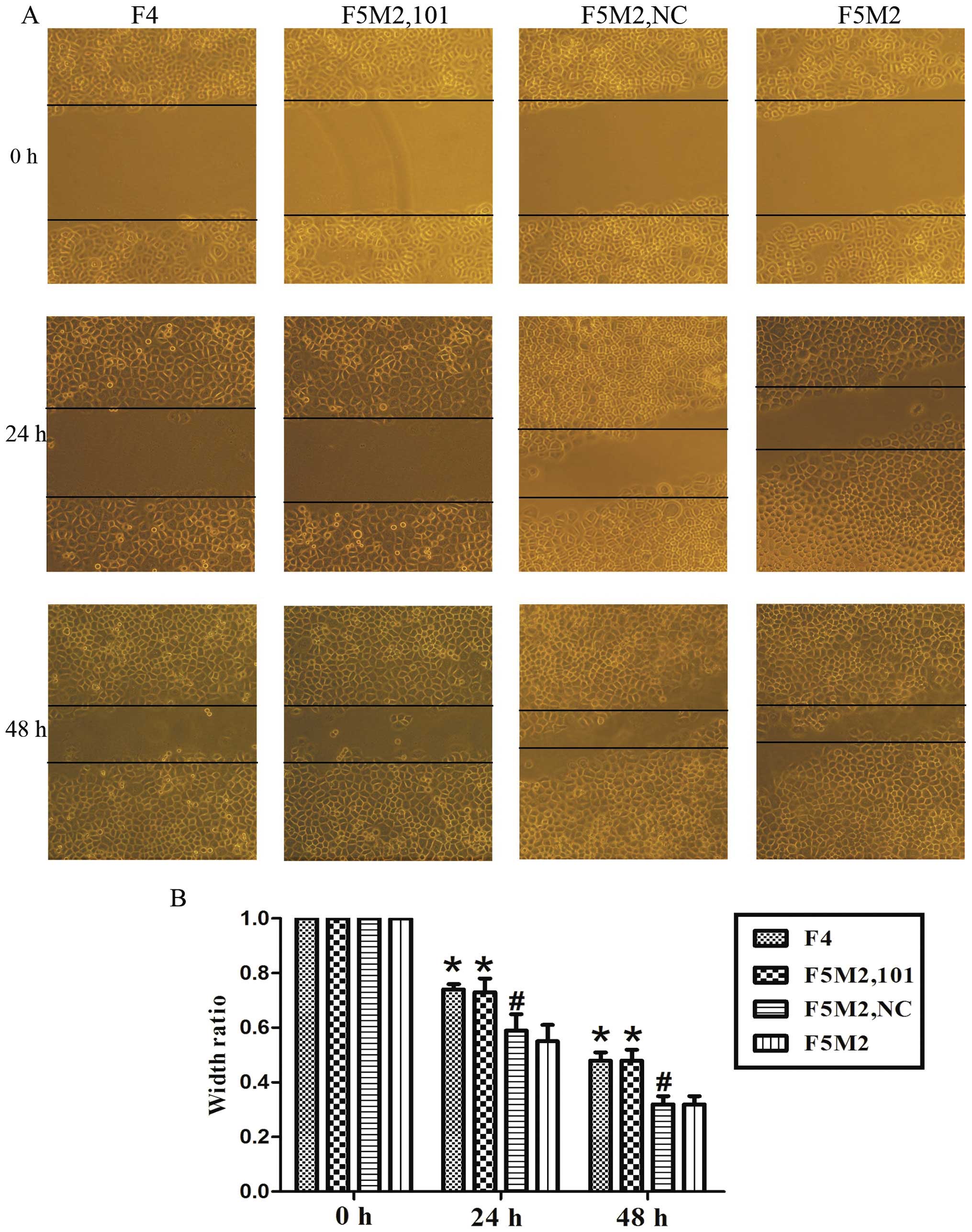
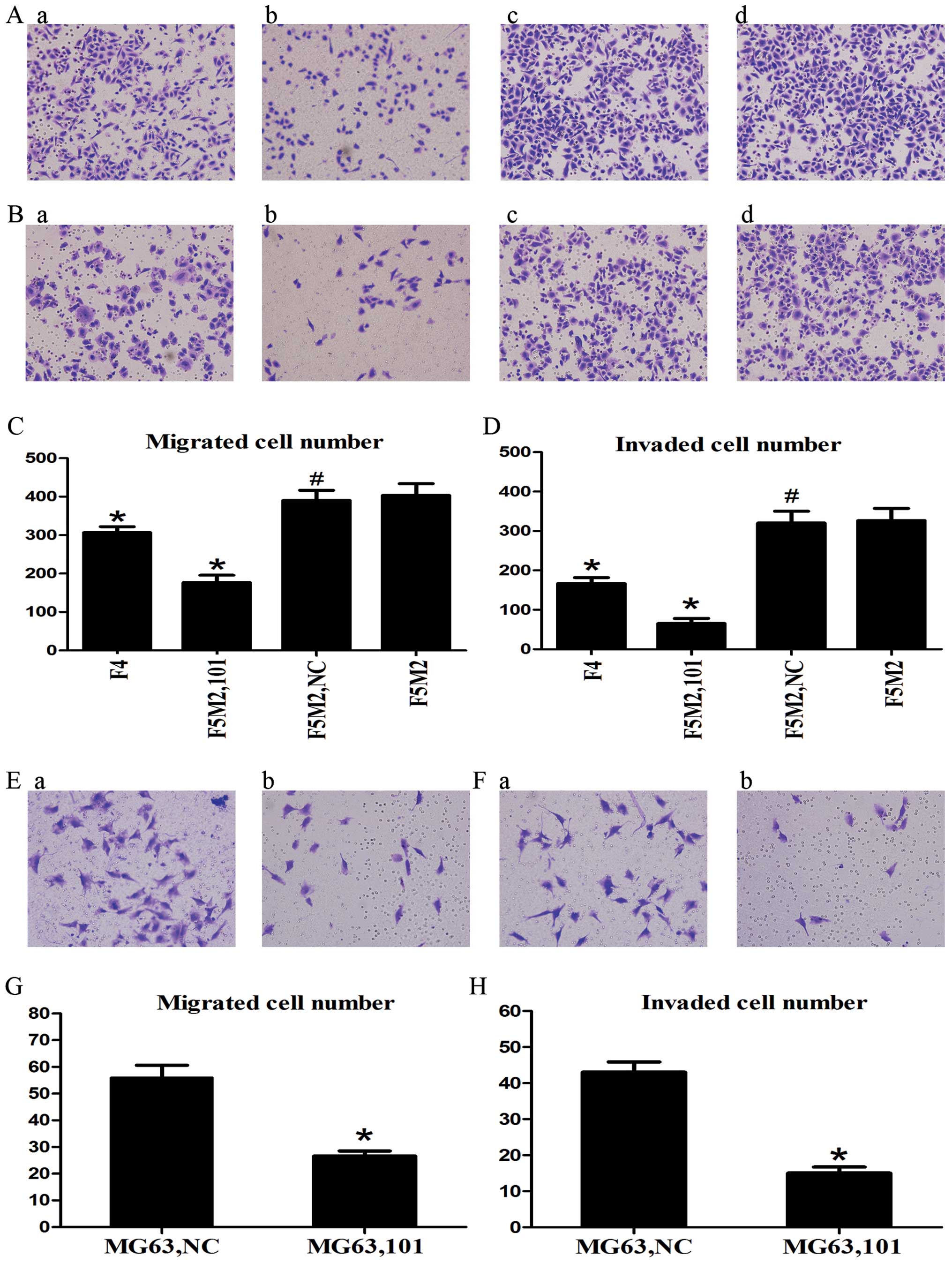 | Figure 2miR-101 inhibits migration and
invasion of osteosarcoma cells in vitro. (A and B)
Representative images of migrated and invaded SOSP-9607 cells under
the microscope (magnification, ×200). a, F4; b, F5M2 with miR-101;
c, F5M2 with NC; d, F5M2. (C and D) F4 and F5M2 cells following
transfection with miR-101 mimics (F5M2,101) showed less migratory
and invasive ability than non-transfected F5M2 cells. (E and F)
Representative images of migrated and invaded MG63 cells under a
microscope (magnification, ×200). a, MG63 with NC; b, MG63 with
miR-101. (G and H) MG63 cells following transfection with miR-101
mimics (MG63,101) showed less migratory and invasive ability than
cells transfected with NC (MG63,NC). *P<0.01,
compared with F5M2 or MG63 with NC; #P>0.05, compared
with F5M2. |
miR-101 significantly inhibits the wound
healing ability of osteosarcoma cells
We performed a wound healing assay and found that
F5M2 cells closed the wounds faster than F4 cells (P<0.01,
Fig. 3). Meanwhile, the migratory
potential of the F5M2 cells treated with miR-101 mimics was
significantly decreased when compared with that of the cells
untreated or treated with the NC mimics (P<0.01, Fig. 3).
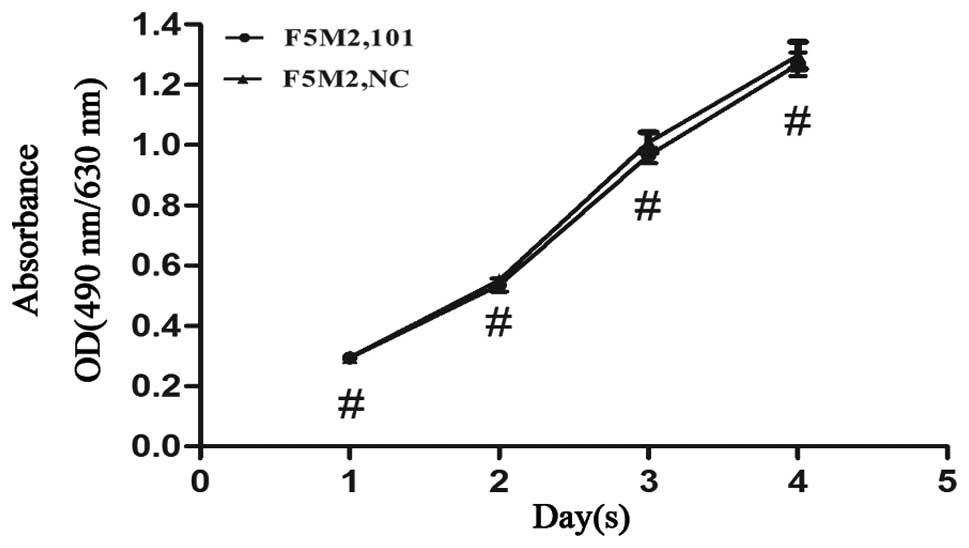
miR-101 does not affect the proliferation
of osteosarcoma cells
MTT assay was conducted to detect the potential role
of miR-101 in the proliferation of osteosarcoma cells. The results
indicated that there was no statistically significant difference
between the F5M2 cells transfected with the miR-101 mimics and the
F5M2 cells transfected with the NC mimics. Therefore, miR-101 had
little effect on the proliferation of osteosarcoma cells
(P>0.05, Fig. 4).
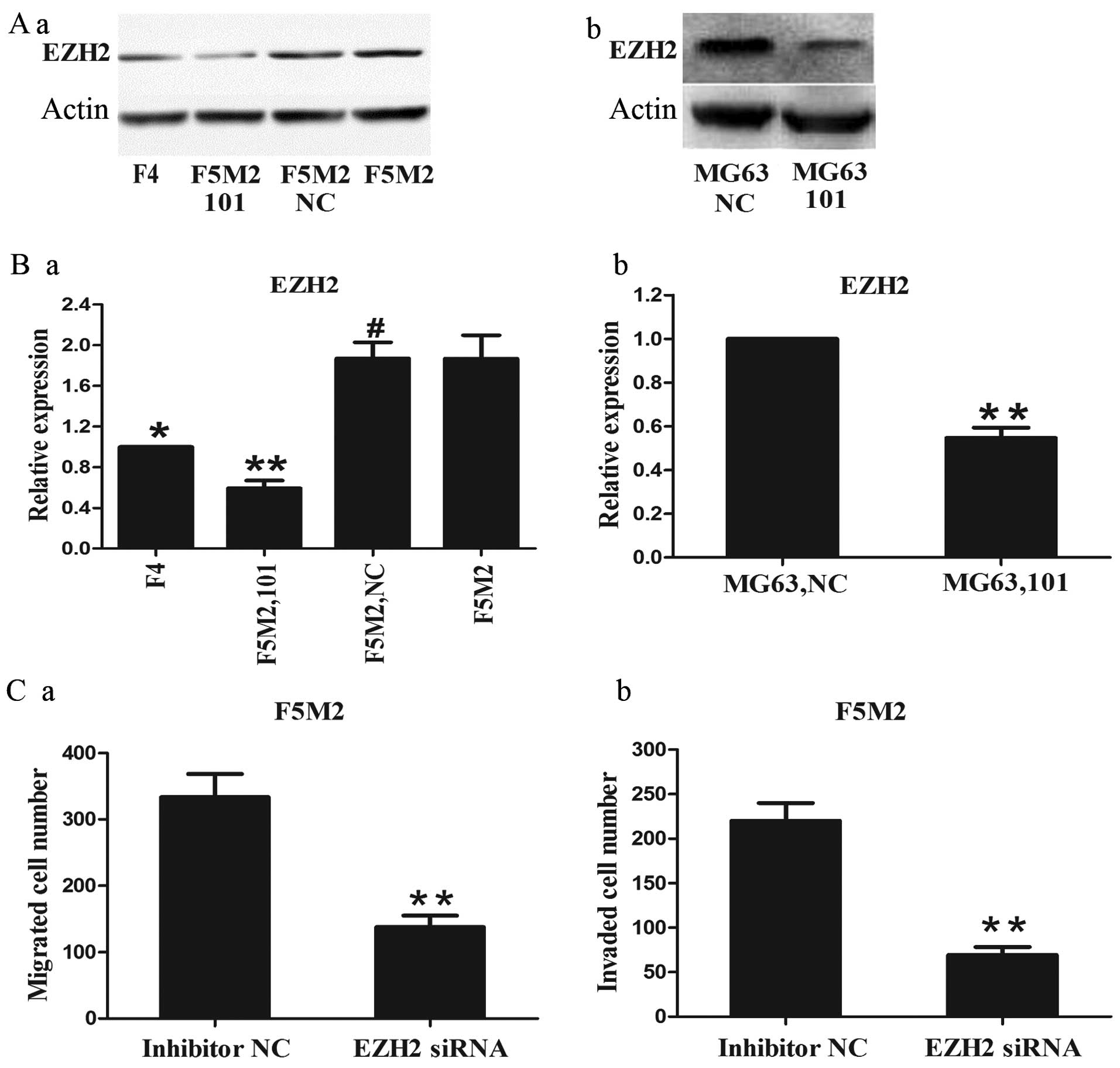
EZH2 is significantly downregulated by
miR-101 and regulates the migration and invasion of osteosarcoma
cells
To analyze the expression of EZH2 in osteosarcoma,
western blotting was performed on SOSP-9607 and MG63 cells. The
results showed that the protein expression level of EZH2 in the
F5M2 cells was higher than that in the F4 cells (Fig. 5A-a). Moreover, EZH2 expression was
downregulated in the F5M2 cells treated with the miR-101 mimics
compared to cells treated with the NC mimics or the untreated cells
(Fig. 5A-a). MG63 cells treated
with the miR-101 mimics also exhibited a lower expression level of
EZH2 than cells treated with the NC mimics (Fig. 5A-b). In addition, qRT-PCR analysis
displayed the same tendency at the mRNA expression level in both
osteosarcoma cell lines (Fig. 5B).
Then as determined in the Transwell assay, EZH2 siRNA significantly
inhibited the migration and invasion of osteosarcoma F5M2 cells
(P<0.01, Fig. 5C). These results
together reveal a negative correlation between miR-101 and EZH2 in
osteosarcoma, and knockdown of EZH2 by siRNA inhibits the migration
and invasion of osteosarcoma cells.
Discussion
In recent years, it has been widely demonstrated
that miRNAs play profound roles in cancer pathogenesis and are
implicated in numerous biological processes of a wide range of
cancers, including metastasis. Aggressive tumor cell metastasis is
a key step during cancer progression, and it contributes to
secondary tumor formation at distant sites. Studies have reported
that several miRNAs, such as miR-126, miR-335 and miR-145, are
metastasis suppressors (29,30).
These data bring new insights into novel treatments of
high-metastatic tumors including osteosarcoma, in which pulmonary
metastasis is the leading cause of death (31).
Accumulating evidence indicates that miR-101 is
significantly underexpressed and functions as a tumor suppressor in
the migration and invasion of various types of cancers, such as
lung cancer, gastric cancer, breast cancer and glioblastoma
(19–22). However, little is known concerning
the role of miR-101 in osteosarcoma. To investigate the potential
role of miR-101 in osteosarcoma metastasis, we compared the
expression levels of miR-101 in F5M2 and F4 cells, which are
respectively high-metastatic and low-metastatic sublines of the
human osteosarcoma cell line SOSP-9607 (26). Moreover, wound healing and Transwell
assays were performed to assess the suppressive role of miR-101 in
the migration and invasion of SOSP-9607 and MG63 cells. As
expected, the F4 cells expressed a higher level of miR-101 than
that in the F5M2 cells (Fig. 1A).
Furthermore, miR-101 was significantly upregulated after
transfection with the miR-101 mimics in both the SOSP-9607 and
MG-63 cell lines (Fig. 1), and
ectopic miR-101 significantly inhibited the migratory and invasive
abilities of the osteosarcoma cells (Figs. 2 and 3). On the basis of these results, this
study demonstrated the tumor-suppressive role of miR-101 in
osteosarcoma metastasis for the first time, and miR-101 might be
proven to be a promising therapeutic target in osteosarcoma.
It has been shown that miR-101 has multiple targets
through which it has regulatory roles in the biological behaviors
of various cancer cells. A previous study by Cho et al
(21) showed that overexpression of
miR-101 inhibited the invasive ability of lung cancer cells through
suppression of EZH2. Furthermore, Wang et al (22) demonstrated that miR-101 expression
in gastric tumor tissues and cells was higher than that in
non-tumor gastric tissues and cells, and exogenous miR-101
inhibited the proliferation, migration and invasion of gastric
cancer cells by downregulating the expression of EZH2, COX-2, Mcl-1
and FOS.
Predicting and identifying miR-101 target genes is
important to further study the regulatory role of miR-101 in
tumorigenesis and can offer a potential new therapeutic strategy
for osteosarcoma. To investigate the suppressive mechanism of
miR-101 in osteosarcoma metastasis, several prediction programs
were used to seek the potential targets of miR-101. Markedly, the
programs (including Target Scan, Pic Tar, Micro Inspector and Mir
Target 2) predicted that EZH2 was one of the targets of
miR-101.
Enhancer of zeste homlog 2 (EZH2) functions in a
protein complex called the polycomb repressive complex 2 (PRC2),
which is involved in the trimethylation of histone3 lysine27
(H3K27) and may lead to epigenetic silencing of target genes
implicated in many cellular processes, including cell cycle
regulation, cell differentiation and tumorigenesis (32). Enforced expression of EZH2 was
initially found in prostate cancer, and EZH2 overexpression is
closely correlated to aggressive and metastatic diseases (33). Subsequently, EZH2 was also found to
be broadly overexpressed in a wide range of cancer types, such as
breast cancer, bladder cancer, colon cancer, lymphomas and
osteosarcoma (34–39). EZH2 has properties of oncogenes, as
its overexpression has been correlated with the metastatic
potential of several aggressive tumors (40–42).
As for the effect of EZH2 in osteosarcoma, it has been reported
that osteosarcoma patient biopsy specimens have higher expression
levels of EZH2 than normal bone tissues, and overexpression of EZH2
was found in osteosarcoma cells by immunohistochemical assay.
However, the aberrant expression of EZH2 is not correlated to
osteosarcoma growth in vivo and in vitro (37). In addition, miR-26a has been
reported to inhibit cell migration and invasion by targeting the
EZH2 gene (43).
miR-101/EZH2 were reported to be deregulated in
several types of cancer, including renal cancer (44), prostate cancer (45), gastric cancer (22), invasive squamous cell carcinoma
(46), glioblastoma (24) and bladder transitional cell
carcinoma (47), and were found to
be significantly correlated with migration, invasion, and
metastasis (22,24,45).
In our study, we evaluated whether ectopic
expression of miR-101 could inhibit the metastasis of osteosarcoma
by suppressing the expression level of EZH2 in osteosarcoma cell
lines. We revealed that substantial EZH2 suppression by miR-101 was
detected at the mRNA and protein levels in SOSP-9607 and MG63 cells
(Fig. 5A and B). In addition,
knockdown of EZH2 by siRNA showed the same tendency as the effect
of miR-101 on migration and invasion (Fig. 5C). We also revealed that
overexpression of miR-101 had little role in cell proliferation
(Fig. 4), which was consistent with
the role of EZH2 in osteosarcoma cells (37). Taken together, we showed that EZH2
expression was inversely correlated with miR-101, and the
expression level of EZH2 was positively correlated with the
capacity of cellular migration and invasion. It is reasonable to
conclude that miR-101 inhibits the migration and invasion of
osteosarcoma cells by downregulating EZH2 expression.
In conclusion, this is the first in vitro
study to provide new insights into the role of miR-101 in
osteosarcoma. It shows that by downregulating the EZH2 expression
level, miR-101 plays a suppressive role in cellular migration and
invasion of osteosarcoma. Further studies in vivo are being
conducted, and miR-101 may be a promising candidate prognostic
biomarker and gene therapeutic agent for osteosarcoma
treatment.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (no. 81072194).
Abbreviations:
|
miRNAs
|
microRNAs
|
|
EZH2
|
enhancer of zeste homolog 2
|
|
miR-101
|
microRNA-101
|
|
FBS
|
fetal bovine serum
|
|
NC
|
negative control
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
DMSO
|
dimethyl sulfoxide
|
|
OD
|
optical density
|
|
PRC2
|
polycomb repressive complex 2
|
|
H3K27
|
histone3 lysine27
|
References
|
1
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peters L and Meister G: Argonaute
proteins: mediators of RNA silencing. Mol Cell. 26:611–623. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar
|
|
5
|
Chen PS, Su JL and Hung MC: Dysregulation
of microRNAs in cancer. J Biomed Sci. 19:902012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
7
|
Hammond SM: MicroRNAs as tumor
suppressors. Nat Genet. 39:582–583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin X, Yan L, Zhao X, Li C and Fu Y:
microRNA-21 overexpression contributes to cell proliferation by
targeting PTEN in endometrioid endometrial cancer. Oncol Lett.
4:1290–1296. 2012.PubMed/NCBI
|
|
9
|
Rather MI, Nagashri MN, Swamy SS, Gopinath
KS and Kumar A: Oncogenic microRNA-155 downregulates tumor
suppressor CDC73 and promotes oral squamous cell carcinoma cell
proliferation: implications for cancer therapeutics. J Biol Chem.
288:608–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Yu X, Guo X, et al: miR-143 is
downregulated in cervical cancer and promotes apoptosis and
inhibits tumor formation by targeting Bcl-2. Mol Med Rep.
5:753–760. 2012.PubMed/NCBI
|
|
11
|
Frampton AE, Krell J, Jacob J, Stebbing J,
Castellano L and Jiao LR: Loss of miR-126 is crucial to pancreatic
cancer progression. Expert Rev Anticancer Ther. 12:881–884. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Jiang L, Wang A, Yu J, Shi F and
Zhou X: MicroRNA-138 suppresses invasion and promotes apoptosis in
head and neck squamous cell carcinoma cell lines. Cancer Lett.
286:217–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Link MP: Osteosarcoma in adolescents and
young adults: new developments and controversies. Commentary on the
use of presurgical chemotherapy. Cancer Treat Res. 62:383–385.
1993.PubMed/NCBI
|
|
14
|
Jaffe N: Osteosarcoma: review of the past,
impact on the future. The American experience. Cancer Treat Res.
152:239–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferguson WS and Goorin AM: Current
treatment of osteosarcoma. Cancer Invest. 19:292–315. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Novello C, Pazzaglia L, Cingolani C, et
al: miRNA expression profile in human osteosarcoma: role of miR-1
and miR-133b in proliferation and cell cycle control. Int J Oncol.
42:667–675. 2013.PubMed/NCBI
|
|
17
|
Zhao H, Guo M, Zhao G, et al: miR-183
inhibits the metastasis of osteosarcoma via downregulation of the
expression of Ezrin in F5M2 cells. Int J Mol Med. 30:1013–1020.
2012.PubMed/NCBI
|
|
18
|
Yan K, Gao J, Yang T, et al: MicroRNA-34a
inhibits the proliferation and metastasis of osteosarcoma cells
both in vitro and in vivo. PLoS One. 7:e337782012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan L, Wu Q, Xing X, Wei Y and Shao Z:
MicroRNA-145 targets vascular endothelial growth factor and
inhibits invasion and metastasis of osteosarcoma cells. Acta
Biochim Biophys Sin. 44:407–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osaki M, Takeshita F, Sugimoto Y, et al:
MicroRNA-143 regulates human osteosarcoma metastasis by regulating
matrix metalloprotease-13 expression. Mol Ther. 19:1123–1130. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho HM, Jeon HS, Lee SY, et al:
microRNA-101 inhibits lung cancer invasion through the regulation
of enhancer of zeste homolog 2. Exp Ther Med. 2:963–967.
2011.PubMed/NCBI
|
|
22
|
Wang HJ, Ruan HJ, He XJ, et al:
MicroRNA-101 is downregulated in gastric cancer and involved in
cell migration and invasion. Eur J Cancer. 46:2295–2303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang R, Wang HB, Hao CJ, et al: MiR-101 is
involved in human breast carcinogenesis by targeting Stathmin1.
PLoS One. 7:e461732012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smits M, Nilsson J, Mir SE, et al: miR-101
is downregulated in glioblastoma resulting in EZH2-induced
proliferation, migration, and angiogenesis. Oncotarget. 1:710–720.
2010.PubMed/NCBI
|
|
25
|
He LJ, Cai MY, Xu GL, et al: Prognostic
significance of overexpression of EZH2 and H3k27me3 proteins in
gastric cancer. Asian Pac J Cancer Prev. 13:3173–3178. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ren G, Baritaki S, Marathe H, et al:
Polycomb protein EZH2 regulates tumor invasion via the
transcriptional repression of the metastasis suppressor RKIP in
breast and prostate cancer. Cancer Res. 72:3091–3104. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rao ZY, Cai MY, Yang GF, et al: EZH2
supports ovarian carcinoma cell invasion and/or metastasis via
regulation of TGF-beta1 and is a predictor of outcome in ovarian
carcinoma patients. Carcinogenesis. 31:1576–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Yang TT, Wang W, et al:
Establishment and characterization of human osteosarcoma cell lines
with different pulmonary metastatic potentials. Cytotechnology.
61:37–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Negrini M and Calin GA: Breast cancer
metastasis: a microRNA story. Breast Cancer Res. 10:2032008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watahiki A, Wang Y, Morris J, et al:
MicroRNAs associated with metastatic prostate cancer. PLoS One.
6:e249502011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wada T, Isu K, Takeda N, Usui M, Ishii S
and Yamawaki S: A preliminary report of neoadjuvant chemotherapy
NSH-7 study in osteosarcoma: preoperative salvage chemotherapy
based on clinical tumor response and the use of granulocyte
colony-stimulating factor. Oncology. 53:221–227. 1996. View Article : Google Scholar
|
|
32
|
Sauvageau M and Sauvageau G: Polycomb
group proteins: multi-faceted regulators of somatic stem cells and
cancer. Cell Stem Cell. 7:299–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Varambally S, Dhanasekaran SM, Zhou M, et
al: The polycomb group protein EZH2 is involved in progression of
prostate cancer. Nature. 419:624–629. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hussein YR, Sood AK, Bandyopadhyay S, et
al: Clinical and biological relevance of enhancer of zeste homolog
2 in triple-negative breast cancer. Hum Pathol. 43:1638–1644. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Changchien YC, Tatrai P, Papp G, et al:
Poorly differentiated synovial sarcoma is associated with high
expression of enhancer of zeste homologue 2 (EZH2). J Transl Med.
10:2162012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang H, Albadine R, Magheli A, et al:
Increased EZH2 protein expression is associated with invasive
urothelial carcinoma of the bladder. Urol Oncol. 30:428–433. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fluge Ø, Gravdal K, Carlsen E, et al:
Expression of EZH2 and Ki-67 in colorectal cancer and associations
with treatment response and prognosis. Br J Cancer. 101:1282–1289.
2009.PubMed/NCBI
|
|
38
|
Guo SQ and Zhang YZ: Overexpression of
enhancer of zests homolog 2 in lymphoma. Chin Med J. 125:3735–3739.
2012.PubMed/NCBI
|
|
39
|
Sasaki H, Setoguchi T, Matsunoshita Y, Gao
H, Hirotsu M and Komiya S: The knock-down of overexpressed EZH2 and
BMI-1 does not prevent osteosarcoma growth. Oncol Rep. 23:677–684.
2010.PubMed/NCBI
|
|
40
|
Tong ZT, Cai MY, Wang XG, et al: EZH2
supports nasopharyngeal carcinoma cell aggressiveness by forming a
co-repressor complex with HDAC1/HDAC2 and Snail to inhibit
E-cadherin. Oncogene. 31:583–594. 2012.PubMed/NCBI
|
|
41
|
Liu DC and Yang ZL: Overexpression of EZH2
and loss of expression of PTEN is associated with invasion,
metastasis, and poor progression of gallbladder adenocarcinoma.
Pathol Res Pract. 207:472–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Alford SH, Toy K, Merajver SD and Kleer
CG: Increased risk for distant metastasis in patients with familial
early-stage breast cancer and high EZH2 expression. Breast Cancer
Res Treat. 132:429–437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song QC, Shi ZB, Zhang YT, et al:
Downregulation of microRNA-26a is associated with metastatic
potential and the poor prognosis of osteosarcoma patients. Oncol
Rep. 31:1263–1270. 2014.PubMed/NCBI
|
|
44
|
Sakurai T, Bilim VN, Ugolkov AV, et al:
The enhancer of zeste homolog 2 (EZH2), a potential therapeutic
target, is regulated by miR-101 in renal cancer cells. Biochem
Biophys Res Commun. 422:607–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Varambally S, Cao Q, Mani RS, et al:
Genomic loss of microRNA-101 leads to overexpression of histone
methyltransferase EZH2 in cancer. Science. 322:1695–1699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Banerjee R, Mani RS, Russo N, et al: The
tumor suppressor gene rap1GAP is silenced by miR-101-mediated EZH2
overexpression in invasive squamous cell carcinoma. Oncogene.
30:4339–4349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Friedman JM, Liang G, Liu CC, et al: The
putative tumor suppressor microRNA-101 modulates the cancer
epigenome by repressing the polycomb group protein EZH2. Cancer
Res. 69:2623–2629. 2009. View Article : Google Scholar : PubMed/NCBI
|